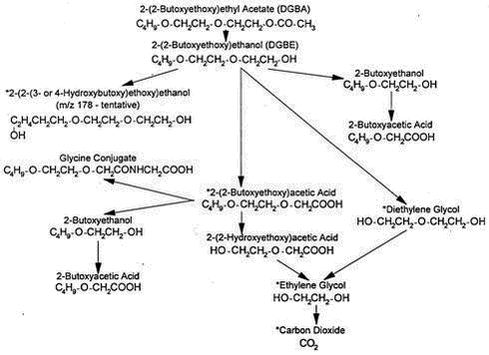
Appendices 1-18 to: Report on the Health Effects of Selected Pesticide Coformulants97 Toxicokinetics97.1 Absorption, distribution and excretion97.2 Mode of action 97.1 Absorption, distribution and excretionNo data regarding the absorption and excretion of DEGBE following inhalation or oral intake, or the distribution of DEGBE have been found. The in vitro percutaneous absorption of DEGBE has been measured in rat and human skin. The absorption rate through rat skin (incubation time 8 hours) was 0.506 ± 0.193 mg/cm2/hour (n = 12); a permeability constant was calculated to be 5.30 ± 2.02 x 10-4 cm/hour. Under the same experimental conditions, the absorption rate through human skin was 0.292 ± 0.133 mg/cm2/hour (n = 10); a permeability constant was calculated to be 3.06 ± 1.42 x 10-4 cm/hour. (Barber et al. 1992 – quoted from DECOS 1996). When 14C-DEGBEA was given as a single oral dose (200 or 2000 mg/kg) to male Sprague-Dawley rats (5 animals per group), about 85% of the total 14C-dose was excreted in the urine, about 2 to 3% in the faeces, and about 5% as carbon dioxide in expired air by 72 hours after the administration. A proposed metabolic pathway for DEGBEA in the rat is shown in Figure 2.1 below. The asterisks indicate metabolites identified by enzymatic, chromatographic and/or mass spectrometric techniques. The major urinary metabolite was 2-(2-butoxyethoxy)acetic acid, comprising 58.9% and 53.4% of the total urinary radioactivity after the low and the high dose, respectively. No unchanged DEGBEA or DEGBE was detected in rat urine at either dose level and no evidence was found for excretion of 2-butoxyacetic acid (BAA). (Deisinger & Guest 1989 – quoted from DECOS 1996). Groups of 4 Sprague-Dawley rats of each sex had 0.2 or 2.0 g/kg of undiluted or 0.2 g/kg of a 10% aqueous solution of 14C-labeled DEGBE or its acetate 14C-DEGBEA (only undiluted) applied under occlusion to shaved skin for 24 hours. At the low dose, females excreted about 42/51% (diluted/undiluted) DEGBE and about 40% DEGBEA, and males about 31/27% (diluted/undiluted) DEGBE and about 48% DEGBEA. At the high dose, females excreted about 18% DEGBE and about 12% DEGBEA, and males about 3% DEGBE and about 13% DEGBEA. The excretion in faeces was only a few percent (about 0.2-3%); excretion via expiration was not measured. The main urinary metabolite was 2-(2-butoxyethoxy)acetic acid, which accounted for 61 to 80% of the total urinary radioactivity. The glucuronide of DEGBE was present at levels of about 5 to 8% of the urinary radioactivity. Trace levels of 2-butoxyacetic acid were present in all 8-hour and 24-hour urine samples following application of DEGBE, but not DEGBEA. The dermal absorption rates for DEGBEA were estimated to be 1.58 and 1.28 mg/cm2/hour for males and females, respectively, and for DEGBE to 0.73 and 1.46 mg/cm2/hour for males and females, respectively. (Boatman et al. 1993). 97.2 Mode of actionNo specific data regarding the toxicological mechanism(s) of DEGBE have been found. A number of glycol ethers and their acetates have been shown to cause haematological, immunological, testicular, and/or developmental toxicity; these toxic effects appear to be dependent on the formation of alkoxy acetic or alkoxy propionic acids (ECETOC 1995). Ethylene glycol mono-n-butyl ether (EGBE) causes haemolysis; significant species differences have been reported with the rat being the most sensitive species whereas human erythrocytes appear to be the least sensitive of those investigated. The mechanism(s) behind the haemolytic effect is not fully understood, but the metabolite 2-butoxyacetic acid (BAA) has shown a significantly greater haemolytic effect in vitro than EGBE whereas DEGBE has shown a considerably less haemolytic activity than EGBE. (ECETOC 1995). The toxic metabolite 2-butoxyacetic acid (BAA) can be formed via metabolism of DEGBE.
Figure 2.1. A proposed metabolic pathway for DEGBEA in the rat according to Deisinger & Guest (1989 –from DECOS 1996).
|