|
| Front page | | Contents | | Previous | | Next |
Survey of lip care products with fragrance and flavour
9 Exposure assessment
For the exposure assessment, the substances' toxicological profiles, analysis results and guidelines for e.g. application frequency and amount as stated in EU's Technical Guidance Document (TGD) (20) and
the SCCNFP guidelines (39) are used to estimate the actual exposure in a worst case scenario for one or more standard persons.
9.1 Exposure calculations
Table 9.2 gives an overview of the results of the worst case calculations of the oral and dermal exposure using EUSES. EUSES is a data base programme used for exposure calculations. Summaries of the
exposure data can be seen in table 8.1. The modelling results are supplemented by manual calculations for all 26 fragrances + Methyl eugenol, cf. table 9.2.
Exposure through the use of lip care products can occur by absorption through the skin and oral intake, but it is relevant to include exposure through inhalation as fragrances are volatile at room and skin
temperature and thus may be inhaled through evaporation from the lips close to the nose.
It has not been possible to establish exposure scenarios for exposure through inhalation and for skin exposure to fragrance due to a lack of data in the literature for the individual substances. The EUSES
exposure evaluations have calculated with 100 % absorption through both types of exposure. This means that the two scenarios correspond to an ingestion of the entire product.
Therefore, it has been chosen to use oral absorption as the total absorption in the body as this is the exposure supported by most data in the literature. In addition, the model assumes exposure of the hands
when dermal exposure is modelled. The skin of the lips and thus absorption through them differs from the skin on the hands and it is hard to accurately estimate the impact of this route of exposure.
Table 9.1. Summary of exposure data calculated by EUSES.
| Exposure |
|
| Amount of substance on the skin (lips), all substances |
60 mg/day |
| Max. concentration in the ingested product: |
|
| Linalool |
0.845 mg/cm³ |
| Geraniol |
0.725 mg/cm³ |
| Benzyl Benzoate |
6.7 mg/cm³ |
| Eugenol |
0.0865 mg/cm³ |
| Methyl eugenol |
0.036 mg/cm³ |
Total absorption in the body
(Corresponds to exposure calculated for absorption through ingestion or the potential skin absorption): |
|
| Linalool |
7.24 x 10-4 mg/kg bw/d |
| Geraniol |
6.21 x 10-4 mg/kg bw/d |
| Benzyl Benzoate |
0.00574 mg/kg bw/d |
| Eugenol |
7.41 x 10-5 mg/kg bw/d |
| Methyl eugenol |
3.09 x 10-5 mg/kg bw/d |
| Margin of safety (MoS), repeated exposure, oral or dermal exposure: |
|
| Linalool |
6.9 x 104 |
| Geraniol |
1.26 x 105 |
| Benzyl Benzoate, oral exposure |
9.68 x 104 |
| Eugenol |
1.07 x 106 |
| Methyl eugenol, oral exposure |
3.24 x 105 |
When comparing the NOAEL and LOAEL values found for the analysed fragrances it can be seen that the calculated total intake in the body is very low. For the substances evaluated, the lowest ratio
between the NOAEL value found and the calculated value for total absorption in the body has been found for Linalool and this value is 69.000. It can also be seen from the MoS values that the health risk
from obtaining the most critical effects seen in literature by oral ingestion of the substances is very low.
The daily exposure to the 26 fragrances, which EU has declared to be allergenic, as well as Methyl eugenol has been calculated for two standard persons. A 60 kg woman and a 18 kg child (3-5 years old).
The daily exposure has been calculated as the highest measured content of the fragrance and the persons have been chosen based on the assumption that women use lip care products more frequently than
men and that the use may start as early as kindergarten due to dry lips or play with cosmetic products. Furthermore, sensitizing is rarely seen in children during the first 2-3 years of life, but exposure to
perfume containing contact allergen fragrances early in life may lead to a clinical problem later in life (40).
The following calculation method shows the worst case scenario with oral intake as the only route of exposure:
| Person weight, woman: |
60 kilos |
| Person weight, child, 3-5 years (19): |
18 kilos |
| Number of daily applications (20): |
6 |
| Amount used per application (20): |
10 mg |
Highest measurement of the fragrance
(example: Benzyl benzoate cf. table 9.2): |
6650 mg/kg |
| Absorption by oral ingestion: |
100 % |
Daily exposure, Benzyl benzoate, woman:
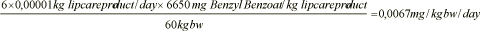
Daily exposure, Benzyl benzoate, child, 3-5 years:
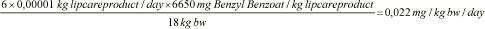
The daily exposure to the 17 fragrances found in the analysis is calculated per kg body weight per day for the two standard persons. The results can be seen in table 9.2.
Table 9.2. Daily exposure for two standard persons to the 17 fragrances found in the lip care products analysed.
| Fragrance |
Weight-%
(highest value
measured) |
Daily exposure,
woman, 60 kg
(mg/kg bw/day) |
Daily exposure,
child, 18 kg,
(mg/kg bw/d) |
| Benzyl alcohol |
0.18* |
0.0018 |
0.006 |
| Benzyl benzoate |
0.67* |
0.0067 |
0.022 |
| Benzylcinnamat |
0.0055* |
0.000055 |
0.00018 |
| Benzyl salicylat |
0.00065 |
0.0000065 |
0.000022 |
| Cinnamal |
0.00015 |
0.0000015 |
0.000005 |
| Citral |
0.12* |
0.0012 |
0.004 |
| Citronellol |
0.075* |
0.00074 |
0.0025 |
| Coumarin |
0.00055 |
0.0000055 |
0.000018 |
| Eugenol |
0.0087* |
0.000087 |
0.00029 |
| Farnesol |
0.0011* |
0.000011 |
0.000035 |
| Geraniol |
0.073* |
0.00073 |
0.0024 |
| α-Isomethylionon |
0.0069* |
0.000069 |
0.00023 |
| D-limonen |
2.25* |
0.0225 |
0.075 |
| Linalool |
0.085* |
0.000845 |
0.0028 |
| Isoeugenol |
0.015* |
0.000145 |
0.00048 |
| Methyl eugenol |
0.0036** |
0.000036 |
0.00012 |
* Maximum weight% measured is above 0.001% which is the declaration limit for the 26 allergenic fragrances.
** Maximum weight% measured is above 0.0002% which is the limit for allowed content of Methyl eugenol in this type of products.
The results of the analysis show (table 9.2) that the total content of the 26 allergenic fragrances is in concentrations from 0-2.45 weight-% with an average of 0.32 weight-% and a median value of 0.042
weight-%. The median value is lopsided towards lower concentration and it can be seen that few products have a very high content of perfume. A comparison with other cosmetic products than lip care
products shows an average value close to the perfume content in face creams (including make up and foundation). Face creams are stated as having the lowest content of perfume among 11 other types of
cosmetic products (41).
Exposure to the combined amount of fragrances through the use of a lip care product is estimated to be equal to exposure at the highest value measured as the contribution from other fragrances are
negligible, cf. table 9.2.
When comparing the calculations of the daily exposure per kg body weight for linalool, geraniol, benzyl benzoate, eugenol and methyl eugenol with the model calculated values using EUSES for total
absorption in the body it can be seen that the numbers are basically identical, cf. tables 8.1 and 8.2. We therefore estimate that the calculated values for d-limonene, benzyl alcohol and children give a realistic
worse case picture of the exposure to these fragrances when using lip care products.
9.2 Exposure assessment of the selected substances
D-limonen and benzyl alcohol have previously been evaluated by the EPA mapping projects. These state a NOAEL value for d-limonen of 10 mg/kg bw/day, which compared to the calculated daily
exposure gives an MoS of > 133 for children and > 444 for women. MoS should be above 100 in order for the product to be considered safe and the calculation for children shows that the margin of safety
is close to being exceeded. SCCNFP has listed d-limonen as a fragrance that causes allergy. Oxidation products of d-limonen are very potent allergens with a frequency of contact allergy of 1-2% in eczema
patients. According to SCCNFP, benzyl alcohol is a documented allergen causing allergy in 1.2-15% (2-4 cases in each study) in patients with cosmetic eczema (29). The substance has not been evaluated
further. Both substances are included on the EPA list of unwanted substances (24).
Due to the carcinogenic effect of methyl eugenol, SCCNFP has established a limit for content of methyl eugenol in leave-on cosmetic products of 0.0002 weight% (5, 36). For 2 of the 3 products analysed
for Methyl eugenol, the content was above the detection limit of 0.0012 and 0.0036 weight%, respectively. This means that both products (no. 80 and 93) are above the legislative maximum limit for methyl
eugenol in leave-on products. Therefore, the content of methyl eugenol may pose a potential health risk when using the products in question.
Linalool, geraniol, benzyl benzoate and eugenol have been evaluated by SCCNFP as being allergenic by skin contact and as no ”zero effect level” exists for this effect it is important to point out that skin
contact with these substances should be avoided (29).
Despite the fact that the exposure calculations show low daily exposure for both children and adults when using the lip care products, it has to be concluded that as the substances in question are allergenic
the use of products with a high content of perfume may pose a health risk. This view is supported by the fact that EU has established a mandatory declaration of these fragrances when present above a given
concentration in the finished cosmetic product.
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 March 2006, © Danish Environmental Protection Agency
|