| 7. | 2,2-Bis(bromomethyl)propane-1,3-diol | |||||||||
| 7.1 | Identification of the substance | |||||||||
| 7.1.1 | CAS No. | 3296-90-0 | ||||||||
| 7.1.2 | EINECS No. | 221-967-7 | ||||||||
| 7.1.3 | EINECS Name | 2,2-Bis(bromomethyl)propane-1,3-diol | ||||||||
| 7.1.4 | Synonyms | 2,2-Bis(bromomethyl)-1,3-propanediol
1,3-Dibromo-2,2-dimethylolpropane 2,2-Dibromomethyl-1,3-propanediol Dibromoneopentyl glycol Dibromopentaerythritol FR-1138 FR-522 Pentaerythritol dibromide Pentaerythritol dibromohydrin DBNPG Some of the studies are performed on a flame retardant, FR-1138, composed of 79-88% DBNPG, 3-8% monobromoneopentyl triol (CAS No. 19184-65-7), 8-15% tribromoneopentyl alcohol (CAS No. 36483-57-5 or 1522-92-5) and traces of various esters, ethers and other derivatives (5). Other studies were performed on another flame retardant, FR-522, with an approx. purity of 100% (7). |
||||||||
| 7.1.5 | Molecular Formula | C5H10Br2O2 | ||||||||
| 7.1.6 | Structural Formula | 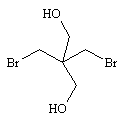 |
||||||||
| 7.1.7 | Known uses | Fire retardant in unsaturated polyester resins, in molded products, and in rigid polyurethane foam (13) | ||||||||
| 7.1.8 | EU Classification | Not included in Annex I | ||||||||
| 7.2 | Physico-chemical Characteristics | |||||||||
| 7.2.1 | Physical Form | Off-white powder (FR-1138)
(9)
White solid or free flowing powder (FR-522) Brown crystalline material ("Dynol" ~ FR-522)(7) |
||||||||
| 7.2.2 | Molecular Weight | 261.94 | ||||||||
| 7.2.3 | Melting Point/range (°C) | 75-95 (FR-1138) (5)
109-111 (FR-522) (7) |
||||||||
| 7.2.4 | Boiling Point/range (°C) | 134 (at 133.322 Pa) (FR-522) (7) | ||||||||
| 7.2.5 | Decomposition Temperature (°C) | Decomposes at 235°C, with the release of toxic, irritant HBr and bromine fumes (7) | ||||||||
| 7.2.6 | Vapour Pressure (Pa (°C)) | 133.322 (129.7) (FR-1138)
5332.88 (213.6) (FR-1138) (5) 1,333.22 (178) (FR-522) 3,333.05 (200) (FR-522) (7) |
||||||||
| 7.2.7 | Relative Density (D420) | No data were available | ||||||||
| 7.2.8 | Vapour Density (air=1) | No data were available | ||||||||
| 7.2.9 | Conversion Factor (1011 hPa at 25 °C) | No data were available | ||||||||
| 7.2.10 | Solubility | Water: 21 g/l at 25°C
(FR-1138)
Water: 1000 g/l at 100°C (FR-1138) Acetone: 825 g/l at 25°C (FR-1138) Benzene: 70 g/l at 25°C (FR-1138) (5) Water: 20 g/l at 25°C (FR-522) (7) |
||||||||
| 7.2.11 | Partition Coefficient (log P ow) | 1.1 (5)
1.06 (14) |
||||||||
| 7.2.12 | Flammability | Not flammable (7) | ||||||||
| 7.2.13 | Explosivity | Will not support combustion (7) | ||||||||
| 7.2.14 | Oxidising properties | No data were available | ||||||||
| 7.3 | Toxicological Data | |||||||||
| 7.3.1 | Observations in humans | No data were available | ||||||||
| 7.3.2 | Acute Toxicity | |||||||||
| 7.3.2.1 | Oral | Purity: 81.1% (FR-1138)
Impurities: 7.6% monobromoneopentyl glycol and 11.3% tribromoneopentyl alcohol. LD50, male rats: 3458 (2810-4257) mg/kg b.w. No significant untoward effects were observed at dose levels as high as 3160 mg/kg b.w. (4, 6) Purity: approx. 100% ("Dynol" ~ FR-522) LD50, rats: 1,880 (1,691-2,120) mg/kg b.w. Treatment related signs included reduced motor activity, ataxia, and prostration preceding unconsciousness within 30 min of dosing and death. All animals that survived appeared normal within 3 days. Fluid dissention and irritation of the gastro-intestinal tract was observed at macroscopic examination of decedents. (7) Purity: approx. 100% (FR-522) LD50, mice: 1,200 mg/kg b.w.(7) |
||||||||
| 7.3.2.2 | Dermal | No data were available | ||||||||
| 7.3.2.3 | Inhalation | Purity: 81.1% (FR-1138)
Impurities: 7.6% monobromoneopentyl glycol and 11.3% tribromoneopentyl alcohol. Exposure of rats to vapours of DBNPG maintained at 100°C, at a nominal concentration of 2.49 mg/l for a period of 7 hours resulted in slightly laboured breathing and slight nasal irritation but no mortality.(6, 4) |
||||||||
| 7.3.2.4 | Other Routes | No data were available | ||||||||
| 7.3.2.5 | Skin Irritation | Grading of dermal reactions
(mean of the 24 and 72 hours examinations/max. score) of "Dynol"
~ FR-522 on intact skin of 6 rabbits (7):
|
||||||||
| 7.3.2.6 | Eye Irritation | Grading of ocular lesions
(mean of the 24, 48 and 72 hours examinations/max. score) after
installation of 100 mg test material ("Dynol" ~ FR-522)
into the conjunctival sac:
The effects appeared reversible, but at the end of the observation period of 8 days, 2 out of 6 rabbits still had slight conjuntival redness. The 4/6 worst affected rabbits had a conjunctival redness score of 0.92/3 (7) Installation of DBNPG (FR-1138) into the conjunctival sac of rabbits resulted in slight pain, slight conjunctival inflammation, inflammation of iris, and corneal injury. The effects had disappeared at one week after exposure (1). |
||||||||
| 7.3.2 7 | Irritation of Respiratory Tract | No data were available | ||||||||
| 7.3.2.8 | Skin Sensitisation | The skin sensitising potential of DBNPG (FR-1138) was evaluated in male guinea pigs by two methods very sparsely reported. No evidence of FR-1138 related skin sensitisation was observed (1). | ||||||||
| 7.3.2.9 | Sensitisation by Inhalation | No data were available | ||||||||
| 7.3.3 | Subchronic Toxicity | |||||||||
| 7.3.3.1 | Oral | 1) DBNPG was administered
to F344/N rats (10/sex/dose level) by oral gavage in corn oil 5
days/week for 13 weeks at doses of 0, 50, 100, 200, 400, and 800
mg/kg b.w. NOAEL was 200 mg/kg/day for males and 400 mg/kg/day for
females
2) DBNPG was administered in the feed to F344/N rats (10/sex/dose level) for 13 weeks at concentrations of 0, 1,250, 2,500, 5,000, 10,000, and 20,000 ppm. NOAEL was approx. 135 mg/kg/day for males and 148 mg/kg/day for females 3) DBNPG was administered orally to B6C3F1 mice (10/sex/dose level) by gavage in corn oil 5 days/week for 13 weeks at doses of 0, 25, 50, 100, 200, and 400 mg/kg b.w. NOAEL was 100 mg/kg/day for both males and females 4) DBNPG was administered in the feed to B6C3F1 mice (10/sex/dose level) for 13 weeks at concentrations of 0, 625, 1,250, 2,500, 5,000, and 10,000 ppm. NOAEL was approx. 113 mg/kg/day for males and could not be established for females (< approx. 174 mg/kg/day). 1-4) The kidney and urinary bladder were target organs when DBNPG was administered by gavage or the dosed-feed route; mice were more sensitive than rats for the development of kidney and bladder lesions. Male rats and mice were more sensitive than females for the development of renal papillary degeneration or necrosis (10) |
||||||||
| 7.3.3.2 | Inhalation | No data were available | ||||||||
| 7.3.3.3 | Dermal | No data were available | ||||||||
| 7.3.4 | Chronic Toxicity and Carcinogenicity | Groups of Sprague-Dawley
rats were maintained on diets supplying nominal doses of 0, 5 or 100
mg DBNPG (FR-1138)/kg b.w./day for up to two years. Toxic effects
were observed at the high dose level in liver, lenses of the eyes,
and thyroids. The NOAEL was 5 mg/kg b.w./day, and no evidence of
FR-1138 related carcinogenicity was observed (8).
1) DBNPG was administered in the feed to F344/N rats (60/sex/dose level) for 2 years at concentrations of 0, 2,500, 5,000, and 10,000 ppm. Up to 10 in each group were used for interim evaluation at 15 months. An additional high dose, 20,000 ppm, was selected for a recovery group consisting of 60 males treated for 3 months and subsequently not exposed for the remainder of the 2 year study period. Ten additional control and 20,000 ppm males were used for interim evaluation at 3 months.A NOAEL could not be established because of treatment related neoplastic and hyperplastic effects observed from the lowest dosage level in subcutaneous tissue, mammary gland, oral cavity, pancreas and kidneys. 2) DBNPG was administered in the feed to B6C3F1 mice (60/sex/dose level) for 2 years at concentrations of 0, 312, 625, and 1,250 ppm.NOAEL was 312 ppm for males and could not be established for females because of treatment related neoplastic effects observed from the lowest dosage level in the Harderian gland. 1-2) The batch of DBNPG used in these studies was also used in some of the 13-week studies. The purity of DBNPG was analysed to 78.6%, and the impurities identified were 6.6% 2,2-bis(hydroxymethyl)-1-bromo-3-hydroxypropane (CAS No. 19184-65-7), 6.9% 2,2-bis(bromomethyl)-1-bromo-3-hydroxypropane (CAS No.1522-92-5), 0.2% pentaerythritol (CAS No. 115-77-5), and 7.7% dimers and structural isomers. Chemical exposure caused neoplasms of the skin, subcutaneous tissue, mammary gland, Zymbal's gland, oral cavity, oesophagus, forestomach, small intestine, large intestine, mesothelium, kidney, urinary bladder, lung, thyroid gland, seminal vesicle, haematopoietic system, and pancreas in the male rat; mammary gland, oral cavity, oesophagus, and thyroid gland in the female rat; lung, kidney, and Harderian gland in male mice; and subcutaneous tissue, lung, and Harderian gland in the female mouse. The recovery group of male rats presented with the same spectrum of treatment-related neoplasms as in the core study. In this recovery group, DBNPG (at 20,000 ppm) caused irreversible effects at numerous sites after 13 weeks of exposure that was not detectable by histological examination, but without further exposure eventually resulted in the development of neoplasms at multiple sites (9, 13) |
||||||||
| 7.3.5 | Mutagenicity | |||||||||
| 7.3.5.1 | Gene Mutation | In vitro assay with Salmonella
typhimurium strains TA-1535, TA-1537 and TA-1538 and Saccharomyces
cerevisiae strain D4, +/- S-9 mix. DBNPG ("pure" and
"plant") was considered negative in this test (2,
3).
In vitro assay with Salmonella typhimurium strains TA-98, TA-100, TA-1535, and TA-1537, +/- S-9 mix. DBNPG (purity unknown) was considered negative in this preliminary test (7). In vitro gene mutation Salmonella: Negative (12) Purity of DBNPG: approx. 84%. In vitro gene mutation assay with Salmonella typhimurium strains TA-98 and TA-100, +/- S-9 mix derived from Aroclor-induced hamster or rat liver. Negative in both strains without S-9 mix. Negative in both strains with rat S-9 mix. In the presence of hamster S-9 mix, positive in TA-100 but negative in TA-98 (16). |
||||||||
| 7.3.5.2 | Chromosome Abnormalities | In vitro cytogenetics
Positive (chromosome aberrations in Chinese Hamster Ovary cells +/-
S-9 mix) (11)
In vivo, DBNPG induced significant increases in the frequencies of micronucleated erythrocytes in male and female mice. Significant increases in micronuclei were observed in peripheral blood samples from male and female mice exposed to DBNPG for 13 weeks via dosed feed. Results of a bone marrow micronucleus test in male mice, where DBNPG was administered by gavage, were considered to be equivocal due to inconsistent results obtained in two trials. An additional bone marrow micronucleus test was performed with male and female mice and DBNPG was administered as a single intraperitoneal injection; results of this test were positive in females and negative in males (13). |
||||||||
| 7.3.5.3 | Other Genotoxic Effects | In vitro cytogenetics (sister chromatid exchanges in Chinese Hamster Ovary cells +/- S-9 mix): Inconclusive (11) | ||||||||
| 7.3.6 | Reproductive Toxicity, Embryotoxicity, and Teratogenicity | |||||||||
| 7.3.6.1 | Reproductive Toxicity | DBNPG was tested for its effects on reproduction and fertility in CD-1 mice using the reproductive assessment by continuous breeding (RACB) protocol. The mice were fed diet containing 0, 0.1, 0.2, and 0.4% DBNPG. Based on body weights and food consumption, the estimated doses were approx. 141, 274, and 589 mg/kg/day.Body weight gain for all treated groups was less than in controls. Both male and female F0 mice (20/sex/dose level, 40/sex in control group) were dosed 7 days prior to and during a 98-day cohabitation period. Although the fertility index was unchanged in the high-dose group, DBNPG exposure significantly decreased the numbers of litters per pair, pups born alive per litter, and pup weight when adjusted for litter size. Crossover mating between treated and control F0 animals indicated a specific effect only on female reproductive capacity. At the highest dose, DBNPG caused a body weight decrease in the F0 animals of both sexes with no effect on relative organ weights. Sperm concentration, motility, morphology, and oestrous cyclicity were unaffected by DBNPG exposure. Histopathology in the F0 animals revealed specific kidney lesions in both sexes; males were more sensitive than females. The last litter born in the 98-day breeding phase was reared to age 74 days and then mated to non-siblings of the same treatment group. The effect of high-dose DBNPG exposure on F1 fertility, body and organ weights, sperm parameters, and oestrous cyclicity was the same as that for the F0 animals, with the exception of the lack of renal lesions seen in the F1 females. The study showed that DBNPG impaired fertility in female mice in both generations in the absence of an effect on reproductive organ weights and oestrous cyclicity. DBNPG is not a selective reproductive toxicant, as these effects were seen concomitant with the general toxicity (15). | ||||||||
| 7.3.6.2 | Teratogenicity | No data were available | ||||||||
| 7.3.7 | Other Toxicity Studies | No data were available | ||||||||
| 7.3.8 | Toxicokinetics | No data were available | ||||||||
| 7.4 | Ecotoxicity | The octanol/water partition coefficient reported was 1.1 (5) | ||||||||
| 7.5 | Environmental Fate | No data were available | ||||||||
| 7.6 | Environmental Concentrations | No data were available | ||||||||
| 7.7 | Conclusion | |||||||||
| 7.7.1 | Health Assessment | Sufficient toxicological
data were identified for a health assessment of DBNPG. Most of the
data were found in documents submitted to the U.S. EPA. These
documents contain results from tests not performed according to
presently accepted international guidelines and Good Laboratory
Practice. Some of the studies are performed on a flame retardant
called, FR-1138, which was composed of 79-88% DBNPG and the
structurally related impurities monobromoneopentyl triol (3-8%),
tribromoneopentyl alcohol (8-15%) and traces of various esters,
ethers and other derivatives. Other studies were performed on
another flame retardant, FR-522, with an approx. purity of 100%. No
data on humans were identified.
The available data indicate that DBNPG is acute toxic after oral exposure and should be classified as harmful. DBNPG is very slightly skin irritating and slightly eye irritating. Based on a preliminary test, it does not seem to be a skin sensitiser. The results from the in vitro gene mutation tests varied, but in most cases DBNPG was not mutagenic. An in vitro test for chromosome aberrations was positive, and in vivo tests also showed some evidence of clastogenicity. The tests for subchronic oral toxicity did not reveal any clear indications of possible danger or risks of irreversible health effects by prolonged exposure. Under the conditions of these 2-year feed studies with mice and rats, there was clear evidence of multi-site carcinogenic activity of DBNPG (FR-1138). Based on available data DBNPG should be considered potential carcinogenic. |
||||||||
| 7.7.2 | Environmental Assessment | No ecotoxicity or environmental fate data were available for environmental assessment. | ||||||||
| 7.8 | References | 1. Eye irritation and skin
sensitization properties of a sample of FR-1138 (dibromoneopentyl
glycol). EPA/OTS; Doc #86-870001217 1900. NTIS/OTS0516120.
2. Mutagenic evaluation of compound 236-2-60 C Plant dibromoneopentyl glycol. EPA/OTS; Doc #86-870001219 1900. NTIS/OTS0516122. 3. Mutagenic evaluation of compound 236-2-60 Pure dibromoneopentyl glycol. EPA/OTS; Doc #86-870001218 1900. NTIS/OTS0516121. 4. Acute oral lethality and acute vapor inhalation toxicity of FR-1138 (dibromoneopentyl glycol). EPA/OTS; Doc #86-870001214 1972. NTIS/OTS0516117. 5. The environmental properties of FR-1138 (dibromoneopentyl glycol). EPA/OTS; Doc #86-870001215 1978. NTIS/OTS0516118. 6. Acute oral lethality and acute vapor inhalation toxicity of dibromoneopentyl glycol. EPA/OTS; Doc #86-870002201 1987. NTIS/OTS0515991. 7. Dibromoneopentyl glycol health and safety studies in rats and rabbits with cover letter dated 041590. EPA/OTS; Doc #86-900000440 1990. NTIS/OTS0524337. 8. Initial submission: Two-year chronic toxicity & oncogenicity study in rats with dibromoneopentyl glycol administered via the diet with cover letter dated 051492. EPA/OTS; Doc #88-920003191 1992. NTIS/OTS0539779. 9. Dunnick JK, Heath JE, Farnell DR, Prejean JD, Haseman JK, Elwell MR. Carcinogenic activity of the flame retardant, 2,2-bis(bromomethyl)-1,3-propanediol in rodents, and comparison with the carcinogenicity of other NTP brominated chemicals. Toxicologic Pathology 1997; 25(6):541-8. 10. Elwell MR, Dunnick JK, Brown HR, Montgomery CA. Kidney and urinary bladder lesions in F344/N rats and B6C3F1 mice after 13 weeks of 2,2-bis(bromomethyl)-1,3-propanediol administration. Fundamental and Applied Pharmacology 1989; 12(3):480-90. 11. Galloway SM, Armstrong MJ, Reuben C et al. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: Evaluations of 108 chemicals. Environmental and Molecular Mutagenesis 1987; 10(Suppl 10):1-175. 12. Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. Salmonella mutagenicity tests: 2. Results from the testing of 270 chemicals. Environmental and Molecular Mutagenesis 1986; 8(Suppl 7):1-119. 13. National Toxicology Program. TR-452 - Toxicology and Carcinogenesis Studies of 2,2-Bis(Bromomethyl)-1,3-Propanediol (FR-1138 ®) (CAS No. 3296-90-0) in F344 Rats and B6C3F1 Mice (Feed Studies) [Web Page]. May 1996; Available at http://ntp-server.niehs.nih.gov/htdocs/LT-studies/tr452.html. (Accessed 3 June 1999). 14. The Environmental Science Center of Syracuse Research Corporation. Experimental Log P (Octanol-Water) Database [Web Page]. 9 December 1998; Available at http://esc.syrres.com/~esc1/kowexpdb.htm. (Accessed 3 September 1999). 15. Treinen KA, Chapin RE, Gulati DK, Mounce R, Morris LZ, Lamb JC. Reproductive toxicity of 2,2-bis(bromomethyl)-1,3-propanediol in a continuous breeding protocol in Swiss (CD-1) mice. Fundamental and Applied Pharmacology 1989; 13(2):245-55. 16. Zeiger E, Anderson B, Haworth S, Mortelman K. Salmonella Mutagenicity Tests V. Results from the Testing of 311 Chemicals. Environmental and Molecular Mutagenesis 1992; 19(Suppl 21):2-141. |