|
More environmentally friendly alternatives to PFOS-compounds and PFOA Summary and conclusions
Background and purposePerfluorooctane sulfonate (PFOS) and a range of related perfluorinated compounds are used in numerous industrial products and consumer products because of their special chemical properties, for instance the ability to repel both water and oil. Perfluorooctanoic acid (PFOA) is used as a processing aid in the manufacture of fluoropolymers. PFOA is a well-documented contaminant in PFOS-related chemicals and telomers and is hence found as an impurity in various products. It is a growing concern that these potential harmful compounds now are found as widespread global environmental pollutants distributed in air, water, soils and biota, which indicates that perfluoroalkyl substances are environmentally persistent and bioaccumulate in wildlife and in humans. The implications of these occurrences and exposures are still not fully explored and understood but preliminary results have in some cases revealed unwanted adverse effects. Many more studies are underway, and in the future the knowledge will be improved considerably. If the substances are considered harmful, substitution with less harmful substances should take place, if possible. However, are there fit-for-use and less-hazardous alternatives available? That is what this report is about. The usages of PFOS and related substances in Denmark and elsewhere are described, and possible alternative substances to PFOA and PFOS-related substances are identified. The environmental properties and health risks are assessed both for PFOS and PFOA related substances and for potential substitutes. However, for most of the alternatives the knowledge level was rather scarcely. The project has mainly been based on scientific and technical literature, national reports and easily accessible information from the Internet or from personal interviews. Where relevant, the information has been supplemented with searches in the Danish Product Register of specific compounds and with contact to relevant producers and suppliers of products containing PFOS, PFOA, fluorotelomers or other related compounds. Furthermore, the Internet has been searched for possible alternatives, and the relevant companies have been contacted for further information. Project resultsSummary of the use of PFOS-related substances and alternativesDifferent recent surveys from different countries have mapped the use of PFOS-related substances. These surveys and information from the industry and producers of the substances have provided the picture of the use of PFOS-related substances and their alternatives as shown in Table 0.1. Table 0.1: Summary of the use areas of PFOS-related substances.
In most cases the alternatives to PFOS-related substances are other fluorinated chemicals with shorter chain length, such as C6-fluorotelomers or perfluorobutane sulfonate (PFBS). These chemicals fall under the larger chemical family called perfluoroalkyl substances (PFAS). The reason for this continuous use of fluorinated compounds is that polyfluorinated surfactants have superior properties compared to other and less expensive surfactants. Today the largest use areas of PFOS-based compounds seem to be:
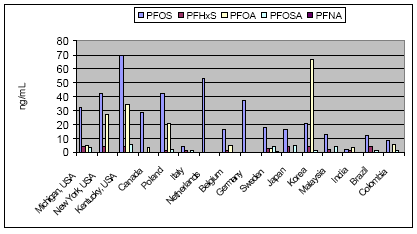
Not many alternatives to polyfluorinated compounds have been identified during this or other projects. The identified alternatives were primarily silicone-based products or hydrocarbon based surfactants for the paint and varnish area. In this area silicone-based products and hydrocarbon surfactants (such as aliphatic alcohols, sulfosuccinates, and propylated aromatics) are also used as alternatives, but in general it seems that these alternatives cannot be used, where extreme demands regarding low surface tension are needed. In these cases fluorinated surfactants seem to be the only substances that can reach the very low surface tension levels. Summary of the use of PFOA-related substances todayPFOA and its salts are used as a processing aid in the manufacture of fluoropolymers such as polytetrafluoroethylene (PTFE), a process not occurring in Denmark. DuPont has for the last 30 years investigated possible alternatives to PFOA as processing aid in the production of fluoropolymers. Several fluorohydrocarbons have been tested, but the results showed that the presence of hydrogen in the surfactant resulted in problems with the polymerisation. Supercritical carbon dioxide as solvent has also been tested in a pilot-scale facility. However, DuPont does not expect that this process will ever evolve into a technology that would have the capability to totally replace the current water-based polymerisation process. The conclusion so far from testing over the last 30 years is that there are no viable alternatives to PFOA. In Denmark only the ammonium salt of PFOA is found in very small quantities in a few products. Other PFOA-related substances were not found via a search in the Danish Product Register. The PFOA ammonium salt was registered for fluxing agents (used in plumbing) and in a primer and topcoat used for fluoroplastic coating. The use in fluxing agents is very limited and will cease, when leaded plumbing are banned from the year 2006, as lead-free plumbing do not require the use of these fluxing agents. Most important use area from an environmental perspectiveEmissions to the environment (air, soil and water) of PFOS and other polyfluorinated substances may happen directly from the production and processing plants. However, most important is releases during use (indoor or outdoor) and disposal of products containing these substances. Other PFAS-based chemicals such as PFBS and perfluorohexane sulfonate (PFHxS) with shorter chain length, precursor of PFOS such as perfluorooctane sulfonamide (PFOSA) and perfluorinated carboxylic acids (PFCA), including PFOA and perfluorononanoic acid (PFNA), are also found in the environment. Furthermore, the fluorotelomers, which in some cases are used as alternatives to PFOS-based compounds are also found in the environment and they seem to be long-range transported and degraded to PFOA and other PFCAs in the environment. Environmental sources of fluorinated telomers are currently unknown but these substances may be released at manufacturing of polyfluorinated compounds and at the decomposition of polymeric materials and consumer products that incorporate telomers. The most important of the above use areas for PFOS-related compounds is from an environmental perspective the use as surfactant in waxes, and floor polish. In this area only some substitution has been carried out, whereas a more substantial substitution has been taken place within the cleaning agent area. Common for the cleaning agents, waxes and floor polishes are that the PFOS-related compounds are ingredients of the cleaning agents. The widespread use of these products therefore also results in a widespread emission to the environment of these substances. Impregnation products, fire-fighting foams, the photographic industry and hydraulic oils within the airplane industry are still use areas that contribute to the total PFOS/PFOA concentration in the environment as these uses predominantly seem to use fluorotelomers or PFAS-compounds with shorter chain length (like PFBS) as alternatives to the former PFOS-compounds. However, the photographic industry does not seem to be the biggest problem, as this industry represents a smaller use area, and as the PFAS compounds are not a part of the final products. No information has been found on the size of the hydraulic oil use area. However, this area is not expected to be that large. Fire-fighting foams represent the area with the largest risk of a huge accidential leak directly to the environment. These foams are primarily used for oil-gasoline related fires, including at airports, air bases, offshore oil platforms, oil refineries and oil storage tanks at harbours. Use of fire-fighting foams to fight large fires and accidental spills may cause considerable local, persistent contamination of ground and surface waters. A shift to the non-fluorinated training foams, which some organisations are beginning to make, is therefore a way forward to avoid unnecessary emissions to the environment of the fluorinated compounds. The use of impregnation agents to protect domestic products such as clothes and carpets may be one of the most important exposure ways for the human population. Measurements have confirmed that PFOA and PFOS can be found in vacuum cleaner dust in private households. The most important human exposure may be through inhalation of air and the dust in private homes and offices. Health and environmental exposure and effects of polyfluorinated substancesPerfluoroalkylated substances are present in the environment primarily in the form of the most stable PFOS and PFOA, which are the final degradation products of various perfluorooctyl compounds. Recently, when the many PFOS uses have been phased out, the environmental concentrations of other related substances such as PFBS, PFHxS and PFNA have been increasing. PFOS, PFOA and other perfluorinated compounds are now considered as global environmental contaminants. They have been found in indoor air, outdoor air, soil, ground water, surface waters and even at 1000 m depth in the Pacific Ocean. Perfluorinated compounds are widely distributed in wildlife. PFOS has been detected in blood and liver samples from various species of aquatic animals (seal, otter, sea lion, dolphin, polar bear, mink), birds, fish and amphibians. Some samples also contained other related substances such as PFOSA, PFHxS, PFNA and PFOA. Some perfluorinated substances have even been found in blood and liver samples from the general human population (see Figure 0.1). Whereas PFOS and PFOA and perfluorinated acids with longer alkyl chain bioaccumulate in wildlife and human tissue, perfluorinated acids with fully fluorinated chain lengths of C5 and below do not seem to accumulate significantly in wildlife and human tissues. . Figure 0.1: Human blood levels of PFOS-related substances in various countries
One of the puzzling aspects about the PFAS contamination in the environment has been that substances such as PFOS and PFOA have been identified in remote Arctic areas, for example in polar bears, and even in relatively high concentration compared to the concentration levels found for other contaminants. The long-range transportation of specifically PFOA has been puzzling, as the low volatility and the ability of the substances to bind to water does not make it probable that PFOA will spread easily in the environment. However, the latest research indicates that the long-range air transportation of PFOA can be explained by the fact that fluorotelomer alcohols (FTOH) and some other non-polar PFAS are more volatile and less water-soluble and therefore have a greater tendency to escape the water phase. Short-chained FTOHs have an atmospheric lifetime of 20 days, which makes them able to travel about 7000 km. They may consequently be long-range transported and hereby reach remote arctic areas, where they can degrade to the most stable compounds PFOA and PFOS. In addition to the air transportation seawater, wildlife and humans may also move these chemicals to the arctic. Studies show that PFOS and other polyfluorinated chemicals are readily absorbed in the body. Both PFOA and PFOS are considered to be metabolically inert, and other perfluorocarboxylic acids and perfluorosulfonic acids do have similar properties, which means that their functional derivatives may be transformed to the parent compound. For example, the fluorotelomer alcohol 8:2 FTOH is transformed to PFOA in rats. Once absorbed in the body, PFOA and PFOS may bind to proteins and accumulate in various body tissues, including blood and liver; for PFOS also in testis and brain. The half-life of PFOA is about 2-4 years in humans and 1 month in monkeys. The half-life of PFOS is longer than for PFOA – about 200 days in monkeys. The half-life value in humans was not found. The acute lethal toxicities of PFOS and PFOA are moderate corresponding to a classification as harmful, if swallowed. PFOS is more toxic than PFOA, and the toxicity of related substances increases with the length of the alkyl chain. The liver is the primary target organ for polyfluorinated compounds, and these chemicals cause peroxisome proliferation in the rodent liver as well as induction of various enzymes involved in lipid metabolism. PFOS seems to be more active than PFOA concerning this effect but again PFDA with a longer alkyl chain is even more active. Toxic effects have been reported, such as induction of fatty liver and uncoupling of the mitochondrial respiratory chain. PFOA also affects the serum levels of various hormones, i.e. reducing testosterone and increasing estradiol in rats. Thus, it can be considered as an endocrine disruptor. Although the fluorinated chemicals do not seem to be mutagenic, PFOA has induced testis cancer, and PFOS and EtFOSE have induced liver cancer in experimental animals. USEPA classifies PFOA as a carcinogen in animals. PFOS and PFOA cause developmental effects, including reduction of foetal weight, cleft palate, oedema, delayed ossification of bones, and cardiac abnormalities. However, the structural abnormalities were only found in the highest PFOS dose groups, where significant reductions of weight gain and food consumption were also observed in the pregnant dams. Thus the relevance may be questioned. Other tested PFAS (PFBS and PFHxS) had no significant effect on reproduction even at high doses. In general, the information in open literature about the toxicology of the polyfluorinated compounds is rather sparse, and it will take some time and efforts, before sufficient information for evaluation of the full impact of the present levels in humans is available. The experience from the work environment has not indicated any important adverse health effects among exposed workers, besides a retrospective cohort mortality study of a perfluorooctanesulfonyl fluoride (PFOSF) production workforce, which reported an excess of bladder cancer at high-exposure jobs. With respect to aquatic toxicity PFOS is considered to be moderately acute toxic and slightly chronically toxic to aquatic organisms. PFOA is practically non-toxic. EtFOSA is slightly acute toxic to daphnids. There seems to be large species difference in the biological response, because PFOS was three orders of magnitude more toxic to the aquatic midge Chironomus tentans than to most other aquatic organisms. The scarce database indicates a need for further studies. Environmental exposure and effects of non-fluorinated alternativesIn general very little information about the specific substances was available. The alternative hydrocarbon surfactants seem to be ready biodegradable. The fatty alcohol polyglycolether sulfate is readily biodegradable and does not seem to be toxic to aquatic organisms. The sulfosuccinates are likewise easily biodegradable, do not seem to bioconcentrate, but are harmful to aquatic organisms. The biphenyls and the naphthalene derivatives are potentially bioaccumulative. The biphenyl moiety seems to be easily biodegradable, whereas the naphthalene moiety only slowly biodegrade. The sparse information suggests that the biphenyls are acutely toxic to aquatic organisms, whereas the naphthalene's have no acute toxic effects in the investigated fish species. Of the investigated alternatives, the silicone polymers seem to have the more adverse environmental effects. The specific compound investigated is classified as environmentally harmful (R51/53 "Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment"), as the substance is toxic to aquatic organisms and is bioaccumulative. Health effects of non-fluorinated alternativesIn general very sparse information about the health effects of the alternative substances is found. Therefore, most of the information is based on the material safety data sheets (MSDS). The fatty alcohol polyglycolether sulfate are acutely toxic by ingestion but are not considered to be irritating. The sulfosuccinates are irritants to eyes, skin and the respiratory system. Dermatitis has been observed as a long-term effect as well as CNS depression. The substance is mildly harmful to toxic, if swallowed. The naphthalene- and biphenyl derivatives are irritating substances, and the biphenyl compounds may produce skin sensitisation or dermatitis. Furthermore, one of the biphenyl compounds is known to cause CNS damage as well as liver and kidney damage. The parent compound naphthalene is classified as possible carcinogenic in humans (IARC Group 2B). However, no carcinogenicity has been identified for the specific naphthalene derivatives used as alternatives to polyfluorinated compounds. The silicone polymers are irritating substances and are harmful by inhalation. Main conclusionsThe information obtained from the different surveys have shown that PFOS-related substances have been replaced in most application areas, or at least in the largest application areas. Still some use areas exist, where until now it has not been possible to identify possible alternatives to the PFOS-related substances, and in these areas it is predicted by industry that it may take as long as 5-10 years to find suitable alternatives. Even though non-fluorinated alternatives, such as different hydrocarbon surfactants and silicone products, have been identified and are in use within specific areas, the general picture is that in most cases or at least in the larger application areas, other fluorinated compounds are used instead. Generally, the reason for this is that the non-fluorinated alternatives do not work as well, especially in situations, where extremely low surface tension is needed. The fluorinated compounds, used as alternatives, are typically fluorinated compounds with shorter chain length such as fluorotelomer alcohols (mainly C6 chain length), PFBS (perfluorobutane sulfonate), or perfluorinated polyethers based on a CF3 or a C2F5 structure. Even though the alternative telomer alcohols mainly are based on a chain length of C6, the products are a mixture of telomer alcohols of different chain length, including C8 and C10 compounds. The new finding that telomer alcohols may break down to PFOA in the environment means that the use of telomer alcohols still may be a source of PFOA (and other PFCA) in the environment. Among the polyfluorinated alkyl compounds the bioaccumulation potential and hazard increase by increasing length of the alkylated alkyl group. Polyfluorinated compounds with an alkyl chain length of C5 or below do not seem to be significant bioaccumulative and toxic. They are, however, still substances that will persist in the environment for decades, and the implications for human health and the environment are unclear, as the toxicity and ecotoxicity of these shorter chained fluorinated compounds are yet to be examined.
|