| 1. | Tetrabromobisphenol A | |
| 1.1 | Identification of the substance | |
| 1.1.1 | CAS No. | 79-94-7 |
| 1.1.2 | EINECS No. | 201-236-9 |
| 1.1.3 | EINECS Name | Phenol, 4,4' (1-metholethylidene)bis[2,6-dibromo-] |
| 1.1.4 | Synonyms | Tetrabromobisphenol A
TBBPA 2,2-bis(3,5-bromo-4-hydroxyphenyl)propane 4,4'-isopropylidenebis(2,6-dibromophenol) 4,4'-(1-methylethylidene)bis(2,6-dibromophenol) Tetrabromodihydroxydiphenyl propane |
| 1.1.5 | Molecular Formula | C15H12Br4O2 |
| 1.1.6 | Structural Formula | 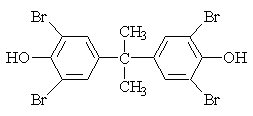 |
| 1.1.7 | Known uses | Used as flame retardant for plastics, paper, textiles and used as a plasticiser (1) |
| 1.1.8 | EU Classification | Not included in Annex I to Directive 67/548/EEC |
| 1.2 | Physico-chemical Characteristics | |
| 1.2.1 | Physical Form | Off-white powder (1) |
| 1.2.2 | Molecular Weight | 543.92 |
| 1.2.3 | Melting Point/range (°C) | 180-184(1), 181-182 (2) |
| 1.2.4 | Boiling Point/range (°C) | Approx. 316°C |
| 1.2.5 | Decomposition Temperature (°C) | No data were found |
| 1.2.6 | Vapour Pressure (Pa (°C)) | < 1 mmHg at 20 °C (2) |
| 1.2.7 | Relative Density (D420) | 2.1 g/ml (3) |
| 1.2.8 | Vapour Density (air=1) | No data were found |
| 1.2.9 | Conversion Factor (1011 hPa at 25 °C) | 1 ppm=mg/l (No data
available)
1 mg/l=ppm |
| 1.2.10 | Solubility | Water: 0.72 mg/l at 15 °C
(2)
Water: 4.16 mg/l at 25 °C (2) Water: 1.77 mg/l at 35 °C (2) Methanol: 920 mg/l at 25 °C (2) Acetone: 2400 mg/l at 25 °C (2) |
| 1.2.11 | Partition Coefficient (log P ow) | 4.5-5.3 (2) |
| 1.2.12 | Flammability | No data were found |
| 1.2.13 | Explosivity | No data were found |
| 1.2.14 | Oxidising properties | No data were found |
| 1.3 | Toxicological Data | |
| 1.3.1 | Observations in humans | No signs of sensitisation
were observed in tests with human volunteers (3, 4)
Forty plasma samples from a "random" population in Sweden were examined. Preliminary results from analyses indicated the presence of TBBPA at low ppb level in all samples (5) |
| 1.3.2 | Acute Toxicity | |
| 1.3.2.1 | Oral | Oral LD50, rats: > 5
g/kg body weight (b.w.) (2)
Oral LD50, mice: 3.2 g/kg b.w. (2) |
| 1.3.2.2 | Dermal | Dermal LD50, rabbits: > 2 g/kg b.w. (2) |
| 1.3.2.3 | Inhalation | Inhalation LC50, rats: > 0.5 mg aerosols/kg b.w./8 hours (2) |
| 1.3.2.4 | Other Routes | |
| 1.3.2.5 | Skin Irritation | TBBPA was tested in several skin irritation assays in rats and rabbits and did not reveal skin irritation (2) |
| 1.3.2.6 | Eye Irritation | TBBPA is not considered to be eye irritating Eye irritation assays in rabbits occasionally showed mild irritation of the conjunctiva, and TBBPA (2). |
| 1.3.2 7 | Irritation of Respiratory Tract | Groups of rats were exposed to an atmosphere containing 2, 6 or 18 mg micronised TBBPA/litre air, 4 hours/day, 5 days/week for 2 weeks. Some or all animals in the two highest dose groups had excessive salivation, lacrimation and nasal discharge, which indicated some irritation of conjunctiva and the mucous membranes of the upper respi-ratory tract (2). |
| 1.3.2.8 | Skin Sensitisation | TBBPA was not a skin sensitiser in two guinea pig sensitisation tests (2). |
| 1.3.2.9 | Sensitisation by Inhalation | No data were available |
| 1.3.3 | Subchronic Toxicity | |
| 1.3.3.1 | Oral | 1) Charles River CD rats
(25 rats/sex/dose level) were exposed to TBBPA in the diet (0, 1,
10, 100, or 1,000 mg/kg diet (ppm, ~ 0, 0.05, 0.5, 5 or 50 mg/kg b.w./day))
for 28 days. Bromine contents of the liver and in fat were higher in
the 1,000 ppm group as compared with the controls, but not
statistically significant. The no observed adverse effect level (NOAEL)
was 50 mg/kg b.w./day (2).
2) Sprague-Dawley rats were exposed to TBBPA for 90 days in the diet corresponding to the following doses: 0 (21 males + 21 females), 0.3 (7 males + 7 females), 3 (21 males + 21 females), 30 (7 males + 7 females) and 100 (7 males + 7 females) mg/kg b.w./day. The NOAEL was 100 mg/kg b.w./day. (2, 6). 3) In a Japanese study B6C3F1 mice (10 mice/sex/group) were fed TBBPA in the diet at 0, 500, 4,900, 15,600 or 50,000 mg/kg diet (ppm, ~ 0, 71, 700, 2,200, or 7,100 mg/kg b.w.). All mice died at the highest dose level, proba-bly because of malnutrition and anaemia. No deaths were observed at lower doses. At 15,600 mg/kg the following signs were noted: decreased weight gain (food intake was not affected), decreased number of red blood cells, decreased haemoglobin, haematocrit, serum triglycerides, and total serum proteins. The NOAEL was 700 mg/kg b.w./day (2) |
| 1.3.3.2 | Inhalation | Groups of rats were exposed to an atmosphere containing 2, 6 or 18 mg micronised TBBPA/litre air, 4 hours/day, 5 days/week for 2 weeks. As mentioned before, some or all animals in the two highest dose groups had excessive salivation, lacrimation and nasal discharge. A decrease in the relative liver weight of the female rats from the three dose levels might have been compound related; but otherwise, no changes in weight gain, food consumption, haematological, biochemical, or urine analytical parameters, gross or microscopic observations were noted. The no observed adverse effect concentration (NOAEC) was 2mg/l (2). |
| 1.3.3.3 | Dermal | Groups of 4 rabbits/sex/dose level were exposed to 0, 100, 500, or 2,500 mg TBBPA/kg b.w. for 6 hours/day, 5 days/week, and for 3 weeks. The exposed skin area of one-half of the rabbits in a group was abraded twice each week. No animals died, and no overt toxicity or unusual behaviour was observed. No changes in haematology, biochemistry, urinalysis or in body weight gain were noted. Very light erythema was occasionally seen in the 100 mg/kg dose group and in every animal in the 500 and 2,500 mg/kg dose groups (2). |
| 1.3.4 | Chronic Toxicity and Carcinogenicity | No data were available |
| 1.3.5 | Mutagenicity | |
| 1.3.5.1 | Gene Mutation | TBBPA was tested in several in vitro gene mutation assays using Salmonella typhimurium or Saccharomyces cerevisiae, with or without metabolic activation. All tests were negative (2). |
| 1.3.5.2 | Chromosome Abnormalities | No data were available |
| 1.3.5.3 | Other Genotoxic Effects | TBBPA was tested in two recently developed in vitro assays for intragenic recombination in mammalian cells, the Sp5/V79 recombination assay and the SPD8 recombination assay. TBBPA did not induce statistically significant increases in recombination frequencies in the Sp5 or SPD8 assay system (7) |
| 1.3.6 | Reproductive Toxicity, Embryotoxicity, and Teratogenicity | |
| 1.3.6.1 | Reproductive Toxicity | No data were available |
| 1.3.6.2 | Teratogenicity | TBBPA was administrated, by gavage, at dose levels of 0, 30, 100, 300, 1000, 3000, or 10,000 mg/kg b.w., on gestation days 6-15, to groups of 5 Charles River CD female rats. They were sacrificed on day 20. Three out of 5 dams given 10,000 mg/kg b.w. died, and the two other rats of the group showed slightly reduced weight gain, green, soft faeces, and an increase in matted hair in the anogenital area. There were no embryotoxic or teratogenic effects. The maternal NOAEL was 3,000 mg/kg b.w., and the foetal NOAEL was 10,000 mg/kg b.w. (2). |
| 1.3.7 | Other Toxicity Studies | No data were available |
| 1.3.8 | Toxicokinetics | TBBPA is very soluble in
fat and was previously thought to be poorly absorbed by the
gastro-intestinal tract (see below). The uptake by skin and lungs is
not known. The following tissue half-lives have been reported after
a single oral dose of radioactive labelled TBBPA to rats: 19.9 hours
(h) in blood, 70.8 h in fat, 17.1 h in kidneys, 10.8 h in liver,
48.0 in muscle and 60.5 h in gonads (2)
A single oral dose of 2 mg 14C-ring labelled TBBPA/kg b.w. was administered to male Sprague-Dawley rats. Some of the rats were bile duct cannulated.48% and 71% of the radioactivity was excreted in bile 24 and 72 hours after the treatment, respectively. In the conventional, 95% was eliminated via faeces within 72 hours, and most of the faecal excretion occurred in the 24-48 hour period, indicating an extensive gastrointestinal absorption and enterohepatic circulation. The biliary metabolites characterised were various glucuronide conjugates. About 2% of the radioactivity remained in the rat 72 hours after dosing with the highest levels in the large (1.0%) and small (0.6%) intestine, lung (0.2%) and carcass (0.2%). No residues were found in fat tissue (8). |
| 1.4 | Ecotoxicity | TBBPA is very toxic to
aquatic organisms. EC/LC50 is below 1 mg/l: Fish (96h) LC50=0.4
mg/l; Daphnia (48h) LC50=0.96 mg/l; Algae (72h) EC50= 0.09
mg/l (10).
NOEC (96h) to fish was 0.1 mg/l for Lepomis macrochirus, 0.18 mg/l for Salmo gairdneri and 0.26 mg/l for Pimephales promelas (10). Bioaccumulation studies with aquatic invertebrates and vertebrates indicate bioconcentration factors ranging from 20 to 3200 (2). The bioconcentration in fish given as BCF was 1200 for Pimephales promelas (10). |
| 1.5 | Environmental Fate | Biodegradation was 0% after 14 days (BOD) activated sludge. The biodegradation in soil (time not indicated) was 36-82% and 44-91% in anaerobic soil (highest in clay loam and lowest in sandy loam soil) (2,10). |
| 1.6 | Environmental Concentrations | TBBPA was detected in
sediments in Japan and Sweden (µg/kg levels) (2). TBBPA residue
level in a sediment from Japan was about 20 µg/kg (ppb) on dry
weight basis by GC determination (9).
The TBBPA dimethyl ether could be identified in mussels and sediment. (2). The residue level of the TBBPA di-methyl ether was 5 µg/kg (w.w) in mussels, Japan (9). |
| 1.7 | Conclusion | |
| 1.7.1 | Health Assessment | Sufficient toxicological
data were identified for a health assessment of TBBPA. Most of the
data are found in reviews, and many tests have probably not been
performed according to internationally accepted guidelines. No data
on chronic toxicity, carcinogenicity or reproductive toxicity in
multi-generation studies were identified. No chromosome aberration
tests or any other mutagenicity tests except the gene mutation tests
were found.
The available data lead to the conclusion that TBBPA seems to be only slightly acute toxic after oral, dermal or inhalation exposure. TBBPA has slightly irritating effects to eyes, skin and respiratory tract, and was not sensitising when tested on guinea pigs and human volunteers. TBBPA was not mutagenic when tested in in vitro gene mutation tests. The tests for subchronic toxicity and teratogenicity did not show any indications of possible danger or risks of irreversible health effects by prolonged exposure. There may be a risk of cumulative effects, because of the long half-life of TBBPA in some body tissues. |
| 1.7.2 | Environmental Assessment | Based on the available information about TBBPA (it is considered not readily biodegradable, log Pow is 4.5-5.3 and BCF is in the range 20-3200 and L(E)C50 for aquatic organisms < 1 mg/l) the substance is considered to be toxic to aquatic organisms, and it may cause long-term adverse effects in the aquatic environment. |
| 1.8 | References | 1. Hazardous Substances
Data Bank (HSDB) (through July 1998). CHEMBANK version. Published by
the National Library of Medicine (NLM), USA. HSDB Accession No.:
5232. Update Code: 9705.
2. WHO working group. International Programme on Chemical Safety. Tetrabromobisphenol A and derivatives. Environmental Health Criteria 1995; 172: 23-64. 3. Laboratory report on DRL-100 and tetrabromobisphenol A. Haskell Laboratory, DuPont. SEP 1972. EPA/OTS no. 86-910000399S (NTIS/OTS no. 0530158). 4.Modified Draize multiple insult test in humans: tetrabromobisphenol A. International Research and Development Corporation, AUG 1978. EPA/OTS no. 878216113 (NTIS/OTS no. 0206828). 5. Klasson Wehler E, Hovander L, Bergman Å. New organohalogens in human plasma - Identification and quantification. Organohalogen Compounds 1997; 33:420-5. 6. Results of a 90-day toxicological study in rats given tetrabromobisphenol A in the diet. Toxicological Research Laboratory, Dow Chemical Company, JUL 1975. EPA/OTS no. 878216066 (NTIS/OTS no. 0206824). 7. Helleday T, Tuominen K-L, Bergman Å, Jenssen D. Brominated flame retardants induce intragenic recombination in mammalian cells. Mutation Research - Genetic Toxicology and Environmental Mutagenesis 1999; 439(2):137-47. 8. Larsen GL, Hakk H, Klasson Wehler E, Örn U, Bergman Å. Metabolism and disposition of the flame retardant tetrabromobisphenol A in conventional rats and rats with cannulated bile ducts. Organohalogen Compounds 1998; 37:413-6. 9. Watanabe, I.; T. Kashimoto; R. Tatsukawa. Identification of the Flame Retardant Tetrabromobisphenol-A in the river Sediment and mussel Collected in Osaka. Bull. Environ.Contam.Toxicol.31, 48-52 (1983). 10. International Uniform Chemical Information Database (IUCLID). Edition 1. Existing Chemicals - 1996. European Commission Joint Research Centre. Environment Institute. European Chemicals Bureau. IUCLID Datasheet. 08-FEB-96. CAS-No.: 79-94-7. |