| 9. | Pentabromodiphenyl ether | |
| 9.1 | Identification of the substance | |
| 9.1.1 | CAS No. | 32534-81-9
60348-60-9 (2,2',4,4',5-PeBDE) 182346-21-0 (2,2',3,4,4'-PeBDE) 189084-64-8 (2,2',4,4',6-PeBDE) |
| 9.1.2 | EINECS No. | 251-084-2 |
| 9.1.3 | EINECS Name | Benzene, 1,1'-oxybis-, pentabromo derivative |
| 9.1.4 | Synonyms | Pentabromodiphenyl
oxide
Diphenyl ether, pentabromo derivative Pentabromophenoxybenzene PeBDE Saytex 125 2,2',4,4',5-PeBDE Benzene, 1,2,4-tribromo-5-(2,4-dibromophenoxy)- 2,2',4,4',5-Pentabromodiphenyl ether 2,2',4,4',5-Pentabromodiphenyl oxide BDE 99 PBDE 99 Tardex 50 Tardex 50L 2,2',3,4,4'-PeBDE Benzene, 1,2,3-tribromo-4-(2,4-dibromophenoxy)- BDE 85 2,2',4,4',6-PeBDE Benzene, 1,3,5-tribromo-2-(2,4-dibromophenoxy)- 2,2',4,4',6-Pentabromodiphenyl ether BDE 100 PBDE 100 Commercial PeBDE is a mixture of approx. 0-1% TrBDE (tribromodiphenyl ether, CAS No. 49690-94-0), 24-38% TeBDE (tetrabromodiphenyl ether, CAS No. 40088-47-9), 50-60% PeBDE and 4-8% HxBDE (hexabromodiphenyl ether, CAS No. 36483-60-0) (8). There are 46 possible isomers of PeBDE and 42 possible isomers of TeBDE. The commercial products seem to contain 3 main components, i.e., 2,2',4,4',5-PeBDE, 2,2',4,4'-TeBDE (CAS No. 5436-43-1) and an unidentified congener containing 5 bromines (23). A commercial PeBDE product (Tardex 50) was reported to consist of 25-35% TeBDE, 55-70% PeBDE, 0-5% HxBDE and 0-1% HpBDE (heptabromodiphenyl ethers, CAS No. 68928-80-3) (20) DE-71 is primary a mixture of TeBDE, PeBDE and HxBDE containing low levels of TrBDE (< 1%) and HpBDE (< 2%) (23) |
| 9.1.5 | Molecular Formula | C12H5Br5O |
| 9.1.6 | Structural Formula | 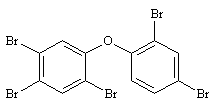 2,2',4,4',5-PeBDE 2,2',4,4',5-PeBDE
|
| 9.1.7 | Known uses | PeBDE is used as an additive in epoxy resins, phenol resins, polyesters and polyurethane, and textiles (23) |
| 9.1.8 | EU Classification | Not included in Annex I to Directive 67/548/EEC |
| 9.2 | Physico-chemical Characteristics | |
| 9.2.1 | Physical Form | Amber solid |
| 9.2.2 | Molecular Weight | 564.8 |
| 9.2.3 | Melting Point/range (°C) | 202 ºC (estimated) (19) |
| 9.2.4 | Boiling Point/range (°C) | > 300 °C (decomposition starts above 200 °C) (19) |
| 9.2.5 | Decomposition Temperature (°C) | Pyrolysis studies with commercial PeBDE showed that polybrominated dibenzofurans and polybrominated dibenzodioxins was formed (700-800°C). When PeBDE was pyrolysed in the absence of oxygen, polybromobenzenes, polybromophenols, and polybrominated dibenzofurans were formed (23). |
| 9.2.6 | Vapour Pressure (Pa (°C)) | 1240 (22) (19)
835-888 (25) (19) |
| 9.2.7 | Relative Density (D420) | 2.28 at 25 °C; 1.78 at 40 °C (19) |
| 9.2.8 | Vapour Density (air=1) | No data available |
| 9.2.9 | Conversion Factor (1011 hPa at 25 °C) | No data available |
| 9.2.10 | Solubility | Water: 9 x 10-7 mg/l at 20 °C (19) |
| 9.2.11 | Partition Coefficient (log P ow) | 6.5 - 7.0 (8)
Log Pow>6, measured (23) 7.66 (QSAR estimation) |
| 9.2.12 | Flammability | No data available |
| 9.2.13 | Explosivity | No data available |
| 9.2.14 | Oxidising properties | No data available |
| 9.3 | Toxicological Data | |
| 9.3.1 | Observations in humans | Forty plasma
samples from a "random" population in Sweden were
examined. The mean concentration of polybrominated diphenyl ethers
was 2.1 +/- 1.4 ng/g lipid weight, which was at least two orders of
magnitude lower than polychlorinated diphenyls. 2,2',4,4'-TeBDE and
2,2',4,4',5-PeBDE were the most abundant and constituted
approximately 70% of the total mean polybrominated diphenyl ethers
in each sample (14)
Milk samples from 39 primiparous mothers (22 to 36 years old) from the Uppsala county in Sweden were analysed for the content of the five most frequently found polybrominated diphenyl ethers. The mean of total polybrominated diphenyl ethers was 4.5 ng/g lipid weight. 2,2',4,4'-TeBDE was the major congener in breast milk, comprising approx. 57%, while 2,2',4,4',5-PeBDE and 2,2',4,4',6-PeBDE represented approx. 16% and 11%, respectively (7) Samples of milk from mothers living in the Stockholm region have been analysed for the presence of polybrominated diphenyl ethers. The samples analysed covered the years 1972-1997. The main congener found in the samples was 2,2',4,4'-TeBDE (60-70% of total), but other congeners found were 2,4,4'-TrBDE, 2,3',4,4'-TeBDE, 2,2',4,4',5-PeBDE, 2,2',4,4',6-PeBDE, 2,2',3,4,4'-PeBDE, 2,2',4,4',5,5'-HxBDE and 2,2',4,4',5,6'-HxBDE. The levels of polybrominated diphenyl ether were shown to increase exponentially over the time period, with a doubling time of around 5 years. The total levels found in the 1997 samples were 4 µg/kg lipid compared with 0.072 µg/kg in 1972, (17, 18) Levels of polybrominated diphenyl ethers (probably TeBDE and PeBDE congeners) of 0.6-11 µg/kg lipid have been found in human breast milk from Germany (9). The levels of the components of commercial PeBDE have been measured in adipose tissue and blood a 21 year old Israeli man, and also in cows milk and poultry fat from Israel. The levels found in adipose tissue were: 2 µg 2,2',4,4'-TeBDE/kg wet wt, 4 µg 2,2',4,4'5-PeBDE/kg wet wt and 1 µg/kg wet wt of an unknown PeBDE. The substances were not detected in blood, cows milk or poultry fat (9). |
| 9.3.2 | Acute Toxicity | |
| 9.3.2.1 | Oral | Oral LD50,
rats: 5 g/kg b.w. (4)
Oral LD50, Wistar rats: 6,200 (5,391 - 7,130) mg/kg b.w. Oral LD50, male rats: 7,400 mg/kg b.w. Oral LD50, female rats: 5,800 mg/kg b.w. Groups of male and female rats were administered single doses of up to 9,600 mg/kg of PBDPE (commercial grade) in corn oil by gavage. Signs of toxicity observed included diarrhoea, piloerection, reduced weight gain, reduced activity, tremors and red staining around the nose and eyes. Animals which died showed pale, enlarged, necrotic livers and multiple small ulcerations of the gastric mucosa (3, 23) In a study designed to assess the immunological and endocrine effects of DE-71 (a commercial PeBDE mixture), groups of 6 female C57BL/6J mice were dosed once by gavage with 0, 0.8, 4, 20, 100 or 500 mg/kg DE-71 in peanut oil. Two days post-dosing all animals were given an intraperitoneal injection of sheep erythrocytes (SRBC). The potential immunotoxicity of DE-71 was assessed by measuring the plaque-forming cell (PFC) response to SRBC and also natural killer cell (NKC) activity in vitro. All animals were sacrificed 8 days post-treatment. No clinical signs of toxicity were reported. Relative liver weight and hepatic cytochrome P450 activity were increased at 500 mg/kg, compared with controls, with no effects being observed at any other doses. The serum concentrations of total thyroxin (T4) were decreased at all dose-levels, but no dose-response relationship was apparent. No conclusions regarding the immunotoxicant potential of PBDPE could be drawn from this study (12) In a behavioural study, groups of neonatal male NMRI-mice were given a single oral dose of 0.8 or 12 mg 2,2',4,4',5-PeBDE/kg on postnatal day 10. Spontaneous motor behaviour was assessed at 2 and 4 months and a swim maze study performed at 5 months post-exposure. Minor behavioural changes were noted in this sparsely reported study. No conclusions as to the significance to human health of these minor changes can be drawn (10). |
| 9.3.2.2 | Dermal | Dermal LDLo,
rats: 5,500 mg/kg (4)
Dermal LD50, rabbits: > 2,000 mg/kg b.w.(3, 23) |
| 9.3.2.3 | Inhalation | Inhalation
LC50, CD rats: > 200 mg/l/1 hour
Groups of 5 rats/sex were exposed to an aerosol mist of 2 or 200 mg/l PBDPE in corn oil for 1 hour in a whole body exposures chamber. Aerosol droplet size was not given. No treatment-related mortalities occurred at either concentration. Animals in the high concentration group showed general signs of toxicity such as lacrimation, salivation and tachypnoea. Animals in both groups displayed increased, followed by decreased motor activity, eye squint and erythema (site not stated) during exposure. Nasal and respiratory "congestion" were noted in 3 rats at 200 mg/l up to day 3. Animals appeared normal by 24 hours after the lower dose and by day 4 after the higher dose.(3) |
| 9.3.2.4 | Other Routes | No data were available |
| 9.3.2.5 | Skin Irritation | Application of PeBDE to rabbit skin caused mild to moderate irritative effects (3, 4, 23) |
| 9.3.2.6 | Eye Irritation | The application of PeBDE to the conjunctival sac in rabbits caused only mild, transient effects (3, 23) |
| 9.3.2 7 | Irritation of Respiratory Tract | Evidence of tachypnoea and nasal and respiratory congestion are reported in rats following single inhalation exposures to very high concentrations 200 mg/l (8333 ppm) PBDPE aerosol mist for 1 hour (3) |
| 9.3.2.8 | Skin Sensitisation | No data were available |
| 9.3.2.9 | Sensitisation by Inhalation | No data were available |
| 9.3.3 | Subchronic Toxicity | |
| 9.3.3.1 | Oral | Groups of 20
male and 20 female Charles River COBS CD rats were administered 0,
0.01, 0.05, 0.1, 0.5 or 1.0 mg/kg/day of a commercial PeBDE mixture
of unknown composition, in the diet daily for 30 days. Groups of 5
rats per sex at each dose level were sacrificed at 30 days, and
after recovery periods of 6, 12 and 24 weeks. No treatment-related
changes in survival, body weight, food consumption, behavioural or
clinical signs, haematology, clinical chemistry, macroscopic or
histopathological changes were observed. There were no
treatment-related changes in liver and urinary porphyrins. No test
material-related effects were noted in this study, except for the
elevated bromine levels in the thyroid gland and liver after 4 weeks
of treatment. The NOAEL was 1 mg/kg/day (1)
Groups of six male Sprague-Dawley rats were administered PeBDE (commercial grade) in corn oil by gavage for 90 days. Two dosing regimens were used: a high-dose series of 0, 6.25, 12.5, or 25 mmol/kg/day (equivalent to 0, 3.53, 7.06, or 14.12 mg/kg/day, respectively) and a low-dose series of 0, 0.78, 1.56, or 3.13 mmol/kg/day (equivalent to 0, 0.44, 0.88, or 1.77 mg/kg/day, respectively). Liver enzyme induction occurred at all dose levels, and some of these changes were persistent, lasting for 30-60 days after the cessation of treatment. No histologic liver abnormalities were observed in rats administered the low-dose series. Histologic evaluation was not performed on the high-dose rats. The NOAEL for PeBDE is considered to be 1.77 mg/kg/day, the highest dose for which liver enzyme induction occurred, but no histologic liver abnormalities were found (5) Groups of 30 Sprague-Dawley rats/sex were administered 0, 2, 10 or 100 mg/kg/day DE-71 (a commercial PeBDE mixture) in corn oil, in the diet for up to 90 days. Ten animals per sex from each group were sacrificed on day 28 of dosing and a further 10 per sex at the end of the 90-day dosing period. Of the remaining animals, 5 per sex per group were sacrificed after recovery periods of 6 and 24 weeks. The results indicated that the liver is the target organ. The effects included increased liver weight associated with microscopic cytoplasmic changes, together with disturbances in porphyrin and cholesterol synthesis. Porphyrin levels in urine and liver were increased in both sexes at 100 mg/kg/day at week 4 and 13. By week 13, urinary porphyrins were increased by 2-fold and 13-fold in males and females respectively, and liver porphyrins were correspondingly increased by 8- and 400-fold. Slight thyroid hyperplasia and reductions in plasma T4 levels were also observed, but these effects are considered to be indirect consequences of the induction of liver enzymes, and due to species differences in thyroid metabolism are not likely to be of relevance to human health. In view of the effects on the liver, a clear NOAEL cannot be identified from this study (< 2 mg DE-71/kg/day) (3, 23) In a study designed to assess the immunological and endocrine effects of DE-71 (a commercial PeBDE mixture), groups of 6-8 female C57BL/6J mice were dosed by gavage with 0, 18, 36, or 72 mg/kg DE-71 in peanut oil for 14 days. The potential immunotoxicity of DE-71 was assessed by measuring the plaque-forming cell (PFC) response to an intraperitoneal injection of sheep erythrocytes (SRBC), natural killer cell (NKC) activity in vitro and cytochrome P450 IA1 and IIB1 activity. All animals were sacrificed on day 15 of the study and spleen, thymus, liver and body weights were measured. There was evidence of dose-related increase in relative liver weight, reduced relative thymus weight at the top dose, increased cytochrome P450 activity and reduced serum T4 levels (as seen in studies in rats). No conclusions regarding the immunotoxic potential of PeBDE could be drawn from this study (12) |
| 9.3.3.2 | Inhalation | No data are available |
| 9.3.3.3 | Dermal | No data are available |
| 9.3.4 | Chronic Toxicity and Carcinogenicity | No data are available |
| 9.3.5 | Mutagenicity | |
| 9.3.5.1 | Gene Mutation | PeBDE (purity
unknown) was not mutagenic in a Salmonella typhimurium assay,
in which four strains (TA-98, TA-100, TA-1535, and TA-1537) were
utilized both with and without metabolic activation (24)
PeBDE was evaluated for mutagenicity by plate assay in two microorganisms, Saccharomyces cerevisiae, strain D4, and Salmonella typhimurium, strains TA-1535, TA-1537, TA-1538, TA-98, and TA-100, both in the presence and absence of metabolic activation. No evidence of mutagenic activity from PeBDE was seen in any of the assays conducted in this evaluation (2) |
| 9.3.5.2 | Chromosome Abnormalities | No data are available |
| 9.3.5.3 | Other Genotoxic Effects | No data are available |
| 9.3.6 | Reproductive Toxicity, Embryotoxicity, and Teratogenicity | |
| 9.3.6.1 | Reproductive Toxicity | No data are available |
| 9.3.6.2 | Teratogenicity | Groups of 25 pregnant Sprague-Dawley rats were administered doses of 0, 10, 100 or 200 mg/kg/day of Saytex 115 (commercial preparation of PeBDE, CAS No. 117148-85-3, unknown composition) in corn oil, on days 6 to 15 of gestation. Caesarean sections were conducted on day 20 of gestation and the foetuses examined for external, visceral and skeletal alterations. The only test material related sign of maternal toxicity observed was a reduced body weight gain of 20 and 30% compared to controls, and the test material was not teratogenic. The maternal NOAEL was 10 mg/kg/day and the foetal NOAEL was 100 mg/kg/day (13, 23) |
| 9.3.7 | Other Toxicity Studies | Mitogen-induced
DNA synthesis and immunoglobulin synthesis by lymphocytes from blood
donors were examined following 2,2',3,4,4'-PeBDE (purity >= 98%)
exposure in vitro in order to determine the immunotoxic potential.
Despite rather high concentrations, 2,2',3,4,4'-PeBDE did not affect
human peripheral lymphocyte proliferation or immunoglobulin
synthesis in vitro. The negative findings in this study indicate
that certain functions of human peripheral lymphocytes, i.e.
proliferation and immunoglobulin synthesis, are insensitive to the
direct action of polybrominated diphenyl ethers and polychlorinated
biphenyls (11)
The potency of some pure polybrominated diphenyl ethers as Ah-receptor (ant)agonists was investigated. 2,2',3,4,4'-PeBDE, 2,2',4,4',5-PeBDE, and 2,3',4,4',6-PeBDE (not 2,2',4,4',6-PeBDE) were reported to exhibit varying degrees of partial Ah-receptor agonist and antagonist activities in an in vitro study in H4IIE rat hepatoma cells (CALUX assay). No signs of cytotoxicity were reported to be observed. No conclusions with regard to the significance of these findings can be drawn from the limited information reported (16). |
| 9.3.8 | Toxicokinetics | The half-life
of PeBDE has been investigated in the perirenal fat in rats after a
single oral dose of 300 mg Bromkal 70 (mainly PeBDE)/kg b.w. The
average half-life of two different PeBDE congeners were between 25
and 47 days, depending on the sex of the animal and the type of
isomer determined (23)
Preliminary results from a distribution study with 14C-labelled 2,2',4,4',5-PeBDE and 2,2',3,3',4-PeBDE (No CAS No. available) in mice indicated that relatively high concentrations of radioactivity was accumulated in fat depots, liver, adrenal, ovary, lung and initially the brain. Absorption from the gastrointestinal tract appeared to be effective. The radioactivity was slowly eliminated from adipose tissue and milk from lactating mice. Studies on pregnant animals indicated low foetal uptake (8) PeBDE behave as mixed-type inducers of cytochrome P-450 types (8, 23) |
| 9.4 | Ecotoxicity | No toxicity
data for fish, daphnia or algae were available.
Different bioconcentration factors in fish have been reported: 10,200-11,700 (10 µg/l test conc, 8w); <3.4 (10 µg/l test conc., 8w) and 20 (3.5 mg/kg food/day, 3.5 month) (6). The following Log Pow>6, measured (23) and 7.66 (QSAR estimation) were reported. |
| 9.5 | Environmental Fate | Only one test result available. No biodegradation of pentabromobiphenyl ether was found in an OECD 301B ready biodegradation test (29d, CO2, GLP) (21). Pentabromodiphenyl ether was found not readily biodegradable (2.4% CO2 evolved after 93 days) (21). |
| 9.6 | Environmental Concentrations | Pentabromobiphenyl
ether in sediment samples from rivers and estuaries in Japan showed
levels ranging from <2 µg/kg (detection limit) up to 28 µg/kg
dry weight. In Sweden, the concentrations in sediment samples from
rivers were up to 1200 µg 2,2',4,4','5-PeBDE/kg (23). Highest
concentration near a producer of pentabromobiphenyl ether was 560.7
µg/kg d.w (15). In Japan, (1981-85), concentrations of 0.4 and 2.8
µg/kg ww were found in mussel. But no pentabromobiphenyl ether was
detected in fish (< 0.2 µg/kg). In cod (liver) from the North
Sea concentrations of 1.9-22 µg/kg, fresh weight, were reported.
Concentrations in freshwater whitefish and herring (Sweden,
different places) were 7.2 and 64 µg 2,2',4,4',5-PeBDE/kg fat (23).
Highest conc., in fish, UK was 108 µg/kg ww (15) and 9400 µg/kg
lipid weight, Sweden (22).
Pooled blubber of ringed seal and grey seal, Sweden (1979-85) contained average concentrations of 1.7 and 40 µg 2,2',4,4',5-PeBDE/kg fat respectively (23). |
| 9.7 | Conclusion | |
| 9.7.1 | Health Assessment | Sufficient
toxicological data were identified for a health assessment of PeBDE.
Most of the data are found in reviews, and many tests have probably
not been performed according to internationally accepted guidelines.
No data on allergenicity, chronic toxicity, carcinogenicity or
reproductive toxicity in multi-generation studies were identified.
No chromosome aberration tests or any other mutagenicity tests
except the gene mutation tests were found. There are 46 possible
isomers of PeBDE, and most of the studies found in literature were
made on various commercial formulations of PeBDE, which contain one
or a few of these isomers plus some other polybrominated diphenyl
ethers. This makes it difficult to make generalised safety
evaluation of PeBDE.
Studies in rats with commercial preparations containing PeBDE indicate that these preparations are of low acute toxicity via inhalation or via the oral and dermal routes of exposure. The available data indicate that PeBDE produces only minimal to mild signs of dermal and eye irritation in animals following single exposure. PeBDE did not cause any substantial skin or eye irritancy, and respiratory tract irritation was seen in animals only following exposure to very high concentrations of PeBDE (>8000 ppm). Repeated oral exposure of rats and mice to PeBDE indicated that the liver is the key target organ affected. The effects observed included increases in liver weight and hepatocytomegaly, cellular microscopic changes, induction of a range of liver enzymes, and disturbances in cholesterol and porphyrin synthesis. Probably as a consequence of the induction of liver enzymes, T4 levels were reduced in rats and mice leading to increases in thyroid gland weight. However, due to species differences in thyroid metabolism the effects on thyroid status are of unclear relevance to human health. The liver and thyroid changes produced by PeBDE are apparent within 4 weeks of repeated oral dosing, with effects on the liver at 2 mg/kg/day and above, and changes in thyroid status at 10 mg/kg/day and above. A NOAEL of 1.77 mg/kg/day was identified. PeBDE was not a bacterial cell mutagen. From the limited data available there is no evidence for developmental toxicity with PeBDE. Toxicokinetic studies in rats and mice indicate a moderate retention in the organism, and traces have recently been detected in human plasma, milk and fat tissue. |
| 9.7.2 | Environmental Assessment | Only few data were available for environmental classification. Based on the features log Pow>6 and not readily biodegradable, PeBDE may cause long-term adverse effects in the aquatic environment. |
| 9.8 | References | 1. Letter from
Great Lakes Chem Corp to US EPA submitting studies for 7 chemical
compounds with attachments. EPA/OTS; Doc #86-900000220 1990. NTIS/OTS0526423.
2. Mutagenicity evaluation of compound 345-76A (final report) with cover sheet and letter dated 030890 (Abstract only). EPA/OTS; Doc #86-900000215 1990. NTIS/OTS0522285. 3. Initial submission: Letter from great lakes chem corp to USEPA regarding ITC request for information on brominated flame retardants (53 FR 5466) with attachments, dated 05/17/88. EPA/OTS; Doc #FYI-OTS-0794-1106 1994. NTIS/OTS0001106. 4. The Registry of Toxic Effects of Chemical Substances (RTECS). Benzene, 1,1'-oxybis-, pentabromo deriv. Update Code: 199807. USA: The National Institute of Occupational Health and Safety (NIOSH), U. S. Department of Health and Human Services; 1998. CD-ROM. 5. Integrated Risk Information System (IRIS). Pentabromodiphenyl ether. Update Code: 9008. USA: U.S. Environmental Protection Agency (U.S. EPA); 1998. CD-ROM. 6. Chemicals Inspection and Testing Institute Japan (CITI). Biodegradation and bioaccumulation data for existing chemicals based on the CSCL Japan. Japan Chemical Ecology-Toxicology and Information Centre, 1992. 7. Darnerud PO, Atuma S, Aune M, Cnattingius S. Polybrominated diphenyl ethers (PBDEs) in breast milk from primiparous women in Uppsala county, Sweden. Organohalogen Compounds 1998; 35:411-4. 8. Darnerud PO, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M. Polybrominated Diphenyl Ethers: Food Contamination and Potential Risks. Copenhagen: Nordic Council of Ministers, 1998. (TemaNord. 9. de Boer J, Robertson LW, Dettmer F, Wichmann H, Bahadir M. Polybrominated diphenylethers in human adipose tissue and relation with watching television - a case study. Organohalogen Compounds 1998; 35:407-10. 10. Eriksson P, Jakobsson E, Fredriksson A. Developmental neurotoxicity of brominated flame-retardants, polybrominated diphenyl ethers and tetrabromo-bis-phenol A. Organohalogen Compounds 1998; 35:375-7. 11. Fernlöf G, Gadhasson I, Pödra K, Darnerud PO, Thuvander A. Lack of effects of some individual polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) congeners on human lymphocyte functions in vitro. Toxicology Letters 1997; 90(2-3):189-97. 12. Fowles JR, Fairbrother A, Baecher-Steppan L, Kerkvliet NI. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology 1994; 86(1-2):49-61. 13. Hoberman AM, Lochry EA, Pinkerton MN, Christian MS. Comparison of the developmental toxicity of octabromodipenyloxide and pentabromodiphenyloxide in Crl:CD(SD)BR rats. Toxicologist 1988; 8(64):Abstract 254 page 64. 14. Klasson Wehler E, Hovander L, Bergman Å. New organohalogens in human plasma - Identification and quantification. Organohalogen Compounds 1997; 33:420-5. 15. Law RJ, Allchin CR, Morris S, Reed J. Analysis of brominated Flame Retardants in Environmental Samples. Ministry of Agriculture, Fisheries and Food, Directorate of Fisheries Research, Burnham-on Crouch, 1996. 16. Meerts IATM, Luijks EAC, Marsh G, Jakobsson E, Bergman Å, Brouwer A. Polybrominated diphenyl ethers as Ah-receptor agonists and antagonists. Organohalogen Compounds 1998; 35:147-50. 17. Meironyté D, Bergman Å, Norén K. Analysis of polybrominated diphenyl ethers in human milk . Organohalogen Compounds 1998; 35:387-90. 18. Norén K, Meironyté D. Contaminants in Swedish human milk. Decreasing levels of organochlorine and increasing levels of organobromine compounds. Organohalogen Compounds 1998; 38:1-4. 19. Organisation for Economic Co-Operation and Development (OECD). Selected Brominated Flame Retardants. Background and National Experience with Reducing Risk. OECD Environment Monograph Series No. 102. Risk Reduction monograph no. 3 edition. Paris: OECD, 1994. 20. Prescott W. Pentabromodiphenyl oxide - Tardex 50. Aspects of its use as a flame retardant additive for plastics. Polymers Paint Colour Journal 1978; 168:1077-81. 21. Schaefer EC, Haberlein D. Pentabromodiphenyl oxide (PeBDPO): Ready biodegradability by the carbon dioxide evolution test method. Wildlife International Ltd., 1997. 22. Sellström U, Kierkegaard A, De Wit C, Jansson B. Polybrominated diphenyl ethers and hexabromocyclododecane in sediment and fish from a Swedish River. Environmental Toxicology and Chemistry 1998; 17(6):1065-72. 23. WHO working group. Brominated diphenyl ethers. Environmental Health Criteria 1994; 162:187-211. 24. Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. Salmonella mutagenicity tests. 3. Results from the testing of 255 chemicals. Environmental Mutagenesis 1987; 9(Suppl 9):1-110. |
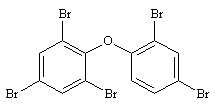 2,2',4,4',6-PeBDE
2,2',4,4',6-PeBDE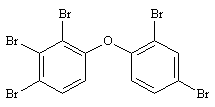 2,2',3,4,4'-PeBDE
2,2',3,4,4'-PeBDE