| 6. | Hexabromocyclododecane, isomers | |||||||||||||||||||||
| 6.1 | Identification of the substance | |||||||||||||||||||||
| 6.1.1 | CAS Nos. | 3194-55-6
(1,2,5,6,9,10-HBCD)
25637-99-4 (HBCD) |
||||||||||||||||||||
| 6.1.2 | EINECS Nos. | 221-695-9
(1,2,5,6,9,10-HBCD)
247-148-4 (HBCD) |
||||||||||||||||||||
| 6.1.3 | EINECS Names | 1,2,5,6,9,10-Hexabromocyclodecane
(1,2,5,6,9,10-HBCD)
Hexabromocyclododecane (HBCD) |
||||||||||||||||||||
| 6.1.4 | Synonyms | None were available | ||||||||||||||||||||
| 6.1.5 | Molecular Formula | C12H18Br6 | ||||||||||||||||||||
| 6.1.6 | Structural Formula | 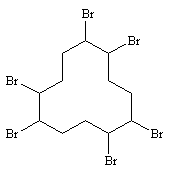 (1,2,5,6,9,10-HBCD
) (1,2,5,6,9,10-HBCD
)
[Purity: min. 96 % w/w. Impurities: Tetrabromocyclododecane and other brominated cyclododecanes. Technical HBCD is manufactured in two forms, high-melting (HM) and low-melting (LM). It consists of three isomers (a-, b-, and g-isomers) each. The low-melting HBCD consists of 70-80 % g-isomer and 20-30 % of a- and b-isomers. The high-melting HBCD consists of 90 % or more of the g-isomer (13)] |
||||||||||||||||||||
| 6.1.7 | Known uses | Flame retardant in polystyrene (15, 20) | ||||||||||||||||||||
| 6.1.8 | EU Classification | Not included in Annex I | ||||||||||||||||||||
| 6.2 | Physico-chemical Characteristics | |||||||||||||||||||||
| 6.2.1 | Physical Form | White to off-white odourless solid or crystalline powder | ||||||||||||||||||||
| 6.2.2 | Molecular Weight | 641.7 | ||||||||||||||||||||
| 6.2.3 | Melting Point/range (°C) | 175 - 195 (14)
178-183 (15) |
||||||||||||||||||||
| 6.2.4 | Boiling Point/range (°C) | Decomposition occurs at 230 ºC (15) | ||||||||||||||||||||
| 6.2.5 | Decomposition Temperature (°C) | 230 ºC (15) | ||||||||||||||||||||
| 6.2.6 | Vapour Pressure (Pa (°C)) | 1.6 x 10-9 (20)
(calculated)
1.7 x 10-8 (50) 1.3 x 10-7 (80) 3.8 x 10-7 (100) (15) |
||||||||||||||||||||
| 6.2.7 | Relative Density (D420) | 2.38 (14) | ||||||||||||||||||||
| 6.2.8 | Vapour Density (air=1) | |||||||||||||||||||||
| 6.2.9 | Conversion Factor (1011 hPa at 25 °C) | 1 ppm = 0.026 mg/l
1 mg/l = 38.1 ppm |
||||||||||||||||||||
| 6.2.10 | Solubility | Water: 0.008 mg/l
(temperature not stated) (14)
Water: 0.12 mg/l (23°C) (15) |
||||||||||||||||||||
| 6.2.11 | Partition Coefficient (log P ow) | 5.81 (calculated) (14)
7.59 (calculated) (15) |
||||||||||||||||||||
| 6.2.12 | Flammability | No data available | ||||||||||||||||||||
| 6.2.13 | Explosivity | No data available | ||||||||||||||||||||
| 6.2.14 | Oxidising properties | No data available | ||||||||||||||||||||
| 6.3 | Toxicological Data | |||||||||||||||||||||
| 6.3.1 | Observations in humans | In a human patch test
fibres (Tyvek T-12 with 10% 1,2,5,6,9,10-HBCD) was tested. One
square inch of the test sample was applied to the arms of ten men
and arms and legs of ten women and held in place for 6 days. After a
two-week rest period new patches were applied for 48 hours. No skin
reactions were observed on any subject (9).
No other data were available. |
||||||||||||||||||||
| 6.3.2 | Acute Toxicity | |||||||||||||||||||||
| 6.3.2.1 | Oral | Oral LD50, rats: >10,000
mg/kg
None of the CD rats died in this limit test. At 2,5-4 hours, 3/5 males and 1/5 females were hypoactive, and the female had diarrhoea. Thereafter, the females showed signs of toxicity. From day 6, 3/5 males had corneal opacity, which persisted through the 14 days observation period, and ptosis that persisted in 1 male at the end of the observation period (1). Results from several other studies were available (15) |
||||||||||||||||||||
| 6.3.2.2 | Dermal | Dermal LD50, rabbits: > 20,000 mg/kg (1) | ||||||||||||||||||||
| 6.3.2.3 | Inhalation | Inhalation LC50, rats: >
202 mg/l/4 hours
Limit test study. Ten animals (5 males & 5 females) were exposed to HBCD as dust (202 mg/l, calculated). No information on particle size distribution was available. The animals responded by preening the first 10 minutes, then settled down. From 1.5 hours to the end of the exposure, the animals showed slight dyspnoea. The animals were observed for 14 days. No animals died (1). Results from other studies are available (15) |
||||||||||||||||||||
| 6.3.2.4 | Other Routes | No data available | ||||||||||||||||||||
| 6.3.2.5 | Skin Irritation | In a study performed in accordance with standard EU-guideline, slight erythema was seen in 1 out of 3 females 30-60 minutes after the treatment. No visible signs at 24, 48 or 72 hours (11). Similar results from several other studies were available (15) | ||||||||||||||||||||
| 6.3.2.6 | Eye Irritation | Grading of ocular lesions
(mean of the 24, 48 and 72 hours examinations/max. score) (1):
All signs had disappeared after 7 days (1). Another study showed similar results (12) |
||||||||||||||||||||
| 6.3.2 7 | Irritation of Respiratory Tract | No data were available. | ||||||||||||||||||||
| 6.3.2.8 | Skin Sensitisation | The induction
concentrations in a Guinea Pig Maximization Test were 0.05, 0.5 or
5% HBCD in olive oil. Challenge concentrations were 0.005, 0.05, 0.5
or 5% HBCD in acetone. Induction concentrations higher than 0.5% and
challenge concentrations higher than 0.05% gave positive responses.
Increase in the induction or challenge concentrations did not
further increase the percentage of positive responders or the
intensity of the responses (17) (based on an abstract in English).
For the induction in another guinea pig maximisation test 5,000 or 50,000 ppm HBCD in olive oil was used for the intra-dermal injection and 250,000 ppm HBCD in petrolatum for the topical application on shaved skin. For the challenge, 21 days after the intra-dermal injection, 500, 5000 or 50 000 ppm HBCD in acetone was used in an open patch test on shaved skin. At the highest concentration of induction and challenge 9/10 animals were sensitised and there was a clear dose-effect relationship (18) |
||||||||||||||||||||
| 6.3.2.9 | Sensitisation by Inhalation | No data were available | ||||||||||||||||||||
| 6.3.3 | Subchronic Toxicity | |||||||||||||||||||||
| 6.3.3.1 | Oral | Sprague-Dawley rats were
fed a diet containing 0, 1.0, 2.5, or 5.0% 1,2,5,6,9,10-HBCD for 28
days (~ ca. 833, 2083, and 4167 mg/kg/day). Twenty animals (10/sex)
at each dose level. At the two highest dose levels, reduced body
weight gain and reduced food intake were seen after 14 days. At all
3 dose levels, absolute and relative liver weights were increased;
but no histological changes were seen. In the thyroid gland, dose
dependent micro-follicular hyperplasia proliferating into
adenomatous hyperplasia and epithelial hyperactivity was seen. At
the highest dose level, oogenesis was reduced. No changes in blood
biochemistry were seen. A NOAEL could not be established. (6).
Rats were fed a diet containing 0, 0.16, 0.32, 0.64 or 1.28% 1,2,5,6,9,10-HBCD for 13 weeks (~ ca. 133 - 1067 mg/kg/day). Forty Sprague-Dawley rats (20/sex) at each dose level, and further twenty animals (10/sex) at the zero and the highest dose level for a 42-days reconstitution period. One male at the highest dose level died on the 43rd study day. At the 0.32%-level and higher dose levels, the absolute liver weight was increased; a dose dependent focal lipid phanerosis was the only histological change. At the 1.28%-level, diminished body weight increase and reduced food intake were seen in the males. The increased liver weight and the lipid phanerosis diminished during the reconstitution period, but were not fully normalized. A NOAEL could not be established. (6, 7) |
||||||||||||||||||||
| 6.3.3.2 | Inhalation | No data were available | ||||||||||||||||||||
| 6.3.3.3 | Dermal | No data were available | ||||||||||||||||||||
| 6.3.4 | Chronic Toxicity and Carcinogenicity | In a Japanese test on carcinogenicity on carcinogenicity B6C3F1 mice were exposed orally to HBCD for 18 months. There were four exposure levels, 100, 1000 and 10 000 ppm and a control group (this is equivalent to about: 13, 130, 1300, and 0 mg/kg b.w., respectively), with 50 males and 50 females at each level. The study was not performed according to current guidelines, it has not been published in an international journal and it is poorly documented and poorly reported. It is impossible to assess the carcinogenic potential of HBCD based on the available study, a long-term study in mice (13). | ||||||||||||||||||||
| 6.3.5 | Mutagenicity | |||||||||||||||||||||
| 6.3.5.1 | Gene Mutation | HBCD was tested in several in vitro gene mutation assays with Salmonella typhimurium strains, both in the absence and presence of a metabolic activating system (S-9 mix). The results were generally negative but on one occasion HBCD induced frame shift mutations (TA100 and TA1535) with and without S9-mix (3, 2, 5, 8, 10, 22) | ||||||||||||||||||||
| 6.3.5.2 | Chromosome Abnormalities | No data were available | ||||||||||||||||||||
| 6.3.5.3 | Other Genotoxic Effects | HBCD was tested in primary
rat hepatocyte culture for unscheduled DNA synthesis (UDS). Highest
dose (500 mg/l) was cytotoxic. UDS was counted by autoradiography. A
dose-dependent increase in silver grains was seen from 2.5 mg/l.
HBCD induced more cells with UDS and higher UDS activity (more than
5 silver grain per cell) than vehicle controls. HBCD was positive in
this UDS test (4).
1,2,5,6,9,10-HBCD was tested in two recently developed in vitro assays for intragenic recombination in mammalian cells, the Sp5/V79 recombination assay and the SPD8 recombination assay. HBCD induced statistically significant increases in recombination frequencies in both the Sp5 and SPD8 assay system (16) |
||||||||||||||||||||
| 6.3.6 | Reproductive Toxicity, Embryotoxicity, and Teratogenicity | |||||||||||||||||||||
| 6.3.6.1 | Reproductive Toxicity | No data were available | ||||||||||||||||||||
| 6.3.6.2 | Teratogenicity | Wistar rats were fed a diet containing 0.01, 0.1 or 1% HBCD (~ ca. 6.7, 69, and 658 mg/kg/day) from day 0 through day 20 of pregnancy. No embryo- or foetotoxicity nor teratogenicity observed. At the highest dose, reduced food intake and increased liver weight of the dams were found. NOAEL for maternal toxicity was 69 mg/kg/day, and NOAEL for teratogenicity was 658 mg/kg/day (15) | ||||||||||||||||||||
| 6.3.7 | Other Toxicity Studies | |||||||||||||||||||||
| 6.3.8 | Toxicokinetics | Radiolabelled material was rapidly absorbed from the gastrointestinal tract after a single oral dosage of 14C-HBCD to rats. HBCD was rapidly metabolised, and 70% of the radioactive dose was eliminated via the faeces and 16% via the urine within 72 hours of dosing. The elimination from fat tissue was slower than from other body compartments (15). | ||||||||||||||||||||
| 6.4 | Ecotoxicity | The toxicity data for algae
were EC50:
For daphnia the EC50 were:
The LC50 for fish were:
Bioconcentration factors in fish (Pimephales promelas) have been reported as:
LogPow=5.81 were reported (14) |
||||||||||||||||||||
| 6.5 | Environmental Fate | No test results available | ||||||||||||||||||||
| 6.6 | Environmental Concentrations | HBCD in fish samples, river, Sweden, showed levels ranging from <50 to 8000 ng/g (lipid weight in muscle) and <1-7600 ng/g in sediments (ignition loss). HBCD was not further specified with CAS-number (19) | ||||||||||||||||||||
| 6.7 | Conclusion | |||||||||||||||||||||
| 6.7.1 | Health Assessment | Sufficient toxicological
data were identified for a health assessment of HBCD. No adequate
data on chronic toxicity, carcinogenicity, or reproductive toxicity
over several generations were identified. No chromosome aberration
tests were found, and none in vivo mutagenicity tests were
identified. Few relevant data on humans were identified.
The available data lead to the conclusion that HBCD is not an acute toxicant after oral, dermal or inhalation exposure. HBCD is slightly irritating to the eyes and the skin. Animal testing showed indications of a skin sensitising potential; but this was not confirmed in a preliminary human patch test. In subacute and subchronic studies with exposures to fairly high doses, HBCD caused reversible lipid phanerosis in the liver and increase in liver weight. In one of the studies, pathological changes were observed in the thyroidea. HBCD did not induce embryo toxicity (teratogenicity). It was generally not mutagenic in Salmonella typhimurium, but induced unscheduled DNA synthesis in rat hepatocytes and intragenic recombination in mammalian cells. Toxicokinetic data and the high n-octanol-water-partition coefficient indicate a risk of accumulation in adipose tissue in case of repeated exposure. |
||||||||||||||||||||
| 6.7.2 | Environmental Assessment | The EC50 for HBCD was below 1 mg/l for some algae species; log Pow>3; BCF>100 and the substance was found not readily biodegradable under aerobic conditions. Based on this hexabromocyclododecane is considered to be toxic for aquatic organisms and may also cause long-term adverse effects in the aquatic environment. | ||||||||||||||||||||
| 6.8 | References | 1. Acute toxicity studies
in rabbits and rats with test data and cover letter dated 03-08-90.
EPA/OTS; Doc #86-900000266 1990. NTIS/OTS0523258.
2. Ames metabolic activation test to assess the potential mutagenic effect of UND no. 49 with cover letter dated 031290. EPA/OTS; Doc #86-900000385 1990. NTIS/OTS0522948. 3. Ames test with hexabromides with cover letter dated 031290. EPA/OTS; Doc #86-900000379 1990. NTIS/OTS0522942. 4. Genetic toxicology rat hepatocyte primary culture/DNA repair test on hexabromocyclododecane with cover letter dated 030890. EPA/OTS; Doc #86-900000163 1990. NTIS/OTS0522234. 5. Genetic toxicology salmonella/microsomal assay on hexabromocyclododecane with cover letter dated 030890. EPA/OTS; Doc #86-900000164 1990. NTIS/OTS0522235. 6. Hexabromocyclododecane 28-day feeding trials with rats with test data and cover letter. EPA/OTS; Doc #86-900000274 1990. NTIS/OTS0523266. 7. Hexabromocyclododecane: 90-day feeding trials with rats with attachments and cover letter dated 031290. EPA/OTS; Doc #86-900000380 1990. NTIS/OTS0522943. 8. In vitro microbiological mutagenicity studies of four Ciba-Geigy corporation compounds (final report) with test data and cover letter. EPA/OTS; Doc #86-900000262 1990. NTIS/OTS0523254. 9. Letter from E I Dupont De Nemours & Co to USEPA concerning enclosed studies on decabromodiphenyl ether, hexabromocyclododecane and 4-vinylcyclohexane with attachments (sanitized). EPA/OTS; Doc #86-900000119S 1990. NTIS/OTS0522190. 10. Mutagenicity of two lots of FM-100 lot 53 and residue of lot 3322 in the absence and presence of metabolic activation with test data and cover letter. EPA/OTS; Doc #86-900000267 1990. NTIS/OTS0523259. 11. Primary dermal irritation study in rabbits with attachments and cover letter dated 030890. EPA/OTS; Doc #86-900000168 1990. NTIS/OTS0522239. 12. Primary eye irritation test epa/82 with attachments and cover letter dated 030890. EPA/OTS; Doc #86-900000165 1990. NTIS/OTS0522236. 13. Anderson Y, Bengtsson L, Filipsson AF, Palmquist M. Risk assessment - Hexachlorocyclododecane. Sweden, 1999. 14. International Uniform Chemical Information Database (IUCLID). Hexabromocyclododecane . 1. European Commission. Joint Research Centre. Environment Institute. European Chemicals Bureau; 1996. 15. Beratergremium für umweltrelevante Altstoffe (BUA) der Gesellschaft Deutscher Chemiker. Hexabromcyclododecan. S. Hirzel. Wissenschaftliche Verlagsgesellschaft, 1995. (BUA-Stoffbericht; 165). 16. Helleday T, Tuominen K-L, Bergman Å, Jenssen D. Brominated flame retardants induce intragenic recombination in mammalian cells. Mutation Research - Genetic Toxicology and Environmental Mutagenesis 1999; 439(2):137-47. 17. Momma J, Kaniwa M, Sekiguchi H et al. [Dermatological evaluation of a flame retardant, hexabromocyclododecane (HBCD) on guinea pig by using the primary irritation, sensitization, phototoxicity and photosensitization of skin]. Eisei Shikenjo Hokoku 1993; (111):18-24. 18. Nakamura A, Momma J, Sekiguchi H et al. A new protocol and criteria for quantitative determination of sensitization potencies of chemicals by guinea pig maximization test. Contact Dermatitis 1994; 31(2):72-85. 19. Sellström U, Kierkegaard A, De Wit C, Jansson B. Polybrominated diphenyl ethers and hexabromocyclododecane in sediment and fish from a Swedish River. Environmental Toxicology and Chemistry 1998; 17(6):1065-72. 20. The Swedish National Chemicals Inspectorate. The Flame Retardants Project - Final Report. Vol. 5. (KEMI Report). 21. Chemicals Evaluation and Research Institute, Japan. Biodegradation and bioaccumulation of existing chemicals. http://www.citi.or.jp/. 22. Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. Salmonella mutagenicity tests: 3 Results from the testing of 255 chemicals. Environmental Mutagenesis 1987; 9(Suppl 9):1-110. |