| 3. | 2,4,6-Tribromophenol | |||||||||
| 3.1 | Identification of the substance | |||||||||
| 3.1.1 | CAS No. | 118-79-6 | ||||||||
| 3.1.2 | EINECS No. | 204-278-6 | ||||||||
| 3.1.3 | EINECS Name | 2,4,6-Tribromophenol | ||||||||
| 3.1.4 | Synonyms | Bromol | ||||||||
| 3.1.5 | Molecular Formula | C6H3Br3O | ||||||||
| 3.1.6 | Structural Formula | 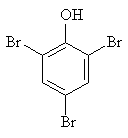 |
||||||||
| 3.1.7 | Known uses | Antiseptic & germicide (e.g., in pharmaceutical prepns). Flame retardant in thermoplastic polyester & epoxy resins, in acrylonitrile-butadiene-styrene resins, in phenolic resins & polystyrene. Chemical intermediate for its bismuth salt (antiseptic), for pentachlorophenol and for 2,4,6-tribromophenoxy compounds (19). | ||||||||
| 3.1.8 | EU Classification | Not included in Annex I to Directive 67/548/EEC | ||||||||
| 3.2 | Physico-chemical Characteristics | |||||||||
| 3.2.1 | Physical Form | Long crystals (20). Long crystals or needles from ethanol, and prisms from benzene (22). The odour is penetrating bromine, and the taste is sweet (19). | ||||||||
| 3.2.2 | Molecular Weight | 330.80 | ||||||||
| 3.2.3 | Melting Point/range (°C) | 87-89 (22)
94-96 (20) 95.5 (23) Sublimes at 95°C (22) |
||||||||
| 3.2.4 | Boiling Point/range (°C) | 244 (20)
282-290 (26) 286 (23) Sublimes at 282-290°C, 764 mmHg (19) |
||||||||
| 3.2.5 | Decomposition Temperature (°C) | When heated to decomposition it emits toxic fumes of Br- (22). | ||||||||
| 3.2.6 | Vapour Pressure (Pa (°C)) | No data were available | ||||||||
| 3.2.7 | Relative Density (D420) | No data were available | ||||||||
| 3.2.8 | Vapour Density (air=1) | 11.4 (26) | ||||||||
| 3.2.9 | Conversion Factor (1011 hPa at 25 °C) | No data were available | ||||||||
| 3.2.10 | Solubility | Water:
Slightly soluble (23)
Water: 71 mg/l at 15°C (26) Water: 996 ppm (15°C), 969 ppm (25°C), and 884 (35°C) (18) Ethanol: Very soluble (23) Ether: Soluble (23) Benzene: Soluble (23) |
||||||||
| 3.2.11 | Partition Coefficient (log P ow) | 4.020 (26)
3.3 (18) |
||||||||
| 3.2.12 | Flammability | No data were available | ||||||||
| 3.2.13 | Explosivity | No data were available | ||||||||
| 3.2.14 | Oxidising properties | No data were available | ||||||||
| 3.3 | Toxicological Data | |||||||||
| 3.3.1 | Observations in humans | No data were available | ||||||||
| 3.3.2 | Acute Toxicity | |||||||||
| 3.3.2.1 | Oral | Oral LD50, male rats: 1,995
(1,728-2,304) mg/kg body weight (b.w.) Oral LD50, female rats: 1,819
(1,513-2,187) mg/kg b.w. (3, 7).
Oral LD50, male rats: 500 - 5,000 mg/kg b.w. None of the rats (5) died after the administration of 500 mg/kg b.w., and all five rats died at the 5,000 mg/kg dosage level (8). Oral LD50, male rats: 5,012 (4,034-6,227) mg/kg b.w. Oral LD50, female rats: 5,012 (3,863-6,503) mg/kg b.w. Signs of toxicity included decreased motor activity, nasal discharge, lacrimation, decreased motor activity, tremors, prostration, clonic convulsions and death (6). |
||||||||
| 3.3.2.2 | Dermal | Dermal LD50, rabbits:
>2,000 mg/kg b.w.(8)
Dermal LD50, rabbits: >8,000 mg/kg b.w. All four rabbits appeared normal during the 24 hours exposure to 8 g/kg b.w. (occluded) and the 14 days observation period (4). |
||||||||
| 3.3.2.3 | Inhalation | Dust inhalation LC50, rats:
>1.63 mg/l/4 hours. 65% of the particles were less than 6 microns
(16) (IBT)
Dust inhalation LC50, rats: >200 mg/l/1 hour; 10 rats were exposed in a whole-body-exposure chamber to atmospheric dust concentrations of 2 or 200 mg/l. Signs at both concentrations included nasal discharge, eye squint, increased followed by decreased respiratory rates, prostration, salivation, lacrimation, erythema, increased followed by decreased motor activity, and ocular and nasal porphyrin discharge. No details were available about f.ex. particle size distribution. All rats appeared normal from day 7 post exposure, except on day 10 of the 14 day observation period when one rat at low exposure level exhibited nasal porphyrin discharge (8) Dust inhalation LC50, rats: >50 mg/l/4 hours; 10 rats were exposed in a whole-body-exposure chamber to atmospheric dust concentrations of 50 mg/l for 4 hours. Signs included eye squint, slight dyspnoea, erythema, decreased motor activity, ocular porphyrin discharge, nasal discharge and diarrhoea. From 5 days to 14 days post exposure several rats continued to show diarrhoea and nasal discharge. Particle size distribution was not measured (5). |
||||||||
| 3.3.2.4 | Other Routes | No data were available | ||||||||
| 3.3.2.5 | Skin Irritation | Grading of dermal reactions
(mean of the 24 and 72 hours examinations/max. score) (8, 15):
|
||||||||
| 3.3.2.6 | Eye Irritation | Grading of ocular lesions
(mean of the 24, 48 and 72 hours examinations/max. score) (8,
11):
The effects appeared reversible, but at the end of the observation period of 7 days, 3 out of 6 rabbits had slight or very slight conjunctival redness. The 4/6 worst affected rabbits had a conjunctival redness score of 2.08/3. |
||||||||
| 3.3.2 7 | Irritation of Respiratory Tract | Irritating to mucous membranes (26) | ||||||||
| 3.3.2.8 | Skin Sensitisation | Eight guinea pigs were induced by 10 intradermal injections with 0.05-0.10 ml 0.1% 2,4,6-tribromophenol in salt water. A group of 4 guinea pigs received 2,4-dinitro-1-chlorobenzene as a positive control. Two weeks after the 10th sensitising dose a challenge dose of 0.05 ml 0.1% 2,4,6-tribromophenol in salt water was given to all animals. The reactions were read 24 and 48 hours after the challenge. Four of the eight guinea pigs responded to the challenge dose, exhibiting a flare response slightly greater than that obtained in the sensitising doses. Two of the four animals in the control group exhibited a flare response slightly greater than that obtained in the sensitising doses. The vehicle was negative. The test compound was considered positive under the conditions of this preliminary test (10) | ||||||||
| 3.3.2.9 | Sensitisation by Inhalation | No data were available | ||||||||
| 3.3.3 | Subchronic Toxicity | |||||||||
| 3.3.3.1 | Oral | No data were available | ||||||||
| 3.3.3.2 | Inhalation | Three groups of rats each
consisting of 5 males and 5 females were exposed (whole-body) to
atmospheric dust concentrations (analytical) of 0, 0.10 and 0.92
mg/l, respectively, for 6 hours/day, 5 days/week, for 3 weeks. The
particle size distribution was not measured.
Two high-dose animals died after 10-11 exposures. The clinical signs in both treatment groups included hypoactivity, salivation, lacrimation and red nasal discharge. Reduced weight gain was observed in low and high dose females and in high dose males. No test material related changes in haematology, clinical chemistry or urinalysis were observed. Gross and histopathological changes were observed in liver and kidneys of high dose rats. The NOAEL in this study appears to be <0.10 mg/l for females and 0.10 mg/l for males (16). (IBT) |
||||||||
| 3.3.3.3 | Dermal | A 28-day subacute dermal toxicity study was con-ducted with 2,4,6-tribromophenol in albino rabbits. The test material was applied to the clipped, unoccluded skin as a 50% suspension in aqueous methyl cellulose. This test suspension was applied 5 days/week for a period of 4 weeks at dose levels of 0, 100, 300 and 1,000 mg/kg/day to 4 rabbits/sex/dose level. The test skin sites of 2 rabbits/sex/dose level were abraded. The only treatment related effects ob-served were local skin reactions of all animals. One high dose animal died of unknown reasons. (1, 2) and (9, 17) (IBT) | ||||||||
| 3.3.4 | Chronic Toxicity and Carcinogenicity | No data were available | ||||||||
| 3.3.5 | Mutagenicity | |||||||||
| 3.3.5.1 | Gene Mutation | 2,4,6-Tribromophenol was
tested for mutagenicity in the Salmonella/microsome preincubation
assay using a protocol approved by the National Toxicology Program.
A wide range of doses (0, 3, 10, 33, 100, and 333 mg/plate) was
tested in four Salmonella typhimurium strains (TA98, TA100,
TA1535, and TA1537) in the presence and absence of Aroclor-induced
rat or hamster liver S9. These tests were negative and the highest
ineffective dose level tested (not causing a slight clearing of the
background lawn) in any Salmonella tester strain was 100 mg/plate
(19).
2,4,6-Tribromophenol was tested for mutagenicity in Salmonella typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, and in Saccharomyces cerevisiae strain D4 in the presence and absence a metabolic activation system. 2,4,6-Tribromophenol was negative in all test assays. (12) |
||||||||
| 3.3.5.2 | Chromosome Abnormalities | No data were available | ||||||||
| 3.3.5.3 | Other Genotoxic Effects | No data were available | ||||||||
| 3.3.6 | Reproductive Toxicity, Embryotoxicity, and Teratogenicity | |||||||||
| 3.3.6.1 | Reproductive Toxicity | No data were available | ||||||||
| 3.3.6.2 | Teratogenicity | In a pilot study, mated
Charles River CD female rats were dosed with 2,4,6-tribromophenol by
gavage at 0, 10, 30, 100, 300, 1,000 and 3,000 mg/kg/day from
gestation day 6 through day 15. The study was performed to determine
dosage levels for a teratology study. All animals died at the
highest dose group after one day of treatment. There were slight
decreases in body weight gains between days 6 and 12, an increase in
post implantation losses, and a slight decrease in the number of
viable foetuses at the 1,000 mg/kg/day dose group. The NOAEL appears
to have been 300 mg/kg/day for both dams and foetuses (embryotoxicity)
(14).
In order to investigate the developmental neurotoxicity and immunotoxicity, pregnant Wistar rats were exposed to 2,4,6-tribromophenol by inhalation in a whole body exposure chamber (0, 0.03, 0.1, 0.3 and 1.0 mg/m3, 24 hours/day, and 7 days/week, from day 1 to 21 of gestation. The results suggested that 2,4,6-tribromophenol during this exposure regime may be a developmental neurotoxicant, embryotoxicant and foetotoxicant but not immunotoxicant. The NOAEL for developmental neurotoxicity could not be established (<0.03 mg/m3), and the NOAEL for maternal neurotoxicity was 0.3 mg/m3 (24). |
||||||||
| 3.3.7 | Other Toxicity Studies | No data were available | ||||||||
| 3.3.8 | Toxicokinetics | A single oral dose of
14C-labeled 2,4,6-tribromophenol (4.0-5.3 mg/kg b.w.) was rapidly
absorbed in rats. 48 hours after the administration, 77% of the
radioactivity was excreted via the urine and 2-14% via faeces, and
detectable radioactivity was measured in kidneys, lungs and liver.
Blood concentrations peaked 1 hour after dosing at 4.57 ppm and then
plunged to 0.002 ppm by 24 hours (half-life in blood: 2.03 hours).
The pharmacokinetics appeared to follow a one-compartment open
model. 2,4,6-tribromophenol was rapidly distributed in the body, and
the elimination in the urine was proportional to the concentration
in the blood (13).
May be absorbed dermally (22). |
||||||||
| 3.4 | Ecotoxicity | For tribromophenol the LC50 (fish) was 6.5-6.8 mg/l (96h, Pimephales promelas) (25). LC50 1.1 mg/l (96h, fathead minnow, flow through bioassay) (26) | ||||||||
| 3.5 | Environmental Fate | Tribromophenol is not readily biodegradable (49% of BOD, 4w, 100 mg/l substance, 30 mg/l sludge) (21) | ||||||||
| 3.6 | Environmental Concentrations | 2,4,6-tribromophenol was measured in wild-harvested prawns, and ocean fish, Eastern Australia. The levels ranged from 0.1 to 230 ng/g in fish (gut, flesh) and 0.12-460 ng/g in prawns (head, tail) (27, 28). | ||||||||
| 3.7 | Conclusion | |||||||||
| 3.7.1 | Health Assessment | Sufficient toxicological
data were identified for a health assessment of
2,4,6-tribromophenol. Most of the data were found in documents
submitted to the U.S. EPA. These documents contain study reports of
tests performed in the late nineteen seventies, not according
presently accepted international guidelines and Good Laboratory
Practice. A few of the tests were conducted by Industrial Bio-Test
Laboratories, a concern later found to have submitted many flawed or
fraudulent reports on its procedures and results. No data on chronic
toxicity, carcinogenicity or reproductive toxicity in
multi-generation studies were identified. No chromosome aberration
tests or any other mutagenicity tests except the gene mutation tests
were found. No data on humans were identified.
Although the results may sometimes be conflicting, the available data lead to the conclusion that 2,4,6-tribromophenol seems to be only slightly acute toxic after oral, dermal or inhalation exposure. 2,4,6-tribromophenol is slightly skin irritating and moderately eye irritating. Based on a preliminary test, it may have a skin sensitising potential. 2,4,6-Tribromophenol was not mutagenic when tested in in vitro gene mutation tests. The tests for subchronic inhalation and dermal toxicity did not reveal any indications of possible danger or risks of irreversible health effects by prolonged exposure. A preliminary study indicated that 2,4,6-tribromophenol might be embryotoxic at oral dosage levels causing only a slight decrease in maternal weight gain during the gestational exposure period. A study published in 1998 suggested that inhalation of 2,4,6-tribromophenol may cause specific developmental neurotoxicity, embryotoxicity and foetotoxicity. Despite a high n-octanol-water partition coefficient (3.3-4.0), toxicokinetic studies did not indicate a cumulative or persistent effect of 2,4,6-tribromophenol in mammalian systems. |
||||||||
| 3.7.2 | Environmental Assessment | The features 1 mg/l < LC50< 10 mg/l, Log Pow>3 and not readily biodegradable indicate that tribromophenol may be considered to be toxic to aquatic organisms and may also be able to cause long-term adverse effects in the aquatic environment. | ||||||||
| 3.8 | References | 1. 28 Day subacute dermal
toxicity study with 2 4 6 tribromo-phenol in albino rabbits. EPA/OTS;
Doc #88-7700024 1977. NTIS/OTS0200423.
2. 28-Day subacute dermal toxicity study with 2,4,6-tribromophenol in albino rabbits with attachments and cover letter dated 011978. EPA/OTS; Doc #88-7800069 1978. NTIS/OTS0200055. 3. Acute oral toxicity (ld50) study in rats with cover letter and attachment. EPA/OTS; Doc #88-7800198 1978. NTIS/OTS0200382. 4. Acute dermal toxicity in male and female albino rabbits with test data and cover letter dated 03-08-90. EPA/OTS; Doc #86-900000306 1990. NTIS/OTS0523298. 5. Acute inhalation toxicity in the albino rat with test data and cover letter. EPA/OTS; Doc #86-900000305 1990. NTIS/OTS0523297. 6. Acute oral toxicity ld50 study in albino rats with test data and cover letter dated 03-08-90. EPA/OTS; Doc #86-900000307 1990. NTIS/OTS0523299. 7. Acute oral toxicity (ld50) study in rats with test data and cover letter dated 03-08-90. EPA/OTS; Doc #86-900000318 1990. NTIS/OTS0523310. 8. Acute toxicity studies in rats and rabbits with test data and cover letter dated 03-08-90. EPA/OTS; Doc #86-900000302 1990. NTIS/OTS0523294. 9. Addendum report to Michigan chemical company 28-day subacute dermal toxicity study with 2,4,6-tribromophenol in albino rabbits with test data and cover letter. EPA/OTS; Doc #86-900000315 1990. NTIS/OTS0523307. 10. Dermal sensitization study in the albino guinea pig with test data and cover letter. EPA/OTS; Doc #86-900000308 1990. NTIS/OTS0523300. 11. Eye irritation study in albino rabbits with test data and cover letter. EPA/OTS; Doc #86-900000304 1990. NTIS/OTS0523296. 12. Mutagenicity evaluation of 2,4,6-tribromophenol lot #3287 in the Ames salmonella/microsome plate test (final) with test data and cover letter. EPA/OTS; Doc #86-900000317 1990. NTIS/OTS0523309. 13. Pharmacokinetic study of 2,4,6-tribromophenol in rats with test data and cover letter. EPA/OTS; Doc #86-900000322 1990. NTIS/OTS0523314. 14. Pilot teratology study in rats with test data and cover letter. EPA/OTS; Doc #86-900000316 1990. NTIS/OTS0523308. 15. Primary skin irritation study in albino rabbits with test data and cover letter. EPA/OTS; Doc #86-900000303 1990. NTIS/OTS0523295. 16. Report to Michigan Chemical Company 21-day subacute dust inhalation toxicity study with 2,4,6-tribromophenol in albino rats with test data and cover letters. EPA/OTS; Doc #86-900000313 1990. NTIS/OTS0523305. 17. Report to Michigan Chemical Company 28-day subacute dermal toxicity study with 2,4,6-tribromophenol in albino rabbits with test data and cover letter. EPA/OTS; Doc #86-900000314 1990. NTIS/OTS0523306. 18. Voluntary submissions from great lakes chemical corporation regarding brominated flame retardants with memo dated 041890. EPA/OTS; Doc #FYI-OTS-0490-0756 1990. NTIS/OTS0000756. 19. Hazardous Substances Data Bank (HSDB). 2,4,6-Tribromophenol. Update Code: 9806. U. S. National Library of Medicine (NLM); 1998. HSDB Accession No.: 5584. SilverPlatter Information. CHEM-BANK (November 1998). SP-018-047. 20. Budavari S, O'Neil MJ, Smith A, Heckelman PE, Kinneary JF Editors. The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 12th edition. Whitehouse Station, N. J., U.S.A.: Merck Research Laboratories. Division of Merck & Co., Inc., 1996. 21. Chemical Inspection and Testing Institute, Bureau Ministry of International trade and industry Japan (MITI). Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan, compiled under the supervision of chemical products safety Division Basic industries. Japan: Japanese Chemical Industry Ecology-Toxicology & Information Center, 1992. 22. Lewis RJ Editor. Sax's Dangerous Properties of Industrial Materials. 9th edition. New York: Van Nostrand Reinhold, 1996. 23. Lide DR, Frederikse HPR Editors. CRC Handbook of Chemistry and Physics. 78th edition. Boca Raton, New York: CRC Press, Inc., 1997: 3-58. 24. Lyubimov AV, Babin VV, Kartashov AI. Developmental neurotoxicity and immunotoxicity of 2,4,6-tribromophenol in Wistar rats. Neurotoxicology 1998; 19(2):303-12. 25. Nikunen E, Leinonen R, Kultamaa A. Environmental Properties of Chemicals. Research report 91 edition. Finland: Ministry of the Environment, Environmental Protection Department, 1990. 26. Richardson ML, Gangolli S. The Dictionary of Substances and their Effects. Cambridge: The Royal Society of Chemistry, 1994: 521-3. 27. Whitfield FB, Helidoniotis F, Shaw KJ, Svoronos D. Distribution of bromophenols in Australian wild harvested and cultivated prawns (shrimp). Journal of Agricultural & Food Chemistry 1997; 45(11):4398-405. 28. Whitfield FB, Helidoniotis F, Shaw KJ, Svoronos D. Distribution of bromophenols in species of ocean fish from eastern Australia. Journal of Agricultural & Food Chemistry 1998; 46(9):3750-7. |