|
| Front page | | Contents | | Previous | | Next |
More environmentally friendly alternatives to PFOS-compounds and PFOA
7 Environment and health assessment of alternatives to PFOS/PFOA related compounds
A search for different ecotox and human tox information has been performed for the identified alternatives to PFOS/PFOA related compounds. The search has been performed in different common
databases, such as Toxnet, NTP, IARC, Ecotox, Chemfate, and N-class. The search results are presented in Table 7.1 below.
As can be seen from the table very little information about the environmental and health effects of the substances is available. A few abstracts were identified for most of the substances, but none of the
abstracts gives relevant information. For three of the substances (two groups of alternatives) a record in HSDB (Hazardous Substances Data Bank) was identified. For a single substance two records were
found in the Ecotox database, but no records of aquatic toxicity (LC50 values) were available. For about half of the substances a few results about the chemical fate of the substances were available in the
Chemfate database. The sparse information found for the different alternatives is presented below.
Table 7.1: Search results in different common ecotox and human tox databases.
| Alternative compound |
CAS-No. |
Trade name |
TOXNET |
NTP, IARC |
ECOTOX |
N-class |
Chemfate |
| Fluorinated polyether |
Classified |
PolyFox™ |
Abstract |
- |
- |
- |
- |
| Fatty alcohol polyglycolethersulphate |
Classified |
Emulphor® |
- |
- |
- |
- |
- |
| Silicone polymers |
67674-67-3 |
WorléeAdd® |
- |
- |
- |
- |
- |
| Di-2-ethylhexyl sulfosuccinate, sodium salt |
577-11-7 |
Hydropalat®
Lutensit® A-BO
|
HSDB
Abstracts
|
- |
- |
- |
Yes |
| Propylated aromatic: Ruetasolv DI (Isopropylnaphthalene) |
38640-62-9 |
Ruetasolv |
HSDB
Abstracts
|
- |
2 |
- |
Yes |
| Propylated aromatic: Ruetasolv BP 4201 |
69009-90-1 |
Ruetasolv |
Abstracts |
- |
- |
- |
Yes |
| Propylated aromatic: Ruetasolv BF 4103 |
25640-78-2 |
Ruetasolv |
HSDB
Abstracts
|
- |
- |
- |
Yes |
| Propylated aromatic: Ruetasolv TPPN |
35860-37-8 |
Ruetasolv |
Abstract |
- |
- |
- |
- |
7.1.1 Fluorinated polyether
OMNOVA Solutions Inc. produces series of fluorinated polyethers (PolyFox™ products), which can be used as a surfactant and as flow, level, and wetting additive for coating formulations. The PolyFox
formulation is currently being used as a surfactant in floor polish products in the USA, Europe and Asia. The entire PolyFox family of fluorosurfactants are polymers with a molecular weight greater than
1,000. The PolyFox polymers are based on ether links – both the polymer backbone linkages and the link between the backbone and the perfluoroalkyl pendant. The PolyFox fluorosurfactants are
synthesized from perfluoroalkyl starting materials with a fully fluorinated carbon chain length of C4 or less. The current first generation products are all made with C2F5 or CF3 perfluoroalkyl side chain
structures (Personal communication OMNOVA 2004).
The basic structure of two of the different PolyFox™ compounds is illustrated in the figures below. Other and further details can be found in appendix F (Personal communication, PoraTek 2004).
Figure 7.1: The basic structure of PolyFox™ 3320 compound (Personal communication, PoraTek 2004). x+y equals about 20.
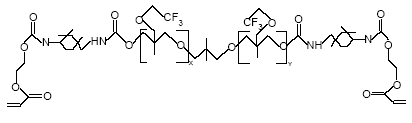
Figure 7.2: The basic structure of PolyFox™ 656 with C2F5 as the basic perfluoralkyl group. (Personal communication, PoraTek 2004). x+y equals about 6.
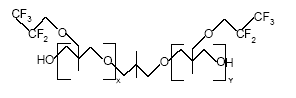
7.1.1.1 Health effects
No information other than the data given in the MSDS's has been found for these fluorinated polyethers (CAS numbers are proprietary).
According to the MSDS's the PolyFox products must be labelled R43: "May cause sensitisation by skin contact". Furthermore, the products may be irritating to the respiratory system (by inhalation) and the
gastrointestinal tract (if swallowed). A LD50 (oral, rat) value of > 2.0 g/kg indicates that the products have low acute toxicity by ingestion.
7.1.1.2 Environmental effects
No information other than the data given in the MSDS's has been found for these fluorinated polyethers.
For some of the PolyFox products 48hLC50 values > 100 mg/L for daphnias were reported, which indicates that the products have low acute toxicity to aquatic organisms (MSDS's from PolyFox products
2004).
However, OMNOVA states that their fluorinated polyethers have a high molecular weight that makes them less available for transport across bio-membranes and therefore less biologically available (in
contrast to the relatively small molecules of PFOS and telomer-based fluorosurfactants). Furthermore, the polymer backbone linkage of the PolyFox molecules is an ether link, which is more environmentally
stable than e.g. the ester linkages of PFOS and telomer-based fluorosurfactants. This makes the PolyFox molecule more resistant to degradation to lower molecular carboxylic acids. Additionally, the
PolyFox products are made with shorter fluorochain lengths (C2F5 or CF3 structures), which means they cannot produce the longer perfluorinated acids such as PFOA but only the less hazardous
perfluoroacetic and perfluoropropionic acids (Personal communication OMNOVA 2004).
The paper by Martin et al. (2003) confirms that perfluorinated acids with fully fluorinated chain lengths of C5 and below do not accumulate (see Chapter 5).
7.1.2 Fatty alcohol polyglycolether sulfate
BASF produces a fatty alcohol polyglycolether sulfate (Emulphor FAS 30), which can be used as levelling and wetting agent in paints and coatings (See further details in Appendix F).
7.1.2.1 Health effects
No information other than the data given in the MSDS has been found for this fatty alcohol polyglycolether sulfate (no CAS number available).
According to the MSDS this fatty alcohol polyglycolether sulfate is a non-irritating and non-hazardous substance. A LD50 (oral, rat) value > 2.0 g/kg is given, which indicates that the substance is acute toxic
by ingestion. No other information is available (MSDS Emulphor® FAS 30 2003).
7.1.2.2 Environmental effects
No information other than the data given in the MSDS has been found for this fatty alcohol polyglycolether sulfate.
According to the MSDS the fatty alcohol polyglycolether sulfate is readily biodegradable (>70% elimination according to OECD 301E), and it does not seem to be acute toxic to aquatic organisms as the
reported 96hLC50 value for fish (Leuciscus idus) is > 100 mg/L (MSDS Emulphor® FAS 30 2003).
7.1.3 Silicone polymers
Worlée-Chemie produces silicon polymers, which can be used as wetting agents as replacement for fluorinated surfactants in several cases in the paint and ink industry. Their products contain
3-(polyoxyethylene) propylheptamethyl trisiloxane (CAS No 67674-67-3) (See further details in Appendix F).
7.1.3.1 Health effects
No information other than the data given in the MSDS has been found for the silicone polymers.
According to the MSDS the silicone polymer must be classified as irritating and harmful with the R-phrases, R20 ("Harmful by inhalation") and R41 ("Risk of serious damage to the eyes"). Prolonged and
frequent skin contact can cause skin irritation (MSDS Worlée Add® 340 2004).
7.1.3.2 Environmental effects
No information other than the data given in the MSDS has been found for the silicone polymers.
According to the MSDS the silicone polymer must be classified as environmentallay dangerous with the R-phrases R51/53 ("Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic
environment"). The R-phrase R53 indicates that the substance is bioaccumulative (MSDS Worlée Add® 340 2004).
7.1.4 Sulfosuccinate
BASF and Cognis both produce a surfactant based on 50-75% of the sodium salt of di(2-ethylhexyl) sulfosuccinate (see Appendix F: "Identified alternatives" for further details), which can be used as a
wetting agent for paints and coatings. In one product the sulfosuccinate is mixed with water and ethanol, and in the other the sulfosuccinate is mixed with water and 2,2-dimethylpropane-1,3-diol (Personal
communication BASF 2004; Personal communication Cognis 2004).
The product with the lowest content of sulfosuccinate (containing water and 2,2-dimethylpropane-1,3-diol) is classified as a local irritant (Xi) with R-phrases R38 ("Irritating to skin") and R41 ("Risk of
serious damage to eyes") (MSDS Lutensit® A-BO 2003).
Münzing Chemie also produces a surfactant based on a sulfosuccinate derivative in ethanol and water, which also can be used as a wetting agent for paints and coatings. The type of sulfosuccinate (or CAS
No.) is not specified.
Figure 7.3: The chemical structure of the sodium salt of di(2-ethylhexyl) sulfosuccinate (CAS No. 577-11-7).
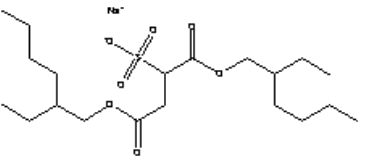
7.1.4.1 Health effects
According to the MSDS's of the sulfosuccinate products, the products can cause skin, eye and respiratory irritation, especially on prolonged or repeated contact. Dermatitis has been observed as a
long-term effect. Other possible long-term effects are central nervous system (CNS) depression, as well as injuryto heart, liver and blood-forming organs. The acute toxicity of the di-2-ethylhexyl
sulfosuccinate (LD50 (oral, rat) = 1.9 g/kg) indicates that the substance is mildly harmful, if swallowed. A LD50 (oral, rat) value of > 2.0 g/kg is also reported, but this value may be for the entire product
and not for the single substance di(2-ethylhexyl) sulfosuccinate (MSDS Hydropalat 875 2002; MSDS Lutensit® A-BO 2003).
Information found in the HSDB database suggests that di(2-ethylhexyl) sulfosuccinate is mildly toxic (by ingestion) to humans with a probable oral lethal dose (human) of 0.5-5 g/kg. The substance is in rare
cases reported to produce skin rash and allergies (HSDB database 2004).
7.1.4.2 Environmental effects
According to the MSDS's, and the Chemfate and HSDB databases the substance di-2-ethylhexyl sulfosuccinate is easily biodegradable and not likely to bioconcentrate. Only one LC50 value of the
substances has been found in a MSDS (MSDS Lutensit A-BO 2003). This 96hLC50 value of 10-100 mg/l for Leuciscus idus (small fresh-water cyprinoid fish) shows that the sulfosuccinate is harmful to
aquatic organisms.
Table 7.2: Environmental data for di(2-ethylhexyl) sulfosuccinate (Chemfate database 2004; HSDB database 2004; MSDS Lutensit A-BO 2003).
| Substance |
Di(2-ethylhexyl) sulfosuccinate |
| CAS No. |
577-11-7 |
| Log KOW |
- |
| BCF |
1.13 (estimated), < 0.9 – < 9.3 (experimentally) |
| LC50 (fish) |
LC50 (96h) of 10-100 mg/l (leuciscus idus) |
| Biodegradability |
Easily biodegradable (91-97% in 17 days, >90% OECD 303A) |
7.1.5 Propylated aromatics
As presented in Appendix F: "Identified alternatives", Rütgers Kureha Solvents produces different propylated aromatics (naphthalenes and biphenyls), which can be used as water repelling agents for
different applications, such as corrosion protection systems, marine paints, resins, printing inks, coatings, electrical, electronical and mechanical applications (Personal communication RKS 2004).
The presented propylated aromatic products are all colourless liquids with a boiling point at about 300 °C. Their flash point lies all about 140 °C. The substances have a very low solubility in water. Common
for the substances is that none of them is classified as hazardous substances.
Figure 7.4: The chemical structure of Ruetasolv DI (CAS No. 38640-62-9) – to the left, and Ruetasolv TTPN (CAS No. 35860-37-8) – to the right.
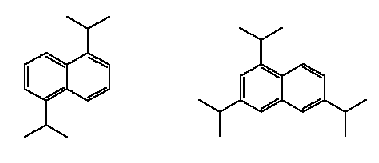
Figure 7.5: The chemical structure of Ruetasolv BP 4103 (CAS No. 25640-78-2) – to the left, and Ruetasolv BP 4201 (CAS No. 69009-90-1) to the left. Both products are mixtures of isomers.
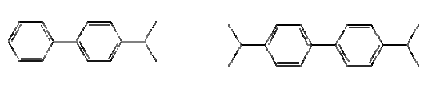
7.1.5.1 Health effects
According to the MSDS of one of the products (MSDS Ruetasolv DI 2003), the acute toxicity towards rats (LD50) of the substance bis(1-methylethyl)-naphthalene (CAS No. 38640-62-9) is higher than
3900 mg/kg, which means no classification of the substance as harmful. Neither irritating nor sensitising effects of the substance have been identified. Furthermore, the substances have been found without
mutagenic effects in the Ames test and in vivo in mice, no carcinogenic effects in rats, teratogenic effects in mice or foetotoxicity in mice.
Somewhat conflicting information was found for the substance in the HSDB database, where naphthalenes in general are found to be possibly irritating to eyes, skin and mucous membrane. Repeated
exposure may cause symptoms like headache and vomiting. Prenatal exposure to naphthalene has been harmful to the unborn child. According to IARC (International Agency for Research on Cancer)
naphthalene is possibly carcinogenic to humans (Group 2B). Ruetasolv DI (bis(1-methylethyl)naphthalene) is belonging to the chemical group of napthalenes, but no carcinogenicity has been identified for this
specific naphthalene compound (HSDB database 2004).
The substances diisopropyl-1,1'-biphenyl (CAS No. 69009-90-1) and (1-methylethyl)-1,1'-biphenyl (CAS No. 25640-78-2) can according to the MSDS cause irritation of the skin at long-term exposure.
Furthermore, irritation of the mucous membrane of the eyes is possible. No sensitising effect is known for the substance (MSDS Ruetasolv BP 4201 2003; MSDS Ruetasolv BP 4103 2004).
The HSDB database confirms that (1-methylethyl)-1,1'-biphenyl is an irritant of eyes, nose, throat, mucous membrane and the respiratory tract. At repeated skin contact the substance may produce skin
sensitisation or dermatitis. Central nervous system (CNS) damage, liver and kidney damage have been reported as chronic effects. Oral LD50 values for rats of 4.7 and 8.5 g/kg indicate that the chemical
has low acute toxicity and does not need a classification as harmful (HSDB database 2004).
The substance tris(1-methylethyl)naphthalene (CAS No. 35860-37-8) has according to the MSDS no irritating or sensitising effects (MSDS Ruetasolv TPPN 2004).
7.1.5.2 Environmental effects
Ruetasolv DI (bis(1-methylethyl)naphthalene; CAS No. 38640-62-9) is according to the MSDS easily biodegradable and has a low bioaccumulation potential. The acute aquatic toxicity for fishes is
measured above the saturation point. After 96 hours the concentration of 0.5 mg/L (above saturation) showed no lethal effect. Therefore, the substance has no acute toxic effect in the investigated fish
species.
Conflicting information was found in the HSDB and Chemfate databases. According to this information (see Table 7.3), the substance bis(1-methylethyl)naphthalene only slowly biodegrades and has a
tendency to bioaccumulate.
In the Ecotox database a LD50 value of 2 ml/kg (14 days) for yellow tails (Seriola quinqueradiata) is reported for bis(1-methylethyl)naphthalene.
As can be seen from Table 7.3 that environmental data about the substances ability to bioaccumulate was found for three of the propylated aromatics in the Chemfate database. All three substances have an
octanol/water partition coefficient (log KOW) higher than three, and since the bioconcentration factor (BCF) for the substances is greater than 100, the substances are considered to be potential
bioaccumulative. Furthermore, the biphenyl moiety seems to be easily biodegradable, whereas the naphthalene moiety only slowly biodegrades. The LC50 value for the biphenyl compound shows that the
compound is acutely toxic to aquatic organisms.
Table 7.3: Environmental data for some of the propylated aromatics. (Chemfate database 2004; HSDB database 2004).
| Trade name |
Ruetasolv DI |
Ruetasolv BP 4201 |
Ruetasolv BF 4103 |
| Substance |
Bis(1-methylethyl)-naphthalene |
Diisopropyl-1,1'-biphenyl |
(1-Methylethyl)-1,1'-biphenyl |
| CAS No. |
38640-62-9 |
69009-90-1 |
25640-78-2 |
| Log KOW |
4.90 |
7.184 |
5.2 |
| BCF |
370 - 3930 |
- |
5300 |
| LC50 (fish) |
LD50 of 2 ml/kg for yellowtail |
- |
LC50 (96h) of 2.5 ppm (rainbow trout)
LC50 (96h) of > 75 ppm (flagfish)
|
| Biodegradability |
Slow biodegradation (35% in 4 weeks) |
- |
Rapidly biodegrade under aerobic condition (80% in 48 h) |
No other ecotoxicological information was found for the other products either in databases or given in the MSDS.
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 June 2005, © Danish Environmental Protection Agency
|