Occurrence and fate of antibiotic resistant bacteria in sewage
2. Methodology
2.1 Sampling sites, times and methods
As part of the study samples of sewage were collected from sewers and sewage treatment plants. The following two sections detail the sites sampled, sampling times and methods used.
2.1.1 Sampling at sewers
Samples of sewage were collected from two separate sewers receiving waste effluent from a hospital and a pharmaceutical plant manufacturing products containing antibiotics (Paper 1). Sampling sites were situated upstream (site A) and downstream (sites B and C) from the effluent discharge points of the hospital and the pharmaceutical plant (Fig. 2.1). At the hospital, site A was situated 350 m upstream from the discharge point, site B was 60 m downstream and site C was 500 m downstream. At the pharmaceutical plant, site A was located 70 m upstream from the discharge point, site B was 30 m downstream and site C was 250 m downstream.
The selection of the sampling sites was restricted by the access to the sewer system for the collection of samples. No important sources of waste effluent were present between sites A and sites B. Therefore, differences in the occurrence of resistant bacteria between these sites could be used to evaluate the impact associated with the discharge of waste effluent.
Samples were collected between June and September 1997, for a total of four sampling times. Samples of sewage mixed with sediment particles were aspirated from the bottom of sewers using sterile catheters applied to 150 ml sterile syringes. Samples were delivered to the laboratory within one hour after their collection.
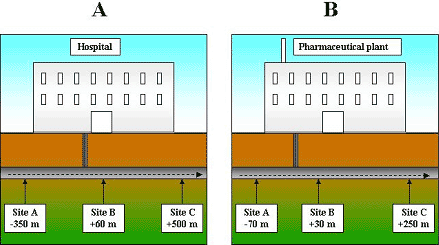
Figure 2.1.
Schematic representation of the sampling sites at the sewers receiving waste
effluent from the hospital (A) and the pharmaceutical plant (B)
2.1.2 Sampling at sewage treatment plants
Samples of raw sewage, treated sewage and anaerobically digested sludge were collected at two large-scale treatment plants in Denmark, Avedøre Spillevandscenter I/S and Lynettefællesskabet I/S (Paper 4). The catchment areas of the two plants have a population of approximately 240,000 and 500,000 inhabitants, respectively. At both plants, sewage undergoes tertiary (advanced) treatment. The treatment process includes: retention of large solids by mechanical screens; separation of sand, grit and grease in aerated chambers; sludge sedimentation in primary settling tanks; biological removal of nitrogen and phosphorus by activated sludge units (supplemented by chemical phosphorus precipitation), and secondary clarifiers.
Sampling was performed monthly from August 1999 to January 2000, for a total of six sampling times at each plant. Twenty-four hour flow-proportional samplers were used to collect raw sewage from the influent, and samples of treated sewage from the final effluent. Grab samples of digested sludge were collected from the digesters following centrifugation. All samples were processed in the laboratory within 6 hours of their collection.
2.2 Measurement of antibiotic resistance
Two different methods were used for measurement of antibiotic resistance of bacteria in sewage samples. Acinetobacter isolates were randomly isolated without inclusion of antibiotics in the medium (non-selective method) and the prevalences of antibiotic resistance were evaluated based on antibiotic susceptibility testing of large numbers of isolates. In addition, total and relative numbers of resistant bacteria were calculated based on bacteriological counts on media containing antibiotics. The latter method differs from the former, in that bacteria are selectively isolated with regard to their antibiotic resistance properties (selective method).
2.2.1 Use of Acinetobacter as a bacterial indicator
One of the main innovative aspects of this project was the use of Acinetobacter as a bacterial indicator. In fact, most previous studies investigating the occurrence of resistant bacteria in sewage were performed using coliforms as bacterial indicators. The choice of Acinetobacter was prompted by the natural occurrence of this organism in the aquatic environment. Based on this characteristic, Acinetobacter was considered a more representative indicator of the aquatic bacterial flora in contrast to coliforms, which occur in sewage mainly through human and animal contamination.
In the last decade, Acinetobacter has assumed an increasing importance as an opportunistic human pathogen, causing infections often refractory to antibiotic treatment 52. The particular ability of Acinetobacter to develop antibiotic resistance suggested us that this organism that could be used as a sensitive indicator for monitoring antibiotic resistance. Furthermore, Acinetobacter represents a good model for studying the ecology of antibiotic resistance genes, as it occurs ubiquitously in a large variety of habitats, including water, soil, food, animals and humans (201).
Several practical features also contributed to the choice of Acinetobacter as a bacterial indicator. Acinetobacter is a non-fastidious organism in the laboratory and therefore allows antibiotic susceptibility testing by standardised procedures. Furthermore, the availability of a pre-established selective enrichment medium and a genus-specific DNA probe allowed us to develop a rapid method for isolation and identification (see section 2.3.1). This aspect was of primary importance in the choice of the bacterial indicator, as large numbers of isolates are required for statistically validating data on antibiotic resistance.
2.2.2 Antibiotic susceptibility testing of Acinetobacter isolates
Antibiotic susceptibility testing of Acinetobacter isolates was performed by the disc-diffusion method in accordance with the Swedish Reference Group for Antibiotics 53. All isolates were tested against antimicrobial compounds representative of six antibiotic classes: amoxicillin or ampicillin (penicillins), chloramphenicol (phenicols), ciprofloxacin (quinolones), gentamicin (aminoglycosides), tetracycline or oxytetracycline (tetracyclines), and sulfamethoxazole (sulfonamides) or sulfamethoxazole/trimethoprim (potentiated sulfonamides). Acinetobacter isolates from the two sewage treatment plants were additionally tested against aztreonam (monocyclic beta-lactam), cefoxitin (2nd generation cephalosporin), cefotaxime (3rd generation cephalosporin), imipenem (carbapenem), nalidixic acid (older quinolone), piperacillin (piperazine-penicillin), amikacin and tobramycin (aminoglycosides).
The breakpoints for classification of resistant and susceptible isolates were empirically selected based on the distribution of the inhibition zone diameters. The selection of the breakpoints was facilitated by the demonstration of two distinct sub-populations constituting resistance and susceptibility. In most cases, the elected breakpoints did not differ significantly from those used for defining resistance in clinical Acinetobacter isolates. In the few instances where they did not correlate, both empirical and clinical breakpoints were used (Paper 4).
2.2.3 Enumeration of resistant coliforms in sewage
Bacteriological counts of total and resistant coliforms were performed by the streak plate method. Total coliforms were enumerated on MacConkey agar following 24 hours incubation at 37° C, without confirmation of presumptive coliforms for gas production. Resistant coliforms were enumerated on the same medium containing ampicillin (16 m g/ml), gentamicin (8 m g/ml), tetracycline (8 m g/ml), or all three antibiotics using the same incubation conditions. The percentages of antibiotic resistance were then calculated for each sample as the number (CFU/ml) of resistant coliforms divided by the number of total coliforms.
Ampicillin, gentamicin and tetracycline were selected as representatives of important classes of antibiotics: beta-lactams, aminoglycosides and tetracyclines, respectively. The antibiotic concentrations added to the medium were in accordance with the minimum inhibitory concentration (MIC) breakpoint values for definition of resistance in clinical practice 53.
2.2.4 Enumeration of resistant acinetobacters in sewage
Total numbers and percentages of resistant acinetobacters (i.e. presumptive Acinetobacter spp. not identified by the genus-specific probe) in sewage were determined as described above, with the exception of the agar (Baumann agar) and the incubation conditions (48 hours at 30° C) used.
2.2.5 Enumeration of total culturable resistant bacteria in blue mussels
Blue mussels were homogenised in a stomacher (5 g blue mussels in 10 ml sterile water for 30 s) and the obtained homogenate serially diluted ten-fold. Total numbers and percentages of culturable antibiotic-resistant bacteria were then determined as described above, with the exception of a different agar (Mueller-Hinton agar) and different incubation conditions (48 hours at 30° C). Mueller-Hinton agar was considered particularly suitable for enumeration of total culturable resistant bacteria, as it does not contain substances that could adversely affect the activity of antibiotics incorporated and permits satisfactory growth of most culturable bacterial species.
2.2.6 Enumeration of resistant E. coli in blue mussels
Total numbers and percentages of antibiotic-resistant E. coli in blue mussels were determined as detailed above, with the exception of a different agar (tryptone bile agar with X-glucoronide) and different incubation conditions (24 hours at 44°C).
2.2.7 Statistical analysis
Statistical analysis of data on antibiotic resistance was performed using either Statistix (Analytical Software, USA)(Paper 1) or SAS version 6.12 (SAS Institute Inc., USA)(Paper 4). The analyses were used to determine any statistically significant associations between antibiotic resistance (outcome variable) and sampling sites (independent variable), including the variable sampling time to allow for any confounding effect of time.
Data derived from antibiotic susceptibility testing of Acinetobacter isolates (dichotomous data) were analysed by logistic regression analysis. When such analysis was not appropriate due to excessive variability of the data, chi-square analysis was performed separately for each sampling time (Paper 1). Data derived from bacteriological counts (continuous data) were analysed by linear regression analysis, after testing whether the residuals were normally distributed.
In the study relating to the effects of waste effluent from the hospital and the pharmaceutical plant, comparisons between sites A and B were carried out to determine whether the discharge of waste effluent was associated with an increase in the occurrence of resistant isolates. Comparisons between sites B and C were carried out to provide information concerning variations in the prevalence of resistant Acinetobacter depending on the distance from the discharge point.
In the study concerning the effects of sewage treatment, the statistical analysis was performed separately for each plant. Data pertaining to raw and treated sewage were compared to assess the effect of sewage treatment on the prevalence of resistant bacteria. Data concerning raw sewage and digested sludge were compared to assess the effect of sludge treatment on the prevalence of resistant bacteria.
2.3 Identification and typing of bacteria and resistance genes
During the project, bacteria were characterised at various levels using phenotypic and genotypic methods. Acinetobacter isolates were identified at the genus level prior to antibiotic susceptibility testing. A number of isolates was characterised by phenotypically and their plasmid content determined to detect possible effects on strain distribution caused by the discharge of waste effluent from the pharmaceutical plant. Ribotyping was used to confirm the identity of strains introduced into membrane diffusion chambers during the in situ experiment on survival of multiple-resistant bacteria. Finally, tetracycline resistance genes were also typed to determine whether the same classes of resistance genes occurred in both clinical and aquatic Acinetobacter strains.
2.3.1 Identification of Acinetobacter at the genus level
Acinetobacter isolates were identified at the genus level by colony hybridisation using a genus-specific DNA probe. In order to enhance the detection of Acinetobacter, colony hybridisation was performed in combination with the use of the Baumann medium, which is a selective medium based on the ability of Acinetobacter to grow using acetate as the only carbon source 54. The protocol used for colony hybridisation is described in Paper 1.
2.3.2 Identification of Acinetobacter at the species level
A subset of Acinetobacter isolates from the sewers at the hospital and the pharmaceutical plant (n=43) was identified at the species level by phenotypic tests. The following tests were performed: growth at 37°C in Brain Hearth Infusion broth (BHI), acidification of glucose, haemolysis of sheep blood, utilisation of citrate, azelate, glutarate, L-histidine, DL-lactate, L-leucine, L-phenylalanine and L-arginine. The methods used for each test and the criteria used for identification are described in Paper 2.
2.3.3 Plasmid profiles
The same subset of Acinetobacter isolates from the sewers at the pharmaceutical plant and the hospital was characterised by plasmid profiling. Plasmids were isolated by a hot alkaline method 55 modified by the addition of lysozyme, and then detected by gel electrophoresis in 0.8% agarose gels.
2.3.4 Ribotyping
Due to its high discriminatory power, ribotyping was considered particularly suitable to confirm the identity of strains inoculated into membrane-filter chambers during the performance of the in situ pond experiment (see section 2.5.3).
Ribotyping was performed as previously described 56 using the restriction enzyme Hind III for DNA digestion. The method entails digestion of bacterial DNA by restriction enzymes followed by DNA hybridisation with rRNA-based probes.
2.3.5 Typing of tetracycline resistance genes
Fifty tetracycline-resistant Acinetobacter isolates from clinical specimens (n=35), sewage (n=10) and aquaculture habitats (n=5) were analysed by PCR for the occurrence of tetracycline resistance genes of classes A to E, which are the predominant classes among Gram-negative bacteria. The PCR primers and protocols used for the typing of tetracycline resistance genes are described in Paper 3.
2.4 Experiments on transfer of tetracycline resistance
In vitro experiments on the transfer of tetracycline resistance between Acinetobacter strains isolated from different environments and belonging to different species were carried out to assess their ability to transfer tetracycline resistance genes. We decided to focus our attention on tetracycline resistance due to the frequent occurrence of this resistance observed in Acinetobacter isolates originating from sewage and aquaculture habitats. Furthermore, tetracycline resistance was considered particularly suitable for studying the ability to transfer antibiotic resistance among aquatic bacteria as it is usually mediated by genetic transfer and only rarely determined by chromosomal mutations.
2.4.1 Bacterial strains
Twenty tetracycline-resistant Acinetobacter strains were used as donors in the mating experiments, including 10 strains from sewage, 5 strains from aquacultural habitats and 5 strains resulting from clinical outbreaks. The five clinical strains belonged to the species A. baumannii and originated from different European countries. Most aquatic strains (8/15) were identified phenotypically as A. lwoffii and A. johnsonii, the two species prevalent in the aquatic environment. The remaining strains belonged to A. junii (n=3), A. haemolyticus (n=1), the unnamed genomic species 16BJ (n=1) or had atypical phenotypic traits precluding speciation (n=2).
Rifampicin-resistant mutants obtained by the gradient plate method 57 were used as recipients. These were strains derived from tetracycline-sensitive Acinetobacter strains isolated from an unpolluted stream (recipient A) and sewage (recipients B and C). According to phenotypic identification, recipients A and B belong to unknown Acinetobacter species and recipient C belongs to A. calcoaceticus.
2.4.2. Mating experiments
Mating experiments were performed on solid media (Luria Bertani agar) as described in Paper 3. The acquisition of tetracycline resistance by recipient strains was confirmed by phenotypic tests differentiating between donors and recipients. Plasmid profiling was used to detect relocation of plasmid DNA from donor to recipient strains. Mating experiments in which transfer of tetracycline resistance was demonstrated, were studied to determine whether transfer was mediated by conjugation or transformation. In order to exclude transfer mediated by transformation, experiments were repeated in the presence of deoxyribonuclease I, an enzyme destroying extracellular DNA. In addition, the ability of the recipient strains to acquire resistance by transformation was tested by experiments in which DNA extracted from the donor strains was used as a source of resistance genes.
2.5 Experiments on survival of multiple-resistant bacteria in natural waters
Three multiple-resistant strains isolated from treated sewage were investigated for their ability to survive in natural waters and retain antibiotic resistance. This was tested using laboratory seawater microcosms and membrane-filter chambers immersed in a pond. The multiple resistance phenotypes characteristic of these strains were used as selective markers for their detection in the presence of indigenous bacteria. The experiments were performed using low bacterial inoculums (103 to 104 CFU/ml) with the scope to reproduce the actual conditions occurring when treated sewage is released into natural aquatic recipients.
2.5.1 Bacterial strains
Three multiple-resistant strains previously isolated from the final effluent of the Lynetten treatment plant, were used for the survival experiments. The strains were identified phenotypically as Acinetobacter johnsonii (strain B1), Escherichia coli (strain M1) and Citrobacter freundiii (strain M2) by the API identification system (Biomerieux, France). All three strains were resistant to ampicillin, gentamicin and tetracycline. In addition, strain B1 was resistant to tobramycin, nalidixic acid, potentiated sulfonamides, piperacillin and aztreonam. Strain M1 was resistant to nalidixic acid, ciprofloxacin and intermediate resistant to chloramphenicol. Strain M2 was resistant to chloramphenicol, potentiated sulfonamides and cefoxitin.
The strains were detected using Baumann agar (strain B1) and MacConkey agar (strains M1 and M2) containing ampicillin (16 m g/ml), gentamicin (8 m g/ml) and tetracycline (8 m g/ml). No bacterial growth was observed when these media were inoculated with either seawater or pondwater used for the survival experiments, indicating that bacteria with this multiple resistance phenotype were not present in the indigenous microflora.
2.5.2 Laboratory seawater microcosms
The first survival experiment was performed in microcosms containing seawater collected from Øresund. Microcosms (n=7) consisted of 200 ml Erlenmeyer flasks containing 100 ml of either untreated seawater (n=3) or autoclaved seawater (n=3). The three strains were inoculated into separate flasks to reach the final concentration of approximately 5 ´ 103 CFU/ml. One flask containing untreated seawater was not inoculated with any of the strains and served as a control to observe the behaviour of the indigenous microflora alone.
Flasks were maintained at room temperature under gentle agitation. Samples were collected from each flask immediately after inoculation (day 0) and after 8 h, 24 h (day 1), 48 h (day 2), 1 week (day 7), 2 weeks (day 14), 3 weeks (day 21), and 4 weeks (day 28). For each sampling time, counts of the strains under study were performed with the antibiotic-selective media described above. In addition, numbers of total culturable bacteria were enumerated on Tryptic Soya agar following 48 hours of incubation at 30° C.
2.5.3 In situ pond experiment
The in situ experiment was performed in a freshwater pond using membrane-filter chambers (Technical Services, Montana State University, USA). These chambers are designed to allow diffusion of water and solutes but without diffusion of the bacteria contained in the chambers (Fig. 2.2). Two bacterial suspensions containing approximately 104 CFU/ml of each strain were prepared using water collected from the pond, either without any previous treatment (chamber 1) or autoclaved (sterilised) (chamber 2). Immediately after preparation, 100 ml of the suspensions were inoculated into the chambers, which were then immersed into the pond.
During the experimental period (November-December 2000), the water temperature in the daytime varied from 6 to 9° C, the pH was approximately 7.5 and the concentration of dissolved oxygen was between 11 and 12 mg/L. Samples were collected from the chambers and the pond immediately after inoculation (day 0) and after 4 days (day 4), 1 week (day 7), 2 weeks (day 14), 3 weeks (day 21), and 4 weeks (day 28). Enumeration of the strains under study and total culturable bacteria was performed as described previously. At day 28, representative colonies were isolated from the media used for enumeration of the strains and characterised by phenotypic tests (i.e. colony morphology, cell morphology, glucose O/F and cytochrome oxydase test) and ribotyping to confirm strain identity.
An enrichment procedure was used for detection of stressed cells following 28 days of incubation into the chambers. For this purpose, 1 ml of water was collected from each of the chambers and serial ten-fold dilutions were prepared using peptone buffered water. Dilution tubes were incubated for 48 h at 30° C under gentle agitation and, subsequently analysed for bacterial growth visually.

Figure 2.2.
A membrane-filter chamber used in the in situ pond experiment.