| 10. | Octabromodiphenyl ether | |||||||||||||||||||||||
| 10.1 | Identification of the substance | |||||||||||||||||||||||
| 10.1.1 | CAS No. | 32536-52-0 | ||||||||||||||||||||||
| 10.1.2 | EINECS No. | 251-087-9 | ||||||||||||||||||||||
| 10.1.3 | EINECS Name | Diphenyl ether, octabromo derivative | ||||||||||||||||||||||
| 10.1.4 | Synonyms | Benzene, 1,1'-oxybis-,
octabromo deriv.
Phenyl ether, octabromo deriv. Octabromobiphenyl ether Octabromodiphenyl oxide OBDE The commercial product is a mixture of polybrominated diphenyl ethers (10):
Saytex 111 (a commercial product) (10)
DE-79 (another commercial product of unknown composition) |
||||||||||||||||||||||
| 10.1.5 | Molecular Formula | C12H2Br8O | ||||||||||||||||||||||
| 10.1.6 | Structural Formula | 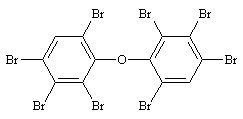
1,1'-oxybis[2,3,4,6-tetrabromobenzene], CAS No. 117964-21-3 Based on the chemical structure, there are 12 possible isomers of OBDE. |
||||||||||||||||||||||
| 10.1.7 | Known uses | Used as a flame retardant in nylon, high impact polystyrene, low density polyethylene, polypropylene copolymer, adhesives and coatings (10) | ||||||||||||||||||||||
| 10.1.8 | EU Classification | Not included in Annex I to Directive 67/548/EEC | ||||||||||||||||||||||
| 10.2 | Physico-chemical Characteristics | |||||||||||||||||||||||
| 10.2.1 | Physical Form | Off-white powders with faint odour (10) | ||||||||||||||||||||||
| 10.2.2 | Molecular Weight | 801.38 | ||||||||||||||||||||||
| 10.2.3 | Melting Point/range (°C) | 75 - 257 depending on the product (10) | ||||||||||||||||||||||
| 10.2.4 | Boiling Point/range (°C) | |||||||||||||||||||||||
| 10.2.5 | Decomposition Temperature (°C) | |||||||||||||||||||||||
| 10.2.6 | Vapour Pressure (Pa (°C)) | < 1.33 x 10-5 (25)(10) | ||||||||||||||||||||||
| 10.2.7 | Relative Density (D420) | 2.76 (10) | ||||||||||||||||||||||
| 10.2.8 | Vapour Density (air=1) | |||||||||||||||||||||||
| 10.2.9 | Conversion Factor (1011 hPa at 25 °C) | |||||||||||||||||||||||
| 10.2.10 | Solubility | Water: < 1 g/l (25°C)
Benzene: 200 g/l (25°C) (10) |
||||||||||||||||||||||
| 10.2.11 | Partition Coefficient (log P ow) | 5.5 or 8.35-8.90 (10) | ||||||||||||||||||||||
| 10.2.12 | Flammability | Not applicable | ||||||||||||||||||||||
| 10.2.13 | Explosivity | None | ||||||||||||||||||||||
| 10.2.14 | Oxidising properties | None | ||||||||||||||||||||||
| 10.3 | Toxicological Data | |||||||||||||||||||||||
| 10.3.1 | Observations in humans | OBDE has been found in human adipose tissue. The levels were from "not detected" to 8 mg/kg fat (8) | ||||||||||||||||||||||
| 10.3.2 | Acute Toxicity | |||||||||||||||||||||||
| 10.3.2.1 | Oral | Oral LD50, rats: > 5,000
mg/kg (DE-79). No rats died during the 14-day observation period (1)
Oral LD50, rats: > 10,000 mg/kg (Saytex 111). Twenty rats (2/sex) were orally administered OBDE at 500, 2,500, 5,000, 7,500, and 10,000 mg/kg. None of the animals died during the study (72 hr) (3) |
||||||||||||||||||||||
| 10.3.2.2 | Dermal | Dermal LD50, rabbits: > 2,000 mg/kg (DE-79). No rabbits died during the 14-day observation period (1, 3) | ||||||||||||||||||||||
| 10.3.2.3 | Inhalation | Inhalation LC50, CD rats:
> 60 mg/l/1 hour (DE-79).Groups of male and female rats were
exposed to 2 or 60 mg OBDE/l air for 1 hour in a whole body exposure
chamber. Particle size distribution was not characterised. No
treatment-related mortalities occurred at either concentration.
Animals in the high concentration group showed tachypnoea, and
animals in both groups displayed decreased motor activity, eye
squint, and erythema (site not stated) during exposure (1).
Inhalation LC50, rats: >52.8 mg/l/1 hour (Saytex 111).One group of 5 male and 5 female rats was exposed to a dust atmosphere of milled OBDE for 1 hour followed by a 14 day observation period. None of the rats died on the study. No gross lesions related to test article were found at gross necropsy (3). |
||||||||||||||||||||||
| 10.3.2.4 | Other Routes | No data were available | ||||||||||||||||||||||
| 10.3.2.5 | Skin Irritation | OBDE (Saytex 111) was not skin irritating (3, 10) | ||||||||||||||||||||||
| 10.3.2.6 | Eye Irritation | OBDE (Saytex 111) was not eye irritating (3, 10) | ||||||||||||||||||||||
| 10.3.2 7 | Irritation of Respiratory Tract | No data were available | ||||||||||||||||||||||
| 10.3.2.8 | Skin Sensitisation | No data were available | ||||||||||||||||||||||
| 10.3.2.9 | Sensitisation by Inhalation | No data were available | ||||||||||||||||||||||
| 10.3.3 | Subchronic Toxicity | |||||||||||||||||||||||
| 10.3.3.1 | Oral | Groups of 35 Charles River
CD rats/sex were fed a diet containing 0, 100, 1,000 or 10,000 ppm
commercial OBDE for 90 days.
Clinical signs, body weight, food consumption, haematology, biochemical and urinalysis were studied after 1 and 2 months and at the end of the study in groups of 5 rats/sex per group. The remaining 20 rats per group were used to study the recovery and 5 rats/sex/group were sacrificed after 13 and 21 weeks and 6 months of withdrawal. A few rats died on test; mainly as a result of blood collection. In the 100 ppm group, absolute and relative liver weight was increased. Hepatic changes in 4 of 10 rats were characterized by granular cytoplasmic changes. Liver total bromine was increased at 13 weeks, but declined during the recovery period. In the 1,000 ppm group a decrease in body weight gain was found, but haematology, blood chemistry and urinalysis were comparable to control. Absolute and relative liver and thyroid weights were increased at 13 weeks, but not at recovery. Hepatic changes included centrolobular and midzonal vacuolisation and hyaline intracytoplasmic inclusions. In the 10,000 ppm group, a decrease in body weight gain was found during treatment and recovery. Changes in some haematology and serum chemistry values were detected. Absolute and relative liver, kidney, thyroid weights were observed. Hepatic changes included granular cytoplasmic changes, cytoplasmic vacuolisation, scattered necrosis, centrilobular fibrosis and pigmented Kupfer cells. Renal changes included the occurrence of small to moderate numbers of cortical regenerative tubules. Lesions in the thyroid were also found. During recovery, the histologic changes decreased in severity and frequency. The total bromine content in the liver increased during the 13 week treatment period and decreased during the recovery period. At the end of the recovery period, bromine levels remained higher than the control values for the liver. A NOAEL could not be established (< 100 ppm ~ approx. 5 mg/kg/day) (3, 10) |
||||||||||||||||||||||
| 10.3.3.2 | Inhalation | Groups of 5 rats/sex were
exposed to dust of commercial OBDE introduced into a inhalation
chamber at nominal concentrations of 0, 0.0012, 0.012, 0.12, and 1.2
mg/l air for 8 hours/day for 14 days. The actual concentrations were
about 15-45% of the nominal concentrations. Particle size
distribution was not characterised.
No animals died on test. Food consumption, body weight gain, haematology, blood chemistry and urinalysis in all dose groups were normal. The total bromine concentrations in lung, liver and fat were statistically significantly higher than in the controls. The average total bromine in lung and fat ranged from about 1.5 to 12.5 times higher than in the liver. The relative liver weights in the 0.012, 0.12, and 1.2 mg/l dose groups were statistically significantly increased in a dose-related manner. These changes were accompanied by histologic lesions consisting of focal to multifocal cytoplasmic enlargement of the hepatocytes, and focal acidophilic degeneration of individual and small groups of liver cells. At the two highest dose levels, the enlargement of the hepatocytes was multifocal to diffuse in distribution and small to large areas had necrosis in the centrolobular regions of the affected liver lobules, especially in the 1.2 mg/l group. The NOAEL was 0.0012 mg/l (nominal concentration) (3, 10) |
||||||||||||||||||||||
| 10.3.3.3 | Dermal | No data were available | ||||||||||||||||||||||
| 10.3.4 | Chronic Toxicity and Carcinogenicity | No data were available | ||||||||||||||||||||||
| 10.3.5 | Mutagenicity | |||||||||||||||||||||||
| 10.3.5.1 | Gene Mutation | Commercial OBDE was examined in vitro for mutagenic activity at a number of concentrations in the Ames assay using Salmonella typhimurium and Saccharomyces cerevisiae with and without metabolic activation. The results of these tests were all negative. (2, 3, 10) | ||||||||||||||||||||||
| 10.3.5.2 | Chromosome Abnormalities | In an in vitro assay for sister chromatid exchange, Chinese hamster ovary cells were exposed to several concentrations of commercial OBDE for 2 hr in the presence or absence of a metabolic activation system. The exposure period was followed by a 24 hr expression period. No statistically significant increase in the number of exchanges per chromosome or the number of exchanges per cell was seen at any dose level tested (3, 10). | ||||||||||||||||||||||
| 10.3.5.3 | Other Genotoxic Effects | An unscheduled DNA synthesis (UDS) assay (in vitro), a test to induce DNA damage followed by repair in mammalian cells, was carried out with WI-38 human fibroblast cells which were exposed to commercial OBDE in the presence of radiolabelled thymidine. OBDE was tested in 5 concentrations with and without metabolic activation. OBDE was negative in this test (3, 10). | ||||||||||||||||||||||
| 10.3.6 | Reproductive Toxicity, Embryotoxicity, and Teratogenicity | |||||||||||||||||||||||
| 10.3.6.1 | Reproductive Toxicity | No data were available | ||||||||||||||||||||||
| 10.3.6.2 | Teratogenicity | Female rats (number not
specified) were dosed daily by gavage from days 6 through 15 of
gestation with 0 (vehicle), 2.5, 10, 15, 25 and 50 mg commercial
OBDE (DE-79)/kg b.w. in a range-finding study. All animals survived
to gestation day 20, when sacrificed. Mean maternal body weight gain
was reduced at 50 mg/kg. Increased number of late resorptions and
statistically significantly reduced mean foetal weight were observed
at the highest dose level. No compound-related microscopic findings
were observed in the liver and kidneys of the dams. No compound
related effects were observed at 15 mg/kg or lower. Malformations
and developmental variations observed in the 50 mg/kg groups were
associated with maternal toxicity. These included foetal anasarca,
bent limb bones, reduced ossification of the skull, various
unossified bones, and two instances of bent ribs.
NOAEL Maternal: 25 mg/kg b.w. NOAEL Teratogenicity: 15 mg/kg b.w.(3, 10). Four groups of 25 pregnant Charles River Crb:COBS CD (SD) BR rats were administered by gavage corn oil suspensions of commercial OBDE (Saytex 111) at doses of 0, 2.5, 10, or 25 mg/kg bw/day on gestation days 6-15. The dams were sacrificed at day 20 of gestation and the foetuses were examined for gross visceral and skeletal abnormalities.The substance was more toxic to the conceptus than to the dam. At the 25 mg/kg dose level, effects on the conceptus included reduce average foetal b.w., increased embryo/foetal deaths (resorptions), foetal malformations such as enlarged heart, rear limb malformation, and delayed skeletal ossification. At 10 mg/kg, the only observed effect was a statistically reduction in average foetal body weight.The maternal NOAEL was 25 mg/kg. The embryo/foetal NOAEL was 2.5 mg/kg. The NOAEL for teratogenicity was 10 mg/kg.(3, 10). Groups of 26 inseminated adult New Zealand white rabbits (weight 3.5-4.5 kg) were treated with 0 (corn oil), 2, 5 or 15 mg commercial OBDPO (Saytex 111)/kg bw/day by gavage on days 7-19 of gestation. Body weight gain was recorded on gestation day 0, 7, 10, 13, 16, 20 and 28. Maternal liver, kidneys and gravid uterine weights were measured at sacrifice. The offspring were examined on day 28 of gestation.A statistically significant increase in liver weight and a decrease in body weight gain was observed in the 15 mg/kg group. There was no statistically significant deviation in maternal mortality, number of pregnancies, number of litters with viable pups, corpora lutea/dam, implantations/dam, liver foetuses/litter, percentage of resorptions and foetal body weight. Slight foetal toxicity was observed in the 15 mg/kg group as evidenced by a significant increase in delayed ossification of the sternebrae. There was an increase in the incidence of retrocaval ureter in the 5 and 15 mg/kg group and fused sternebrae in the 5 mg/kg group. These increases were not dose related. It was concluded by the authors that there was no evidence for teratogenic activity but slight foetotoxicity at the maternally toxic dose level, (e.g., 15 mg/kg bw), was seen. The maternal NOAEL was 5 mg/kg. The embryo/foetal NOAEL was 2 mg/kg. The NOAEL for teratogenicity was 15 mg/kg.(3, 10). |
||||||||||||||||||||||
| 10.3.7 | Other Toxicity Studies | Liver enzymes was induced
by OBDE (commercial products) in a dose and time dependent manner
(10)
When investigated in cultured chick embryo liver cells, OBDE was strongly pophyrinogenic (10) |
||||||||||||||||||||||
| 10.3.8 | Toxicokinetics | Measurement of total bromine content in various tissues after repeated oral or inhalation exposure to OBDE (commercial products) indicate some absorption by these routes (10) | ||||||||||||||||||||||
| 10.4 | Ecotoxicity | Only few data were available. LC50 in fish was > 0.5 mg/l (Oryzias latipes, 48h) in 20 g/l dispersing agent (4). NOEC daphnia was >0.002 mg/l (Daphnia magna, 21d) in a OECD 202, GLP, flow through test (5). The bioconcentration Factor (BCF) in fish was <4 (carp, 8 week exposure) at 10 µg/l and 100 µg/l exposure concentration (9). Different Log Pow 8.35-8.9 (9) and 10.33 (QSAR estimation) have been reported. | ||||||||||||||||||||||
| 10.5 | Environmental Fate | No biodegradation of octabromobiphenyl ether was found in a closed bottle test (OECD 301D; 28d) (7). | ||||||||||||||||||||||
| 10.6 | Environmental Concentrations | Octabromobiphenyl ether was found in sediments in concentrations of 0.008 to 0.53 mg/kg ww highest close to manufacturer site. In fish the highest concentration of 0.325 mg/kg was measured in dab (liver), UK. <0.001 - 0.179 mg/kg were reported in fish liver and muscle, UK (6). | ||||||||||||||||||||||
| 10.7 | Conclusion | |||||||||||||||||||||||
| 10.7.1 | Health Assessment | Sufficient toxicological
data were identified for a health assessment of OBDE. Most of the
data were taken from a review performed by WHO. Many of the
toxicological studies were performed on old commercial OBDE products
of low OBDE purity and high HpBDE content, and they were not
performed according to today's standards. No data on sensitisation
and long term toxicity and carcinogenicity were identified. Few
relevant data on humans were identified.
OBDE has a low acute toxicity and low irritative potential. Repeated doses of OBDE induced liver changes, indicative of an inducer effect. OBDE is not considered mutagenic. Exposure of pregnant rats and rabbits indicated that the foetuses were more sensitive than the dams. Evidence of teratogenicity was found in one rat study. |
||||||||||||||||||||||
| 10.7.2 | Environmental Assessment | Only few data were available for environmental classification. OBDE is suspected to cause long-term adverse effects in the aquatic environment. | ||||||||||||||||||||||
| 10.8 | References | 1. Initial submission:
Letter from great lakes chem corp to USEPA regarding ITC request for
information on brominated flame retardants (53 FR 5466) with
attachments, dated 05/17/88. EPA/OTS; Doc #FYI-OTS-0794-1106 1994.
NTIS/OTS0001106.
2. Final report, bacterial reverse mutation assay with octabromodiphenyl oxide, with cover letter dated 9/25/96. EPA/OTS; Doc #86960000603 1996. NTIS/OTS0558804. 3. Anonymous. Diphenyl ether, octabromo derivative. International Uniform Chemical Information Data-base (IUCLID). Version 1. European Commission. Joint Research Centre. Environment Institute. European Chemicals Bureau; 1996. CD-ROM. 4. Chemicals Inspection and Testing Institute Japan (CITI). Biodegradation and bioaccumulation data for existing chemicals based on the CSCL Japan. Japan Chemical Ecology-Toxicology and Information Centre, 1992. 5. Grawes WC, Mank MA, Swigert JP. Octobromodiphenyl oxide (OBDPO): A flow-through life-cycle toxicity test with the Cladoceran (Daphnia magna). Wildlife International Ltd, 1997. 6. Law RJ, Allchin CR, Morris S, Reed J. Analysis of brominated Flame Retardants in Environmental Samples. Ministry of Agriculture, Fisheries and Food, Directorate of Fisheries Research, Burnham-on Crouch, 1996. 7. Schaefer EC, Harberlien D. Octabromodiphenyl oxide (OBDPO): Closed Bottle Test. Wildlife Inter-national Ltd, Maryland, United States, 1996. 8. Stanley JS, Cramer PH, Thornburg KR, Remmers JC, Breen JJ, Schwemberger J. Mass spectral confirmation of chlorinated and brominated diphenylethers in human adipose tissues. Chemosphere 1991; 23(8-10):1185-95. 9. Watanabe I, Tatsukawa R. Anthropogenic brominated aromatics in the Japanese environment. Work-shop on Brominated Aromatic Flame Retardants. Swedish National Chemicals Inspectorate (KemI), Solna Sweden, 1990. 10. WHO working group. Brominated diphenyl ethers. Environmental Health Criteria 1994; 162. |