|
| Front page | | Contents | | Previous | | Next |
Survey of lip care products with fragrance and flavour
8 Health assessment
In the following toxicological profiles have been drawn up of the 5 fragrances comprised by the health assessment.
8.1 Linalool
Occurrence and use
Linalool is used as a fragrance in perfumes, most often as a substitute for bergamot or lavender oil. Occurs naturally in e.g. orange juice, peach, tomatoes and carrot, as well as in a number of flower oils such
as orange flower and bergamot. Linalool is the mail component of rosewood oil (21). Flavour characterization of linalool is stated as fruity, citrus-like and woody (22).
Identification
Linalool is an acyclic terpenalcohol (23).
| Chemical name |
Linalool |
| Synonyms |
3,7-dimethyl-1,6-octadien-3-ol |
| CAS-No. |
78-70-6 |
| EINECS No. |
201-134-4 |
| Molecular formula |
C10H18O |
| Molecular structure |
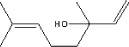 |
Legislation:
Classification according to the List of hazardous substances (Stat.
Order 439 of 3 June 2002)
The List of unwanted substances. Danish Environmental Protection
Agency (24).
Cosmetics (25) |
Not classified
On the list as the substance is considered to be dangerous to the health and the environment
As of 11 March 2005, the fragrance must be declared in cosmetics if it is used in amounts above 0.01 % in products that are cleaned off
and 0.001 % in products that are not cleaned off. |
Physical-chemical properties (26)
| Physical state |
Liquid |
| Molecular weight (g/mol) |
154.25 |
| Melting point, °C |
- |
| Boiling point, °C |
195-199°C (720 mmHg) |
| Evaporation (Pa) |
0.16 mmHg (21 Pa) at 22-25°C
21 Pa. at 25°C (21) |
| Octanol-water distribution, (log Pow) |
2.84 at 25°C (22)
Log Kow = 2.97 |
| Water solubility (g/L) |
1.45 g/l at 25°C (22) |
WHO has laid down a NOEL (No Observed Effect Level) for linalool of 50 mg/kg bw/d (27).
Acute toxicity
LD50-values (the exposure dose at which half the experimental animals die) at oral exposure of rats has been found to be 2790 and 4180 mg/kg bw (body weight) (21, 22). LD50 at oral exposure of mice
is approximately 3500 mg/kg bw when administered in oil and 1700 mg/kg bw when administered in aqueous solution. Observed toxic effects were dose dependent and included anaesthetization, depression
and hyper mobility with uncoordinated movements (26).
At exposure of rabbit skin, LD50 has been stated at 5610 mg/kg bw (21, 22).
Inhalation experiments on mice, where 20-50 mg linalool is inhaled in combined amount during 1 hour of exposure, reduced mobility was observed and linalool was measured in the blood in a concentration
of 4.22 ng/ml (22).
Linalool is absorbed through the skin and is effectively excreted, mainly through the kidneys. 10 minutes of massage with lavender oil of a 376 cm² skin area of a 60 kg man resulted in a calculated
percutanous absorption of 7.23 mg linalool. After 5 minutes, linalool could be detected in the blood, after 20 minutes a blood concentration of 211 ng/ml was measured and after 90 minutes, all linalool was
eliminated from the bloodstream (22). It has not been evaluated whether inhalation took place in connection with the massage.
Chronic, repeated exposure
Several oral experiments on rats are stated (22):
Exposure to 1500 mg/kg bw/d for 5 days led to enzyme changes in peroxisomes and increased liver weight.
Exposure to 600 mg/kg bw/d for 3 days led to enzyme changes in the liver.
Exposure to 500 mg/kg bw/d for 64 days led to enzyme changes in the liver and increased liver weight.
A No Observed Adverse Effect Level (NOAEL), the highest exposure dose not to cause observed critical effects on the animals, of 50 mg/kg bw/d has been laid down based on a 90-day oral exposure
study with rats where a reduced food intake and growth was observed. These effects were attributed to poor tasting food (28).
At oral exposure of mice of 375 mg/kg bw/d for 5 days, no effects were observed on body weight, spleen and thymus weight and in addition no clinical signs of toxicity (22).
Linalool has been stated as possibly having effects on the liver in humans at chronic or repeated exposure (21).
A 13-week dermal exposure experiment on rats established a NOAEL of 250 mg/kg bw/d with effects such as momentary blushing and reduced activity as critical effects. At an exposure of 1000 mg/kg
bw/d, reduced growth, reduced activity and blushing was observed (28). No dose level was mentioned at which a critical effect occurs or what the critical effect was assessed to be.
Upon exposure to linalool in a concentration of 1% of the diet for 20 weeks in rats, no changes in the development of tumors in the animals was observed (22).
In vitro mutagenicity test for chromosome changes in mammalian cells is negative in doses of up to 0.25 mg/ml (21). Linalool has tested negative in Ames test, mouse micronucleus test, Chinese hamster
ovary or fibro blast cells and in DNA assay with rat liver cells (28).
No data has been found for inhalation of linalool.
Local irritation
0.5 ml on shaved rabbit skin in concentrations of 100 %, 30 %, 10 % and 3 % for 24 hours lead to a slight irritation for the 100% and 30% solutions and no irritation for the 10% and 3% solutions (26). The
substance has been stated as being mildly irritating in patch tests on human skin in a concentration of 32% (21).
No irritation and sensitization has been observed at application of 20% linalool in petrolatum (28).
0.1 ml in rabbit eyes in concentrations of 100%, 30%, 10% and 3% for an unspecified time period lead to moderate irritation at 100%, slight irritation at 30%, very slight irritation at 10% and no irritation at
3% (26). The substance has been stated as being irritating to human eyes, concentration not stated (21).
Linalool and its esters do not absorb UV light at wave lengths in the interval 290-400 nm and does therefore not display potential for photo irritation or allergy when used as a fragrance (28).
Allergy
The EU scientific committee, SCCNFP, has included linalool on the list of fragrances that are known allergens but for which not many reports are found of allergy in consumers. 1 and 3 cases respectively of
contact allergy have been reported from two examinations of 119 and 75 patients corresponding to 0.8 and 5 % of the patients with cosmetic eczema (29).
Critical effect
The critical effect of linalool is estimated to be liver damage. Due to the allergenic effect of linalool, people who are allergic to the substance should avoid skin contact as there is no lower limit to this side
effect.
Table 8.1. Summary of data used to calculate MoS for linalool using EUSES.
| Toxicological data (animals) |
|
| LD50, (mg/kg bw), oral, rat |
2790 (22) |
| NOAEL, (mg/kg bw/d), ingestion |
50 (28) |
| LOAEL*, (mg/kg bw/d), ingestion liver damage |
500 (22) |
*Lowest Observed Adverse Effect Level (LOAEL), the lowest exposure dose where effects were seen in the animals.
8.2 Geraniol
Occurrence and use
Geraniol occurs in many ethereal oils, e.g. rose oil, geranium oil, palmarosa oil, lemon grass and it is the main component in citronella oil (21, 30). The substance smells sweetly of roses or flowers and can
bring out lemon scents (26, 31). One source states that it tastes like apple (26). Geraniol is used as fragrance in perfumes as well as in cosmetics, cleaning and washing agents and in perfumed air fresheners
as well as an insect lure (21, 22).
Identification
Geraniol is an acyclic terpenalcohol (23).
| Chemical name: |
Geraniol |
| Synonyms: |
(E)-3,7-dimethyl-2,6-octadien-1-ol; 2,6-dimethyl-2,6-ectadien-8-ol |
| CAS-No.: |
106-24-1 |
| EINECS No.: |
203-377-1 |
| Molecular formula: |
C10H18O |
| Molecular structure: |
 |
Legislation:
Classification according to the List of hazardous substances (Stat.
Order 439 of 3 June 2002)
The List of unwanted substances. Danish Environmental Protection
Agency (24).
Cosmetics (25) |
Not classified
On the list as the substance is considered to be dangerous to the health and the environment
As of 11 March 2005, the fragrance must be declared in cosmetics if it is used in amounts above 0.01 % in products that are cleaned off
and 0.001 % in products that are not cleaned off. |
Physical-chemical properties (26)
| Physical state |
Oily liquid |
| Molecular weight (g/mol) |
154.24 |
| Melting point, °C |
< -15°C |
| Boiling point, °C |
230°C (760 mmHg) |
| Evaporation (Pa) |
< 1 hPa at 20°C (22). A more specific specification of evaporation has not been found. |
| Octanol-water distribution, (log Pow) |
|
| Water solubility (mg/L) |
Slightly soluble.
100 mg/l at 25°C
686 mg/l at 20°C (22) |
Acute toxicity
Geraniol is low acute toxic with an LD50 of 3600 mg/kg bw at oral exposure of rats (21). Inhalation studies with dogs at an exposure of 500 mg/m³ for 4 hours did not lead to any enzymatic changes in the
olfactory epithelium (21). At dermal exposure of rabbit skin, LD50 is > 5000 mg/kg bw.
Furthermore, acute toxicity experiments have been carried out with intramuscular, intraperitoneal and subcutaneous administration to mice and intravenous administration to rabbits. The lowest value is LD50
of 50 mg/kg bw with intravenous administration to rabbits and an LD50 of 1090 mg/kg bw at subcutaneous administration to mice (22).
The substance has been stated as moderately toxic to humans with an estimated deadly dose of 0.5–5 g/kg bw (26). This corresponds to a deadly dose in the interval of 35-350 g for a person weighing 70
kg. The unintentional ingestion by a child of an unknown amount of citronella oil containing 93% geraniol lead to vomiting, shock, seizures and death. The mucous membrane of the stomach was severely
damaged (26).
Chronic, repeated exposure
A NOAEL of 10,000 ppm (corresponding to 10 g/kg feed) has been stated for oral exposure of rats for 16 weeks and a NOAEL of 1000 ppm has been stated for oral exposure of rats for 28 weeks (22).
The critical effect has not been stated. Average weight of 16 and 28 week old rats is approximately 275 g and daily feed intake is approximately 22 g (20). Ingestion of 10000 ppm corresponds to 1 % of
geraniol in the feed, i.e. a dose of 0.22 g geraniol per 275 g rat and thus equal to a dose of 800 mg/kg bw/d.
A NOAEL of 1000 ppm corresponds to 78.3 mg/kg bw/d. The critical effect has not been stated.
Geraniol has tested negative in Ames test. In vitro tests with mammalian cells has been positive to chromosome changes in Chinese hamster fibroblast cells and negative in mouse micronucleus test (21, 22).
No data has been found for geraniol and cancer. No data has been found regarding reproduction toxicology for mammals but chicken embryos injected on the 3rd incubation day with 190 µg geraniol
showed weak embryo-toxic effects, mainly malformations in the skeletal or limb structure (21, 22).
No data has been found for inhalation of geraniol.
Local irritation
Literature states four studies regarding skin irritation. Two of the studies conclude that geraniol is not irritating and two of the studies that geraniol is irritating in contact with rabbit skin. One of the studies,
which conclude that geraniol is irritating in contact with rabbit skin, has stated a complex test substance in which geraniol comprises 50 %, nerol 30% and citronellol 20 % (22).
No data has been found discussing geraniol's effects on eyes.
Allergy
The EU scientific committee, SCCNFP, has included geraniol on the list of fragrances that are known allergens Geraniol is found on the list of well-known and often reported consumer allergens and is a
component in Fragrance mix (29).
Geraniol leads to sensitization in 0.4% eczema patients and is responsible for 3-7% of the reactions to Fragrance mix. Geraniol has lead to contact allergy reactions in 1.2-30% of patients with cosmetic
eczema (29).
Critical effect
The critical effect for geraniol is estimated to be the extensive sensitizing effect. Due to geraniol's allergenic effect, people who are allergic to the substance should, however, completely avoid skin contact as
there is no lower limit for this side effect.
Table 8.2. Summary of data used to calculate MoS for geraniol using EUSES.
| Toxicological data (animals) |
|
| LD50, (mg/kg bw), oral, rat |
3600 mg/kg bw (22) |
NOAEL, (mg/kg bw/d), ingestion, 28 weeks
NOAEL, (mg/kg bw/d), ingestion, 16 weeks |
78.3 (22)
783 (22) |
| LOAEL, (mg/kg bw/d), ingestion |
- |
8.3 Benzyl Benzoate
Occurrence and use
Benzyl benzoate is one of the mail components in Peru Balsam (29) and is also found in leaves from cinnamon, in the flowers carnation, tuberose, hyacinth and in ylang-ylang. The substance is used as
perfume and aroma substance and as a fixative and it is often found in heavy flower scents (29). The substance has a balsamic scent and a sweet and sharp, burning taste (8). Is used in i.e. cosmetics,
pharmaceuticals and pesticides (8, 32).
Identification
Benzyl benzoate is an ester of Benzyl alcohol and an aromatic acid, Benzoic acid (23).
| Chemical name |
Benzyl Benzoate |
| Synonyms |
Phenylmethyl Benzoate, Benylate, Benzoic acid, Benzyl ester |
| CAS-No. |
120-51-4 |
| EINECS No. |
204-402-9 |
| Molecular formula |
C14H12O2 |
| Molecular structure |
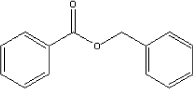 |
Legislation:
Classification according to the List of hazardous substances (Stat.
Order 439 of 3 June 2002)
The List of unwanted substances. Danish Environmental Protection
Agency (24).
Cosmetics (25) |
Xn;R22
On the list as the substance is considered to be dangerous to the health and the environment
As of 11 March 2005, the fragrance must be declared in cosmetics if it is used in amounts above 0.01 % in products that are cleaned
off and 0.001 % in products that are not cleaned off. |
Physical-chemical properties
| Physical state |
Colourless liquid with a weak aromatic scent (8) |
| Molecular weight (g/mol) |
212.25 (21) |
| Melting point, °C |
18 - 20°C (21) |
| Boiling point, °C |
323°C (21) |
| Evaporation (Pa) |
0,000224 mm Hg (0,03 Pa) at 25°C (26),
1.3 mm Hg (173 Pa) at 44°C (21), |
| Octanol-water distribution, (log Pow) |
Log Pow: 3.97 (21) |
| Water solubility (mg/L) |
15.3 mg/L (20°C) (22) |
Acute toxicity
Benzyl benzoate is hazardous to health by ingestion. LD50 oral rat has been measured from 500 mg/kg bw to >2000 mg/kg bw in rats (22). Symptoms of poisoning of rats, cats and rabbits is impact on the
central nervous system, uncoordinated muscle movements, paralyzing of hind legs, convulsions, trouble when breathing and death (22). The substance penetrates the skin; 54% of the applied dose penetrated
human skin in a 24 h occlusive test (26).
Chronic, repeated exposure
A 90-day test where 556 mg/kg bw/d was applied to rabbit skin has shown slight dermatitis and weakening, at higher doses slight to moderate atrophy of the testicles was observed as well as elevated
leucocyte numbers. There were suggestions of kidney damage. Lethal dose was measured to be > 2,2 g/kg bw/d (22).
There are no signs of mutagenic effects in Ames test (22).
Tests on pregnant rats with a daily dose of 595 mg/kg bw from gestation day 0 until day 21 after birth showed no harmful effects to foetuses and young (22).
No data was found for inhalation of benzyl benzoate.
Local irritation
Benzyl benzoate is irritating to eyes and mucous membranes and may lead to irritation of the skin (21, 26).
Allergy
The EU scientific committee, SCCNFP, has included benzyl benzoate on the list of fragrances that are known allergens The substance may cause allergy by skin contact in humans (21, 29). Most reports
about allergy are caused by allergy to Peru Balsam in which the substance is the main component. In addition, Peru Balsam contains benzylcinnamat which is also a known allergen (29).
Critical effect
Dermatitis and atrophy of tests are described by doses, which animals survived.
Due to the allergenic effects of benzyl benzoate, people who are allergic to the substance should, however, completely avoid skin contact as there is no lower limit for this side effect.
Table 8.3. Summary of data used for calculation of MoS for benzyl benzoate using EUSES.
| Toxicological data (animals) |
|
| LD50, oral |
1000 - 1700 (rat, mouse, rabbit, guinea pig) (21)
500 (rat) (22)
1891 (rat) (22)
> 2000 (rat, OECD 401)(22)
2800 (rat)(22) |
| LD50 (mg/kg bw), dermal |
4000 - 4448 (rabbit) (21, 22)
4000 (rat) (22) |
| NOAEL, (mg/kg bw/d), ingestion, embryonic damage (rat) |
595 (22) |
| LOAEL, (mg/kg bw/d), skin contact, dermatitis (rabbit) |
556 (22) |
8.4 Eugenol
Occurrence and use
Eugenol is found in many ethereal oils from plants. The substance is the main component of clove oil and is also found in cinnamon and rose oil. The substance is used as perfume and aroma substance and
gives off a clove-like, spicy and oriental scent and flavour (8, 32). Is also used as a pain killer in dental care, as insect allurement and in chemical syntheses (21).
Identification
The substance is a substituted phenol (23).
| Chemical name |
Eugenol |
| Synonyms |
2-Methoxy-4-(2-Propenyl)Phenol;
4-Allyl-2-Mehoxyphenol; Caryophyllic Acid; Eugenic Acid; 2-Methoxy-1-Hydroxy-4-Allylbenzene; Allylguaiacol |
| CAS-No. |
97-53-0 |
| EINECS No. |
202-589-1 |
| Molecular formula |
C10H12O2 |
| Molecular structure |
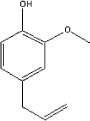 |
Legislation:
Classification according to the List of hazardous substances (Stat.
Order 439 of 3 June 2002)
The List of unwanted substances. Danish Environmental Protection
Agency (24).
Cosmetics (25) |
Xn;R22
On the list as the substance is considered to be dangerous to the health and the environment
As of 11 March 2005, the fragrance must be declared in cosmetics if it is used in amounts above 0.01 % in products that are cleaned off
and 0.001 % in products that are not cleaned off. |
Physical-chemical properties (26)
| Physical state |
Clear, colourless or slightly yellowish liquid. Characteristic fragrance. |
| Molecular weight (g/mol) |
164.20 (21) |
| Melting point, °C |
-9.2 – 9.1°C (21) |
| Boiling point, °C |
254°C (760 mmHg) (21) |
| Evaporation (Pa) |
0.009 mm Hg (1,2 Pa) at 20°C (21) |
| Octanol-water distribution, (log Pow) |
Log Kow: 2.27 |
| Water solubility (mg/L) |
Almost insoluble in water |
Acute toxicity
Eugenol poses a health hazard by inhalation. LD50 oral rat has been measured to be 1930 mg/kg bw. Poisoned rats have shown signs of kidney damage, demonstrated by urine incontinence as well as slight
paralysis of hind legs and jaw, exhaustion and coma (26). Ingestion of aqueous emulsions of the substance may lead to nausea and promote the secretion of mucin, in dogs vomiting has been observed at
ingestion of 250 mg/kg bw and 500 mg/kg bw (26). The substance is strongly irritating to the mucous membrane of the stomach. By oral ingestion in rats and guinea pigs, peeling of the epithelium of the
stomach and punctiform bleeding in pyloric and glandular areas of the stomach have been observed (26).
Chronic, repeated exposure
20 rats' ingestion of eugenol in doses from 1.4 g eugenol/kg gradually increasing to 4.0 g/kg, 8 rats survived for 34 days and 12 rats long enough to obtain the maximum dose. It has not been described how
the dose gradient was increased. Damage to the liver and gastrointestinal tract was seen, particularly to the fore stomach (33). Rats that ingested doses of eugenol of 6000 ppm in the feed for 13 weeks
showed no side effects. Ingestion of 12.000 ppm lead to loss of weight loss (21). As a 13 week rats weighs approximately 275 g and eats approximately 22 g feed per day (20), their ingestion is 12000 ppm
which is the same as 1.2% of the feed corresponding to a dose of 0.264 g eugenol per 275 g rat, thus equal to a dose of 0.96 g/kg bw. There were no side effects in groups of 15 male rats and 15 female
rats fed with eugenol in doses of 79.3 mg/kg bw/d for 12 weeks (33).
IARC has classified eugenol in group 3: insufficient evidence of carcinogenic effect in humans, limited evidence of carcinogenic effects in animals (34).
Eugenol has not shown any mutagenic effects in several bacteria tests. In vitro tests have show chromosome changes in Chinese hamster ovary cells and a slight increase in sister chromatide changes (34).
No data has been found for inhalation of eugenol.
Local irritation
Eugenol may irritate the air passage by inhalation. The substance has shown slight skin irritation in patch tests on humans (21).
Allergy
Eugenol is a well-known contact allergen and the substance forms part of Fragrance Mix which is used for screening of patients for perfume allergy (29). Numerous tests have been carried out of the
sensitizing properties of the substance and the EU has informed that eugenol has caused allergic reactions in 0.7-20% of patients with eczema from cosmetic products (26, 29).
Critical effect
Based on the above, the critical effect for eugenol is estimated to be liver damage. However, due to the allergenic properties of eugenol, people who are allergic to the substance should completely avoid skin
contact as there is no lower limit for this side effect.
Table 8.4. Summary of data used for calculation of MoS for eugenol using EUSES.
| Toxicological data (animals) |
|
| LD50, (mg/kg bw), oral, rat |
1930 (rat) (26) |
| LD50 (mg/kg bw), dermal |
1200 (21) |
| NOAEL, (mg/kg bw/d), ingestion, liver damage |
79.3 (33) |
| LOAEL, (mg/kg bw/d), ingestion, liver damage |
960 mg/kg bw/d (12.000 ppm in feed) (21) |
8.5 Methyl eugenol
Occurrence and use
Methyl eugenol is used as fragrance in perfumes. It is not added directly in cosmetic products but is only added as a fragrance raw material. In addition, it is used as an aromatic substance in food and drinks
and as an insect allurement (21, 35, 36). Methyl eugenol is found in many ethereal oils, in concentrations of up to 90% or more in oil extracts from pine trees. The substance smells mildly spicy with a slight
herbal-like scent and is used e.g. in scent compositions of clove and lilac. The substance can be produced synthetically through methylation of eugenol (31).
Identification
Methyl eugenol is a phenylether.
| Chemical name |
Methyl eugenol |
| Synonyms |
4-allyl-1,2-dimethoxybenzen |
| CAS-No. |
93-15-2 |
| EINECS No. |
202-223-0 |
| Molecular formula |
C11H14O2 |
| Molecular structure |
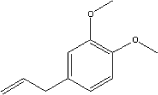 |
Legislation:
Classification according to the List of hazardous substances (Stat. Order 439 of 3 June 2002)
The List of unwanted substances. Danish Environmental Protection Agency (24).
Cosmetics (25) |
Not classified
Not listed
Recommended maximum concentration of 0.0002% in leave-on products (36) |
Physical-chemical properties (26)
| Physical state |
Liquid |
| Molecular weight (g/mol) |
178.23 |
| Melting point, °C |
-4°C |
| Boiling point, °C |
255°C (760 mmHg) |
| Evaporation (Pa) |
1 mm Hg (133 Pa) at 85°C
0,02 mm Hg (2,7 Pa) at 20°C (21) |
| Octanol-water distribution, (log Pow) |
2.9 (37) |
| Water solubility (mg/L) |
500 mg/l
< 1 mg/ml (21) |
Acute toxicity
LD50-values by oral exposure of rats and mice has been found from 850-1560 mg/kg bw for rats and 540 mg/kg bw for mice (37).
LD50 by dermal exposure of rabbits has been found to be > 2025 mg/kg bw (21) and > 5000 mg/kg bw (26).
A single exposure of 88 mg/kg bw lead to neurological effects in mice and a single exposure of 200 mg/kg bw for mice and rats (strain not specified) lead to the animals falling asleep but not dying (26).
LD50 for administration in the abdominal cavity in mice has been stated at 540 mg/kg bw (21).
Methyl eugenol is absorbed quickly after oral exposure, converted in the liver and excreted with the urine (35).
Chronic, repeated exposure
Daily dosage in the abdominal cavity at an exposure of 100 mg/kg bw/d in rats and mice for 26 and 42 days, respectively, did not lead to any effects in mice but a reduced growth in rats (26).
Methyl eugenol has been tested by NTP (National Toxicology Programme) for toxicity and carcinogenicity based on the structural similarity with safrol (CAS No. 94-59-7) which is a known carcinogen
(35). These tests have been reported below:
Tests have been carried out with oral exposure of rats in doses of 0, 10, 30, 100, 300 and 1000 mg/kg bw, 5 days/week for 14 weeks. At an exposure of 100 mg/kg bw/d, effects such as increased liver
weight in male animals and enzyme changes and liver cell damage in both sexes were observed. Furthermore, at a dose of 300 mg/kg bw/d, reduced body weight, increased bile amount, inflammation of the
mucous membrane of the stomach was seen in both sexes along with an increased liver weight in females. Exposure to 1000 mg/kg bw/d furthermore lead to an increased testicular weight (35). The same
tests on mice showed the same type of effects (35). NOEL for methyl eugenol has been set at 10 mg/kg bw/d for both species with effects on the liver and not cancer as the critical effect (37).
Tests carried out during 2 years with oral exposure of rats in doses of 37, 75, 150 and 300 mg/kg bw, 5 days/week for 105 weeks showed reduced body weight at all doses. 100% of the male animals died
before the end of the test at exposures of 150 and 300 mg/kg bw/d. At these dosage levels, an increased mortality was also observed in the female animals. Pathological examinations showed effects from
exposures of 37 mg/kg bw/d. These effects include neoplasms and cell changes in the liver, atrophy of the mucous membranes, kidney failure and adenomas in the kidneys. At 75 mg/kg bw/d,
neuroendoctrine tumours were seen in females, an effect observed at 150 mg/kg bw/d in males (35). When carrying out the same test on mice, the same type of effects was seen (35).
Based on the above, NTP concludes that there is clear evidence of carcinogenic effect of methyl eugenol in rats and mice (35).
Methyl eugenol is not mutagenic in Ames test at the highest test concentration of 0.33 mg/plate. The substance has shown that it may introduce sister chromatide exchange in Chinese hamster ovary cells with
activation, but it did not introduce chromosome changes in Chinese hamster ovary cells with and without activation. 14-week in vivo micronucleus tests with blood cells in mice were negative.
No data have been found regarding reproduction toxicology (26, 35, 37, 38).
The SCCNFP evaluation regarding the use of methyl eugenol in cosmetics is that the substance should not continue to be added to cosmetics as an ingredient. If the substance occurs naturally, e.g. in
fragrances, the highest concentration of methyl eugenol in the finished product should not exceed 0.01% in fine fragrance, 0.004% in eau de toilette, 0.002% in a fragrance lotion, 0.0002% in other leave-on
products and oral care product and 0.001% in rinse-off products (36). It is therefore evaluated that lip care products are allowed to contain up to 0.0002% methyl eugenol.
The European Medicines Agency for evaluation of medical products (EMEA) estimates that ingestion of natural medicine containing methyl eugenol due to the widespread occurrence of the substance in
plant oils does not pose a significant cancer risk in adults. It is, however, recommended that exposure of children as well as pregnant and nursing women should be minimised and that the absorption of
methyl eugenol through the skin be investigated (37).
No data has been found on inhalation of methyl eugenol.
Local irritation
No data has been found on the irritating properties of methyl eugenol.
Allergy
No data has been found on the sensitizing properties of methyl eugenol. The substance has not been listed by the SCCNFP as one of the substances for which there are reports on allergenic effects in
consumers (29).
Critical effect
The critical effects for methyl eugenol are effects on the liver and the carcinogenic effect. As the SCCNFP has set out guidelines for the use of the substance in cosmetic products, exposure must be evaluated
according to these limits (0.0002 %) as exposure below these must be expected to be of no concern to health.
Table 8.5. Summary of data used for calculation of MoS for methyl eugenol using EUSES.
| Toxicological data (animals) |
|
| LD, (mg/kg bw), oral, rat |
850 (37) |
| NOAEL, (mg/kg bw/d), ingestion, liver damage |
10 (37) |
| LOAEL, (mg/kg bw/d), ingestion, liver damage |
37 (35) |
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 March 2006, © Danish Environmental Protection Agency
|