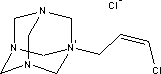|
| Front page | | Contents | | Previous | | Next |
Survey and assessmens of chemical substances in glass and porcelain colours
7 Toxicological profile of selected substances
7.1 Toxicological profile of anthraquinone
7.1.1 Identification of the substance and physical-chemical properties
7.1.2 Toxicological properties
7.1.3 Ecotoxicological properties
7.1.4 Fate in the environment
7.1.5 Conclusion
7.2 Toxicological profile of 2-butanoneoxime
7.2.1 Identification of the substance and physical-chemical properties
7.2.2 Toxicological properties
7.2.3 Ecotoxicological properties
7.2.4 Fate in the environment
7.2.5 Conclusion
7.3 Toxicological profile of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride
7.3.1 Identification of the substance and physical-chemical properties
7.3.2 Toxicological properties
7.3.3 Ecotoxicological properties
7.3.4 Fate in the environment
7.3.5 Conclusion
7.4 Toxicological profile of diarylide
7.4.1 Identification of the substance and physical-chemical properties
7.4.2 Toxicological properties
7.4.3 Ecotoxicological properties
7.4.4 Fate in the environment
7.4.5 Conclusion
7.5 Toxicological profile of 2-naphthol
7.5.1 Identification of the substance and physical-chemical properties
7.5.2 Toxicological properties
7.5.3 Ecotoxicological properties
7.5.4 Fate in the environment
7.5.5 Conclusion
7.6 Toxicological profile of quinacridone
7.6.1 Identification of the substance and physical-chemical properties
7.6.2 Toxicological properties
7.6.3 Ecotoxicological properties
7.6.4 Fate in the environment
7.6.5 Conclusion
7.7 Toxicological profile of 1,2,3,4,5,6,7,8-octahydroacridine
7.7.1 Identification of the substance and physical-chemical properties
7.7.2 Toxicological properties
7.7.3 Ecotoxicological properties
7.7.4 Fate in the environment
7.7.5 Conclusion
7.8 Toxicological profile of hexamethylene-1,6-diisocyanate
7.8.1 Identification of the substance and physical-chemical properties
7.8.2 Toxicological properties
7.8.3 Ecotoxicological properties
7.8.4 Fate in the environment
7.8.5 Conclusion
7.9 Toxicological profile of chloroisocyanate benzene
7.9.1 Identification of the substance and physical-chemical properties
7.9.2 Toxicological properties
7.9.3 Ecotoxicological properties
7.9.4 Fate in the environment
7.9.5 Conclusion
7.10 Summary
7.1 Toxicological profile of anthraquinone
7.1.1 Identification of the substance and physical-chemical properties
7.1.1.1 Identification
| Chemical name |
anthraquinone |
| EINECS name |
anthraquinone |
| CAS no. |
84-65-1 |
| Molecular formula |
C14H8O2 |
| Structural formula |
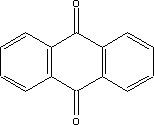 |
Applications
Anthraquinone is used in the manufacture of dyestuffs and pigments, as an organic inhibitor and through the impairment of taste as a bird repellent on seeds (6, 18). The substance is also used as a catalyst in
connection with the manufacture of paper pulp (2).
Synonyms
The following synonyms have been found for anthraquinone (1, 5):
- anthracenedione
- 9,10-anthracenedione
- anthracenequinone
- anthradione
- Anthrapel
- 9,10-anthraquinone
- corbit
- 9,10-dihydro-9,10-dioxoanthracene
- 9,10-dioxoanthracene
- diphenylenketon
- hoelite
- morkit
Regulation
| EU classification |
not classified |
| DK guideline list for self-classification |
not classified |
| Cosmetics Statutory Order |
not regulated |
| Foodstuffs (the positive list) |
not on the positive list |
| Foodstuffs (the flavour list, 2002) |
not on the flavour list 2002 |
The Danish Working Authority
occupational exposure limit list |
not on the EOL list |
7.1.1.2 Physico/chemical properties
Table 7.1. Physico-chemical properties of anthraquinone.
| Physico/chemical properties |
|
Reference |
| Physical form |
solid substance |
1 |
| Molecular weight (g/mol) |
208.22 |
17 |
| Melting point (°C) |
286 |
17 |
| Boiling point (°C) |
377 |
17 |
| Vapour pressure (Pa) |
0.000016 |
17 |
| Specific weight (kg/L) |
1.438 |
1 |
| Log Kow |
3.39 |
17 |
| Water-solubility (mg/l) |
1.35 |
17 |
7.1.2 Toxicological properties
Absorption
No relevant data was found in the literature examined.
Metabolism
No relevant data was found in the references examined.
7.1.2.1 Acute toxicity
Inhalation
Data from the literature points to relatively low toxicity by inhalation, since the LC50 for rats is reported at >1,300 mg/m³ given exposure of more than 4 hours/6,16/.
Ingestion
Studies of acute toxicity indicate that anthraquinone is of low acute toxicity. The literature mentions LD50 values for rats of >5 g/kg by ingestion. A LDLo value for rats has been reported as being 15,000
mg/kg /16/.
Skin contact
The literature reports a LD50 for dermal exposure of >5,000 mg/kg /16/.
Irritation and corrosiveness
The substance was not found to be a skin irritant in tests on rabbits /6/. Eight human male test subjects exposed to a non-specified quantity of the substance showed no signs of irritation after 24-hour
application on the upper arm /16/. Safety datasheets and other secondary literature often describe the substance as being a possible irritant.
The application of the substance in powder form to the eyes of rabbits has caused irritation. This effect is assumed to be principally mechanical in nature, as the substance cannot be dissolved in the eye.
Other tests with the application of the substance in dissolved form showed no signs of eye irritation /16/.
7.1.2.2 Subacute/chronic toxicity
Allergy and hypersensitivity
No primary data was found documenting the substance's sensitization properties /16/. Secondary literature describes the substance as possibly sensitizing /6/.
Organ damage
In rats subject to inhalation of 0.0052 and 0.0122 mg/l respectively for 5-6 hours/day for four months, bodyweight and blood picture were effected and there were histopathological findings in the lungs of
the high dose group /16/.
Daily oral dosing of rats with 1, 10 or 100 mg/kg for three months resulted in a decline in food intake and bodyweight, liver weight increases and changes in clinical-chemical parameters. NOAEL was
determined at 1 mg/kg /16/.
Daily dosing of rats via stomach pump with 2, 10, 20, 50 or 250 mg/kg for 28 days resulted in a decline in bodyweight and increases in the weight of certain organs including the liver, kidneys, spleen, thyroid
gland, heart, testes and ovaries. NOAEL was determined at 2 mg/kg /16/.
The literature makes reference to acute and chronic effects on the nervous system /9/. Mentioned as acute effects are convulsive fits, paralysis of the spinal cord, while chronic effects mentioned include visual
disorders.
Genetic damage
The substance has been examined in a number of Ames' tests on a variety of strains, both with and without metabolic activation with both negative and positive results.
Cancer
In tests on rats which were administered anthraquinone in their food corresponding to daily doses of 20, 45, 90 or 180 mg/kg for males and 25, 50, 100 or 200 mg/kg for females, increased incidence of
non-neoplastic damage to kidneys, liver, spleen and bone marrow was observed in both males and females. It was concluded that there was some evidence of carcinogenic activity in males, but clear
evidence of carcinogenic activity in females /18/.
In equivalent tests on mice exposed to daily doses of 90, 265 or 825 mg/kg for males and 80, 235 or 745 mg/kg for females, non-neoplastic damage to liver, bladder and spleen was observed in both males
and females and to thyroid gland and kidneys in males. It was concluded that there was clear evidence of carcinogenic activity in both males and females /18/.
In other tests involving dermal and oral administration of the substance to mice no carcinogenic activity was observed /16/.
Damage to the reproductive process and the foetus
No relevant data was found in the references examined.
7.1.3 Ecotoxicological properties
The table below summarises the effects of anthraquinone on aquatic organisms.
Table 7.2. Ecotoxicological data for anthraquinone (16, 20). - No data
Length of test is stated in parentheses immediately after the concentration.
| Group of organisms |
Latin name |
EC50/LC50 (mg/l) |
LOEC (mg/l) |
| Bacteria |
Oscillatoria chalybea |
- |
0.02 (5 d) |
| Active sludge |
|
7264 (3 h) |
- |
| Algae |
Lemna gibba |
0.01-1.5 (7 d) |
- |
| Algae |
Selenastrum capricornutum |
- |
20.8 (5 d) |
| Algae |
Blue-green algae |
- |
20.8 (5 d) |
| Crustaceans |
Americamysis bahia |
0.1-2.2 (48 h) |
- |
| |
Pimephales promelas |
2650 (96 h) |
- |
| Fish |
Tilapia mossambica |
12-26 (48 h) |
- |
7.1.4 Fate in the environment
7.1.4.1 Degradation
Table 7.3. Reported minimum and maximum degradation for anthraquinone (14). - No data. Length of test is stated in parentheses immediately after the degradation ratio.
| |
Minimum (%) |
Maximum (%) |
| Aerobic conditions |
15 (28 d) |
100 (14 d) |
| Anaerobic conditions |
- |
- |
7.1.4.2 Bioaccumulation
No data was found on anthraquinone's bioaccumulation potential. Based on Log Kow, the bioconcentration factor can be calculated using the following equation (21):
log BCF = 0.76 log Kow – 0.23
Based on this equation, log BCF for anthraquinone comes out at 2.35. The BCF value is not considered to be critical with regard to the risk of accumulation in the food chain of living organisms until it
reaches 100.
7.1.5 Conclusion
Anthraquinone is of limited acute toxicity through inhalation, ingestion and skin contact and has not been found to irritate skin and eyes. The substance is described as possibly sensitizing, but additional
documentation of this effect was not found.
The lowest identified NOAEL in connection with repeated exposure was determined to be 1 mg/kg after 3 months' administration of the substance in food. At higher doses, effects observed included weight
increase in liver and other organs.
In tests, the substance showed carcinogenic activity in female rats and in both male and female mice. However, there is insufficient information on cancer studies to constitute the basis for self-classification.
No data was found on reproduction toxic effects.
In the light of the data gathered on anthraquinone's environmental properties, the substance would be classified as a hazard to the environment due to its high acute toxicity relative to aquatic organisms and its
bioaccumulation potential.
The analysis results show that the substance is contained in one of the analysed products in concentrations of 0.0034 and 0.0055% respectively. At such low concentrations it is unlikely the substance will
constitute an environmental or health hazard. However, there are grounds for being attentive to the substance's carcinogenic properties, which may be considered to be the critical effect.
7.2 Toxicological profile of 2-butanoneoxime
7.2.1 Identification of the substance and physical-chemical properties
7.2.1.1 Identification
| Chemical name |
2-butanoneoxime |
| EINECS name |
butanoneoxime |
| CAS no. |
96-29-7 |
| Molecular formula |
C4H9NO |
| Structural formula |
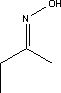 |
Applications
Among other uses, 2-butanoneoxime is used in paint and varnish as an additive for the regulation of viscosity /16/.
Synonyms
The following synonyms have been found for 2-butanoneoxime /1.9/:
- aron m 1
- ethyl methyl ketone oxime
- ethyl methyl ketoxime
- mek-oxime
- methyl ethyl ketone oxime
- methyl ethyl ketoxime
- skino #2
- troykyd anti-skin b
- USAF AM-3
- USAF EK-906
Regulation
| EU classification |
Xn;R21 Carc3;R40 Xi;R41 R43 |
| DK guideline list for self-classification |
not assessed |
| Cosmetics Statutory Order |
not regulated |
| Foodstuffs (the positive list) |
not on the positive list |
| Foodstuffs (the flavour list, 2002) |
not on the flavour list 2002 |
| The Danish Working Environment Authority occupational exposure limit level list |
not OEL list |
7.2.1.2 Physico/chemical properties
Table 7.4. Physico-chemical properties of 2-butanoneoxime.
| Physico/chemical properties |
|
Reference |
| Physical form |
liquid |
1 |
| Molecular weight (g/mol) |
87.12 |
17 |
| Melting point (°C) |
-29.5 |
17 |
| Boiling point (°C) |
152.5 |
17 |
| Vapour pressure (Pa) |
120.5 |
17 |
| Specific weight (kg/L) |
0.923 |
1 |
| Log Kow |
0.63 |
17 |
| Water-solubility (mg/l) |
10,000 |
17 |
7.2.2 Toxicological properties
Absorption
No relevant data was found in the references examined.
Metabolism
2-butanoneoxime is largely metabolised and does not accumulate in the tissues /18/.
7.2.2.1 Acute toxicity
Inhalation
In tests on rats LC50 values of 20 mg/l were observed upon four-hour exposure /16/. TCLo for rats has been recorded as 400 and 1000 ppm respectively upon six-hour exposure (1.4 mg/l and 3.6 mg/l)
/6/.
Ingestion
The literature contained LD50 values for rats of 930 mg/kg body weight for males and between 1620 and 3700 mg/kg bodyweight for females /6, 16/. The majority of values found exceeded the
classification limit of 2000 mg/kg.
Skin contact
The substance is classified as Harmful with R21, Harmful in contact withsSkin, on the List of Dangerous Substances.
Irritation and corrosiveness
Exposure of rabbit skin resulted in no, mild or moderate skin irritation. Moderate irritation was presumed to have occurred in tests following American guidelines, where the exposure time was 24 hours
instead of the 4 hours used under the EU's classification criteria /16/.
The substance has been found to be highly irritating to the eyes of rabbits and is classified as Irritant with R41, Risk of serious damage to eyes, on the List of Dangerous Substances.
7.2.2.2 Subacute/chronic toxicity
Allergy and hypersensitivity
The substance caused sensitization in tests on guinea pigs /16/ and is classified as Sensitizing with R43, May cause sensitization by skincContact, on The List of Dangerous Substances.
Organ damage
In a 28-day inhalation test on rats, significant changes to most of the blood parameters of both males and females were observed at concentrations of 400 ppm (1.4 mg/l). Raised methaemoglobin was
observed in females at 100 ppm (0.3 mg/l). NOAEL was determined at 25 ppm (0.09 mg/l) /16/. In a corresponding study only minor changes to blood parameters were observed at concentrations of 1.92
mg/l and 2.57 mg/l. In this study NOAEL was determined at 1.02 mg/l /16/.
Oral dosing (stomach pump) of rats over 90 days with 25, 75 and 225 mg/kg/day resulted in dose-related changes to blood parameters and a change in the weight of spleen and liver. No neurological effects
were observed and there were no fatalities. Based on the results NOEL was extrapolated to be 10 mg/kg. The blood is the primary target organ with effects such as haemolytic anaemia and the
compensatory formation of blood cells (hematopoiesis).
Changes were observed to blood parameters at all in dose groups in a 90-day neurotoxicity study using oral (stomach pump) dosing of rats with 40, 125 and 400 mg/kg respectively. Transitory behavioural
changes were observed at 400 mg/kg. NOEL for neurotoxicity was established at 125 mg/kg/day /16/.
Genetic damage
2-butanoneoxime has been examined both in in vitro and in vivo test batteries with largely negative results /10, 16/. Positive results were found in an Ames' test with metabolic activation and in an in vitro
sister chromatide exchange test /18/.
Cancer
The substance is suspected of having carcinogenic properties, and in the EU is classified as a category 3 Carcinogenic with R40, Possible risk of cancer.
This is confirmed by inhalation studies on rats and mice where the substance proved to be a liver oncogen at 75 ppm in male rats and at 374 ppm in male mice /16/.
Damage to the reproductive process and the foetus
No damage to the reproductive process was found in a two generation study on rats using oral (stomach pump) doses of 10, 100 and 200 mg/kg. Dose-related effects to the parents were observed in both
generations /16/.
Rats administered 2-butanoneoxime in drinking water (stomach pump) in doses of 60, 200 and 600 mg/kg/day showed no treatment-related effects on the foetuses and NOAEL was established at 600
mg/kg/day /16/. Corresponding tests on rabbits dosed with 8, 14, 24 and 40 mg/kg also failed to cause any treatment-related effects on the foetuses /16/.
7.2.3 Ecotoxicological properties
The table below summarises the effects of 2-butanoneoxime on aquatic organisms.
Table 7.5. Ecotoxicological data for 2-butanoneoxime (16, 20). - No data
Length of test in days (d) is listed in parentheses after the concentration.
| |
Latin name |
EC50/LC50 (mg/l) |
LOEC (mg/l) |
| Bacteria |
Pseudomonas putida |
281 (17 h) |
- |
| Bacteria |
Pseudomonas sp. |
- |
630 (-) |
| Algae |
Scenedesmus subspicatus |
83 (72 h) |
- |
| Crustaceans |
Daphnia magna |
> 500 (48 h) |
- |
| Crustaceans |
Daphnia magna |
750 (48 h) |
- |
| Fish |
Poecilia reticulata |
760 (96 h) |
- |
| Fish |
Leuciscus idus |
320-1000 (96 h) |
320 (96 h) |
| Fish |
Pimephales promelas |
843 (96 h) |
- |
7.2.4 Fate in the environment
Standard tests for determining the aerobic biodegradation of 2-butanoneoxime show that the substance is relatively easily degradable, cf. Table 7.6. No data was found on 2-butanoneoxime's degradability
under anaerobic conditions.
Table 7.6. Reported minimum and maximum degradation for 2-butanoneoxime (16). - No data.
Length of test is listed in parentheses after the degradation ratio.
| |
Minimum (%) |
Maximum (%) |
| Aerobic conditions |
25 (28 d) |
70 (18 d) |
| Anaerobic conditions |
- |
- |
7.2.4.1 Bioaccumulation
2-butanoneoxime's bioaccumulation potential is low. Based on Log Kow, Log BCF may be calculated at 0.25 (see section 7.1.4.2). The bioconcentration factor for freshwater fish has been established at
0.5-0.6 /14/.
7.2.5 Conclusion
2-butanoneoxime is rapidly metabolised and does not accumulate in the tissues. The substance is classified as Harmful with R phrase R21, Harmful in contact with skin, Carcinogenic in Category 3 with
R40, Possible risk of cancer, Local Irritant with R41, Risk of Serious Eye Damage, and Sensitizing with R43, May cause sensitization by skin contact. The blood system (the red blood cells) is the
primary target organ given repeated exposure.
Based on the data on 2-butanoneoxime's environmental properties the substance does not require classification as being an environmental hazard.
Chemical analyses detected 2-butanoneoxime in concentrations of between 1.8 and 9.2% in five products in the first analyses. The second analyses of 2-butanonoxime detected concentrations between 1.4
and 2.1% for the products P1, P2, P10 and P11. Glass and porcelain paints containing such high concentrations of the substance must be considered unsuitable as hobby products for children and adults
both by virtue of the sensitizing and the carcinogenic properties.
7.3 Toxicological profile of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride
7.3.1 Identification of the substance and physical-chemical properties
7.3.1.1 Identification
| Chemical name |
1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride |
| EINECS name |
methenamine 3-chloroallylochloride |
| CAS no. |
4080-31-3 |
| Molecular formula |
C9H16Cl2N4 |
| Structural formula |
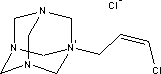 |
Applications
The substance is a formaldehyde releaser used as a biocide in e.g. paint, glue, ink and similar products. It is also used as a preservative in cosmetics.
Synonyms
The following synonyms have been found for 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride
- cis-N-(3-chloroallyl)hexaminium chloride
- 1-(3-chloro-2-propenyl)-3,5,7-triaza-1-azoniatricyclo[3.3.1.1(3,7)]decan chloride
- cinartc 200
- dexamethylenetetramine chloroallyl chloride
- dowicide 184
- dowicide Q
- dowicil 75
- dowicil 100
- dowco 184
- Quaternium-15
Regulation
| EU classification |
not classified |
| DK guideline list for self-classification |
not classified |
| Cosmetics Statutory Order |
preservative permitted to max. 0.2% |
| Foodstuffs (the positive list) |
not on the positive list |
| Foodstuffs (the flavour list, 2002) |
not on the flavour list 2002 |
| The Danish Working Environment Authority occupational exposure limit list |
not on the OEL list |
7.3.1.2 Physico/chemical properties
Table 7.7. The physico-chemical properties of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride -no data was found
| Physico/chemical properties |
|
Reference |
| Physical form |
- |
|
| Molecular weight (g/mol) |
251.12 |
17 |
| Melting point (°C) |
- |
|
| Boiling point (°C) |
- |
|
| Vapour pressure (Pa) |
5.586e-7 |
17 |
| Specific weight (kg/L) |
- |
|
| Log Kow |
-5.92 |
17 |
| Water-solubility (mg/l) |
1E+006 |
17 |
7.3.2 Toxicological properties
Absorption
No relevant data was found in the references examined.
Metabolism
No relevant data was found in the references examined.
7.3.2.1 Acute toxicity
Inhalation
No relevant data was found in the references examined.
Ingestion
The reviewed literature contains an old (1966) oral LD50 value in rats of 500 mg/kg /6/. This value lies within the concentration limits which according to the classification criteria result in classification as
Harmful with R22, Harmful is swallowed. No other LD50 values were found.
Skin contact
In the references examined we found a single dermal LD50 value for rabbits of 565 mg/kg /6/. This value also lies within the concentration limits which according to the classification criteria result in
classification as Harmful to Health with R21, Harmful in contact with skin.
Irritation and corrosiveness
1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride is described as a moderate skin irritant in test animals in concentrations of over 5% and is not considered to be a primary skin irritant in humans.
In tests on rabbits the substance did not show significant eye irritating properties /7/.
7.3.2.2 Subacute/chronic toxicity
Allergy and hypersensitivity
The substance, which is a formaldehyde releaser, is described in the literature as potentially skin sensitizing. Results from test animals are ambiguous, but partly depend on test method. Cross sensitization
with formaldehyde can occur. From its widespread use as preservative in e.g. cosmetics it is well known that the substance may result in skin allergies. However, it is considered to be safe for use in
concentrations of less than 0.2% as stipulated by the Cosmetics Statutory Order.
Organ damage
No relevant data was found in the references examined.
Genetic damage
1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride has not been found to be a mutagen in various Ames' tests with and without metabolic activation. Point mutations have been shown in mice
lymphoma tests with and without metabolic activation /10/.
Cancer
No relevant data was found in the references examined.
Damage to the reproductive process and the foetus
1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride administered orally (stomach pump) to gestating rabbits produced teratogenic effects at doses in excess of 25 mg/kg/day. NOAEL for teratogenic
effects was established at 5 mg/kg/day. Dermal application of the substance did not result in toxic effects either in the parent animal or foetuses, nor were there other teratogenic effects at doses up to and
including 500 mg/kg/day /7/. Testicular atrophy and reduced spermatogenesis has been reported in connection with a sub-chronic, dermal test on incompletely developed rabbits, but no further information
was found in the test conditions in the reference used /7/.
7.3.3 Ecotoxicological properties
Table 7.8 shows data on the aquatic toxicity of the substance. No EC50 value was found for algae or NOEC for any of the organism groups.
Table 7.8. Ecotoxicological data for 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride /20/. Length of test (in hours (h)) is stated in parentheses after the concentration.
| Group of organisms |
Latin name |
EC50/LC50 (mg/l) |
| Molluscs |
Crassostrea virginica |
180 (48 h) |
| Molluscs |
Crassostrea virginica |
11 (96 h) |
| Crustaceans |
Daphnia magna |
27-40 (48 h) |
| Crustaceans |
Penaeus duorarum |
182 (96 h) |
| Crustaceans (crab) |
Uca pugilator |
560 (96 h) |
| Fish |
Pimephales promelas |
29-34 (96 h) |
| Fish |
Menidia beryllina |
34 (96 h) |
| Fish |
Lepomis macrochirus |
28-148 (48 h) |
| Fish |
Oncorhynchus mykiss |
20-144 (96 h) |
7.3.4 Fate in the environment
Table 7.9. Reported minimum and maximum degradation of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantan chloride /14/ - no data.
| |
Minimum (%) |
Maximum (%) |
| Aerobic conditions |
35 (1.5 d) |
95 (7 d) |
| Anaerobic conditions |
- |
- |
The little data found on aerobic degradability of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantan chloride shows that the substance degrades relatively rapidly. The minimum and maximum values given in
Table 7.9 have been determined for a substance concentration of 10 mg/l with active sludge as inoculum. At 50 mg/l the layer phase was longer, but the degradation ratio after 7 days corresponded to that of
the lower concentration.
7.3.4.1 Bioaccumulation
No experimental data was found on the bioaccumulation potential of 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride. Based on Log Kow the bioconcentration factor (log BCF) may be
calculated to – 4.7 (see paragraph 7.1.4.2), and the substance is not expected to bioaccumulate in aquatic organisms.
7.3.5 Conclusion
The literature describes the substance as moderately toxic depending on animal species, concentration and exposure pathway. Only one oral LD50 value was found for rats and one dermal LD50 value for
rabbits. According to the classification criteria both values would require classification as Harmful to Health, but they constitute too flimsy a basis for classification.
The substance has displayed sensitizing potential, which is confirmed by clinical experience. Cross reaction with formaldehyde can occur.
The substance does not appear to possess significant mutagenic activity. Foetal damage effects have been observed in rabbits following oral administration. One test established NOAEL for foetal-damaging
effects at 5 mg/kg/day.
However, in general the data found does not provide sufficient basis for a classification or its health hazard properties.
Based on the data collected on 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantan chloride's environmental properties the substance does not require classification relative to environmental hazards.
Reservations stem from uncertainty about the degradability profile and rate.
Based on the results of the survey, the substance is expected to occur in concentrations of less than 0.5%. The substance is permitted in cosmetics and thus considered safe in concentrations up to 0.2%. At
higher concentrations the risk of sensitization cannot be ruled out. However, no data has been found that would throw light on the substance's sensitization potential. There is too little information on the
reproduction toxic properties of the substance for ingestion and skin contact to draw conclusions about any risks to children and pregnant women at the concentration levels used.
7.4 Toxicological profile of diarylide
7.4.1 Identification of the substance and physical-chemical properties
7.4.1.1 Identification
| Chemical name |
diarylide |
| EINECS name |
2,2'-[(3,3'-dichloro[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[3-oxo-N-phenylbutyramid] |
| CAS no. |
6358-85-6 |
| Molecular formula |
C32H26Cl2N6O4 |
| Structural formula |
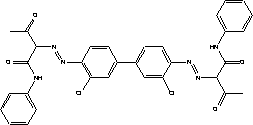 |
Applications
Diarylide is an azo dyestuff used in a large number of products, including printer ink, where heat resistance and colour intensity is required. The pigment is also used in paper products, in connection with
textile printing and in linoleum /9/.
Synonyms
There are a large number of synonyms for diarylide /1, 9/. The following have been selected as examples:
- amazon yellow x2485
- benzidine yellow
- C.I. 21090
- C.I. pigment yellow 12
- dainichi benzidine yellow gt
- diarylanilide yellow
- isol benzidine yellow gbpropyl
- monolite yellow
Regulation
| EU classification |
not classified |
| DK guideline list for self-classification |
not classified |
| Cosmetics Statutory Order |
not regulated |
| Foodstuffs (the positive list) |
not on the positive list |
| Foodstuffs (the flavour list, 2002) |
not on the flavour list 2002 |
| The Danish Working Environment Authority's occupational exposure limit list |
not on the OEL list |
7.4.1.2 Physico/chemical properties
Table 7.10. Physico-chemical properties of diarylide - no data was found.
| Physico/chemical properties |
|
Reference |
| Physical form |
solid |
1 |
| Molecular weight (g/mol) |
629.51 |
17 |
| Melting point (°C) |
320 |
17 |
| Boiling point (°C) |
- |
|
| Vapour pressure (Pa) |
≈0 |
17 |
| Specific weight (kg/L) |
- |
|
| Log Kow |
6.80 |
17 |
| Water-solubility (mg/l) |
3.61E-5 |
17 |
7.4.2 Toxicological properties
Absorption
Diarylide is not absorbed through the skin or from the gastro- and intestinal tract of rats, but is excreted in the faeces following oral ingestion /16/.
Metabolism
Tests on rats detected no signs of metabolic reduction and segregation via the urine of 3,3'-dichlorobenzidine, which is an experimental carcinogen /16/. One single study on rabbits observed
3,3'-dichlorobenzidine in the urine following oral administration of 20 mg/kg. After 48 hours the quantity excreted amounted to approx. 0.05% of the administered dose /16/.
7.4.2.1 Acute toxicity
Inhalation
No relevant data was found in the references examined.
Ingestion
The data examined points to a limited acute toxicity upon ingestion, as oral LD50 values in rats in the order of >2000 mg/kg and up to >15,000 mg/kg have been reported /16/. No exact figures were found.
Skin contact
No data was found on acute toxicity in the case of skin contact in the references examined.
Irritation and corrosiveness
The substance has been found to be mainly non-irritative or slightly irritating to skin and eyes in tests on rabbits /16/. Some individual trade names have result in more pronounced eye irritation /7, 16/.
7.4.2.2 Subacute/chronic toxicity
Allergy and hypersensitivity
A single study was found on a non-specified animal species where a positive reaction on the skin was observed upon patch testing with 1% and 10% solutions respectively /16/. In addition, cases of skin
allergy in humans have been reported /7/.
Organ damage
No relevant data was found in the references examined.
Genetic damage
Diarylide was found to be negative in a large number of Ame's tests with and without metabolic activation. This may be ascribed to the poor solubility of the substance /16, 10, 7/.
Cancer
A number of studies have been carried out on the substance's carcinogenic properties relative to oral dosing of rats and mice, all with negative results /10, 16/.
Damage to the reproductive process and the foetus
No relevant data was found in the references examined.
7.4.3 Ecotoxicological properties
Table 7.11. Ecotoxicological data for diarylide (16, 22). Length of test (in days (d) is stated in parentheses after the concentration.
| Group of organisms |
Latin name |
EC50/LC50 (mg/l) |
NOEC |
| Fish |
Brachydanio rerio |
5-10 (48 h) |
- |
| Fish |
Cyprinus carpio |
> 420 (48 h) |
|
| Fish |
Leuciscus idus |
> 500 (48 h) |
- |
| Fish |
Leuciscus idus |
10-100 (96 h) |
- |
Data on diarylide's acute ecotoxicity has only been found for fish (see Table 7.11). Two results indicate that the substance is potentially acutely toxic. The two other results indicate that the substance is not
toxic to aquatic organisms. Note that the substance has a water-solubility of less than 1 µg/L.
No conclusions can be drawn as to whether diarylide shows negative ecotoxicological effects due to a lack of data on diarylide's chronic toxicity and as data on acute toxicity only comprises a single group of
organisms.
7.4.4 Fate in the environment
7.4.4.1 Degradability
The little data on the degradability of diarylide shows that the substance is either not degradable or is slightly degradable with a degradation ratio of 81 per cent after 15 days /16, 22/. No data was
encountered on the substance's anaerobic degradability.
However, water solubility is so low that the substance must be considered to be non-degradable, as its bioaccessibility is very low.
Table 7.12. Reported minimum and maximum degradation of diarylide /16, 22/. - No data. Test duration is given in parentheses immediately after the degradation ratio.
| |
Minimum (%) |
Maximum (%) |
| Aerobic conditions |
0 (14 d) |
81 (15 d) |
| Anaerobic conditions |
- |
- |
7.4.4.2 Bioaccumulation
According to the Danish EPA (1999), diarylide has a low bioconcentration factor (-0.42-0.51). Based on Log Kow, the bioconcentration factor (log BCF) may be calculated at 7.3 (see section 7.1.4.2).
The large gap between the experimental and the calculated BCF may be due to the fact that azo pigments such as diarylide besides being of low water solubility also have very little potential for accumulating
in fatty tissues /16/.
7.4.5 Conclusion
Diarylide is not absorbed through the stomach and intestinal tract and is excreted mainly unconverted in the faeces. However, under certain circumstances the formation and excretion of
3,3'-dichlorobenzidine, which is an experimental carcinogen, is possible.
The substance has limited acute toxicity by ingestion, but there is no information on other exposure pathways. The substance causes no or very little irritation of the skin and eyes. Cases of skin sensitization
have been reported in humans. The substance has not been found to be mutagenic or carcinogenic in the tests referred to. No data was found on reproduction toxicity.
The data found does not give cause for self-classification of the substance for the effects investigated.
The data materials describing diarylide's fate in the environment is insufficient to make an accurate evaluation of the substance. It will primarily be found in solid form and water solubility is extremely low. The
substance has been assessed to persist in the aquatic environment and its acute toxicity is low.
Based on the data on diarylide's environmental properties the substance does not require classification as being an environmental hazard.
Based on the results of the survey, the substance is expected to occur in concentrations of between 0 and 2%. In animal tests skin sensitization was seen at concentrations of 1% of the substance.
7.5 Toxicological profile of 2-naphthol
7.5.1 Identification of the substance and physical-chemical properties
7.5.1.1 Identification
| Chemical name |
2-naphthol |
| EINECS name |
2-naphthol |
| CAS no. |
135-19-3 |
| Molecular formula |
C10H8O |
| Structural formula |
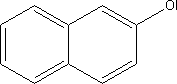 |
Applications
2-Naphthol is used in the manufacture of dyestuffs and pigments. The substance is also used as an antioxidant in e.g. rubber, oils and insecticides and in connection with the production of perfumes and
pharmaceutical products. It is used as a lubricant in electric motors and hydraulic equipment /9/.
Synonyms
The following synonyms are used for 2-naphthol /1/:
- hydroxynaphthalene
- 2-hydroxynaphthalene
- beta-hydroxynaphthalene
- isonaphthol
- naphthalenol
- 2-naphthalenol
- 2-naphthol
- beta-naphthol
- naphthyl alcohol
- naphthyl hydroxide
Regulation
| EU classification |
Xn; R20/22, N; R50 |
| DK guideline list for self-classification |
not assessed |
| Cosmetics Statutory Order |
not permitted in cosmetics products |
| Foodstuffs (the positive list) |
not given |
| Foodstuffs (the flavour list, 2002) |
not regulated |
| The Danish Working Environment Authority occupational exposure limit list |
not on the OEL list |
7.5.1.2 Physico/chemical properties
Table 7.13. Physico-chemical properties of 2-naphthol.
| Physico/chemical properties |
|
Reference |
| Physical form |
solid |
9 |
| Molecular weight (g/mol) |
144.17 |
9 |
| Melting point (°C) |
121.6 |
9 |
| Boiling point (°C) |
285 |
9 |
| Vapour pressure (Pa) |
0.043 |
9 |
| Specific weight (kg/L) |
1.28 |
9 |
| Log Kow |
2.70 |
9 |
| Water-solubility (mg/l) |
756 |
9 |
7.5.2 Toxicological properties
Absorption
2-naphthol can be absorbed through the skin. No data was found on the extent of absorption /7/.
Metabolism
The substance is excreted through the urine, primarily in conjugated form as sulphate or glucuronide. In humans up to 41% is excreted unconjugated and up to 59% in conjugated form following dermal
application /7/.
7.5.2.1 Acute toxicity
Inhalation
LC50 for rats is reported at 2.2 mg/l. The substance is classified as Harmful with R20, Harmful by inhalation, on the List of Dangerous Substances.
Ingestion
Oral LD50 for rats is reported at 1320 mg/kg and above /16/. The substance is classified as Harmful with R22, Harmful if swallowed, on the List of Dangerous Substances.
Skin contact
Tests on rats produced LD50 values for dermal toxicity of >2500 mg/kg /16/.
Irritation and corrosiveness
2-naphthol causes mild or no irritation to rabbit skin. The substance is a moderate eye irritant for rabbits and in a single test it was found to be irritating with risk for serious eye damage.
Turbidity of the cornea and damage to the conjunctiva have been reported in humans who have got the substance in their eyes /7/.
7.5.2.2 Subacute/chronic toxicity
Allergy and hypersensitivity
The substance was found to be sensitizing in a single study on guinea pigs. In addition, there is both positive and negative sensitization data for human sensitization subsequent to patch tests with the substance
/16/.
Organ damage
In an inhalation study, rats were exposed to concentrations of 0.45, 1.35 or 10.1 mg/m³. The highest concentration led to fatalities among 25% of the animals, reduced weight gain, changes in haematological
parameters and histopathological changes in liver and kidneys. Medium concentration resulted in reduced weight gain, changes in the haematological and clinical-chemical parameters and in histopathological
changes in liver and kidneys. The lowest concentration exclusively resulted in a reduction in the nitrogen content of the urine /16/.
Clinical studies have shown that chronic poisoning by the substance may be associated with lesions in the kidneys and effects on the gastro-intestinal tract and nervous system. Effects on kidney function seem
to be the clearest sign of harmful effects /7/.
Genetic damage
The substance has not been found to be mutagenic in a number of in vitro and in vivo mutagenicity tests. The substance was found to be genotoxic in a single DNA repair test /16/.
Cancer
Data was found from a single cancer study in mice in which 2-naphthol failed to show cancer-promoting activity on mouse skin upon application of 25 µl of a 20% solution, twice//week for 12 weeks /7, 16/.
Damage to the reproductive process and the foetus
No relevant data was found in the references examined.
7.5.3 Ecotoxicological properties
Data for 2-naphthol's ecotoxicity is shown in Table 7.14. No figures were found for NOEC.
Table 7.14. Ecotoxicological data for 2-naphthol /15/. Length of test is stated in parentheses after the concentration.
| Group of organisms |
Latin name |
EC50/LC50 (mg/l) |
| Molluscs |
Strongylocentrotus droebachiensis |
1.9 (96 h) |
| Algae |
Nitzschia palea |
6.3 (4 h) |
| |
Selenastrum capricornutum |
18.8 (4 h) |
| Crustaceans |
Daphnia magna |
3.5 (24 h) |
| Fish |
Oncorhynchus mykiss |
0.07 (27 d) |
| |
Micropterus salmoides |
1.0 (7 d) |
| |
Gadus morhua |
3.0 (96 h) |
In the List of Dangerous Substances the substance is classified as Hazardous to the Environment with R-phase R50, Highly toxic to aquatic organisms. No data was found in support of this
classification in the literature examined.
7.5.4 Fate in the environment
7.5.4.1 Degradability
Table 7.15. Reported minimum and maximum degradation of 2-naphthol /16/. - No data Test duration is given in parentheses after the degradation ratio.
| |
Minimum (%) |
Maximum (%) |
| Aerobic conditions |
8 (24 h) |
100 (5 d) |
| Anaerobic conditions |
0 (75 d) |
- |
7.5.4.2 Bioaccumulation
No data was found on 2-naphthol's bioaccumulation properties. Based on Log Kow, the bioconcentration factor (log BCF) may be calculated at 1.8 (see section 7.1.4.2).
7.5.5 Conclusion
2-naphthol can be absorbed through the skin. The substance is classified as Harmful with R-phrases R20/22, Harmful by Inhalation and if Swallowed.
The substance causes mild or no irritation of the skin and moderate eye irritation. The cornea and conjunctiva may be effected. Sensitization has been reported in animals and humans, but data does not
indicate any significant effect. A number of studies have been made of the genotoxic properties of the substance, but no convincing evidence of genotoxic activity has been found. Nor has the substance
shown tumour-promoting activity upon dermal application. The kidneys are the primary target organ given repeated exposure.
In the light of 2-naphthol's relatively broad area of application, the environment will be exposed via a number of dispersal pathways. The substance is classified as Hazardous to the Environment with R
phrase R50, Highly Toxic to Aquatic Organisms.
2-naphthol is an intermediate product in pigment manufacture and no information has been found on concentration of the substance in glass and porcelain paints. The substance was found in none of the ten
analysed products.
7.6 Toxicological profile of quinacridone.
7.6.1 Identification of the substance and physical-chemical properties
7.6.1.1 Identification
| Chemical name |
quinacridone |
| EINECS name |
5,12-dihydroquino[2,3-b]acridine-7,14-dione |
| CAS no. |
1047-16-1 |
| Molecular formula |
C20H12N2O2 |
| Structural formula |
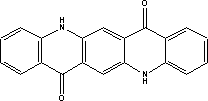 |
Applications
Quinacridone is a red/violet pigment used in e.g. paints, printer ink, plastic and rubber /9/.
Synonyms
The following synonyms are used for quinacridone /9/.
- CI Pigment red 122/CI 46500
- CI Pigment violet 19/CI 73900
- cinquasia violet/ red B
- histaperm red E 3B/ violet ER
- linear trans quinacridone
- monastral red / red B/ red Y/ violet R
- paliogen red BG
- permanent red E3B/E5B
- pigment quinacridone red
- pigment Violet #19
- PV fast red E 3B/E 5B
- quinacridone red/ MC/ violet / violet MC
- quino[2,3-b]acridine-7,14-dione, 5,12-dihydro-
- dihydroquino[2,3-b]acridine-7,14-dione
Regulation
| EU classification |
not classified |
| DK guideline list for self-classification |
not classified |
| Cosmetics Statutory Order |
permitted in cosmetics products which are only intended to be in short-term contact with the skin |
| Foodstuffs (the positive list) |
not on the positive list |
| Foodstuffs (the flavour list, 2002) |
not on the flavour list 2002 |
| The Danish Working Environment Authority occupational exposure limit list |
not on the OEL list |
7.6.1.2 Physico/chemical properties
Table 7.16. Physico-chemical properties of quinacridone. - no data was found.
| Physico/chemical properties |
|
Reference |
| Physical form |
solid |
9 |
| Molecular weight (g/mol) |
312.18 |
9 |
| Melting point (°C) |
- |
|
| Boiling point (°C) |
- |
|
| Vapour pressure (Pa) |
- |
|
| Specific weight (kg/L) |
1.5 |
9 |
| Log Kow |
1.9 |
9 |
| Water-solubility (mg/l) |
insoluble |
9 |
7.6.2 Toxicological properties
Absorption
No relevant data was found in the references examined.
Metabolism
No relevant data was found in the references examined.
7.6.2.1 Acute toxicity
Inhalation
No relevant data was found in the references examined.
Ingestion
The LD50 for oral ingestion in rats is recorded as >20 ml/kg /6/.
Skin contact
The LD50 for skin contact in rabbits is recorded as >2 ml/kg /6/.
Irritation and corrosiveness
Investigations of the pigments used in tattooing have shown that substances in the quinacridone substance group may cause skin inflammations /9/.
7.6.2.2 Subacute/chronic toxicity
Allergy and hypersensitivity
No relevant data was found in the references examined.
Organ damage
No relevant data was found in the references examined.
Genetic damage
Quinacridone was not found to be mutagenic in Ames' tests /6/.
Cancer
No relevant data was found in the references examined.
Damage to the reproductive process and the foetus
No relevant data was found in the references examined.
7.6.3 Ecotoxicological properties
No data was found on quinacridone's ecotoxicological properties.
7.6.4 Fate in the environment
No data was found on the degradability and bioaccumulation properties of quinacridone.
Based on Log Kow, the bioconcentration factor (log BCF) may be calculated at 1.2 (see section 7.1.4.2).
7.6.5 Conclusion
The literature contains very little information on quinacridone. The LD50 values for ingestion and skin contact cannot be used for an evaluation of the toxicity, as there is insufficient information about the test
conditions. Isolated items of information would indicate that the substance may cause skin inflammation when used in tattooing inks. Results of a single Ames' test were negative. Against this background it is
not possible to assess the human toxicological properties of the substance.
Insufficient data was found on quinacridone's environmental properties to assess the environmental hazardousness of the substance.
Based on the results of the survey, the substance is expected to occur in concentrations of between 0 and 2%. The substance was not identified in conjunction with the chemical analysis of the selected
products.
7.7 Toxicological profile of 1,2,3,4,5,6,7,8-octahydroacridine
7.7.1 Identification of the substance and physical-chemical properties
7.7.1.1 Identification
| Chemical name |
1,2,3,4,5,6,7,8-octahydroacridine |
| EINECS name |
1,2,3,4,5,6,7,8-octahydroacridine |
| CAS no. |
1658-08-8 |
| Molecular formula |
C13H17N |
| Structural formula |
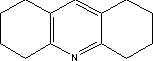 |
Applications
No data was found on octahydroacridine’s areas of application.
Synonyms
No synonyms were found for octahydroacridine.
Regulation
| EU classification |
not classified |
| DK guideline list for self-classification |
not classified |
| Cosmetics Statutory Order |
not regulated |
| Foodstuffs (the positive list) |
not on the positive list |
| Foodstuffs (the flavour list, 2002) |
not on the flavour list 2002 |
| The Danish Working Environment Authority occupational exposure limit list |
not on the OEL list |
7.7.1.2 Physico/chemical properties
Table 7.17. Physico-chemical properties of 1,2,3,4,5,6,7,8-octahydroacridine. - no data was found.
| Physico/chemical properties |
|
Reference |
| Physical form |
- |
|
| Molecular weight (g/mol) |
187.284 |
1 |
| Melting point (°C) |
- |
|
| Boiling point (°C) |
- |
|
| Vapour pressure (Pa) |
- |
|
| Specific weight (kg/L) |
- |
|
| Log Kow |
- |
|
| Water-solubility (mg/l) |
- |
|
7.7.2 Toxicological properties
Absorption
No relevant data was found in the references examined.
Metabolism
No relevant data was found in the references examined.
7.7.2.1 Acute toxicity
Inhalation
No relevant data was found in the references examined.
Ingestion
No relevant data was found in the references examined.
Skin contact
No relevant data was found in the references examined.
Irritation and corrosiveness
No relevant data was found in the references examined.
7.7.2.2 Subacute/chronic toxicity
Allergy and hypersensitivity
No relevant data was found in the references examined.
Organ damage
No relevant data was found in the references examined.
Genetic damage
No relevant data was found in the references examined.
Cancer
No relevant data was found in the references examined.
Damage to the reproductive process and the foetus
No relevant data was found in the references examined.
7.7.3 Ecotoxicological properties
No data was found on octahydroacridine's ecotoxicological properties.
7.7.4 Fate in the environment
No data was found on the degradability and bioaccumulation properties of octahydroacridine.
7.7.5 Conclusion
Insufficient data has been found to allow an assessment of octahydroacridine's toxicological and ecotoxicological properties.
During chemical analysis the substance was found in a single product in concentrations of 0.12 – 0.18%.
7.8 Toxicological profile of hexamethylene-1,6-diisocyanate
7.8.1 Identification of the substance and physical-chemical properties
7.8.1.1 Identification
| Chemical name |
hexamethylene-1,6-diisocyanate |
| EINECS name |
hexamethylene diisocyanate |
| CAS no. |
822-06-0 |
| Molecular formula |
C8H12N2O2 |
| Structural formula |
 |
Applications
Hexamethylene-1,6-diisocyanate is primarily used in the production of polyisocyanate products, which are added to polyurethane-based paint and varnish /9/.
Synonyms
The following synonyms are used for hexamethylene-1,6-diisocyanate /1/:
- desmodur h
- desmodur n
- 1,6-diisocyanatohexan
- evafanol AS-1
- HDI
- hexane 1,6-diisocyanate
- 1,6-hexanediol diisocyanat
- 1,6-hexamethylene diisocyanat
- 1,6-hexylene diisocyanat
- HMDI
- isocyanic acid, hexamethylene ester
- TL 78
Regulation
| EU classification |
T; R23, Xi; R36/37/38, R42/43 |
| DK guideline list for self-classification |
not assessed |
| Cosmetics Statutory Order |
not regulated |
| Foodstuffs (the positive list) |
not on the positive list |
| Foodstuffs (the flavour list, 2002) |
not on the flavour list 2002 |
| The Danish Working Environment Authority exposure limit level list |
Limit level list: 0.005 ppm; 0.035 mg/m³ |
7.8.1.2 Physico/chemical properties
Table 7.18. Physico-chemical properties of hexamethylene-1,6-diisocyanate.
| Physico/chemical properties |
|
Reference |
| Physical form |
liquid |
1 |
| Molecular weight (g/mol) |
168.20 |
17 |
| Melting point (°C) |
-67 |
17 |
| Boiling point (°C) |
255 |
17 |
| Vapour pressure (Pa) |
4 |
17 |
| Specific weight (kg/L) |
1.04 |
1 |
| Log Kow |
3.20 |
17 |
| Water-solubility (mg/l) |
117 |
17 |
7.8.2 Toxicological properties
Absorption
The substance is primarily absorbed through the lungs /9/.
Metabolism
The substance is hydrolysed and 1,6-hexamethylendiamine (HDA) is excreted in the urine. In a volunteer exposed to 0.03 mg/m³ for 7.5 hours (absorbed hexamethylene-1,6-diisocyanate estimated at 0.1
mg) 0.01 mg of HDA was measured in the urine within 28 hours, equivalent to 10% of the absorbed HDA. HDA half life was approx. 1.4 hours and more than 90% was excreted in the urine during the first
hour /16/.
7.8.2.1 Acute toxicity
Inhalation
LC50 values for rats have been reported between 0.15 and 0.35 mg/l/4 hours /16/. On the List of Dangerous Substances the substance is classified as Toxic with R23, Toxic by inhalation.
Ingestion
LD50 values for oral ingestion in rats have been found in the range between 710 mg/kg and 959 mg/kg /16/. These values correspond to a classification as Harmful with R22, Harmful if swallowed.
Skin contact
LD50 for rabbits has been recorded at 570 mg/kg and 599 mg/kg bodyweight, respectively /16/. These values correspond to a classification as Harmful with R21, Harmful in contact with skin.
Irritation and corrosiveness
On the List of Dangerous Substances the substance is classified as a Local Irritant with R36/37/38, Irritating to eyes, respiratory system and skin.
Studies involving 41 car painters employed for an average of seven years and exposed to HDI in concentrations of approx. 0.001 mg/m³ showed an increased incidence of eye, nose and throat irritation and
chronic bronchitis /16/.
7.8.2.2 Subacute/chronic toxicity
Allergy and hypersensitivity
The substance is skin sensitizing in tests on guinea pigs. Experience from the work environment also shows that the substance is sensitizing to human skin and respiratory organs /16/. Hexamethylene
diisocyanate is classified as Sensitizing on the List of Dangerous Substances with R42/43, May cause sensitisation by inhalation and skin contact.
The literature reports several incidences of cross reactivity between various isocyanates in exposed workers /16/.
Organ damage
Repeated inhalation results in damage to the lungs in animal tests with both macroscopic and histopathological signs of severe lung irritation or pneumonia with diffuse bleeding. This latter symptom has been
observed in rats at concentrations of 14 mg/m³ and above /16/
Genetic damage
Hexamethylene diisocyanate was not found to be mutagenic in Ames' tests with and without metabolic activation /10, 16/.
Cancer
In a cancer study in which rats inhaled concentrations of 0.035, 0.175 or 1.2 mg/m³ of the substance, no increase in the incidence of tumours in the animals was observed/16/.
Damage to the reproductive process and the foetus
Gestating rats were exposed to hexamethylene diisocyanate (HDI) in concentrations of 0.005, 0.050 or 0.300 ppm HDI through whole body exposure in a test to investigate foetal damage. Toxic effects
were observed in the mother animals at the highest concentration and to a lesser extent at the medium concentration level. The effects included histopathological changes, and in severe cases, degeneration of
the olfactory epithelium. Pathological changes were not observed in the lungs, throat or trachea in any of the exposed groups. No foetal damage, effects on litter size, the number of foetuses per implantation
site, effects on foetus weight or placenta weight were observed /7/.
7.8.3 Ecotoxicological properties
Table 7.19. Ecotoxicological data for hexamethylene-1,6-diisocyanate /16/. Test times are stated in parentheses immediately after the concentration.
| Group of organisms |
Latin name |
LC100 (mg/l) |
EC0 (mg/l |
| Crustaceans |
Daphnia magna |
|
> 0.33 (24h) |
| Fish |
Brachydanio rerio |
31 (96 h) |
|
7.8.4 Fate in the environment
7.8.4.1 Degradation
Data was only found from one test, which showed that the substance is not degradable under aerobic conditions over 28 days /16/.
7.8.4.2 Bioaccumulation
Based on Log Kow, the bioconcentration factor (log BCF) may be calculated at 2.2 (see section 7.1.4.2).
7.8.5 Conclusion
HDI is classified as Toxic on the List of Dangerous Substances with R23, Toxic by inhalation, Irritant, with R36/37/38, Irritating to eyes, respiratory system and skin, and sensitizing with R42/43,
May cause sensitisation by inhalation and skin contact. The LD50 values found also indicate that the substance should be classified as Harmful to Health R21/22, Harmful in contact with skin and if
swallowed. The eyes are particular sensitive to isocyanates, which also have a primary irritating effect on the respiratory tract. No data was found to indicate that substance has mutagenic, carcinogenic or
reproduction toxic effects.
The information collected on hexamethylene-1,6-diisocyanate is insufficient to assess the extent to which the substance represents a hazard to the environment. The toxicity results found indicate a certain
degree of toxicity to fish, and there may be a bioaccumulation potential.
During chemical analysis the substance was found in four of the products in concentrations between 0.045 and 0.075%. No data was found to document sensitization at these concentration levels, but potent
isocyanates can constitute a problem for sensitive people even at low concentrations.
7.9 Toxicological profile of chloroisocyanate benzene
7.9.1 Identification of the substance and physical-chemical properties
7.9.1.1 Identification
| Chemical name |
chloroisocyanate benzene |
| EINECS name |
2-chlorophenyl isocyanate |
| CAS no. |
3320-83-0 |
| Molecular formula |
C7H4ClNO |
| Structural formula |
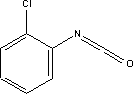 |
Applications
No information was found about the applications of the substance.
Synonyms
The following synonyms were found for chloroisocyanate benzene:
- 2-chlorophenyl isocyanate
- benzene, 1-chloro-2-isocyanato
- isocyanic acid, o-chlorophenyl ester
- o-chlorophenyl isocyanate
Regulation
| EU classification |
not classified |
| DK guideline list for self-classification |
Xn;R22 R43 |
| Cosmetics Statutory Order |
not regulated |
| Foodstuffs (the positive list) |
not on the positive list |
| Foodstuffs (the flavour list, 2002) |
not on the flavour list 2002 |
| The Danish Working Environment Authority occupational exposure limit list |
not on the OEL list |
7.9.1.2 Physico/chemical properties
Table 7.20. Physico-chemical properties of chloroisocyanate benzene /17/. -no data was found
| Physico/chemical properties |
|
Reference |
| Physical form |
- |
|
| Molecular weight (g/mol) |
153.57 |
17 |
| Melting point (°C) |
30.5 |
17 |
| Boiling point (°C) |
200 |
17 |
| Vapour pressure (Pa) |
- |
|
| Specific weight (kg/L) |
1.273 |
1 |
| Log Kow |
3.24 |
17 |
| Water-solubility (mg/l) |
- |
|
7.9.2 Toxicological properties
Absorption
No relevant data was found in the references examined.
Metabolism
No relevant data was found in the references examined.
7.9.2.1 Acute toxicity
Inhalation
No relevant data was found in the references examined.
Ingestion
Based on QSAR models the substance is classified as Harmful to Health on the guideline list for self-classification of hazardous substances with R22, Harmful if Swallowed.
Skin contact
No relevant data was found in the references examined.
Irritation and corrosiveness
No relevant data was found in the references examined.
7.9.2.2 Subacute/chronic toxicity
Allergy and hypersensitivity
Based on QSAR models the substance is classified as Sensitizing on the guideline list for self-classification of hazardous substances with R43, May cause sensitization by skin contact.
Organ damage
No relevant data was found in the references examined.
Genetic damage
No relevant data was found in the references examined.
Cancer
No relevant data was found in the references examined.
Damage to the reproductive process and the foetus
No relevant data was found in the references examined.
7.9.3 Ecotoxicological properties
No data was found on chloroisocyanate benzene's ecotoxicological properties.
7.9.4 Fate in the environment
7.9.4.1 Degradation
No data was found on chloroisocyanate benzene's degradability.
7.9.4.2 Bioaccumulation
Based on Log Kow, the bioconcentration factor (log BCF) may be calculated at 2.2 (see section 7.1.4.2).
7.9.5 Conclusion
Insufficient data has been found to allow an assessment of chloroisocyanate benzene's toxicological and ecotoxicological properties.
Upon chemical analysis the substance was found in just one of the ten products at a concentration of 0.015%. The critical effect of the substance is sensitization, but no documentation was found to show that
concentrations of the magnitude of 0.015% could trigger allergies.
7.10 Summary
Table 7.21 summarises the substances' intrinsic toxicological properties with regard to the key parameters: acute effects, local effects, sensitization, the effects of repeated exposure, and carcinogenicity(C),
mutagenicity (M), and reproduction toxicity (R). Moreover, the most highly critical effects of the substances have been listed based on the available data.
Table 7.22 summarises information on the environmental effects of the substances and their environmental classification.
Table 7.23 summarises information about regulatory requirements applying to the substances together with specification of concentrations of those substances identified through chemical analysis.
In the cases of butanoneoxime, methylpyrrolidone and phthalatic acid anhydride the percentage contents found by chemical analysis are above the lower limits classification. With the exception of
methylpyrrolidone all substances require classification as sensitizing. Both the first and the second analysis of 2- butanoneoxime require classification as category 3 carcinogens. All other detected compounds
are present in concentrations below the classification limits.
As regards the environmental properties of the substances, two of them (anthraquinone and 2-naphthol) were both extremely harmful to aquatic organisms and also persistent in the environment. As a
consequence, there are grounds for limiting discharge into the aquatic environment.
Table 7.21 List of toxicological properties and critical effects of the ten substances.
• Positive test results/data, ° Negative test results/data, and – no data. Exposure pathways are specified as follows: I = ingestion, S =skin contact, E = eyes, R = respiratory organs
| Name of substance |
CAS no. |
Acute
effects |
Local
effects |
Sensiti-
zation |
Repeated
exposure |
CMR |
Critical
effect |
| |
|
I |
S |
R |
E |
S |
R |
S |
R |
I |
S |
R |
C |
M |
R |
|
| Anthraquinone |
84-65-1 |
° |
° |
° |
° |
° |
- |
• |
- |
• |
- |
• |
• |
• |
- |
Carc. activity, sensitizing |
| 2-butanoneoxime |
96-29-7 |
° |
• |
° |
• |
• |
- |
• |
- |
• |
- |
• |
• |
• |
- |
Carc3, sensitizing |
| 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride |
4080-31-3 |
• |
• |
- |
° |
° |
- |
• |
- |
- |
- |
- |
- |
° |
° |
Acute toxicity (I,H), sensi-tizing (H) |
| Diarylide |
6358-85-6 |
° |
° |
- |
° |
° |
- |
• |
- |
- |
- |
- |
° |
° |
- |
Sensitization (S) |
| 2-Naphtol |
135-19-3 |
• |
° |
• |
• |
° |
- |
• |
- |
• |
- |
• |
- |
- |
- |
Acute tox., sensitizing kidney damage |
| Quinacridone |
1047-16-1 |
- |
- |
- |
- |
• |
- |
- |
- |
- |
- |
- |
- |
° |
- |
Skin irritation |
| 1,2,3,4,5,6,7,8-octahydroacridine |
1658-08-8 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
No data |
| Hexamethylene-1,6-diisocyanate |
822-06-0 |
• |
• |
• |
• |
• |
• |
• |
• |
- |
- |
• |
° |
° |
- |
Acute tox., sensitizing (S,R), lung damage (R) |
| chloroisocyanate benzene |
3320-83-0 |
• |
- |
• |
- |
- |
- |
• |
- |
- |
- |
- |
- |
- |
- |
Sensitization (S) |
Table 7.22 Summary of environmental impact and classification
I= Impact, P = Persistence, A = Accumulation
| Name of substance |
CAS no. |
Env.
impact |
Classification |
| |
|
I |
P |
A |
|
| Anthraquinone |
84-65-1 |
• |
• |
• |
N;R50/53 (self evaluation) |
| 2-butanoneoxime |
96-29-7 |
° |
° |
° |
None (several algae tests desirable however) |
| 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride |
4080-31-3 |
• |
° |
° |
None (possibly R52/53) |
| Diarylide |
6358-85-6 |
° |
• |
° |
None |
| 2-Naphthol |
135-19-3 |
• |
° |
° |
N;R50 |
| Quinacridone |
1047-16-1 |
- |
- |
- |
No data |
| 1,2,3,4,5,6,7,8-octahydroacridine |
1658-08-8 |
- |
- |
- |
No data |
| Hexamethylene-1,6-diisocyanate |
822-06-0 |
• |
- |
- |
Very little data |
| Chloroisocyanate benzene |
3320-83-0 |
- |
- |
- |
No data |
Table 7.23 Summary of regulations applying to the ten substances
| Name of substance |
CAS no. |
Conc.
(%) |
Classification) |
Max. permissible value in work environment |
Cosmetic Statutory Order |
The positive list |
The Flavour List 2002 |
| Anthraquinone |
84-65-1 |
- |
Not classified |
No |
Not stated |
No |
No |
| 2-butanoneoxime |
96-29-7 |
<0.5
9.2 |
Xn;R21 Carc3; R40 Xi;R41 R43 |
No |
Not stated |
No |
No |
| 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane
chloride |
4080-31-3 |
<0.5 |
Not classified |
No |
Permitted as preservative, max. 0.2% |
No |
No |
| Diarylide |
6358-85-6 |
0-2 |
Not classified |
No |
Not permitted |
No |
No |
| 2-Naphtol |
135-19-3 |
- |
Xn;R20/22 N;R50 |
No |
Not stated |
No |
No |
| Quinacridone |
1047-16-1 |
0-2 |
Not classified |
No |
Permitted in products in short-term contact with the skin |
No |
No |
| 1,2,3,4,5,6,7,8-octahydroacridine |
1658-08-8 |
0.012-0.18 |
Not classified |
No |
Not stated |
No |
No |
| Hexamethylene-1,6-diisocyanate |
822-06-0 |
0.058-0.075 |
T;R23 Xi;R36/37/38 R42/43 |
0.005 ppm
0.035 mg/m³ |
Not stated |
No |
No |
| Chloroisocyanate benzene |
3320-83-0 |
0.015 |
Xn;R22 R43 |
No |
Not stated |
No |
No |
1) Classifications from the List of Dangerous Substances are specified in bold type and classifications from the Danish EPA's guideline list for self-classification are specified in normal type.
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 July 2005, © Danish Environmental Protection Agency
|