| 5. | Decabromodiphenyl ether | |||||||||||||||||
| 5.1 | Identification of the substance | |||||||||||||||||
| 5.1.1 | CAS No. | 1163-19-5 | ||||||||||||||||
| 5.1.2 | EINECS No. | 214-604-9 | ||||||||||||||||
| 5.1.3 | EINECS Name | Benzene, 1,1'-oxybis[2,3,4,5,6-pentabromo- | ||||||||||||||||
| 5.1.4 | Synonyms | Bis(pentabromophenyl) ether
Decabromobiphenyl oxide Decabromobiphenyl ether Decabromodiphenyl oxide Ether, bis(pentabromophenyl) DeBDE Typical composition for commercial DeBDE products would be 97-98% DeBDE with 0.3-3.0% of other brominated diphenyl ethers, mainly nonabromodiphenyl ether (NBDE, CAS No. 63936-56-1). Older products may contain less DeBDE. For instance a composition of 77.4% DeBDE, 21.8% NBDE and 0.85% octabromodiphenyl ether (OBDE, CAS No. 32536-52-0) has been reported for an older product ((Dow) FR-300-BA) (7). |
||||||||||||||||
| 5.1.5 | Molecular Formula | C12Br10O | ||||||||||||||||
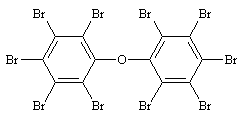
| 5.1.6 | Structural Formula | Based on the chemical
structure, DeBDE is fully brominated and there is only one congener.
|
||||||||||||||||
| 5.1.7 | Known uses | Organic synthesis and flame retardant | ||||||||||||||||
| 5.1.8 | EU Classification | Not included in Annex I to Directive 67/548/EEC | ||||||||||||||||
| 5.2 | Physico-chemical Characteristics | |||||||||||||||||
| 5.2.1 | Physical Form | Off white powder, depending on the manufacturer (7) | ||||||||||||||||
| 5.2.2 | Molecular Weight | 959.22 | ||||||||||||||||
| 5.2.3 | Melting Point/range (°C) | 290-306 | ||||||||||||||||
| 5.2.4 | Boiling Point/range (°C) | Boiling point is not applicable to this substance | ||||||||||||||||
| 5.2.5 | Decomposition Temperature (°C) | > 320 - 425 (different products) (7) | ||||||||||||||||
| 5.2.6 | Vapour Pressure (Pa (°C)) | < 1.3x10-4 (20)
< 133 (250) 271 (278) 661 (306) (1, 7) |
||||||||||||||||
| 5.2.7 | Relative Density (D420) | 3.0 or 3.25 (7) | ||||||||||||||||
| 5.2.8 | Vapour Density (air=1) | No data were available | ||||||||||||||||
| 5.2.9 | Conversion Factor (1011 hPa at 25 °C) | No data were available | ||||||||||||||||
| 5.2.10 | Solubility | Water: ca. 0.002 - 0.003
mg/l (Dow FR-300-BA: 77.4% DeBDE, 21.8% NBDE, and 0.8% OBDE) (1)
Acetone: 0.5 or 1.0 g/l Benzene: 1.0 or 4.8 g/l (7) |
||||||||||||||||
| 5.2.11 | Partition Coefficient (log P ow) | 5.24* or 9.97** (7)
* (Dow FR-300-BA: 77.4% DeBDE, 21.8% NBDE, and 0.8% OBDE) (1) ** (Unknown composition) |
||||||||||||||||
| 5.2.12 | Flammability | Non-flammable (7) | ||||||||||||||||
| 5.2.13 | Explosivity | Not applicable on the basis of its structure and physical properties nor is it known to contribute explosive properties with other materials | ||||||||||||||||
| 5.2.14 | Oxidising properties | Not considered to be an oxidiser | ||||||||||||||||
| 5.3 | Toxicological Data | |||||||||||||||||
| 5.3.1 | Observations in humans | In a human patch test, 5%
DeBDE (FR-300-BA) in petrolatum was repeatedly applied, 3 times a
week for 3 weeks, to skin of 50 human subjects. This treatment did
not result in skin sensitisation reactions during the sensitising
period or on challenge two weeks subsequent to the last induction
application. In 9 out of 50 subjects a slight to moderate skin
irritation was observed (7) and (1).
In a recent Swedish study, DeBDE was detected and quantified in blood serum from 3 categories of workers (median (range) in pmol/g lipid weight):
The serum conc. decreased during summer vacation in the electronics dismantling workers, and results indicated a shorter half-life with increasing degree of brominating. The exposure to DeBDE may occur via contaminated food and inhalation of airborne particulate matter (8). |
||||||||||||||||
| 5.3.2 | Acute Toxicity | |||||||||||||||||
| 5.3.2.1 | Oral | Oral LD50, female Sprague-Dawley
rats: > 2,000 mg/kg b.w. No clinical signs of toxicity were
observed during the 14 day observation period. No gross lesions were
detected at necropsy. The test material was FR-300-BA (7) and
(1).
Oral LD50, male Sprague-Dawley rats: > 5,000 mg/kg b.w. No clinical signs of toxicity were observed during the 14 day observation period (7). |
||||||||||||||||
| 5.3.2.2 | Dermal | Dermal LD50, rabbits: >
2,000 mg/kg b.w.
Commercial DeBDE was applied and occluded on the clipped intact skin of 2 male and 2 female New Zealand white rabbits each at a dosage of 200 or 2000 mg/kg body weight for 24 hours. Animals were observed for 14 days. No mortality occurred.(7) and (1). |
||||||||||||||||
| 5.3.2.3 | Inhalation | Inhalation LC50, Sprague-Dawley
rats: > 48.2 mg/l/1 hour.
Groups of 10 rats/sex were exposed for 1 hour to concentrations of DeBDE of 2 or 48.2 mg/l in air and subsequently observed for 14 days. There was no information on particle size distribution. All rats survived. At 2 mg/l salivation was noted in 2 rats on the first day, but thereafter all the rats of this group appeared normal, except one with respiratory difficulties and another with ocular discharge during the observation period. At 48.1 mg/l eye squint and increased motor activity was noted in the animals through day four. Respiratory difficulties were noted in 2 rats at days 3 to 6 and in one rat on day 8 and one on day 7. A few rats showed eye squint and ocular discharge on days 7 to 12. All rats were normal on day 14 (7) and (1). |
||||||||||||||||
| 5.3.2.4 | Other Routes | No data were available | ||||||||||||||||
| 5.3.2.5 | Skin Irritation | Two commercial DeBDE
products were tested in rabbits, and no evidence of skin irritation
was observed (7) and (1).
The potential for DeBDE to produce chloracne was studied in rabbits. A commercial product (Saytex 102 as a 10% chloroform solution) caused a slight irritation but no chloracnegenic activity (7) and (1). |
||||||||||||||||
| 5.3.2.6 | Eye Irritation | Two commercial DeBDE products were tested in rab-bit eyes, and only a transient mild irritation of the con-junctival membranes was observed (7) and (1). | ||||||||||||||||
| 5.3.2 7 | Irritation of Respiratory Tract | No data were available | ||||||||||||||||
| 5.3.2.8 | Skin Sensitisation | No data were available | ||||||||||||||||
| 5.3.2.9 | Sensitisation by Inhalation | No data were available | ||||||||||||||||
| 5.3.3 | Subchronic Toxicity | |||||||||||||||||
| 5.3.3.1 | Oral | Groups of 10 male and 10 female F344/N rats and B6C3F1 mice were fed diets containing 0, 3.1, 6.2, 12.5, 25.0 or 50.0 g DeBDE/kg diet for 13 weeks. The purity of DeBDE was 94-99%. All rats and mice lived to the end of the study. DeBDE did not adversely affect food consumption or final mean body weights. No compound related clinical signs or gross or microscopic pathologic effects were observed. NOAEL was 50.0 mg/kg diet. Liver weights were not recorded in these studies, but, in subsequent studies, liver weights were significantly increased in F344/N rats at dose levels of 25 and 50 g DeBDE (92%)/kg diet, for 14 days (7) and (1). | ||||||||||||||||
| 5.3.3.2 | Inhalation | Fifty rats were observed up to 556 days after a single intratracheal installation of 20 mg DeBDE (77.4%) dust (length mean diameter 2.65 mm, surface mean diameter 2.91 mm, and volume mean diameter 3.17 mm) suspended in 1 ml of rat serum. The half-life of DeBDE particles in the lung was ca. 150 days. No evidence of proliferative response was detected in the lungs or regional lymph nodes (7). | ||||||||||||||||
| 5.3.3.3 | Dermal | No data available | ||||||||||||||||
| 5.3.4 | Chronic Toxicity and Carcinogenicity | A 2 year study of DeBDE
(FR-300-BA) for chronic toxicity and carcinogenicity to male and
female Sprague-Dawley rats (25 male and 25 females/dose group)
maintained on diets providing 0, 0.01, 0.1 or 1.0 mg/kg body
weight/day indicated no discernable alteration in appearance,
behaviour, body weight, feed consumption, haematological analysis,
urinalysis, clinical chemistry, organ weights, survival, or tumour
incidence. The dose levels selected were too low (6) and (1).
In the U.S. National Toxicology Program (NTP), groups of 50 male and 50 female F344/N rats were exposed to DeBDE (purity 94-99%, no brominated dioxins or furans) in the diet at levels of 0, 25 or 50 g/kg for 103 weeks. The average DeBDE consumption was:
Body weights of dosed male and female rats in the 2 year study were comparable to those of the control animals. No treatment related effect on survival was noted. Minimal chronic toxicity was exhibited. The incidence of neoplastic nodules in the liver of low and high dose male rats (1/50; 7/50; 15/49) and high dose female rats (1/50; 3/49; 9/50) were statistically greater than those in the controls. Under the conditions of this 2 year feed study of DeBDE, there was some evidence of carcinogenicity for male and female F344/N rats as shown by increased incidence of neoplastic nodules of the liver in low dose (25 g/kg diet) males and high dose (50 g/kg diet) groups of each sex. The incidence of acinar-cell adenomas of the pancreas in males was significantly increased (1/49; 0/50; 4/49), but not significantly different from the controls. The incidence of mononuclear-cell leukaemia was also increased in males. Several non-neoplastic lesions were observed at increased incidence (1, 6 and 7). In the U.S. National Toxicology Program (NTP), groups of 50 male and 50 female B6C3F1 mice were exposed to DeBDE (purity 94-99%, no brominated dioxins or furans) in the diet at levels of 0, 25 or 50 g/kg for 103 weeks. The average DeBDE consumption was:
Body weights of dosed male and female mice in the 2 year study were comparable to those of the control animals. No treatment related effect on survival was noted. Minimal chronic toxicity was exhibited. Hepatocellular adenomas or carcinomas occurred at marginally increased incidence in dosed male mice only (8/50; 22/50; 18/50), but not increased in comparison with historical control groups. The incidences of thyroid gland follicular cell adenomas or carcinomas (combined) were increased, but not significantly, in dosed male mice (0/50; 4/50; 3/50) and females (1/50; 3/50; 3/50).Under the conditions of this 2 year feed study of DeBDE, there was equivocal evidence of carcinogenicity for male B6C3F1 mice as shown by increased incidence of hepatocellular adenomas or carcinomas (combined) in the low dose group and of thyroid gland follicular cell adenomas or carcinomas (combined) in both dosed groups. There was no evidence of carcinogenicity for female B6C3F1 mice receiving 25 or 50 g/kg diet. Thyroid gland follicular cell hyperplasia was in creased in both groups of treated male and female mice (7) and (1). |
||||||||||||||||
| 5.3.5 | Mutagenicity | |||||||||||||||||
| 5.3.5.1 | Gene Mutation | DeBDE (in various purities) was tested in two Ames tests with Salmonella typhimurium strains TA1535, TA1537, TA98 and TA100, or Saccharomyces cerevisiae, with and without metabolic activation. No evidence of mutagenic activity was found (1, 2, 7). | ||||||||||||||||
| 5.3.5.2 | Chromosome Abnormalities | Commercial grade DeBDE was not mutagenic in a Mouse lymphoma L5178Y/TK+/- assay or in Chinese Hamster Ovary Cells for Sister Chromatid Exchange or Chromosome Aberrations, all with and without metabolic activation (7) and (1). | ||||||||||||||||
| 5.3.5.3 | Other Genotoxic Effects | No data were available | ||||||||||||||||
| 5.3.6 | Reproductive Toxicity, Embryotoxicity, and Teratogenicity | |||||||||||||||||
| 5.3.6.1 | Reproductive Toxicity | A one-generation reproduction study was performed with 10 male and 20 female Sprague-Dawley rats at the two lower dose levels (3 and 30 mg/kg b.w./day) and 15 male and 30 female rats at the higher dose level (100 mg/kg b.w./day). Twenty males and 40 females served as controls. The reproductive capacity of rats was not affected by diets providing those dose levels of DeBDE (FR-300-BA) given for 60 days prior to mating, 15 days during mating, and throughout gestation and lactation. NOAEL was 100 mg/kg/day. This is the highest dose tested. No adverse effect on fertility was observed (7). | ||||||||||||||||
| 5.3.6.2 | Teratogenicity | Pregnant female rats
(number unknown) were given 0, 10, 100, or 1,000 mg DeBDE
(FR-300-BA)/kg bw suspended in corn oil by intragastric gavage on
days 6-15 of gestation. There was no evidence of toxicity or
teratogenicity at any dose level. Maternal food consumption and body
weight did not differ from controls. Body weights, food consumption,
and liver weights of the treated animals were comparable with those
of the controls. The position and number of foetuses in utero,
the number of corpora lutea, individual pup weight,
crown-rump length and sex ratio comparable with those of the
controls. A significant increase in resorptions was found at the low
dose levels but not at the high dose levels. No gross external
abnormalities were seen in the foetuses of dams treated at any dose
level. Soft tissue and skeletal examinations revealed an increased
number of litters with subcutaneous oedema and delayed ossification
of bones of the skull of foetuses of dams treated with 1,000 mg/kg,
but not at 100 mg/kg bw. Analysis of maternal and foetal livers for
total bromine revealed a significantly increased concentration in
maternal livers of rats treated with 1,000 mg/kg. With 100 mg/kg and
lower no increase in total bromine content was found. In livers of
foetuses from dams receiving any dose level of DeBDE, no increase in
total bromine content was observed.
Maternal NOEAL: 1,000 mg/kg/day Foetal LOAEL: 10 mg/kg/day, since it can not be shown that the observed foetal effects are without toxicological significance (7) |
||||||||||||||||
| 5.3.7 | Other Toxicity Studies | DeBDE seems to have low enzyme-inducing potency (5) | ||||||||||||||||
| 5.3.8 | Toxicokinetics | Based on results from
several studies it may be concluded that (7):
|
||||||||||||||||
| 5.4 | Ecotoxicity | No toxicity data for daphnia were available. EC50 for algae >1 mg/l (72h, Skeletonema costatum; 96h Chlorella sp.) (1). LC50 for fish was >500 mg/l (Killifish, 48h,) in a part of a six week bioconcentration study (4). Little or no DeBDE bioconcentrate in carp (4). BCF<5 (60 µg/l test conc.) and BCF<50 (6 µg/l test conc.) (3). Log Pow=5.24 was reported (7). | ||||||||||||||||
| 5.5 | Environmental Fate | Only one test result available. No biodegradation of DeBDE was found (2 Week), measured by BOD in a test equivalent to MITI I (3). DeBDE was found not readily biodegradable (4). | ||||||||||||||||
| 5.6 | Environmental Concentrations | DeBDE in sediment samples from rivers and estuaries in Japan showed levels ranging from 4 µg/kg up to 11600 µg/kg dry weight. (7). One of 3 mussel samples, Japan 1981-85 contained 1.4 µg/kg ww (7). | ||||||||||||||||
| 5.7 | Conclusion | |||||||||||||||||
| 5.7.1 | Health Assessment | Sufficient toxicological
data were identified for a health assessment of DeBDE. Most of the
data were taken from reviews performed by WHO and IARC. Many of the
toxicological studies were performed on old commercial DeBDE
products of low purity and are not performed according to the new
standards. No data on sensitisation were identified. Few relevant
data on humans were identified.
DeBDE has a low acute and subacute toxicity. Evidence of carcinogenicity was found in rats and mice. The International Agency for Research on Cancer (IARC) concluded DeBDE was not classifiable as to its carcinogenicity to humans, because there was only limited evidence for the carcinogenicity of DeBDE in experimental animals. IARC assigned DeBDE to Group 3. A rat teratogenicity study indicated some developmental effects of an old commercial DeBDE product at a high dose level not affecting the dams. The purity of DeBDE was low (presumably 77.4%), and the data available are, however, too sparse to permit an adequate evaluation. No treatment related effects were observed in a one-generation reproduction study. The dose levels were however too low. The potential for bioaccumulation of DeBDE is considered low because of low gastrointestinal absorption, but the retention of absorbed DeBDE in adipose tissue may be pronounced. |
||||||||||||||||
| 5.7.2 | Environmental Assessment | Few data were available for environmental assessment. Based on the features log Pow=5.24 and not readily biodegradable, DeBDE may be considered to cause long-term adverse effects in the aquatic environment. | ||||||||||||||||
| 5.8 | References | 1. Anonymous. International
Uniform Chemical Information Database (IUCLID). Bis(pentabromophenyl)
ether. European Commission. Joint Research Centre. Environment
Institute. European Chemicals Bureau; 1996. CD-ROM.
2. Ashby J, Tennant RW. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutation Research 1988; 204:17-115. 3. Chemical Inspection and Testing Institute. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan, compiled under the supervision of chemical products safety Division Basic industries. Japan: Japanese Chemical Industry Ecology-Toxicology & Information Center, 1992. 4. Chemicals Inspection and Testing Institute Japan (CITI). Biodegradation and bioaccumulation data for existing chemicals based on the CSCL Japan. Japan Chemical Ecology-Toxicology and Information Centre, 1992. 5. Darnerud PO, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M. Polybrominated Diphenyl Ethers: Food Contamination and Potential Risks. Copenhagen: Nordic Council of Ministers, 1998. (TemaNord). 6. IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Some Flame Retardants and Textile Chemicals, and Exposures in the Textile Manufacturing Industry. Switzerland: International Agency for Research on Cancer, World Health Organisation, 1990: 73-84. (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; 48). 7. WHO working group. Brominated diphenyl ethers. Environmental Health Criteria 1994; 162. 8. Sjödin A, Hagmar L, Klasson-Wehler E, Kronholm-Diab K, Jakobsson E, Bergman Å. Flame Retardant Exposure: Polybrominated Diphenyl Ethers in Blood from Swedish Workers. Environ Health Perspect. 1999 Aug;107(8):643-648. |