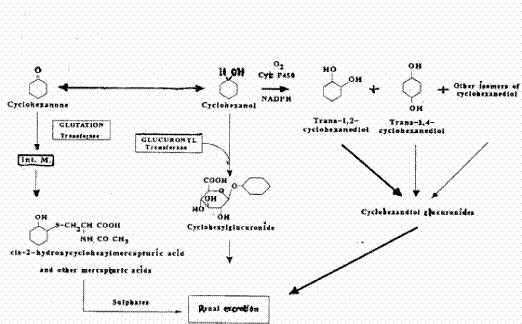
Appendices 1-18 to: Report on the Health Effects of Selected Pesticide Coformulants121 Toxicokinetics121.1 Human data121.2 Animal data 121.3 Mode of action 121.1 Human dataIn humans the inhalation uptake of concentrations of 101, 207 or 406 mg/m3 cyclohexanone over 8 hours was 57-59%. Skin uptake of liquid cyclohexanone after immersion of the hands of three persons for 30 minutes was estimated to be 0.037- 0.069 mg/cm2/h corresponding to 1-2% of the dose absorbed by inhalation during 8 hours of exposure to 200 mg/m3. (Mraz 1994 – quoted from A&H 1999). Following occupational exposure (8 hours) by inhalation to 1- 40 ppm (4 -160 mg/m3, average concentration of 9 ppm, 36 mg/m3) cyclohexanone, the concentration of cyclohexanone in breath was approximately 4 mg/m3 and the urinary concentration of cyclohexanol was 9 mg/g creatinine (Ong et al. 1991 – quoted from DECOS 1993 and from IARC 1999). Analysis of urine collected from three humans exposed for 8 hours to cyclohexanone at a concentration of 415 mg/m3 showed that 3.5% of the cyclohexanone-dose found in urine was cyclohexanol conjugated to glucuronic acid, with the rest being present as trans-1,2-cyclohexanediol glucuronide (68.4%) and trans-1,4-cyclohexanediol (28.1%) (Flek et al. 1989 – quoted from OEL 1993). Four men and four women were exposed to cyclohexanone at concentrations of 101, 207 or 406 mg/m3 for 8 hours. At 207 mg/m3, 57% of the inhaled dose was excreted in urine as cyclohexanol (1%), 1,2 and 1,4-cyclohexanediols (39%), and their glucuronide conjugates (18%). Elimination half-lives of the 1,2 and 1,4-diols were 16 and 18 hours, respectively. Following repeated inhalation exposure to 207 mg/m3 for 5 days, the excretion rate for cylclohexanediol increased, but not for cyclohexanol. (Mráz et al. 1994 – quoted from A&H 1999 and from IARC 1999). End-of-shift urine samples were collected from 24 factory workers occupationally exposed to up to 9 ppm (37 mg/m3) of cyclohexanone in combination with toluene and other solvents, and from 10 non-exposed controls. Calculation of relative quantities of the urinary metabolites identified showed that 0.4% of the inhaled dose was excreted in urine as cyclohexanol and 4.4% as trans-1,2-cyclohexanediol. Very low levels of cyclohexanone and no cis-1,2, cyclohexanediol were detected. (Kawai et al. 1999). In a case of attempted suicide by ingestion of 720 ml sake, containing 10% ethanol and about 100 ml liquid cement containing 39% cyclohexanone, 28% methyl ethyl ketone, 18% acetone and 15% polyvinyl chloride, the cyclohexanone level in plasma at 5 hours after ingestion was 10 mg/ml. At the same time point, the plasma level of cyclohexanol was about 200 mg/ml. Cyclohexanone excretion in urine was minimal (33 mg/ml at 12 hours after ingestion) with the major metabolite excreted by this route being cyclohexanol glucuronide (440 mg/ml) and, to a lesser degree, unconjugated cyclohexanol (51 mg/ml). (Sakata et al 1989 – quoted from DECOS 1993 and from IARC 1999). Accidental exposure of newborn babies to cyclohexanone had occurred in a special care unit from contamination of intravenous nutrient solutions. Isomers of cyclohexanediol (mostly trans-1,2-cyclohexanediol) were found in 101 of 584 urine samples. No conjugates of cyclohexanol or cyclohexanediol were detected. (Mills & Walker 1990 – quoted from DECOS 1993 and from IARC 1999). 121.2 Animal dataResults from acute toxicity studies in rodents indicate that cyclohexanone is absorbed after ingestion, skin contact and inhalation in animals. The blood/air distribution coefficient is 2150, implying high uptake via respiratory passages. (OEL 1993 and A&H 1999). Two rabbits given gavage doses of 890 mg cyclohexanone/kg b.w. excreted glucuronic conjugates in the urine (858 mg at 24 hours, 2632 mg at 48 hours, measured as glucuronic acid), whereas the inorganic sulfate content in urine decreased. This demonstrates, according to the authors, that the metabolism of cyclohexanone includes conjugation to glucuronic acid. (Treon et al. 1943a). Rabbits dosed orally with 270 mg/kg b.w. excreted 66% cyclohexanol glucuronide conjugate and 6% glucuronide conjugate of trans-1,2-cyclohexanediol. Thus, one of the metabolic pathways for cyclohexanone is, according to the authors, P450 dependent. (Elliot et al. 1959 – quoted in OEL 1993 and DECOS 1993). Groups of 10 Wistar and 10 Gunn rats were dosed intravenously with cyclohexanone (0, 50, or 100 mg /kg b.w.) daily for 28 days. Neither cyclohexanone nor its metabolite cyclohexanol could be detected in plasma at 24 hours. Urine was collected over 24 hours after the last injection and analysed for free cyclohexanone, cyclohexanol, and sulfate and glucuronide conjugates of cyclohexanol. Levels of free cyclohexanone or cyclohexanol in urine were less than 1 %. No sulfate conjugates of cyclohexanol were found. Cyclohexanol-glucuronide conjugate accounted for 15-25% in the low dose group and for 19-34% in the high dose group. No analyses for cyclohexane-diol conjugates in the urine, or of breath or faeces were included in the study. (Greener et al. 1982). In dogs, approximately 74-100% of an intravenous dose of 285 mg/kg was converted to cyclohexanol. The distribution half-life was 6.6 min. Sixty percent of the dose was excreted in urine as the conjugate of cyclohexanol. (Martis et al. 1980 – quoted from OEL 1993, DECOS 1993, and from A&H 1999). Overall, the data indicate that both humans and animals rapidly metabolise cyclohexanone to cyclohexanol. Then, the pathway in humans in primarily through cytochrome P-450 oxidation with the formation of diols, which are then conjugated with glucuronic acid before excretion mainly in urine. In animals, it appears that the P-450 system is less important, as the excretion of cyclohexanol conjugates are more predominant than in humans. The formation of cis-2-hydroxylcyclohexylmercapturic acid has also been described. (OEL 1993). The proposed metabolic pathways of cyclohexanone are summarised in Figure 2.
Figure 2. Metabolism of cyclohexanone (modified from OEL 1993). Dominant pathways in humans is indicated by thick arrows. Int. M: labile intermediate 121.3 Mode of actionCyclohexanone is an organic solvent, which causes CNS depression, no details on the mechanism(s) of action have been found.
|