| Front page | | Contents | | Previous | | Next |
Appendices 1-18 to: Report on the Health Effects of Selected Pesticide Coformulants
Appendix 17: 1-Methylpyrrolidone (NMP)
128 General description
128.1 Identity
128.2 Physical / chemical properties
128.1 Identity
Molecular formula: C5H9NO
Structural formula:
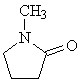
Molecular weight: 99.13
CAS-no.: 872-50-4
Synonyms: N-methyl-2-pyrrolidone
Methylpyrrolidone
N-Methyl-2-pyrrolidinone
1-Methyl-5-pyrrolidinone
N-Methyl-2-oxypyrrolidine
N-Methyl-2-ketopyrrolidine
NMP
Butyrolactam
N-Methyl-gamma-butyrolactone
1-Methylazacyclopentan-2-one
128.2 Physical / chemical properties
Description: NMP is a colourless, hygroscopic liquid with a mild amine odour.
Melting point: -23 to -24.4 °C
Boiling point: 202 °C (at 760 mmHg)
Density: 1.034 g/ml (at 20°C)
Vapour pressure: 0.29 mmHg (39 Pa) (at 20°C)
Concentration of
saturated vapours: 1400 mg/m3 (Åkesson 1994)
1600 mg/m3 (calculated)
Conversion factor: 1 ppm = 4.12 mg/m3 (at 20°C and 760 mmHg)
1 mg/m3 = 0.243 ppm
Solubility: Water: Miscible with water.
Highly soluble in lower alcohols, lower ketones, ether, ethyl acetate, chloroform and benzene. Moderately soluble in aliphatic hydrocarbons.
logPoctanol/water: -0.46 - 0.42
References: Merck Index (1996), Åkesson (1994), IUCLID (2000), HSDB (2001), Trochimowicz et al. (1994).
| Front page | | Contents | | Previous | | Next | | Top |
|