| 2. | Pentabromotoluene | |
| 2.1 | Identification of the substance | |
| 2.1.1 | CAS No. | 87-83-2 |
| 2.1.2 | EINECS No. | 201-774-4 |
| 2.1.3 | EINECS Name | 2,3,4,5,6-Pentabromotoluene |
| 2.1.4 | Synonyms | Pentabromomethylbenzene
Pentabromotoluene |
| 2.1.5 | Molecular Formula | C7H3Br5 |
| 2.1.6 | Structural Formula | 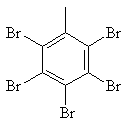 |
| 2.1.7 | Known uses | Used in the formulation of glass reinforced unsaturated polyester compounds used in electrical applications as flame retardant bulk moulding compounds (6) |
| 2.1.8 | EU Classification | Not included in Annex I to Directive 67/548/EEC |
| 2.2 | Physico-chemical Characteristics | |
| 2.2.1 | Physical Form | Colourless powder |
| 2.2.2 | Molecular Weight | 486.62 |
| 2.2.3 | Melting Point/range (°C) | 299 (6)
288 (5) |
| 2.2.4 | Boiling Point/range (°C) | No data were available |
| 2.2.5 | Decomposition Temperature (°C) | No data were available |
| 2.2.6 | Vapour Pressure (Pa (°C)) | No data were available |
| 2.2.7 | Relative Density (D420) | 3.15 at 20 ºC (6) |
| 2.2.8 | Vapour Density (air=1) | No data were available |
| 2.2.9 | Conversion Factor (1011 hPa at 25 °C) | No data were available |
| 2.2.10 | Solubility | Water: Insoluble (6)
Ethanol: Slightly soluble (5) Benzene: Soluble (5) |
| 2.2.11 | Partition Coefficient (log P ow) | 5.43 (3) |
| 2.2.12 | Flammability | Flash point: 280-282 ºF (6) |
| 2.2.13 | Explosivity | No data were available |
| 2.2.14 | Oxidising properties | No data were available |
| 2.3 | Toxicological Data | |
| 2.3.1 | Observations in humans | No data were available |
| 2.3.2 | Acute Toxicity | |
| 2.3.2.1 | Oral | No data were available |
| 2.3.2.2 | Dermal | No data were available |
| 2.3.2.3 | Inhalation | No data were available |
| 2.3.2.4 | Other Routes | No data were available |
| 2.3.2.5 | Skin Irritation | No data were available |
| 2.3.2.6 | Eye Irritation | No data were available |
| 2.3.2 7 | Irritation of Respiratory Tract | No data were available |
| 2.3.2.8 | Skin Sensitisation | No data were available |
| 2.3.2.9 | Sensitisation by Inhalation | No data were available |
| 2.3.3 | Subchronic Toxicity | |
| 2.3.3.1 | Oral | Sprague-Dawley rats (15 rats/sex/dose level) were ex-posed to 5BT in the diet (0, 0.05, 0.5, 5.0, 50.0 or 500.0 mg/kg diet (~ 0, 0.003-0.004, 0.03-0.04, 0.35-0.4, 3.5-4.0 or 34-40 mg/kg b.w./day)) for 91 days. No clinical signs of toxicity was observed, and growth rate and food consumption was not affected. 5BT caused no dramatic changes in biochemistry, haematology and gross pathology. Mild dose-dependent histological changes were observed in the thyroid, liver, and kidney of rats fed 5BT diets. The no observed adverse effect level (NOAEL) was 5.0 mg/kg diet (~ 0.35 mg/kg b.w./day) (4). |
| 2.3.3.2 | Inhalation | No data were available |
| 2.3.3.3 | Dermal | No data were available |
| 2.3.4 | Chronic Toxicity and Carcinogenicity | No data were available |
| 2.3.5 | Mutagenicity | |
| 2.3.5.1 | Gene Mutation | 5BT was tested for mutagenicity in the Salmonella/microsome preincubation assay using a protocol approved by the National Toxicology Program. A wide range of doses (0, 100, 333, 1000, 3333, and 10,000 mg/plate) was tested in four Salmonella typhimurium strains (TA98, TA100, TA1535, and TA1537) in the presence and absence of Aroclor-induced rat or hamster liver S9. These tests were negative and the highest ineffective dose level tested (not causing the formation of a precipitate) in any Salmonella tester strain was 100 mg/plate (1, 2). |
| 2.3.5.2 | Chromosome Abnormalities | No data were available |
| 2.3.5.3 | Other Genotoxic Effects | No data were available |
| 2.3.6 | Reproductive Toxicity, Embryotoxicity, and Teratogenicity | |
| 2.3.6.1 | Reproductive Toxicity | No data were available |
| 2.3.6.2 | Teratogenicity | According to an abstract by Ruddick et al. published in Teratology in 1984, no adverse foetal effects were observed when doses up to 600 mg/kg b.w. were given orally to rats during organogenesis (7) |
| 2.3.7 | Other Toxicity Studies | No data were available |
| 2.3.8 | Toxicokinetics | No data were available |
| 2.4 | Ecotoxicity | The LC50 for fish was >
5 mg/l (48h).
The bioconcentration factor was 4.5-39. Log Pow=5.43 (3) |
| 2.5 | Environmental Fate | 5BT is not readily biodegradable (7% of BOD, 4w, 100mg/l substance, 30 mg/l sludge) (3) |
| 2.6 | Environmental Concentrations | No data were available. |
| 2.7 | Conclusion | |
| 2.7.1 | Health Assessment | Only few toxicological data were identified making an adequate health assessment of 5BT impossible.5BT was not mutagenic when tested in in vitro gene mutation tests. The tests for subchronic toxicity and teratogenicity did not show any indications of possible danger or risks of irreversible health effects by pro-longed exposure. |
| 2.7.2 | Environmental Assessment | The features log Pow>3, LC50 >5 mg/l and not readily biodegradable indicate that 5BT may be able to cause long-term adverse effects in the aquatic environment. |
| 2.8 | References | 1. Hazardous Substances
Data Bank (HSDB). 2,3,4,5,6-Pentabromotoluene. Update Code: 9806. U.
S. National Library of Medicine (NLM); 1998. HSDB Accession No.:
5253. SilverPlatter Information. CHEM-BANK (November 1998).
SP-018-047.
2. Chemical Carcinogenesis Research Information System (CCRIS). Pentabromotoluene. Last revision date 930910. U. S. National Library of Medicine (NLM); 1998. CCRIS Number: 4854. Deutsches Institut für Medizinische Dokumentation und Information (DIMDI). 3. Chemical Inspection and Testing Institute, Bureau Ministry of International trade and industry Japan (MITI). Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan, compiled under the supervision of chemical products safety Division Basic industries. Japan: Japanese Chemical Industry Ecology-Toxicology & Information Center, 1992. 4. Chu I, Villeneuve DC, McDonald B, Secours VE, Valli VE. Pentachlorotoluene and pentabromotoluene: results of a subacute and a subchronic toxicity study in the rat. Journal of Environmental Science and Health B 1987; 22(3):303-17. 5. Lide DR, Frederikse HPR Editors. CRC Handbook of Chemistry and Physics. 78th edition. Boca Raton, New York: CRC Press, Inc., 1997: 3-58. 6. Organisation for Economic Co-Operation and Development (OECD). Selected Brominated Flame Retardants. Background and National Experience with Reducing Risk. OECD Environment Monograph Series No. 102. Risk Reduction monograph no. 3 edition. Paris: OECD, 1994. 7. Shepard TH. Catalogue of Teratogenic Agents. 8th edition. Baltimore and London: The John Hopkins University Press, 1995: 330. |