Feminisation of fish
11. Fate in municipal sewage treatment plants
Influents, effluents and sewage sludge of STPs have been monitored in several countries, e.g. Germany, Switzerland, Italy, the U.K., Spain, the U.S., Canada, Japan, and Denmark, with the purpose to study the occurrence of estrogens as well as xenoestrogens. A considerable amount of these studies have only included analysis of effluents, which is certain to give a picture of the concentration levels and the possible amount of substance discharged to the aquatic environment. However, more detailed monitoring of the material streams around each single process in a STP is needed for assessment of the fate of a substance in the treatment plant.
Results are presented in the scientific literature from examinations of parallelly collected influent and effluent samples from STPs and some times also samples of sewage sludge. However, it should be born in mind that variable procedures of sampling have been used from one study to another and, in some cases, even within the same study. Also the applied analytical methods including their limits of detection and determination vary between different studies. These facts limit the possibilities of an exhaustive evaluation of the fate of the compounds in STPs and of an assessment of the effect of different treatment processes.
A review of the effluent concentrations of the substances of concern is presented in Chapter 5. This chapter will primarily focus on investigations, which allow an assessment of the behaviour of the substances in a STP, i.e. studies which, as a minimum, have included simultaneous monitoring of either influent and final effluent from a STP or in- and outlet of specific treatment processes within a STP.
11.1 Estrogens
11.1.1 Biodegradation in sludge of STPs
The majority of natural estrogens and contraceptive compounds are excreted from humans as a variety of inactive glucuronide or sulfonide conjugates as described in Chapter 10. It has been questioned whether these inactive conjugates are cleaved in the STP and perhaps already in the raw sewage and thereby released to the environment as active estrogens. The detection of several unconjugated estrogens like estradiol, estrone, estriol and ethinylestradiol, in the effluents from STPs supports this hypothesis.
The biodegradability of (4-14C)-estradiol has been examined according to the principle in semicontinous activated sludge test (SCAS test) described in the OECD Guideline No. 302A and the "Activated sludge biodegradability simulations test", Environmental Project No 337 (83). The test simulated an activated sludge basin at municipal STPs. The biodegradation was investigated under both aerobic and anoxic conditions during 120 h at 15 °C. The experiments were made in triplicates. Reactors of one litre were inoculated with activated sludge from Måløv STPs in Denmark achieving a final concentration at 5 g suspended solid (SS)/l. Filtrated supernatant from settled activated sludge was used as substrate at the initiation of the test and later a peptone medium. This medium was supplemented with nitrate in the anoxic experiments. The initial concentration of estradiol was approx. 20 µg/l. The total and dissolved amounts of 14C in the reactors were analyzed by liquid scintillation. Determination of the mineralisation rates of (4-14C)-estradiol under aerobic condition showed a first order rate constant for total and dissolved estradiol of 0.031 ± 0.003 l/d/g SS and 0.052 ± 0.003 l/d/g SS, respectively, when the concentration was less than 2.5 µg/l. No significant degradation of (4-14C)-estradiol was observed in the anoxic test system. Average sludge distribution coefficients Kd for 14C labelled compounds in the aerobic and anoxic test system with Måløv sludge were estimated at 0.25 ± 0.04 l/g SS and 0.96 ± 0.10 l/g SS, respectively (83).
The aerobic transformation of natural estrogens, contraceptives and estradiol glucuronides has recently been studied in a batch experiment using suspensions of activated sludge at 0.26 g SS/l as inoculum (80). The experiment showed that the glucuronide conjungates of estradiol were de-conjugates relatively fast. When spiking the sludge slurry with approx. 1 mg/l of estradiol glucuronides, both estradiol and estrone were detected after only 15 min. Approx. 70 % of the conjungated estradiol were found in the oxidized form, estrone, at the maximum concentration after 20-30 h. However, estradiol was still detected after 28 h indicating that the cleavage of the glucuronide was not completed. That glucuronides are cleaved in slurries of activated sludge was confirmed in another experiment with a spiking level at 1 µg/l and in this case with even higher turnover rates. Furthermore, it was found that, when in contact with activated sludge, the estradiol was oxidized into estrone that was further eliminated. The contraceptive ethinylestradiol was principally persistent under the condition used in the experiment.
Furthermore, the transformation of the inactive excreted glucuronides into the active estrogens in sewage have been documented by Panter et al. (81). They suggested that estradiol-3-glucuronide were converted into active estrogens after having been inoculated with activated sludge. The suggestion was based on studies of in-line degradation systems allowing fish to be exposed before and after biodegradation of conjugated estradiol. The studies demonstrated that solutions after passing through a degradation system with activated sludge microorganisms were highly estrogenic.
Layton et al. (84) have performed a series of biodegradation studies with steroidal hormones in laboratory assays inoculated with biosolids obtained in the period 1998-2000 from aeration basins of four different STPs in the U.S.A. The initial concentrations of the estrogenic hormones, 17b -estradiol and 17a -ethinylestradiol, were 58 and 72 µg/l, respectively. The steroids were 14C-labelled on the C-4 carbon of the steroid backbone. Therefore, release of 14C-CO2 from these compounds would denote ring cleavage and concomitant inactivating of the steroid molecule. In biosolids taken from the aeration basin of a municipal STP, 84 % of 14C-estradiol and 85% of 14C-estrone were mineralized to 14C-CO2 within 24 hours of incubation. The mineralisation of the same compounds in biosolids from an industrial STP was considerably lower (4%). This confirmed the importance of an adapted microbial population in the biological removal of estrogens. There were no statistical differences in the mineralisation in biosolids between municipal STPs with different operations parameters, e.g. different percentage of BOD removal and percentage of suspended solids removal.
Investigation of the mineralisation of ethinylestradiol confirmed the results of Ternes et al. (80). The mineralisation rate of ethinylestradiol was low compared to the rate of estradiol resulting in a removal of 20 % 14C-ethinylestradiol versus 75 % of 14C-estradiol in the same type of biosolid after 24 h of incubation (84). Determination of the first-order rate constant k for removal by mineralisation or removal from the aqueous phase of the 14C-steroids shows no significant differences for ethinylestradiol at temperatures differing by 10-15°C. The observed differences of k values for estradiol at different temperatures were statistically significant. However, it was noted by Layton et al. (84) that the initial mineralisation rates of estradiol at 5-10 °C were 200 ng/l in a minute suggesting that, even at low temperatures, estradiol is rapidly removed. Sorption was not found to be the rate-limiting step at the tested concentrations of estradiol. The obtained rate constants for mineralisation of estradiol were 0.0029 ± 0.0002 min-1 and 0.0042 ± 0.0002 min-1 at 5-10°C and 22-25°C, respectively. The rate constant for mineralisation of ethinylestradiol were 0.0001 ± 0.0000 min-1 and 0.0002 ± 0.0000 min-1 at 5-10°C and 22-25°C, respectively.
Degradation of [3H]-ethinylestradiol has been studied in a batch experiment with activated sludge grown in a laboratory continuously fed activated sludge (CAS) reactor. The reactor was fed with a mineral medium allowing the growth of a highly active nitrifying sludge (50 mg NH4+/g dry weight/h). Ethinylestradiol (50 µg/l) added to sludge from this reactor together with hydrazine was degraded or transformed to hydrophilic products within six days of incubation at 20°C. The degradation products were not identified. In a similar experiment in activated sludge with an ammonium oxidation rate of only 1 mg NH4+/g dry weight/h no detectable degradation of ethinylestradiol was observed (233).
It should be noticed that the steroid estrogens were the sole added carbon source besides the naturally occuring carbon in the activated wastewater sludge in the degradation studies performed by Ternes et al. (80), Layton et al. (84) and Vader et al. (233). The situation is quite different in a full-scale STP, in which the influent consists of a broad spectrum of different carbon sources. It is, therefore, difficult to assess the situation in a real STP based on the degradation rates obtained in the present lab-scale studies.
The possible initial degradation steps of estradiol glucuronides based on the results of the studies presented above are shown in Figure 11.1.
estradiol-glucuronide ¾® estradiol ¾®
Figure 11.1
Initial degradation steps of estradiol-glucuronides in aerated activated
sludge
11.1.2 Sorption to sludge particles
Upon examination of log Pow for the estrogens of concern, one will expect that ethinylestradiol (log Pow = 4 (234)) and estradiol (log Pow = 4.01 (Pomona 1987)) are likely to be sorbed to sludge and possibly also estrone (log Pow = 2.1-3.5 (235), (234)). Estriol with a log Pow at 2.7 (234) is, however, considered to be less hydrophobic and binding to sludge would be more unlikely to dominate the fate of estriol.
11.1.3 Fate in STPs
There are no available data on extensive investigations of the fate of estrogens in STPs covering the different process step throughout the STP and only few studies include analysis of samples from internal streams of STPs. Shore et al. (236) studied samples of raw sewage, reaction fluid after aerobic treatment, the supernatant of activated sludge after digestion and the final effluent taken from a STP in Israel in 1991. Analysis of estradiol using radioimmunoassay showed concentrations of estrogens in raw sewage at 48-141 ng/l and a relative removal of estrogens in the water phase of 20-88 %.
A study of 27 STPs was performed in Japan during 1998-1999. The study was made three times during the season, one in the summer, one in the autumn, and one in the winter (89). The STPs used mostly an activated sludge process. Some of the plants had nitrogen and phosphate removal and the plants were, furthermore, equipped with different disinfection treatments. Samples were taken of the influent of the STPs, the influent of the primary sedimentation tank (i.e. after mixing with reject water from the sludge treatment process), the effluent after primary sedimentation and the effluent after final sedimentation before and after desinfection. Analysis of 17b -estradiol in the samples showed median removal efficiency in the water phase, i.e. from influent to final effluent at 69 % and 64 % in the autumn and the winter studies, respectively. The fate of 17b -estradiol in the sewage treatment process was illustrated with an example in the work of Nasu et al. (89). The median values of these data are shown in Appendix A. The data indicate that there was a minor increase in the influent concentration of 17b -estradiol after mixing with reject water and after the primary sedimentation. However, the differences do not seem to be statistically significant. The biological treatment process and the final sedimentation resulted in a distinct reduction of the 17b -estradiol concentration, while no further reduction was observed after the disinfection steps.
Ternes et al. (86) sampled corresponding influent and effluent samples of a German and a Brazilian municipal STP in 1997. The sampling at the German STP, furthermore, included samples taken from the effluent from the primary sedimentation tank. The Brazilian STP operated an aeration tank and a trickling filter (biological filter) in parallel. Effluent samples were collected from both of these biological treatment processes. The analyses of the samples included estrone, 17b -estradiol, 16a -hydroestrone (only German STP) and 17a -ethinylestradiol. The investigation of the German plant showed a minor elevation of the loads of estrogens and especially of estrone after the primary sedimentation (Appendix A). Ternes et al. (86) suggested that despite the statistically insignificant differences, this increase could be caused by a cleavage of conjungates like glucuronides, which are the principal excreted metabolites, during the STP process.
The investigation of the Brazilian plant showed that there were distinct differences between the reduction rate of the estrogens in the aeration tank and that of the trickling filter (Appendix A). The highest removal efficiency of the estrogens was found in the aeration tank, where removal was in the range of 78-99.9 % whereas the removal efficiency in the trickling filter was 64-92 %. The removal of 17b -estradiol (92-99.9 %) was higher than that of estrone (67-83 %) and 17a -ethinylestradiol (64-78 %) in both treatment processes.
The removal efficiencies in the German plant were remarkably lower than in the Brazilian plant. It was for instance observed that only 64 % of 17b -estradiol and ~ 0 % of the 17a -ethinylestradiol were removed during the overall treatment process. The low temperature in the German sampling period of –2 °C in average compared to above 20 °C in Brazil may have caused this difference.
Lee & Peart (85), Ternes et al. (86), Baronti et al. (87), Johnson et al. (88) and Nasu et al. (89) have all reported concentrations of estrogens in samples taken from either the influent or the effluent of primary sedimentation tanks and the final effluent from different STPs. The five investigations cover examination of 15 different STPs in total (Appendix A). There are no significant differences in the influent concentrations for the five series of investigations taking into account the use of different sampling techniques and analytical methods. They are all within the same concentration range for the four estrogens of concern according to the summarized data in Table 11.1. The result of the analyses of the corresponding effluent samples shows that the concentrations of all the estrogens are reduced during the treatment process in the STPs ( Table 11.2). The removal of 17b -estradiol and estriol are generally more extensive than the removal of estrone and 17a -ethinylestradiol. This is in accordance with the finding in the laboratory studies of the degradation of 17b -estradiol, estrone and ethinylestradiol of Ternes et al. (80) and Layton et al. (84) described above (section 11.1.1). The mean removal efficiencies based on the results from the examination of thirty parallel influent and effluent samples from five STPs in the extensive study of Baronti et al. (87) were following:
| Estrone % removal:61 ± 38 | |
| Estradiol % removal:87 ± 9 | |
| Estriol % removal:96 ± 6 | |
| Ethinylestradiol % removal:85 ± 14 |
Table 11.1
Concentration ranges of estrogens in influent of STPs. Conjugates as e.g.
glucuronides were not included in the analyses.
Substance |
Unit |
Lee & Peart |
Ternes et al. |
Baronti et al. |
Johnson et al. 2 |
Nasu et al. |
Estrone |
(ng/l) |
41-75 |
27; 40 |
25-90 (132)1 |
11-87 (140)1 |
|
17b -estradiol |
(ng/l) |
<5-15 |
15; 21 |
4.0-25 |
11-48 |
20-94 |
Estriol |
(ng/l) |
158-250 |
|
25-188 |
|
|
17a-ethinylestradiol |
(ng/l) |
|
1.2; 6 |
0.4-13 |
<0.2-8.8 |
|
1: a single measurement
2: data from three STPs in the Netherlands
3. data from one STP in Canada
Table 11.2
Concentration ranges of estrogens in effluents of the same STPs as in Table 11.1.
Conjugates as e.g. glucuronides were not included in the analyses.
Substance |
Unit |
Lee & Peart |
Ternes et al. |
Baronti et al. |
Johnson et al. 1 |
Nasu et al. |
Estrone |
(ng/l) |
14-18 |
6.8-23 |
2.5-82 |
2.1-47 |
|
17b -estradiol |
(ng/l) |
<5 |
0.2-5.4 |
0.35-3.5 |
<0.6-12 |
<0.2-55 |
Estriol |
(ng/l) |
30-37 |
|
0.43-18 |
|
|
17a -ethinylestradiol |
(ng/l) |
|
1.3-2.7 |
<0.3-1.7 |
<0.2-<1.8 |
|
1: data from three STPs in the Netherlands
2. data from one STP in Canada
The importance of conjugated estrogens as a potential pool of estrogenically active substances has been widely discussed (e.g., Belfroid et al. (237), Panter et al. (81), Ternes et al. (80), Johnson & Sumpter (95). It has been shown that estradiol-glucuronid conjugates are very easily converted to free active estrogens by microorganisms from activated sludge (Panter et al. (81), Ternes et al. (80)). Furthermore, Johnson et al. (88) found that the deconjugated estrogens detected in influent were close to the expected total based on excretion values (95). During the examination of effluent from five STPs in the Netherlands, Belfroid et al. (237) found concentrations of glucuronids above the detection limit (estrone glucuronides) in the effluent of two municipal STPs (Appendix A). This let to the hypothesis that the hormone glucuronides are degraded or transformed back into hormones in the STPs. Recently, Johnson & Sumpter (95) have suggested that the deconjugation may already occur in the sewage system before entering into the STPs. However, this suggestion is not supported by the results obtained by Adler et al. (82). They examined the content of both un-conjungated and conjungated estrogens in raw sewage and sewage effluent from STPs in Germany and found that the conjungates contributed with up to 50 % of the total steroid concentration in raw sewage. A summary of the results is given in Table 11.3.
Table 11.3
The median of the concentration of un-conjungated and total (un- and
conjungated) estrogens in influent and effluent of German STPs (82).
Substance |
Unit |
Influent |
Effluent |
||
total |
Un- |
Total |
Un- |
||
Estrone |
(ng/l) |
13 |
5.5 |
8 |
2.5 |
17b -estradiol |
(ng/l) |
3 |
1.5 |
0.8 |
0.2 |
17a -ethinylestradiol |
(ng/l) |
9.5 |
7 |
0.5 |
0.3 |
11.2 Alkylphenols
11.2.1 Biodegradation in sludge of STPs
Alkylphenols (AP) in STPs are mainly a result of the biodegradation of alkylphenol polyethoxylates (APEO). Descriptions of the aerobic and anaerobic biotransformation pathways of APEOs show that the degradation is initiated by sequentially cleaving ethoxylate units (e.g. Ahel et al. (93), and the review by Ying et al. (238). Under aerobic conditions, the resulting products are alkylphenol (AP), mono- and diethoxylates (AP1EO, AP2EO), and the more hydrophilic mono- and dicarboxylates (AP1EC, AP2EC) according to Ahel et al. (93). The transformation under anaerobic conditions results in the production of AP1EO, AP2EO and finally AP.
Investigation of the biodegradation of nonylphenol (NP) in lab-scale semi-continuous activated sludge (SCAS) reactors has shown that it is degradable under aerobic conditions and that its degradation is temperature-dependent (239). The influent solution to the reactors consisted of either a synthetic medium or effluent from a full-scale STP. Removal efficiencies of more than 99 % were obtained in 1.2 L SCAS reactors operated for 47 days at a temperature of 28 °C and given doses of approx. 5 mg NP three times a week. Mass balance over the reactors indicated that the added NP was biologically degraded according to Tanghe et al. (239). A subsequent lowering of the temperature from 28 °C to 10-15 °C and an increase of the loading rate from 1 to 2 g COD/l/d resulted in an accumulation of NP in the sludge and a decrease of the NP removal efficiency to 13-86 %. Furthermore, an increase of the NP concentration was observed in the effluent from a reactor receiving only synthetic medium.
Staples et al. (90) have examined the aerobic ultimate biodegradation of NP and OP using the OECD 301B modified Sturm test. The test vessels were inoculated with activated sludge from a STP in the U.S.A. The test substances were added in a concentration of 10 mg AP/l and the vessels were incubated at 22 ± 2 °C. The evolution of carbon dioxide, which is a direct measure of the ultimate biodegradation of the test compound, was followed for a period of 35 days. On day 35, the CO2 formation in the test vessels reached 48 % and 70 % of the theoretically produced CO2 for NP and OP, respectively. Chemical analyses showed no detectable concentration of either NP or OP in the test vessels at the end of the test period.
The degradation of NP2EO and NP1EO has been investigated under anaerobic condition by Ejlertson et al. (91). Anaerobic bottles were amended with 100 % digested sludge at different concentrations of NPnEO (n=1-2). (U-14C)-NPnEO was used to detect any possible decomposition of the aromatic moiety of the NPnEO. NPnEO degraded at 2 mg/l, with nonylphenol as the ultimative degradation products. Both NP and NPnEO interacted with the organic matter, which resulted in sorption to the solid phase.
The possible degradation pathways of APnEO based on the above studies are presented in Figure 11.2.
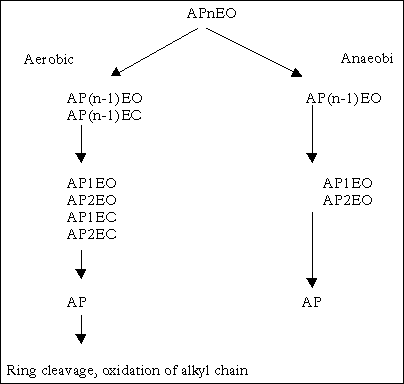
Figure 11.2
Degradation of APnEO (93), Renner 1997 in (238).
11.2.2 Sorption to sludge particles
The most abundant alkylphenols, nonyl- and octylphenol tend to be associated with sludge and other organic particles due to the high hydrophobicity of these substances. The octanol/water coefficient (log POW) for NP and OP at 4.48 and 4.12, respectively, exceed the log POW level for considering a substance bioaccumulative or for having a tendency to sorb to organic matter (log POW ³ 3). The removal of NP and OP from the aquatic phase in a STP may, therefore, not only occur through degradation but also as a result of sorption to the sludge fraction. Measurement of sorption coefficients (Kd) of NPnEO homologues (n = 3-13) onto sewage sludge has showed Kd values ranging from 12,000 to 33,000 L/kg (John et al., 2000 in (238)).
11.2.3 Fate in STPs
It is necessary to have a certain knowledge of the hydrophobic nature of the parent compounds APnEO and APnEC to understand the fate of APs in a STP. The hydrophobicity of APnEO depends of the length of the hydrophilic polyethoxylate moiety, i.e. a shortening of the chain results in substances of increasing hydrophobicity ending up with AP as the most hydrophobic of the metabolites. In contrary, a carboxylate group at the end of an ethoxylate chain giving APnEC increases the hydrophilicity.
Investigations of the fate of AP in STPs have focussed on NP and to a certain extend OP. Ahel et al. (93) performed an extensive investigation of the behaviour of nonylphenol polyethoxylates (NPnEO, n=3-20) and their metabolites in eleven full-scale mechanical-biological STPs in Switzerland from 1983 to 1985. The STPs had capacities ranging from 4,000 to 240,000 population equivalents (PE). The typical processes of the STPs were a primary clarifier for mechanical sewage treatment, an aeration tank and a secondary clarifier for biological sewage treatment, and an anaerobic digester for sludge treatment.
The concentration of NPnEO and their metabolites varied significantly in the examined waste water. The total concentrations of nonylphenol compounds (NP-c) for primary and secondary effluents ranged from 1,090 to 2,060 µg/l and from 240 to 760 µg/l, respectively. Thus, the overall elimination efficiency of NP-c from the water phase was relatively low with an average of 59 ± 18 %. The distribution of NPnEO oligomer and metabolites changed during the different treatment processes towards lower oligomers (nEO < 8), NPnEC and NP. The most dramatic change in the composition occurred during the activated sludge treatment. A model was proposed for the relative mass flow and the average composition of NPnEO based on the obtained data for the eleven STPs (Figure 11.3). The investigation showed that the main part of the remaining NP-c (60 %) was transported to the aquatic recipient via the secondary effluents as un-transformed NPnEO (n: 3-20), NP1EO, NP2EO, NP1EC, NP2EC and NP. The remaining 40 % of the total NP-c load were disposed of to the environment via digested sewage sludge. A significant amount of the NP was discharged from the STPs via digested sludge. This was particularly pronounced for NP, of which 92-96 % were in the anaerobically digested sludge and only 4-8 % in the secondary effluents. The adsorption of NP to the sludge particles in addition to the formation of NP as the end product during anaerobic degradation resulted in high NP concentrations in anaerobically digested sludge (93;240).
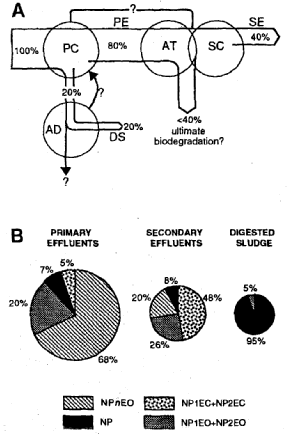
Figure 11.3
Estimated relative mass flow (A) and average composition (B) of nonylphenolic
compounds in 11 sewage treatment plants in Switzerland (calculated on molar basis). PC:
primary clarifier, PE: primary effluent, AT: aeration tank, SC: secondary clarifier, SE.
secondary effluent, AD: anaerobic digestion, DS: digested sludge (93)
Carboxylated NP-c with longer chain of ethoxylates, i.e. > 2, have been found at an examination of influent and effluent samples collected monthly for 12 months (1991-1992) from a mechanical-biological STP in Rome. In average the amounts of NPnEC with n > 3 accounted for approx. 30 % of the total content of carboxylated compounds in the effluent. The concentrations of the total amount of NPnEC were in the range of 10 to 145 µg/l. The highest concentration was found in December (145 µg/l) followed by a concentration in July of 58 µg/l (241). Quantification of NPnEC oligomers (n: 1-4) in effluent samples from STPs near Green Bay, U.S.A., showed the following average proportion of NPnEC: 7 % of NP1EC, 54 % of NP2EC, 31 % of NP3EC, and 8 % of NP4EC (242). The total NPnEC concentration was in a range of 143-272 µg/l. Furthermore, double carboxylated metabolites have been identified in sewage effluent water, in which both the alkyl and ethoxyside chains become carboxylated (CAPnEC). Analysis of a sewage plant effluent in Rome showed a total CAPnEC concentration of 58 µg/l (243). Not all of the mentioned degradation products have been included in the existing fate studies of APnEOs and their metabolites. Therefore, many of the studies give an incomplete understanding of the fate of the compounds in STPs.
Surveys of the concentrations of NPnEO and their metabolites in wastewater samples from Canadian STPs have been described by Bennie et al. (244) and Lee & Peart (94). Bennie et al. (244) have presented a snapshot of the occurrence of some APnEO metabolites in samples from streams from 16 different STPs collected in the period from 1995-1996. 4-tert-octyl phenol (4-t-OP), 4-NP and the mono- and diethoxylates of NP were analysed in samples of influent (9 STPs only), final effluent and sludge. It is not possible to make a mass balance over the fate of NPnEO and their metabolites. However, the study showed that the NP concentrations were higher than the OP concentration in all streams. Furthermore, it was found that the highest concentrations were associated with STPs handling large volumes of textile mill waste effluent. The concentration of NP in influent samples from STPs known to receive textile waste water were high (> 100 µg/l) compared to the range in the influent of the other plants of 0.7-62 µg/l.
The study of Lee & Peart (94) includes analytical data of NPnEO (n: 0-17) as well as NPnEC and OPnEC (n: 1-2) (OPnEO coeluted with NPnEO in the analysis of this study). Samples were collected monthly from influent (untreated water), primary effluent and final effluent for 12 months (1997-1998) of one Canadian STP. The STP received water from a municipality with a population of approx. 140,000 and from local industries. The treatment processes of the waste water were as follows: primary sedimentation, aeration with activated sludge as secondary treatment and secondary sedimentation before discharging to the environment. Chlorinating of the final effluent was used from May to October. NP and OP were found in all type of samples, i.e. influent, primary effluent and final effluent. The OP concentration was lower than the concentrations of NP in all samples. The influent generally had the highest NP concentration varying from 1.81 to 22.69 µg/l. The NP concentrations in the primary effluent and the final effluent were ranging from 1.59 to 10.92 µg/l and from 0.56 to 2.12 µg/l, respectively.
There was no obvious seasonal or temperature dependence of the concentrations in the sample. The concentrations (in nmol/l) of the total alkylphenolics in the sewage samples are shown in Appendix A, Table A.2. Summarising the results showed the same picture of the fate of the compounds throughout the STP treatment process as found by Ahel et al. (93). The primary sedimentation did not significantly alter the distribution between the different groups of compounds (APnEO, APnEC, AP). However, APnEC was the most abundant group of compounds (NpnEC, 64 % and OpnEC, 13 %) after the activated sludge treatment as measured in the final effluent. The elimination rate based on the molarities of the nonylphenolic compounds showed elimination efficiencies ranging from 20 % to 76 % over the year. The highest elimination was seen in April and October and the lowest in September and December. The average reduction of 53 % was similar to the elimination of 59 % obtained by Ahel et al. (93).
The occurrence and fate of NPnEO were examined in two Danish STPs during 1998-1999. One of them Herning STP was loaded with high amounts of industrial sewage (50 %) while the other plant located in Hilleroed mainly received sewage from household (95 %). The investigation of these STPs primarily focussed on the occurrence and fate of the nonylphenolic compounds in the following processes: primary sedimentation, anaerobic digestion of primary and secondary sludge and aerobic treatment of the waste water (245). The analytical programme included analysis of NPnEO (1-18) and NP but not of carboxylates. The sum of the NP-c concentrations in samples taken from the influent and primary effluent was in the range of <20-64 µg/l and 37-51 µg/l, respectively. The primary effluent was only examined at Herning STP in the spring of 1999. The total NP-c concentration in sludge after dewatering, which was the final step in the sludge treatment, was 41-220 µg/kg dry weight. The concentrations in the analysed streams were highest in the STP heavily loaded with industrial sewage (Appendix A, Table A.2). Evaluation of the distribution of the analysed NP-c when passing through the treatment processes within the STPs showed that the amount of NP increased from the influent to the dewatered sludge while the amount of NPnEOs (n = 1-18) decreased. The results indicated that the degradation of NPnEO stops with NP in the anaerobic digester.
Fujita et al. (246) studied the occurrence of NPnEOs, their metabolites including carboxylates and halogenated derivates in fourty full scale STPs in Japan from 1995 to 1996. Almost all the STPs consisted of primary sedimentation, aeration tanks with activated sludge, secondary sedimentation and disinfection with chlorine or ozone. The primary effluents (after primary sedimentation) were dominated of NPnEO (n=4-8). Nonylphenolethoxylates and carboxylates with 1-3 ethoxylates were also present but they accounted for less than 5 % (mol/mol) of the total NP-c of 10.3-1,972 µg/l. NP and halogenated compounds were not found in the primary effluent. The summary of analysis of samples taken from primary effluents, secondary effluents (SE) and final effluents (after disinfection) is shown in Appendix A. Halogenated NP-c were found in concentrations of up to 52.4 µg/l in the final effluent. The average concentration of NP-c in the secondary and the final effluent were 95.4 and 90.0 µg/l, respectively. NP was seen in both the secondary and final effluent in minor concentrations of up to 3.9 µg/l. The average removal of the NP-c from the water phase in the STPs were approx. 60 % and 70 % after biological (SE) and full treatment, respectively.
Several others have examined influent, effluent and in some cases also intermediate streams in STPs. Körner et al. (247) examined the municipal sewage plant in Steinhaule in Germany processing sewage from 200,000 inhabitants. The plant had a total capacity of 350,000 people equivalents (PE) and was equipped with primary sedimentation, activated sludge treatment, biological nitrate removal, biological phosphate removal and final settlement tanks as the main cleaning steps. Influent and effluent samples from four low and eight high technology STPs in the area of Århus, Denmark, were studied by Boutrup & Plesner (98) in 1998-2001. Nasu et al. (89) studied 27 Japanese plants in a period from 1998 to 1999. Planas et al. (248) studied the degradation of NPnEOs in a treatment plant near Barcelone in Spain receiving industrial and domestic sewage from a city with 134,000 PE. Samples were collected from four sampling points during the treatment process. Finally, Sheahan et al. (249) have studied a STP in West Yorkshire, the U.K. The objective of this study was to determine the concentration of nonylphenolic compounds from different trade sources entering the STP and to evaluate the contribution of these compounds to the estrogenic activity of the final effluent. Common to all these studies is that the analyses of NP-c only covered some of the metabolites. There was no analysis of carboxylates NP-c and some of the studies only included NP, NP1EO and NP2EO. Therefore, the data give no additional information to the fate of NP-c and their metabolites in STPs.
11.3 Bisphenol A
11.3.1 Biodegradation in sludge of STPs
Bisphenol A appears to be considered readily biodegradable, possibly after a short period of adaptation as stated in Section 3.3.3.1.
Examination of the biodegradability using a modified SCAS procedure (semi-continuously activated sludge) and microorganisms obtained from a municipal STP showed a removal of bisphenol A of 85-96 % after 24-30 days (Turner & Watkinson 1986 in (221)). The initial concentration of bisphenol A was 20 mg/l and the removal was followed by measurement of % dissolved organic carbon (DOC) removal and UV adsorption spectroscopy. A lag phase of 13 to 17 days was observed before the initiation of the degradation. Another biodegradation study of an activated sludge batch test study using sludge from an industrial STP resulted in a removal of bisphenol A of 72 % chemical oxygen demand (COD) and 57 % total organic carbon (TOC) after 24 hours. The concentration of the activated sludge was 2-3 g SS/l (SS: suspended solid) and the initial test concentration was 58 mg/l of bisphenol A (250).
These results of biodegradation studies using microorganisms from STPs show that bisphenol A should be degradable in STPs. Furthermore, the studies indicate that the degradation rate will increase after a period of adaptation.
Degradation of bisphenol A under anaerobic or anoxic conditions is much more unlikely to occur as explained in Section 5.3.3.2.
11.3.2 Sorption to sludge particles
The reported log Pow of bisphenol A of 3.4 indicates that an amount of the compound in a STP may be removed from the water phase by sorption to sludge particles. An estimation of the fate in STPs according to the principles in the EU's Technical Guidance Document (251) showed the following distribution of bisphenol A: 12 % to water, 6.2 % to sludge, 81.9 % degraded and a negligible fraction to air (221).
11.3.3 Fate in STPs
The investigation by Lee & Peart (96) of samples from influent, effluent, and sludge taken from eight Canadian STPs in 1999 gives an impression of the fate of bisphenol A in STPs. The investigations were performed in connection with the development of analytical methods for bisphenol A. The concentrations in the influent and effluent samples were in the range of 193 to 2,440 ng/l and 35 to 223 ng/l, respectively. The removal efficiency from the water phase was 47-96 %. Analysis of digested sludge showed concentrations between 316 and 12,500 ng/g dry weight. For two of the STPs, data are available on raw sludge and digested sludge. In both cases, the concentration in the raw sludge is approx. 70 % lower than the concentration in the digested sludge. Bisphenol A is not expected to be degradable under anaerobic conditions. Supposing that the digestion process is anaerobic, the observed increase of the concentration could be due to a lower water content in the digested than in the raw sludge.
In Germany, Körner et al. (247) found bisphenol A in two influent samples of 540 and 3,010 ng/l. The concentrations in the corresponding effluent samples were 162 and 258 ng/l giving removal efficiencies of 70 % and 91 %, respectively. Examination of four low technological sewage treatment plants in Denmark showed influent concentrations of <100-1,300 ng/l and effluent concentrations between 50 and 1,800 ng/l. The percentage removal of bisphenol A was between ~ 0 % and 96 % (98). The lowest removal was found in a plant with only mechanical treatment. Recently, Fromme et al. (252) have reported the results from an investigation of sludge and effluent samples collected from 39 German STPs. The concentration in the sludge samples was 4-1,363 ng/g dry weight, which is comparable with some of the concentrations observed by Lee & Peart (96).