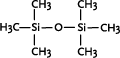|
Environmental Project no. 1031, 2005 Siloxanes - Consumption, Toxicity and AlternativesContents2 Application of silicones in Denmark
3 Health evaluation of siloxanes 4 Environmental fate and effects
Annex 1 Siloxanes listed in the INCI database Annex 2 Siloxanes in hair styling products on the Danish market Annex 3 Siloxanes in the Danish Product Register Annex 5 Siloxanes in cleaning and maintenance products Annex 6 Contacted companies and organisations Annex 7 Database screening for decamethyl cyclopentasiloxane Annex 8 Human toxicity test results for siloxanes PrefaceSiloxanes, the building blocks for silicone products, are widely used chemicals. The siloxanes are characterised by a high stability, physiologic inertness and good release and lubricating properties. The stability of the siloxanes, desirable from a technical point of view, makes the siloxanes very persistent, and once released to the environment the siloxanes remain for many years. In recent years studies indicating that some of the siloxanes may have endocrine disrupting properties, and reproductive effects have caused concern about the possible effects of the siloxanes on humans and the environment. Until now no overview of the use of siloxanes in Denmark has been available. The purpose of the present project initiated by The Danish Environmental Protection Agency is to assess the use of siloxanes in Denmark and identify potential sources of releases of siloxanes to the environment. Besides the toxicity of siloxanes has been reviewed, and alternatives to some of the groups of siloxanes of concern have been identified. The project has been followed by a Steering Group consisting of:
The report has been prepared by Carsten Lassen, Charlotte Libak Hansen, Sonja Hagen Mikkelsen and Jakob Maag, COWI A/S. Summary and conclusionsSiloxanes are chemical compounds with a backbone of alternating silicium (Si) and oxygen (O) atoms, each silicon atom bearing one or several organic groups. Siloxanes are building blocks for silicone products or make part of other products, such as cosmetics or paint. In colloquial language the term silicones is often used synonymously with siloxanes. The properties of the siloxanes and the silicone products depend on the length of the Si-O backbone, the chemical groups attached to the backbone and the presence of cross-links between the backbones. Silicone products are grouped into silicone fluids, elastomers and resins. Silicone fluids are used for a wide range of applications, silicone elastomers are mainly used for sealants and rubbers, and resins are mainly used for paints. The most common siloxanes are polydimethylsiloxane (PDMS) with different modifications. Of particular interest to this study are the relatively small compounds: Siloxanes with a cyclic structure and linear siloxanes with a small Si-O backbone with a few Si-O moieties. The most common and the most investigated as to toxicity are octamethylcyclotetrasiloxane (D4) and decamethylcyclopentasiloxane (D5). These compounds are widely used in cosmetic products and maintenance products (e.g. wax) under the name cyclomethicone - among other names. In the present report different names are used for the same compounds depending on the names typically used in the different contexts. Many of the compounds are volatile, and the users are directly exposed to the compounds when using the products, and the compounds are to a high extent released to the atmosphere or to wastewater. Consumption of siloxanes in Denmark The consumption of siloxanes by application area is shown in Table 1. The estimates are to a large extent based on information on the use of siloxanes in Western Europe under the assumption that the consumption pattern in Denmark of most products will resemble the general consumption pattern in Western Europe. Best estimates and an indication of the uncertainty of the best estimate are given. The total consumption is estimated at approx. 3,100 t/year. Considering the uncertainty on applying the Western European consumption figures, the total consumption in Denmark is estimated to be within the range of 2,400-3,800 t/year. The type of the siloxanes used is indicated in Table 1. The type can roughly indicate the potential for releases of the compounds to the atmosphere and wastewater. Volatile fluids are released to the atmosphere, whereas other fluids may end up in wastewater or released directly to surface water and soil. Elastomers and resins will mainly end up in solid waste. The main application area is silicone sealants for construction, which account for about one third of the consumption. Besides, siloxanes are widespread, used in a vide range of products: In cosmetics and toiletries, paints, cleaning products, clothes, health-care products, etc. Often the siloxanes only account for a small part of the product, e.g. as defoaming agent. A large number of different siloxanes are used within each application area. As an example the Danish Product Register includes 53 different siloxanes (CAS no.) registered as used in sealants and 98 different siloxanes used in paints and lacquers. About 200 siloxanes and siloxane derivatives are listed in the inventory of ingredients used in cosmetic products compiled by the European Commission (INCI 2000). The specific siloxanes are often used in many different product types. The most widely used, polydimethylsiloxane, is in the Danish Product Register registered as being present in 159 product types. The most widely used of the cyclic siloxanes, octamethylcyclotetrasiloxane, is registered in 49 product types: Paints, cleaning agents, dyes, fillers, polishes, adhesives, etc. In most product groups the total registered amount is, however, quite small. Table 1 Consumption of siloxanes in Denmark in 2002
* Uncertainty indication: Releases to the environment The main source of releases of siloxanes to the air is volatile siloxanes used in cosmetics, wax, polishes, and to a minor extent in several other applications. No information of the quantity of volatile siloxanes for these applications has been available, but the volatile siloxanes may account for a significant part of the siloxanes used for cosmetics, and it is roughly estimated that between 50 and 200 t/year is released to the air. Siloxanes disposed of to municipal solid waste incineration are deemed nearly 100% to be mineralised by the incineration, and incineration plants are not considered significant sources of siloxane releases to the atmosphere. Non-volatile silicone fluids used in cosmetics, wax, polishes, cleaning products and for textile applications (softeners) will to a large extent end up in wastewater and be directed to wastewater treatment plants. The total release to wastewater is estimated at 200-700 t/year. By the treatment process the siloxanes mainly follow the sludge and are either spread on agricultural fields, incinerated or disposed of for landfills. The major part of siloxanes used in silicone elastomers and resins in sealants, paints, rubbers, etc. is disposed of to incineration or to landfills with building materials. By the incineration the siloxanes are destructed. Effects on human health and the environment Only few siloxanes are described in the literature with regard to health effects, and it is therefore not possible to make broad conclusions and comparisons of the toxicity related to short-chained linear and cyclic siloxanes based on the present evaluation. Data are primarily found on the cyclic siloxanes D4 (octamethylcyclotetrasiloxane) and D5 (decamethylcyclopentasiloxane) and the short-linear HMDS (hexamethyldisiloxane). These three siloxanes have a relatively low order of acute toxicity by oral, dermal and inhalatory routes and do not require classification for this effect. They are not found to be irritating to skin or eyes and are also not found sensitizing by skin contact. Data on respiratory sensitization have not been identified. Subacute and subchronic toxicity studies show that the liver is the main target organ for D4 which also induces liver cell enzymes. This enzyme induction contributes to the elimination of the substance from the tissues. Primary target organ for D5 exposure by inhalation is the lung. D5 has an enzyme induction profile similar to that of D4. Subacute and subchronic inhalation of HMDS affect in particular the lungs and kidneys in rats. None of the investigated siloxanes show any signs of genotoxic effects in vitro or in vivo. Preliminary results indicate that D5 has a potential carcinogenic effect. D4 is considered to impair fertility in rats by inhalation and is classified as a substance toxic to reproduction in category 3 with the risk phrase R62 ('Possible risk of impaired fertility'). The results of a study to screen for estrogen activity indicate that D4 has very weak estrogenic and antiestrogenic activity and is a partial agonist (enhances the effect of the estrogen). It is not uncommon for compounds that are weakly estrogenic to also have antiestrogenic properties. Comparison of the estrogenic potency of D4 relative to ethinylestradiol (steroid hormone) indicates that D4 is 585,000 times less potent than ethinylestradiol in the rat stain Sprague-Dawley and 3.7 million times less potent than ethinylestradiol in the Fisher-344 rat strain. Because of the lack of effects on other endpoints designated to assess estrogenicity, the estrogenicity as mode of action for the D4 reproductive effects has been questioned. An indirect mode of action causing a delay of the LH (luteinising hormone) surge necessary for optimal timing of ovulation has been suggested as the mechanism. Based on the reviewed information, the critical effects of the siloxanes are impaired fertility (D4) and potential carcinogenic effects (uterine tumours in females). Furthermore there seem to be some effects on various organs following repeated exposures, the liver (D4), kidney (HMDS) and lung (D5 and HMDS) being the target organs. A possible estrogenic effect contributing to the reproductive toxicity of D4 is debated. There seems however to be some indication that this toxicity may be caused by another mechanism than estrogen activity. Effects which based on the reviewed literature do not seem to be problematic are acute toxicity, irritant effects, sensitization and genotoxicity. Siloxanes are in general stable compounds that are very persistent in the environment. The cyclic siloxanes and small-chain linear siloxanes are bioconcentrated (bioconcentration factors for long-chained siloxanes have not been assessed). The estimated bioconcentration factors (BCF) of the small siloxanes range from 340 for HMDS to 40,000 for a phenylated trisiloxane (phenyl trimethicone). The small phenylated siloxanes seem to have very high BCF, and model estimates indicate that these substances are the most toxic for aquatic organisms. Alternatives to siloxanes for cosmetics and maintenance products Traditionally when talking about substitution, the siloxanes have been on the positive side, e.g. as alternatives to PCBs. The development of alternatives to siloxanes has mainly focused on siloxanes used in cosmetics and breast implants. Until now the absence of siloxanes in cosmetics has not been a competition parameter in Denmark, but many - and in particular American producers - use the Internet for advertising "silicone-free" hair care and skin care products. As cosmetics and maintenance products are among the most significant product groups as to consumer exposure and releases to the environment, the assessment of alternatives to siloxanes has focused on these groups. The siloxanes have a number of properties which are not easily matched by alternatives. For soaps and leave-on products (lotions and creams for skin) the siloxanes e.g. can give the product the combination "smooth and soft feeling" on the skin combined with the sense that the product does not feel greasy on the skin after application. In particular the properties of the volatile cyclic siloxanes are difficult to substitute. The price of the alternatives ranges from the same as the price of the siloxanes to approximately the double price. The use of alternatives will in general not require changes in production equipment. Alternatives to siloxanes in cosmetics identified by enquiries to Danish producers and suppliers are listed in Table 2. The substitution of siloxanes has not had the particular attention of the producers of cleaning and maintenance agents. Siloxanes used in cleaning agents, waxes and polishes are in general different from the siloxanes used in cosmetics, although some of the wanted properties are the same, for example shine, spreadability and antifoaming. The identified alternatives are therefore also quite different from the alternatives developed for cosmetic products. As for alternatives to siloxanes in cosmetic products it is the general opinion in the cleaning agent trade that siloxanes have some special qualities that cannot easily be found in alternatives. These qualities are in particular as solvent, emulsifier, anti-soiling and defoaming agents. Identified alternatives to siloxanes antifoaming agents are non-ionic mineral oils (tensides), paraffin oils, vegetable oils and block polymers consisting of polyethylenglycol and polypropylenglycol. Alternatives to amino-functional dimethylsiloxanes in polishes are lipophilic tensides. It is, however, difficult to assess to what extent the alternatives actually match the properties of the siloxanes. Advertisements for silicone-free polishes and waxes can be found, but the reason for mentioning that they are silicone-free is usually technical. Table 2 Identified alternatives to siloxanes in cosmetics from Danish producers and suppliers.
* Is used as alternative to cyclomethicone and paraffin, but do not substitute all properties Sammenfatning og konklusionerDenne undersøgelse har til formål at give et overblik over anvendelsen af siloxaner i Danmark, siloxanernes miljø- og sundhedsmæssige egenskaber og hvilke alternativer, der findes til siloxaner til udvalgte formål. Undersøgelsen er igangsat af Miljøstyrelsen, fordi der de seneste år er opstået en stigende bekymring for, at visse af siloxanerne kan have nogle uønskede effekter på mennesker og i miljøet. Siloxaner er kemiske forbindelser med en "rygrad" af skiftende silicium (Si) og ilt (O) atomer. På hvert af siliciumatomerne sidder der en eller flere organiske grupper. Siloxanerne er byggestenene i silikoneprodukter eller udgør en mindre del af andre produkter, som det fx er tilfældet i kosmetik eller maling. I dagligdags sprog anvendes navnet silikoner ofte som synonym for siloxaner. Siloxanernes og silikoneprodukternes egenskaber er afhængig af længden af Si-O kæderne, de kemiske grupper fæstnet til kæderne og tilstedeværelsen af tværbindinger mellem kæderne. Silikoneprodukter grupperes i silikonevæsker, silikoneelastomerer og silikoneharpikser. Silikonevæsker indgår i en lang række produkter, som fx kosmetik og maling, silikone elastomerer anvendes hovedsageligt til fugemasser og silikonegummier, mens harpikser hovedsageligt anvendes til malinger. De mest almindelige siloxaner er polydimethylsiloxan med forskellige modifikationer. Af særlig interesse for denne undersøgelse er de relativt små forbindelser: siloxaner med en cyklisk struktur og kortkædede lineære siloxaner med kun få Si-O led. De mest udbredte er octamethylcyclotetrasiloxan (D4) og decamethylcyclopentasiloxan (D5). Disse forbindelser er udbredt anvendt i kosmetikprodukter og visse plejemidler som fx voks. Mange af stofferne er flygtige, og brugerne er direkte eksponeret for stofferne, når de bruger produkterne, og en stor del af stofferne bliver udledt til luften eller med spildevand. Forbrug af siloxaner i Denmark Forbruget af siloxaner opdelt på anvendelsesområder er vist i tabel 1. Overslagene er i høj grad baseret på kendskab til forbruget af siloxaner i Vesteuropa under antagelse af at forbrugsmønstret i Danmark for de fleste produkter ligner det generelle forbrugsmønster i Vesteuropa. I tabellen er der for hvert anvendelsesområde angivet et bedste estimat samt en vurdering af usikkerheden på de enkelte estimater. Det samlede forbrug er anslået til omkring 3.100 t/år. Når usikkerheden ved at basere overslagene på oplysninger om det samlede vesteuropæiske forbrug tages i betragtning, vurderes den korrekte angivelse af det samlede forbrug at befinde sig inden for intervallet 2.400-3.800 t/år. Typen af siloxaner/silikoner er også vist i tabellen. Typen giver er grov indikation af potentialet for udledninger til luften og til spildevand. Flygtige væsker udledes til atmosfæren, mens andre væsker kan ende i spildevand eller tabes direkte til overfladevand og jord. Elastomerer og harpikser vil hovedsageligt ende i fast affald, som bortskaffes til affaldsforbrænding eller deponier. Det største anvendelsesområde er silikonefugemasser (mængden, der angives i tabellen, er mængden af silikone i fugemasserne). Herudover anvendes siloxaner i en bred vifte af produkter: kosmetik og toiletprodukter, maling, rengøringsmidler, tøj, produkter til medicinske anvendelser, kølemidler, mm. Ofte udgør siloxanerne kun en lille del af produkterne, bl.a. som skumhindrende middel. Der anvendes inden for hvert enkelt anvendelsesområde et stort antal forskellige siloxaner. For eksempel er der i Produktregistret registreret 53 forskellige siloxaner (CAS-numre), som anvendes i fugemasser, og 98 forskellige siloxaner, som anvendes i maling og lak. Der optræder omkring 200 siloxaner og siloxan-derivater på EUs liste over ingredienser i kosmetiske produkter (INCI 2000). De enkelte siloxaner anvendes ofte i mange typer af produkter. Den mest udbredt anvendte siloxan, polydimethylsiloxan, er således i Produktregistret registreret anvendt til 159 produkttyper. Den mest udbredte af de cykliske siloxaner, octamethylcyclotetrasiloxan, er registreret i 49 produkttyper: Malinger, rengøringsmidler, farvestoffer, spartelmasse, pudsecreme, lime, m.m. Den samlede registrerede mængde er dog for de fleste af produkttyperne meget beskeden. Tabel 1 Forbrug af siloxaner i Danmark i 2002
* Angivelse af usikkerhed: Udledninger til miljøet Den væsentligste kilde til udslip af siloxaner til luften er flygtige siloxaner, som anvendes i kosmetik, voks, polish og i mindre grad en række andre anvendelser. Det har ikke været muligt at få præcis information om mængden af flygtige siloxaner som anvendes til disse formål. Men de flygtige siloxaner synes at udgøre en betydelig del af de siloxaner, som anvendes i kosmetikprodukter, og det anslås groft, at mellem 25 og 200 tons siloxaner hvert år udledes til luft. Siloxaner, der bortskaffes via forbrændingsanlæg regnes at blive næsten 100% destrueret ved forbrændingen, og forbrændingsanlæg betragtes ikke som væsentlige kilder til udledninger af siloxaner til luften. Ikke-flygtige silikonevæsker anvendt i kosmetik, voks, polish, rengøringsmidler og i tekstiler (blødgørere) vil i høj grad ende i spildevand og afledes til renseanlæg. De totale udledninger af siloxaner til renseanlæg anslås groft til 200-700 t/år. Ved renseprocessen vil siloxanerne hovedsageligt ende i slammet og vil med slammet spredes på landbrugsjord, forbrændes eller deponeres. Hovedparten af siloxaner anvendt i silicone elastomerer og harpikser i fugemasser, maling, gummier, m.m. bortskaffes til affaldsforbrænding eller deponi med byggematerialer. Ved affaldsforbrændingen vil siloxanerne blive destrueret. Sundheds- og miljøeffekter Ved screening for sundheds- og miljøeffekter er der fokuseret på de cykliske og korte lineære siloxaner. Kun få af siloxanerner er beskrevet i litteraturen med hensyn til sundhedseffekter, og det er derfor ikke muligt at foretage brede konklusioner og sammenligninger mellem sundhedsfarligheden af de forskellige siloxaner. De fundne data vedrører primært de cykliske forbindelser D4 (octamethylcyclotetrasiloxan) og D5 (decamethylcyclopentasiloxan) og den korte lineære siloxan HMDS (hexamethyldisiloxan). Den akutte giftighed af de tre siloxaner er relativt lille uanset om stofferne indåndes eller indtages gennem munden eller huden, og der er ikke noget krav om klassifikation for akut giftighed. De er ikke påvist at irritere huden eller øjnene, og der er ikke fundet sensibilisering ved hudkontakt. Der er ikke fundet data om sensibilisering ved indånding. Studier af subakut og subkronisk giftighed viser, at leveren er det organ, i hvilket der ses de væsentligste effekter af D4, som også inducerer dannelsen af levercelle enzymer. Denne enzymdannelse bidrager til at fjerne stoffet fra vævet. Det primære organ for effekter af D5 eksponering er lungerne. D5 har samme enzymdannelsesprofil som D4. Subakut og subkronisk indånding af HMDS påvirker i rotter især lungerne og nyrerne. Ingen af de undersøgte siloxaner viser tegn på at skade arveanlæggene hverken i reagensglasforsøg (in vitro) eller ved forsøg i levende organismer (in vivo). Foreløbige undersøgelser viser, at D5 har et potentiale for at være kræftfremkaldende. D4 anses at påvirke frugtbarheden af rotter ved indånding og er klassificeret som et stof der er giftigt i forhold til forplantningsevnen i kategori 3 og er tildelt risikosætningen R62, "Mulig risiko for hæmmet forplantningsevne". Resultaterne af et studie med det formål at screene for østrogen aktivitet indikerer, at D4 har en svag østrogen og anti-østrogen aktivitet og er en delvis agonist (forøger virkningen af østrogen). Det er ikke ualmindeligt, at stoffer der har østrogen virkning også har anti-østrogene egenskaber. Sammenligning af den østrogene styrke af D4 med ethinylestradiol (et steroid hormon) indikerer, at styrken af D4 er henholdsvis 585.000 gange og 3,7 mio. gange mindre end styrken af ethinylestradiol i to forskellige rottestammer. På grund af manglen på påviste effekter af stofferne på andre "endpoints", som anvendes til at vurdere østrogene effekter, er der stillet spørgsmålstegn ved, om effekten af D4 i forhold til forplantningen skyldes en østrogenvirkning. Det er foreslået at mekanismen er en forsinkelse af den såkaldte LH-bølge, som er nødvendig for en optimal timing af ægløsningen. Baseret på de gennemgåede undersøgelser, er de kritiske effekter af de her omtalte siloxaner en hæmmet forplantningsevne (D4) og potentielle kræftvirkninger (svulster i livmoderen). Yderligere ser der ud til at være nogle effekter på en række organer af gentagen eksponering: leveren (D4), nyrerne (HMDS) og lungerne (D5 og HMDS). En mulig østrogen effekt, som bidrager til D4's hæmning af forplantningsevnen er til diskussion. Der er dog en indikation på, at denne hæmning er forårsaget af andre mekanismer. Baseret på den gennemgåede litteratur synes der ikke at være problemer hvad angår akut giftvirkning, irritationer, sensibiliseringer og skader på arveanlæggene. Siloxanerne er generelt meget stabile forbindelser, der er svært nedbrydelige i miljøet. De cykliske siloxaner og kortkædede lineære siloxaner opkoncentreres i organismer (biokoncentreringsfaktorer er ikke undersøgt for de langkædede siloxaner). De beregnede biokoncentreringsfaktorer (BCF) af de små siloxaner varierer fra 340 for HMDS til 40.000 for phenyleret trisiloxan (phenyl trimethicone). De små phenylerede siloxaner synes at have meget høje biokoncentreringsfaktorer, og modelberegningen indikerer, at det også er disse stoffer, som er de mest giftige for organismer i vandmiljøet. Alternativer til siloxaner i kosmetikprodukter og plejemidler Traditionelt har siloxanerne været på positivsiden, når emnet har været substitution, fx som alternativer til PCB. Udviklingen af alternativer til siloxaner har hovedsageligt fokuseret på siloxaner anvendt i kosmetik og brystimplantater. Hidtil har det i Danmark ikke været en konkurrenceparameter, at kosmetik ikke indeholder siloxaner, men mange, især amerikanske producenter, bruger Internettet til at annoncere for silikonefri hår- og hudplejeprodukter. Da kosmetik og plejemidler er blandt de vigtigste produktgrupper, hvad angår forbrugereksponering og udledninger af siloxaner til miljøet, har vurderingen af alternativer fokuseret på disse produktgrupper. Siloxanerne har en række egenskaber som det ikke er nemt at erstatte med brug af alternativer. I sæber og "leave-on" produkter (bl.a. lotions og hudcremer) giver siloxanerne fx produkterne en kombination af glat og blød fornemmelse på huden samtidig med, at produkterne ikke føles fedtede på huden. Især er de flygtige siloxaners egenskaber svære at erstatte. Prisen på alternativerne varierer fra en pris lidt lavere end siloxanernes til omtrent det dobbelte af siloxanernes pris. Brugen af alternativer vil normalt ikke kræve ændringer i produktionsudstyr. Alternativer til siloxaner i kosmetikprodukter, fundet gennem henvendelse til danske producenter og leverandører, er vist i tabel 2. Substitution af siloxaner har ikke tiltrukket sig særlig opmærksomhed fra producenter af rengørings- og plejemidler. Siloxaner som anvendes i rengøringsmidler, voks og polish er i almindelighed forskellige fra siloxanerne, som anvendes i kosmetikprodukter, selvom om nogle af de ønskede egenskaber er de samme fx glans, skumhindring og den egenskab at produkterne let kan fordeles på overfladen. De fundne alternativer er derfor også forskellige fra de alternativer, der er udviklet til kosmetikprodukter. Det er blandt producenter og leverandører den generelle opfattelse, at siloxanerne har visse særlige egenskaber, som det er svært at finde hos alternativerne. Disse egenskaber er især knyttet til siloxanernes brug som opløsningsmiddel, emulgeringsmiddel, skumhindrende middel og anti-smuds middel. De fundne alternativer til siloxan antiskummidler er nonioniske mineralske olier (tensider), paraffinolier og vegetabilske olier samt blokpolymerer bestående af polyetylenglycol og polypropylenglycol. Alternativer til amino-funktionelle dimethylsiloxaner i polish er lipophile tensider. Det er dog vanskeligt at vurdere, i hvilken grad alternativernes egenskaber faktisk svarer til siloxanernes. Der kan findes en del annoncering for "silikone-fri" polish og voks, men det vil normalt være af tekniske årsager, at det fremhæves, at produkterne er silikonefri, da silikoner til visse formål er uønskede. Tabel 2 alternativer til siloxaner i kosmetikprodukter fundet hos danske producenter og leverandører
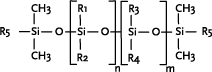
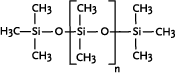
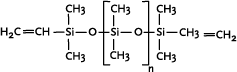
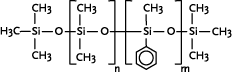
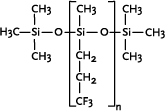
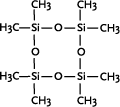
* Anvendes som alternative til cyclomethicone og paraffin, men kan ikke substituere alle siloxanens egenskaber 1 Introduction1.1 What are siloxanes and silicones?Siloxanes are compounds in which silicon atoms (Si) are linked via oxygen atoms (O), each silicon atom bearing one or several organic groups. According to the main rules for classification of chemicals (IUPAC) the compounds are designated siloxanes, but most often the term "silicones" are used. When talking about products and materials, "silicones" are nearly always used, and this term will be used here as well. The term "siloxanes" will be used when we talk about single compounds, or when we have a more chemical approach to the subject. In other words: Siloxanes are building blocks for silicone products. The chemical names of the compounds most often include the string "siloxane", but in particular in cosmetics and toiletries names including methicone are used instead, e.g. dimethicone derived from dimethyl silicone; synonymous with dimethylsiloxane. Another used synonym is poly(oxy(methylsilylene)). To add to the confusion about the terms, several of the compounds recorded in the Danish Product Register, listed in Annex 3, are designated "silicones and siloxanes". The alternating silicon and oxygen atoms form a backbone structure to which different side chains are linked. The side chains may form cross links which influence the properties of the polymer. The silicon and oxygen atoms may be linked into cyclic or linear structures, and we distinguish between linear siloxanes and cyclic siloxanes. Linear siloxanes
The cyclosiloxanes are often designated with reference to the number of silicium atoms: D3 (cyclotrisiloxane), D4 (cyclotetrasiloxane), D5 (cyclopentasiloxane) and D6 (cyclohexasiloxane). Cyclic siloxanes with other functional groups, e.g. methylphenylcyclosiloxanes, are used for fewer applications. Among other applications, cyclic methylsiloxanes are widely used in cosmetic products, in which cyclic siloxanes or mixtures of the compounds are known under the name "cyclomethicone". According to the 2000 version of the INCI list (Annex 1) cyclomethicone is synonymous with octamethyltetrasiloxane. Producers, however, use the term more widely, e.g. the UK producer Basildon Chemicals has four types of cyclomethicone, either as pure D4 or D5 or as different mixtures of D4, D5 and D6 (http://www.baschem.co.uk/downloads/cy56.pdf). Silanes Silanes are silicon compounds, both organic and inorganic. The siloxanes are actually one of five subgroups of silanes, but often the term "silanes" is used for designating the other groups. The other groups are those containing Si–H bonds (hydride functional silanes), Si–X (halosilanes), Si–C (organosilanes), and Si–OR (silicon esters).
Silsesquioxanes
Fluids, elastomers and resins
Silicone fluids Silicone fluids are distinguished from common organic fluids by a number of unique properties (Ullmann 2003):
Because of these properties silicone fluids are widely applied as release agents for moulding operations, medical and cosmetic applications, polishes, coolants and dielectric fluids for electrical systems, metal processing fluids and agents for foam control. The silicone fluid may be formulated into emulsions, dispersions, greases and compounds. Silicone elastomers In general is distinguished between four main systems:
Different cross-linking agents are used depending on temperature and other conditions for the curing process. The marketed products, e.g. silicone sealants, thus both contain the siloxanes and cross-linking agents. The principle is here illustrated by the release of compounds when one-component room-temperature-vulcanising (RTV) silicone sealants are applied. The products are widely applied by both private and professional users.
Well known is the smell of acetic acid, when silicone sealants with ethyltriacetoxysilane as cross-linking agent are applied. The acetic acid is formed by the curing process. Table 1.1 Crosslinking agents and cleavage products for curing of silicone elastomers ( based on Ullmann 2003)
Silicone elastomers are characterised by excellent electrical insulating properties, inertness, low toxicity and resistance to ozone, weathering, oil and moisture. Some grades have the ability to perform at very low temperatures, whereas others retain elastomeric properties at very high temperatures. Unfilled silicone elastomers achieve only low mechanical strength when cured. Adequate strength is obtained by incorporating reinforcing fillers. Small-particle-size, silicas are used almost exclusively for this purpose. Because of the small particle size elastomers with high transparency can be produced. The major application of silicone elastomers is as sealant, but they are also widely used for other applications like electrical fittings, textile coating, paints and rubbers for automotive, medical and dental purposes. Silicone resins The major market for silicone resins is surface coatings, mainly water-repellant coatings for masonry and high-performance paints. For these purposes the silicone resin is often reacted with other polymers to form copolymers. Silicone/alkyd and silicone/polyester are the most frequently used. 1.2 International marketGlobally the consumption of siloxanes totals about 0.85 million tonnes (Will et al 2003). The main producer countries are the USA, Germany, Japan, France and the UK. The American producer DOW Corning Corporation is by far the largest producer of the World with production facilities in many countries. In Western Europe Wacker Group (Germany), Degussa AG (Germany) and Rhodia S.A. (France) are main producers. The total consumption of silicones in Western Europe is according to Will et al. about 296,000 t. The breakdown by application is shown in Table 1.2. The consumption breakdown is by the authors of the report designated "somewhat tentative", but is considered to provide a general view of the market. In this report the breakdown of the Western European consumption will be used as basis for a first rough estimate on the use of silicones in Denmark if nothing more specific is known. Denmark accounts for 1.2% of the W. European population, but for 1.4% of the total industry-related GDP (Gross Domestic Product). For the first estimate the per-capita ratio of 1.2% will be used. For most consumer products and products used in the building industry the consumption pattern of siloxanes in Denmark is most probably not very different from the common W. European market. Differently, the consumption of siloxanes in industrial processes will be very dependent on the extent to which the industrial processes, in which siloxanes are used as processing aids, take place in Denmark. The actual consumption may thus be quite different from the common W. European use pattern. The information on the W. European market will be combined with information about siloxanes registered in the Danish Product Register and information obtained from providers and users of silicones in Denmark. Table 1.2 Consumption of silicones in Western Europe in 2002 (Will et al. 2003)
2 Application of silicones in Denmark
2.1 Raw materials production, import and exportThe statistics from Statistics Denmark (Danmarks Statistik) hold information on one commodity only for which it is explicitly stated that the commodity includes siloxanes or silicones. Import, export and production of unprocessed silicones for the years 1998-2001 are shown in Table 2.1. The main part of unprocessed silicones is sealants packed in Denmark. As silixanes only account for a part of the sealants, the amount of silicones will be less than indicated. Table 2.1 Import,export and production of unprocessed silicones (Statistics Denmark 1988-2002)
* supply = import + production - export 2.2 Fields of application2.2.1 Data from the Danish Product RegisterA list of siloxanes and siloxane-containing substances registered in PROBAS, the database of the Danish Product Register was retrieved. The retrieval comprises substances with chemical names including the text strings 'silox', 'silicone', 'methicon' or 'silsesquio'. For each substance the total content of the substance in imported and exported products as well as in products produced in Denmark by application areas and branch was retrieved. The application areas registered in PROBAS are, however, not fully identical with the application areas defined in this study, and the data from PROBAS is only to some extent immediately applicable. In addition, only a part of the siloxanes in products is registered. Companies only have the obligation to submit information on the turnover of products to PROBAS, if the products contain substances classified dangerous (Bek. 439, 2002). Siloxanes are generally not classified dangerous. If the products contain dangerous substances, however, all constituents of the products are registered. The retrieval thus includes siloxanes in products in which other substances are classified dangerous. In total 175 siloxanes or siloxanes-containing substances are registered as used in Denmark. The substances are listed in Annex 3. The total import, production and export of siloxanes registered in the Danish Product Register by application area is shown in Table 2.2. The total content of siloxanes in registered, imported products is 1,269-1,483 t in 78,000-90,500 t of products. The total content of registered products produced in Denmark is 162-1,143 t in 45,000-293,000 t of products. The data demonstrate the widespread use of siloxanes at low concentration in a very large product volume. It should be noted that the supply calculated as import + production - export is only indicative. Siloxanes imported for production often will be registered in two different categories for import and production, respectively, e.g. imported emollients may be used for production of paints. Table 2.2 The total import, production and export of siloxanes registered in the Danish Product Register by application area
* Supply = import + production - export The substances which are registered with the largest volumes in imported products and products produced in Denmark are shown in Table 2.3. One substance is excluded for reasons of confidentiality. For most of the substances a large number of application areas and branches are registered, and the retrieval demonstrates that many of the siloxanes are used for a large range of different applications. Table 2.3 Content of the 20 most used siloxanes in imported and produced products as recorded in the Danish Product Register. 2.2.2 Sealants used for constructionSilicone elastomers are widely used for sealants and rubbers. The different types of elastomers have been discussed in section 1.1. Siloxane-based sealants can be organised into two main groups:
Besides the pure silicone sealants, a number of different hybrid sealants in which the siloxanes are blended with other polymers like polyurethanes, acrylics and isobutylene exist. In some of the sealants the siloxanes only account for a small part of the product. Typical one-component RTV silicone sealants consist of the following components (Krogh 1999):
The cross-linking agents are tri- or tetrafunctional silanes containing hydrolysable Si-O or Si-N bonds. Most of the cross-linking agents react either spontaneously with the SiOH groups of the siloxane or with water. Curing of one-component RTV silicones starts when the compounds are exposed to atmospheric moisture during application. Depending on the used cross-linking agent different compounds are formed as cleavage product and will be released during the curing process (see section 1.1). Tin catalysts are generally added to these systems to give complete curing and improve the properties. In two-component RTV silicones, tetrafunctional alkoxysilanes are generally used as cross-linking agents in combination with tin catalysts (Ullmann 2003). Consumption The knowledge center "Fugebranchens Samarbejds- og Oplysningsråd" does not hold any statistics on the consumption of silicone sealants in Denmark but estimates that the consumption pattern in Denmark is similar to the general Western European pattern. It is estimated by the organization that more than half of the traded elastomers are used for construction and glassing. Imported products, recorded in the Product Register, contain in total 435-560 t silicones, whereas the content of produced products amounts to 170-1,120 t. Siloxanes used For production in Denmark 0-130 t of dimethicone and 11-35 t siloxane polymers with silsesquioxanes are registered. According to the knowledge center "Fugebranchens Samarbejds- og Oplysningsråd" silicones with acetic acid as cleavage products were formerly the most used, but they are to a large extent replaced by silicones with oxime and alcohol cleavage products. Fate of the siloxanes By the application of the sealants different volatile compounds will be released as mentioned above, whereas the release of siloxanes seems to be insignificant. Surplus sealant will mainly be disposed of to incineration. 2.2.3 Paints, inks and coatingsSiloxanes are widely used for paints and coatings. The main application areas are:
Silicones are used as water repellents on mineral-based products like masonry, concrete and tiles for both interior and outdoor applications. The silicones can be applied as neat materials, in solvents or as water-based emulsions. Other silanes are used for this application area as well. Siloxanes resins are used in high-performance paints to modify paints based on polyester, alkyd, epoxy and acrylic e.g. for anticorrosion. Silicones are used in high-temperature resistant coatings for exhaust pipes and stoves, household appliances and industrial applications. A growing application area of silicones is antifouling paints used as alternative to TBT-containing paints. The silicone coating form a surface to which it is difficult for the fouling organisms to adhere. Silicone liquids are widely used at low concentration in water-based paints in which the siloxanes improve the flow properties of the coating, eliminating wetting problems and thinning the coating edge. Besides, silicones are used in small volumes in inks and dyes. Consumption According to the Product Register the total volume of siloxanes in imported products (46 product types) was 40-78 in 12,000-21,000 t of products. The total volume in produced and exported products was 7-426 t and 5-244 t respectively. Most of the products are registered as paint and lacquer without further specifications. As many water-based paints are not registered, the total volume in paints may be significantly higher. The data from the Product Register demonstrates that the siloxanes are used in low concentration in a very large volume of products. Siloxanes used Besides the compounds with silsesquioxanes, polydimethyl siloxanes are registered as the most used siloxanes for this application area. Silicones with side groups containing among others polyethylene and polypropylene glycol butyl ethers are used at low concentrations (<1%) in many waterborne paintings, in which the siloxanes are used as defoamers, flow control agents and levelling agents. Fate of the siloxanes A few per cent of the siloxanes in waterborne paints may end up in the sewage system by washing of brushes and paint pots. Besides, a minor part may be diffusively released to the surrounding by maintenance of painted surfaces. 2.2.4 Cosmetics and toiletriesSilicone fluids are widely used in cosmetics and toiletries for:
The major use is in hair-care products and antiperspirants. About 200 siloxanes and siloxane derivatives are listed in the inventory of ingredients used in cosmetic products compiled by the European Commission (INCI 2000) (see Annex 1). The siloxanes and derivatives function in the cosmetics as emollients, antifoaming agents, viscosity-controlling agents, antistatic agents, binders, film formers, surfactants, emulsifying agents, humectants, antioxidants and additives. According to a leading producer of silicones the primary benefits for incorpation of different siloxanes into hair-styling products are as follows (Berthiaume 1995):
Besides their technical properties, the siloxanes are used in the products, because they are generally nonsticky, nonoily, nonirritating, do not make marks on clothing and have a relatively low toxicity. Consumption For the American market it is specified that 60% of the silicones used for cosmetics, toiletries and pharmaceutical preparations is used in hair, skin and other personal care products, whereas 32% is used in stick antiperspirants (Will et al. 2003). The Danish branch organization for cosmetics and toiletries, SPT, has no specific information regarding the consumption of siloxanes in cosmetic products made in Denmark. It is however believed that the total consumption of cyclomethicone, which is one of the most used siloxanes in the cosmetic industry, is approximately 5 - 6 t/year in cosmetic products produced in Denmark. The typical content of siloxanes in the products is below 2 % of the final cosmetic product, but the content can according to SPT vary between 0.5-40%, depending on the products in which the siloxanes are used. According to one of the suppliers of silicones, the siloxanes are more widely used in cosmetic products produced by the large international companies, whereas there has been a tendency to avoid the substances in the cosmetic products made in Scandinavia. Siloxanes used Shampoos, conditioners and stick deodorants are the main cosmetic products in which siloxanes are used. The presence of some of the same siloxanes mentioned above in stick deodorants, shampoos and conditioners on the Danish market has been confirmed by a short survey of products carried out as part of this project. Volatile cyclosiloxanes (mainly octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane) are used in many stick deodorant and antiperspirant products. Mixtures of the compounds are in cosmetics designated 'cyclomethicones', but in some cases the term 'cyclomethicone' is also used for pure octamethylcyclotetrasiloxane (D4) or decamethylcyclopentasiloxane (D5). According to Will et al. the use has shifted from D4 towards D5 because of their supposed minor toxicity. The Danish EPA has a database in which the content of chemicals in 766 cosmetic products on the Danish market is registered. Cyclomethicone is present in 64 of the 766 products within the product groups: suntan lotions, body lotions, hair-styling products, creams, lipsticks, children's makeup and deodorants (Pedersen 2004). According to Will et al. 2003, polydimethylsiloxanes, either linear or cyclic are the most widely used silicones in skin-care products. According to producer web-sites (e.g. Basildon Chemicals, UK) the same siloxanes used in hair-care products may also be used for skin care. Cosmetic products are in general not notified to the Product Register, and the database retrieval only revealed a few siloxanes used for this application area. The most common was dimethicone, polydimethyl siloxane, poly(oxy(dimethylsilylene)) and 3-hydroxypropyl methyl etoxylated dimethylsiloxanes/silicones. According to Allan et al. (1997) 20,000 t volatile siloxanes was used in the USA for personal care applications in 1993. If it is assumed that the per capita consumption is the same in Denmark, the consumption of volatile siloxanes for this application area would be about 300 t. However, most probably it is lower. Based on the present information it is not possible to split the total consumption on siloxanes of the different compounds, but the available information indicates a significant consumption of both volatile and non-volatile siloxanes. Fate of the siloxanes The volatile cyclosiloxanes used in cosmetic products are meant to evaporate during use and will mainly be emitted to the air. Based on American experience 92% of the volatile siloxanes should be emitted to the air (Allan et al. 1997). 2.2.5 Cleaning agents and maintenance agentsSilicone fluids are widely used in polishes and waxes for paints (e.g. car wax), rubber, plastics, silverware, leather products, etc. Silicones improve the ease of application of the wax and polish, provide water repellence and produce a quick shine. Besides, siloxanes are used in smaller amounts in different types of cleaning agents and in softeners for laundry. Consumption The market report by Will et al. (2003) does not specifically provide information on the W. European consumption of silicones for waxes and polishes, but paint, coatings and waxes in total are estimated at 130 t/year. If data for the U.S. market is applied on a per capita basis, the consumption with polishes and coatings would be approx. 170 t/year. Based on the available data the consumption of siloxanes with cleaning agents and maintenance agents is roughly estimated at 100 t/year. Table 2.4 Content of siloxanes in imported, produced and exported cleaning agents and maintenance agents according to the Danish Product Register
Siloxanes used According to the data from the Product Register the main compound is poly(dimethylsiloxane). This name may partly be used synonymously with many derivatives of dimethylsiloxane by the companies when notifying on the content of the products, if the exact siloxane compound is not known. Polydimethylsiloxanes with aminofunctional groups are widely used for this application area (Will et al. 2003). The presence of aminoethyl and aminopropyl groups increases the water solubility and forms a bridge to organic substances in the product. The aminofunctional silicone fluids were developed to impart durability and detergent resistance through bonding to the paint film. Twelve different siloxanes with aminofunctional groups are registered in the Product Register for use within this application area. The total registered consumption of these compounds is approx. 10 t/year. Volatile cyclic dimethylsiloxanes are also widely used for polishes and waxes. In a recent Danish study of car polishes, hexamethylcyclotrisiloxane was released to the air from three out of 10 investigated products, whereas decamethylcyclopentasiloxane was released from 2 products. In the Danish Product Register 33 different products including cyclic dimethylsiloxanes (CAS no. 69430-24-6) are registered. The exact chemical compounds are not indicated, but the products most probably contain a mixture of different cyclic dimethylsiloxanes. The total registered consumption of these compounds is approx. 2 t/year. Fate of the siloxanes A minor part of the siloxanes (e.g. the cyclic dimethylsiloxanes) may be released to the air by the application. 2.2.6 Mechanical fluids and heat transfer fluidsSilicones fluids are widely applied for the following applications:
The siloxanes may be formulated into grease (with filler material) or emulsions (mixed with water). For many applications the siloxanes only account for a few per cent of the products. Silicone fluids and greases are widely used in the automotive and the aircraft industries - from hydraulic damping and brake fluids to lubricate bearings, locks, linkages, instruments, etc. Silicone fluids and greases are generally applied when high temperatures, solvents or corrosive materials would destroy petroleum-based lubricants. (Will et al. 2003) Consumption Products within this application area are to a large extent registered in the Danish Product Register. According to the database retrieval, the total consumption of siloxanes with these products was about 35 tonnes, the main part used in lubricants. The data only indicate a minor consumption of siloxanes with metal-working fluids, but a large part of the siloxane-containing fluids for this application may not be included in the register. Siloxanes used According to Will et al. (2003) phenyl methyl siloxanes and fluorosiloxanes are more widely used for these application areas than the simple dimethylsiloxane fluids. Fate of the siloxanes Table 2.5 Siloxanes in imported, exported and produced mechanical fluids and heat transfer fluids according to the Product Register 2003
Note: .. confidential 2.2.7 Textile applicationsThe main uses of silicones for textile applications are as follows:
The main application of the silicone fluids are as softening agent particularly in cotton and polyester/cotton substrates. They are used as emulsions or additives to other softeners. Consumption In the Product Register a total of 50 t is registered in imported "textile impregnation products", whereas the content of produced and exported products were 0.3 and 0.2 t respectively. Siloxanes present in imported textiles will not be registered in the Product Register. Siloxanes used Fate of the siloxanes It has not been possible to identify studies on the fate of siloxanes in textiles. 2.2.8 Process control and plastic additivesSiloxanes are widely used for process control and as plastic additives:
Silicone used as antifoams in textile industry is included in section 2.2.7. The main application within this area is as surfactant used for manufacturing of polyurethane foams. The silicones add stability to the liquid foaming mixture so that collapse of bubbles is retarded, and flowability is increased. Consumption According to the trade organisation Plastindustrien i Danmark, siloxane surfactants are used for production of all PUR products. The siloxane content of the products is approximately 0.75%. In total 35,000-40,000 tonnes PUR was produced in Denmark in 1999, corresponding to 260-300 t siloxanes per year. Based on the information above the total consumption as processing aid is estimated at 470 t of which the main part is used for PUR production. Siloxanes used Fate of the siloxanes 2.2.9 Health-care applicationsSilicones are widely used in the following healthcare applications:
Consumption The Danish Product Register will in general not include information on substances in health-care products. Siloxanes used Simethicone (synonymous with polydimethylsiloxane) is stated to be the most commonly used antiflatulent, and acts by dispersing excess gas in the intestine (http://wiz2.pharm.wayne.edu/module/gastromed.html). For other applications a large number of different siloxanes are applied. Fate of the siloxanes As regards human exposure the use of siloxanes in breast implants, baby-care products (e.g. in nipples) and siloxanes used as antiflatulent is of particular importance. The major part of the siloxanes will be disposed of with municipal solid waste or medical waste for incineration. A minor part, used as antiflatulent, will be released to municipal wastewater. 2.2.10 Paper coatingSilicones are used for paper coating, primaryly to coat release papers, films and foils and as a backing of pressure-sensitive adhesive labels and tapes. Consumption In the Danish Product Register a total of 9 t siloxanes were registered as surface treatment agents for paper, cardboard, etc. Siloxanes used Fate of the siloxanes 2.2.11 Other uses of silicone elastomers and resinsOther uses of silicone elastomers and resins not covered elsewhere include the following:
Consumption Siloxanes used Fate of the siloxanes 2.2.12 Other uses of silicone fluidsSilicone fluids may be used for different applications not included above. These applications include use in reprography, impregnation of water-proof insulation materials and probably other uses. The consumption of silicone fluids for other applications is, with a sidelong glance at Will et al. 2003, roughly estimated to be of the order of magnitude of 50 t/year. 2.3 SummaryThe present information on the use of siloxanes in Denmark in 2001 is summarised in Table 2.6. The main application areas are sealants for construction (29%), processing aids (15%) and textile applications (12%). The type can roughly indicate the potential for releases of the compounds to the atmosphere and wastewater. Volatile fluids are released to the atmosphere, whereas other fluids may end up in wastewater or released directly to surface water and soil. Elastomers and resins will mainly end up in solid waste. Table 2.6 Consumption of siloxanes in Denmark 2002
* Uncertainty indication: In the present study it has not been possible to obtain a detailed split of the different types of siloxanes on application areas. A study of the fate of the siloxanes in the USA may though give an indication of the fate of the siloxanes in Denmark (Allen et al. 1997). The main source of siloxanes to the air is the volatile siloxanes of which 92% is released to the air by use. Polydimethylsiloxane with modifications (silicone oils) are the main source of siloxane releases to wastewater, as about 25% of the total is discharged to wastewater treatment plants. Another 21% is "dispersed" and may end up in water bodies, on the ground, etc. Silicone resins and elastomers mainly end up in solid waste for incineration, landfilling or recycling. Table 2.7 Environmental loadings of industrial siloxanes for the USA in 1993 (based on Allen et al. 1997) (x 1000 t)
* Modified polydimethylsiloxanes include: methyl(hydrido)siloxanes, methyl(vinyl)siloxanes, methyl(alkyl)siloxanes, methyl(phenyl)siloxanes, methyl(trifluoropropyl)siloxanes, and methyl(aminoalkyl)siloxanes. These are treated as a group, because their physico-chemical properties (which dictate their fate) are similar to PDMS and to each other. The major release/disposal routes and risk of consumer exposure is summarised in Table 2.8 below. The main source of releases of siloxanes to the air is volatile siloxanes used in cosmetics, wax, polishes and to a minor extent in several other applications. No information of the quantity of volatile siloxanes for these applications has been available, but data for the USA indicates that volatile siloxanes may account for a significant part of the siloxanes used for cosmetics and it is roughly estimated that between 50 and 200 t/year is released to the air. Siloxanes disposed of for municipal solid waste incineration are deemed nearly 100% to be mineralised by the incineration, and incineration plants are not considered significant sources of siloxane releases to the atmosphere. Non-volatile silicone fluids used in cosmetics, wax, polishes, cleaning products and for textile applications (softeners) will to a large extent end up in wastewater and be directed to wastewater treatment plants. The total release to wastewater is estimated at 200-700 t/year. By the treatment process the siloxanes mainly follow the sludge and is either spread on agricultural fields, incinerated or disposed of to landfills. According to Fendinger et al. (1997) approximately 97% of the polydimetylsiloxane will be bound to the sludge by the wastewater treatment, while the remaining 3% will be discharged to surface waters. It indicates that the main sources of discharge to surface waters in Denmark, as is the case for many heavy metals, are precipitation-dependent discharges which are discharged directly to surface water without treatment. The major part of siloxanes used in silicone elastomers and resins in sealants, paints, rubbers, etc. is disposed of for incineration or landfills with building materials. By the incineration the siloxanes are destructed. Table 2.8 The major release/disposal routes and risk of consumer exposure
3 Health evaluation of siloxanes3.1 Data on toxicity of siloxanesInformation about the toxicity of the siloxanes has been searched in open databases on the Internet and also as a general search based on CAS number, chemical name or just the term “siloxanes”, e.g. in combination with individual terms relevant to toxicity testing and results, using different search engines and meta search engines. Contacts to a few siloxane research university environments have pointed to the same literature as identified from searching the Internet. As a first step in the data search, a preliminary database screening was carried out for decamethyl cyclopentasiloxane (Annex 6). The data search has included the following general databases with information on chemical substances and their toxicological effects:
The screening did not reveal any data on human toxicity, and it was decided not to make similar screenings for other siloxanes, but instead make a short review based on the available original literature. The main source of information has been the Siloxane Research Program. The program is run by The Silicones Environmental, Health and Safety Council of North America (SEHSC) which is a non-profit trade association comprised of North American silicone chemical producers and importers. The programme was started in 1993 and includes a series of studies examining acute and long-term safety of exposure to the fundamental building blocks of many silicone materials (Meeks 1999). Testing under this programme includes the following type of tests: Fundamental research:
Descriptive toxicological studies:
Human clinical studies:
Exposure assessment studies:
Information was specifically searched for the CAS numbers shown in the table below. Table 3.1 Siloxanes searched by CAS number
Information has primarily been identified for D4, D5 and HMDS which besides polydimethylsiloxane (PDMS) is part of the Siloxane Research Program. For the other three siloxanes which are not part of the program, little or no information has been found. This is also the situation as regards more general information on toxicity related to small linear siloxanes and cyclic siloxanes. A few studies are focussing on estrogenic and anti-estrogenic properties of D4 and HMDS, but in general data on endocrine disruption end points are scarce. 3.2 Toxicity of siloxanesAlthough siloxanes are used in many products including consumer products and have been so for many years, there is relatively little information available about their toxicity apart from the information provided by the Siloxane Research Program. However, siloxanes have generally been regarded as safe in consumer products, but new uses, e.g. in breast implants and focus on reproductive toxicity and possible endocrine disrupting effects have focussed attention on this group of substances. Of the six siloxanes mentioned in Table 3.1 only D4 is on Annex I to the Substance Directive (67/548/EEC) with a health classification as toxic to reproduction in category 3. The German justification for classification of D4 with regard to carcinogenic, mutagenic and reprotoxic effects is included in the reference list. D4 is on the list of potential PBT and vPvB (very persistent and very bioaccumulative) substances selected on the basis of screening criteria in the EU (DEPA 2003). In the following a short review of the findings in literature about the substances are presented. An overview of the studies and their results are presented in Annex 7. 3.2.1 ToxicokineticsA number of studies in rats using unlabelled or 14C labelled D4 show that the level of absorption following inhalation of this substance is low and independent of gender and dose. The substance is distributed to most tissues and the highest concentrations were found in fat and the lowest in the reproductive tissues. Parent D4 is eliminated via the lungs and metabolised D4 via urine and faeces. The elimination profile from tissues except from fat and lung resembles that from blood and follows a two-compartment model (EPA DCN 86970000024 1996). The elimination half-life for D4 has been shown to vary from 68 hours in plasma to approximately 150 hours in skin. Higher values are seen in testes. Blood clearance in human volunteers was non-linear and more rapid than by rats, whereas the elimination from the lungs resembled that from rats (BAuA 2001). D4 has unusual distribution properties that have become apparent after examination of the time course data for blood and tissues using a quantitative physiological model (PBPK). Despite the very high lipophilicity, D4 does not show prolonged retention because of high pulmonary and hepatic clearance coupled with induction of metabolising enzymes at high exposure concentrations (Andersen et al. 2001). This avoids accumulation of free D4. Pharmacokinetics of D4 administered to rats by inhalation and dermal route are similar and differs from the intravenous and oral route (Sarangapani et al. 2003). Percutaneous absorption of neat D4 in humans following topical application between 1 and 24 hours has been shown at levels of 0.57 – 1.09% (EPA DCN 86980000153 1998 and EPA DCN 8601000003 2000). In in vitro studies with percutaneous absorption following 24 hours exposure to 14C-D5 the absorption was found to be 0.8 – 1.08% (EPA DCN 86960000593 1996 and EPA DCN 86970000009 1996). In rats administered HMDS orally and intravenously no parent HMDS was found in the urine. Metabolites from this linear siloxane appear to be structurally different from those obtained for cyclic siloxane except for the commonly present Me(2)Si(OH)(2). 3.2.2 Acute toxicityIn general the acute toxicity of siloxanes is considered low. LD50 following oral administration of D4 in rats is reported to be more than 4,500 mg/kg and more that 5,000 mg/kg for HMDS. LC50 in rats exposed to D4 was >12.17 mg/l and >48 mg/l when exposed to HMDS (European Commission 2000). LC50 in rats exposed to HMDS for four hours was 15,956 ppm (EPA DCN 86970000724 1997). LD50 following dermal application of D4 was >2400 mg/kg bw. Several studies with dermal application of HMDS have shown greater LD50 values, but mortality was observed at 10000 mg/kg bw. Toxic effects at 10000 mg/kg included gross pathological findings (lung, kidney, bladder, heart), while clinical findings (altered activity, ataxia, gasping and eschar formation) occurred in small numbers of rabbits. In contrast to rabbits, rats did not produce mortality or signs of toxicity at the dose tested (European Commission 2000). 3.2.3 Irritation and sensitizationD4 and HMDS have been tested on rabbit eyes without signs of irritation. The substances are also found non-irritant on rabbit skin. For both substances one study exists that describes the substances as slightly irritating. There are no further details from these studies (European Commission 2000). D4 was not sensitizing in guinea pig maximisation test and also not sensitizing in 50 human subjects exposed to repeated insult patch test (European Commission 2000). HMDS has also been tested in guinea pig maximisation test without positive result (European Commission 2000). 3.2.4 Subacute / subchronic / chronic toxicityD4 administered by oral gavage to rats over 28 days did not cause any immune suppression at doses between 10 and 300 mg/kg (EPA DCN 86980000072 1997). Human volunteers did not show any immunotoxic or proinflammatory/adjuvant effects following ingestion of 12 mg D4 in corn oil for 7 or 14 days (EPA DCN 86990000015 1998). The same result was obtained after inhalation of 10 ppm for one hour and re-exposure after three months (Loony et al. 1998). Investigation of subacute oral toxicity in rats administered between 25 and 1600 mg/kg per gavage over two week with five applications per week caused increased relative liver weights in female animals at 100 mg/kg and male animals at 400 mg/kg. Absolute liver weight was also increased in female rats at 400 mg/kg. Decreased body weight was seen at the highest concentration in both male and female animals (BAuA 2001). Rats exposed to D4 at 70 and 700 ppm by inhalation for 28 days, 5 days per week and 6 hours per day in different studies show rapid but reversible increase in liver size, induction of several metabolising enzymes, primarily CYP2B1 and induction of hepatic cytochrome P450 enzymes. D4 appears to be a phenobarbital-like inducer of hepatic microsomal enzymes in Fisher-344 rats (EPA DCN 86970000723 1996; EPA DCN 86970000725 1997; McKim et al. 1998). Other studies with D4 in Fisher-344 rats exposed by inhalation over 3 months, 5 days per week and 6 hours per day, showed slight reduction in body weight and food intake at the 10.87 mg/kg dose group, slight dose-related increase in absolute and relative lever weight in female rats at 10.87 mg/kg and slight reduction in thymus and ovarian weight in female rats in the two highest dose groups, 5.91 and 10.87 mg/kg. Ovarian atrophy and vaginal mucification was also seen at the highest dose group (EPA DCN 8690000155 1995; EPA DCN 8690000153 1995). Rats exposed to D5 by inhalation for 28 days, 7 days per week and 6 hours per day at concentrations between 10 and 160 ppm showed no adverse effects on body weight, food consumption or urinalysis. Minor transient changes in haematological serum chemistry and organ weight and a transient increase in liver to body weight and thymus to body weight at 160 ppm. NOEL (histopathological changes) was determined at 10 ppm, NOEL (systemic toxicity) was determined at 75 ppm and NOEL (immunosuppression) was determined at 160 ppm (EPA DCN 86970000385 1996). Subchronic toxicity studies in rats exposed over three months at doses up to 224 ppm show that the lung is the primary target organ following D5 inhalation (Burns et al. 1998). Rats exposed to inhalation of HMDS for one month in concentrations between 0.9 and 59.2 mg/l showed moderate increase in focal inflammatory lesions in the lungs in the highest dose group, increase in incidence and severity of renal tubule regeneration in male rats exposed to 12.7 and 59.2 mg/kg, hyaline droplet accumulation, protein casts and granular casts were present in kidneys in several males in the highest 59.2 mg/kg dose group. Other signs of toxicity included minimal hepatocellular hypertrophy in males of the two highest dose groups and a slight increase in pigment accumulation in bile ducts in the high dose group males (EPA DCN 869000048 1997). Exposure of rats for three months to HMDS in concentrations between 0.33 and 33.3 mg/kg showed also similar histological lesions in the kidneys of males in the three highest dose groups; 4.0, 10.0 and 33.3 mg/kg. NOEL was determined at 1.3 mg/kg for male rats and 33.0 mg/kg for female rats (EPA DCN 86980000182 1998; Cassidy 2001). Multifocal, subpleural, subacute to subchronic interstitial inflammation were seen in lungs of all groups of rats exposed for three months to inhalation of concentrations between 0.9 and 13.64 mg/kg HMDS. After the recovery period an increase of these finding were still seen in the high dose group (EPA DCN 86980000048 1997). 3.2.5 Genetic toxicityBoth D4 and HMDS have been tested in a number of in vitro studies including Ames test in different strains (with and without metabolic activation), DNA damage and repair test in E. Coli, cytogentic assay in Chinese Hamster Ovary cells, chromosome aberration assay and sister chromatide exchange assay in mouse lymphoma cells, all with and without activation with negative result. In vivo tests have included cytogenetic assay in rat (HMDS) and dominant lethal assay in rat (D4). Both tests were negative (European Commission, 2000). An in vivo chromosome aberration test in rats exposed to 700 ppm D4 was also negative (Vergnes et al. 2000). The results gave no indication of a genotoxic potential - neither for D4 nor HMDS. 3.2.6 CarcinogenicityVery little information is available on carcinogenicity of siloxanes. The only information identified is a report from Dow Corning received by EPA with preliminary results from a two-year chronic toxicity and carcinogenicity study in rats exposed to vapour concentrations of 0, 10, 40 or 160 ppm of D5 for 6 hours per day, 5 days per week, for 24 months. The preliminary results show that female rats in the highest dose group had a statistically significant increase of uterine tumours. These findings may indicate that there is a potential carcinogenic hazard associated with D5 (EPA. 2003). Final results are expected in the Spring of 2004. Other relevant information is related to silicone in breast implants, where IARCH has evaluated that there is evidence suggesting lack of carcinogenicity in humans of breast implants, made of silicone, for female breast carcinoma (IARC 1999). 3.2.7 Reproductive toxicityTests to examine reproductive toxicity for siloxanes are primarily available to D4 which is also classified in the EU as Toxic for Reproduction. Cat. 3; R62 (Possible risk of impaired fertility). D4 has been evaluated based on information on toxic effects on the parent animals, toxicity to fertility and developmental toxicity/teratogenicity. Various effects have been observed in both male and female parent animals in both studies to examine reproductive toxicity and other relevant studies. These effects have included decreased body weight, haematological and clinical-chemical effects, increased liver weight, cellular and subcellular changes, induction of liver enzymes, reduced organ weights (adrenals and thymus), ovarian atrophy and mucification of the vagina. The last two effects are indications of changes in the oestrus cycle. However, the results from the different tests have not shown a consistent picture with regard to the listed effects (BAuA, 2001). Data on impairment of fertility are related to a reduction of the number of mean corpora lutea and implantation sites and increased post-implantation losses. The effects are phase specific indicating that D4 influences the hormonal cycle. It is suggested that this effect on the ovary is indirect and that the mode of action is related to neuroendocrine mechanisms in rodents which are quite different than those in humans. The rodent estrous cycle is generally four days long but in humans it is much longer (i.e. 28 days). It is this brevity of the estrous cycle that requires the precise control of the neuroendocrine events, the sensory inputs, and the sexual behaviours (Centre Europeen des Silicones 1999). Studies to investigate developmental toxicity of D4 have not shown significant teratogenic effects (BAuA 2001). A single generation study in rats exposed to inhalation is available for D5 showing no significant toxicological findings and no effects on reproductive parameters (EPA DCN 86970000006 1996). 3.2.8 Endocrine disruptionVery little information is available on endpoints which are considered relevant to endocrine disruption. In a uterotrophic assay in immature rats receiving oral doses of D4 and HMDS for 4 days, D4 exhibited weakly estrogenic effects (dose-related increase in uterine weight and epithelial cell height) in both SP and F-344 rats. The substance also showed weak antiestrogenic properties by partially blocking EE (ethinylestradiol) induced uterine weight increases (competitive inhibition of estrogen receptor binding or D4 acting as a partial estrogen agonist). Estrogenic and antiestrogenic effects of D4 were several orders of magnitude less potent than EE, and many times less potent than the weak phytoestrogen CE (EPA DCN 86990000059 1999). In the same assay HMDS showed no measurable effect on uterine weight when tested as an agonist. When co-administered together with EE, HMDS produced a slight, but statistically significant reduction in absolute uterine weight. The biological relevance of this could not be assessed in the present study (EPA DCN 86990000059 1999). 3.3 ConclusionOnly few siloxanes are described in the literature with regard to health effects, and it is therefore not possible to make broad conclusions and comparisons of the toxicity related to short chained linear and cyclic siloxanes based on this evaluation. Data is primarily found on the cyclic siloxanes D4 and D5 and the small linear HMDS. The three siloxanes have a relatively low order of acute toxicity by oral, dermal and inhalatory routes and do not require classification for this effect. They are not shown to be irritating to skin or eyes and are also not found sensitizing by skin contact. Data on respiratory sensitization have not been identified. Subacute and subchronic toxicity studies show that the liver is the main target organ for D4 which also induces hepatocellular enzymes. This enzyme induction contributes to the elimination of the substance from the tissues. Primary target organ for D5 exposure by inhalation is the lung. D5 has a similar enzyme induction profile as D4. Subacute and subchronic inhalation of HMDS affects in particular the lungs and kidneys in rats. None of the investigated siloxanes show any signs of genotoxic effects in vitro or in vivo. Preliminary results indicate that D5 has a potential carcinogenic effect. D4 is considered to impair fertility in rats by inhalation and is classified as a substance toxic to reproduction in category 3 with the risk phrase R62 ('Possible risk of impaired fertility'). The results of a study to screen for estrogen activity indicate that D4 has very weak estrogenic and antiestrogenic activity and is a partial agonist (enhances the effect of the estrogen). It is not uncommon for compounds that are weakly estrogenic to also have antiestrogenic properties. Comparison of the estrogenic potency of D4 relative to ethinylestradiol (steroid hormone) indicates that D4 is 585,000 times less potent than ethinylestradiol in the rat stain Sprague-Dawley and 3.7 million times less potent than ethinylestradiol in the Fisher-344 rat strain. Because of lack of effects on other endpoints designated to assess estrogenicity, the estrogenicity as mode of action for the D4 reproductive effects has been questioned. An indirect mode of action causing a delay of the LH (luteinising hormone) surge necessary for optimal timing of ovulation has been suggested as the mechanism. Based on the reviewed information, the critical effects of the siloxanes are impaired fertility (D4) and potential carcinogenic effects (uterine tumours in females) (D5). Furthermore there seem to be some effects on various organs following repeated exposures, the liver (D4), kidney (HMDS) and lung (D5 and HMDS) being the target organs. A possible estrogenic effect contributing to the reproductive toxicity of D4 is discussed. There seems however to be some indication that this toxicity may be caused by another mechanism than estrogen activity. Effects which based on the reviewed literature do not seem to be problematic are acute toxicity, irritant effects, sensitization and genotoxicity. 4 Environmental fate and effects
It is beyond the scope of this project to prepare a comprehensive review of environmental effects of the siloxanes, but some data on persistence, bioaccumulation and toxicity are summarised below. 4.1 Initial screening for decamethylcyclopentasiloxane (D5)Information about environmental properties and toxicity of decamethylcyclopentasiloxane (D5) was initially retrieved from the databases Aquire, CambridgeSoft Corporation database, HSDB database, IUCLID-CD, PHYSPROP DEMO-database, SPIN database, DART Special (RTECS) and ToxLine. The screening result for decamethylcyclopentasiloxane is shown in Annex 6. No information on environmental toxicity was found and it was decided not to go further on with similar database screenings for the other substances. 4.2 PBT profiler screeningIn order to make a first comparison between the substances as to persistence, bioaccumulation and toxicity, the substances were screened using the PBT profiler developed by U.S. EPA (U.S. EPA 2003). The profiler uses a procedure to predict persistence, bioaccumulation, and toxicity of organic chemicals on the basis of the chemical structure and physical parameters of the substances combined with experimental parameters for substance with a similar structure, using a QSAR approach. For more information see U.S. EPA (2003). The results for six members of the siloxane family (Table 4.1) predict the highest bioconcentration factors for the two phenyl siloxanes, one order of magnitudes higher than the values for the cyclic siloxanes and two orders of magnitudes higher than the values for the small linear methyl siloxanes. The predicted toxicity is as well significantly higher (lowest ChV values - see description in table notes) for the phenyl siloxanes. The predicted half-life is nearly the same for all substances. Using U.S. EPA's criteria, the screening indicates that all substances are of high concern as to environmental toxicity, and that the phenyl siloxanes are considered very bioaccumulative. Table 4.1 PBT profiler results for selected siloxanes (based on U.S. EPA 2003)
CvH: Chronic Value (ChV) is the same as the chronic NEC (No effect concentration). US EPA uses the following criteria for Fish ChV (mg/l): 4.3 Aquatic toxicity data for octamethylcyclosiloxane and PDMSThe environmental fate and effects of volatile methylsiloxanes (mainly cyclosiloxanes) and polydimethylsiloxane (PDMS) have been reviewed by Hobson et al. (1997) and Fendinger et al. (1997), respectively. A summary of aquatic toxicity data for octamethylcyclosiloxane is shown in Table 4.2 whereas toxicity data for PDMS is shown in Table 4.3. Table 4.2 Summary of octamethylcyclosiloxane aquatic toxicity data (Hobson et al. 1997)
* This concentration is the mean value measured in the highest exposure level. Table 4.3 Soil and sediment testing results used for PDMS risk screening (Fendinger et al. 1997)
* Indicates highest dose tested with no effects observed in test. 5 Alternatives
The use of some types of siloxanes is regarded potentially to cause problems to the aquatic environment and human health. The investigation on alternatives has therefore focussed on product groups where the risk of exposure to humans and releases to the aquatic environment is regarded to be present. The investigation has focused on the following product groups:
The information in this chapter is based on telephone interviews with manufacturers and suppliers of both siloxanes and alternatives to siloxanes. In the following chapter the names of the compounds when they are used in cosmetic products (INCI names) will be used. Dimethicone is synonymous with linear polydimethylsiloxane, cyclopentasiloxane with decamethylcyclopentasiloxane and cyclomethicone with cyclic siloxanes, either pure or in mixtures. 5.1 Alternatives to siloxanes in cosmetic productsThe investigation has primarily focused on the siloxanes cyclomethicone and dimethicone, which are commonly used in the cosmetics industry. Cyclomethicone is volatile and is furthermore suspected to cause different types of health and environmental problems. As mentioned in section 2.2.4 the siloxanes have many applications in the different types of cosmetic products, and the siloxanes have very specific properties depending on the composition of the compounds. Because of this it is not possible to find just one alternative for e.g. cyclomethicone, as cyclomethicone can have many different applications and compositions depending on the product in which the substance is used. The alternatives for siloxanes must therefore be very specific, as they have to comply with the special characteristics that the given siloxane has in the given product. The use of alternatives to siloxanes in cosmetic products is still modest. The Danish suppliers of both siloxanes and alternatives to siloxanes have so far not experienced great demand for alternatives, but most of the contacted suppliers expect an increased demand for alternatives in the coming years. The suppliers have consequently started to develop alternatives to especially cyclomethicone and dimethicone. It has so far been a difficulty that some of the properties of siloxanes are lacking in the developed alternatives. For soaps and leave-on products (lotions and creams for skin) the siloxanes can give the product the combination 'smooth and soft feeling' on the skin combined with the effect that the product does not feel greasy on the skin after application. These properties are obtained because the used siloxanes are volatile and can give extra softness. It has so far been difficult to find alternatives that can match these properties, and especially cyclomethicone is difficult to substitute. Dimethicone is in general easier to substitute, because the same properties can often be obtained with different types of vegetable oils. A test of one of the alternatives in Table 5.1 has shown that the developed alternative can give almost the same properties as cyclomethicone in the tested cosmetic emulsion. Emulsions are regarded to be one of the most problematic products to substitute cyclomethicone from. The functional silicone oils, which for example can be a mix of cyclomethicone and dimethicone, are even more problematic to substitute, as the mixture of the different siloxanes is made to obtain some very specific properties. Polyether-modified silicones and other types of functional silicones will give the same problem, and alternatives have so far not been regarded. The typical content of siloxanes in the products is below 2% of the final cosmetic product, but the content can according to SPT (The Danish trade organization for cosmetics and toiletries) vary between 0.5-40%, depending on the products in which the siloxanes are used. Table 5.1 shows identified alternatives to siloxanes used in cosmetic products. 5.1.1 Neopentylglycol heptanoateNeopentylglycol heptanoate is an alternative to dimethicone in different cosmetic products like conditioners and leave-on products. Neopentylglycol heptanoate has the same good spreadability as dimethicone and can like dimethicone be used as solvent for other substances and emulsifiers. The substance has just been introduced in Denmark, and customers have according to the supplier shown great interest in the alternative, but the substance cannot be found in Danish cosmetic products yet. The price of neopentylglycol heptanoate is approximately the double of the price for dimethicone, as 1 kg of dimethicone costs approximately 50 DKK per kg, while the alternative costs approximately 100 DKK per kg. The use of neopentylglycol heptanoate should according to the supplier not result in changes in the production equipment. It would though be necessary to change the packaging, due to changes in the declaration. 5.1.2 Isodecyl neopentanoateIsodecyl neopentanoate is an alternative to cyclomethicone. It can be used in leave-on products, conditioners and perhaps also in shampoos and cream soaps. Isodecyl neopentanoate has high spreadability like cyclomethicone and gives a soft feeling like cyclomethicone. It can be used as solvent and emulsifier. The alternative is new on the Danish market and has not been sold to Danish customers yet, but customers have shown great interest in the product. Isodecyl neopentanoate is quite expensive compared to cyclomethicone. Cyclomethicone costs approximately 45 DKK/kg, while the price of isodecyl neopentanoate is expected to be approximately 100 DKK/kg. The use of the isodecyl neopentanoate should according to the supplier not result in changes in the production equipment. It would though be necessary to change the packaging, due to the changes in the declaration. Table 5.1 Identified alternatives to siloxanes from Danish producers and suppliers
N/A CAS No. has not been available - the substances are not included in the 1st update of the inventory of ingredients used in cosmetic products (INCI 2000) 5.1.3 Glycol distearateGlycol distearate is an alternative to siloxanes in different types of soaps. Glycol distearate gives the product a "milk-like" appearance and contains wax that gives shine and smooth feeling to cream soaps, shower gels and shampoos. Glycol distearate can typically not directly substitute all properties of dimethicone, cyclomethicone or other types of siloxanes, which often can give a more distinct feeling of softness etc., but it has similar qualities. Glycol distearate has been used in cream soaps sold in Denmark for the last 2 - 3 years, and the use of glycol distearate is increasing. A compound containing glycol distearate costs approximately 20 - 25 DKK/kg, but the compound only contains 20-40% of glycol distearate - the rest are other additives used in soaps. Glycol distearate is therefore economically competitive compared to the siloxanes. The use of glycol distearate instead of siloxanes will according to the supplier not cause changes in the production equipment. It would though be necessary to change the packaging, due to changes in the declaration. 5.1.4 Dicaprylyl carbonate (vegetable oil components)Different vegetable oil components can be used in creams and lotions instead of siloxanes. One particular example of these oils is dicaprylyl carbonate. Dicaprylyl carbonate cannot directly substitute the properties of siloxanes used in creams and lotions, as the alternative does not have the foam reducing effect that the siloxanes have. But except from this dicaprylyl carbonate can be used instead of cyclomethicone, dimethicone and other siloxanes and can add softness and spreadability to the products. The product is in use in products sold in Denmark. The price of dicaprylyl carbonate is approximately the same as for the different types of siloxanes, perhaps a bit more expensive than cyclomethicone and dimethicone. It should be possible to use the same production facilities. It would though be necessary to change the packaging, due to changes in the declaration. 5.1.5 Diethylhexyl carbonateDiethylhexyl carbonate is an extremely spreadable and low-viscous ester oil, which can substitute cyclopentasiloxane in lotions and emulsions. A cream product based on diethylhexyl carbonate has been tested together with a cream product based on cyclopentasiloxane. The test, where 6 people in a sensory panel tested the two creams, showed that the cream based on cyclopentasiloxane was perceived by all panellists, because it feels better with respect to spreadability and soft feeling than the cream based on diethyl carbonate. If an additive was added to the cream with diethylhexyl carbonate, three of the panellists did not find any difference between the two creams, while the three remaining still found that the cream with cyclopentasiloxane was a bit lighter. The conclusion of the test is that it is possible to make a product based on diethylhexylcarbonate that has almost the same qualities as the product based on cyclopentasiloxane. The diethylhexyl carbonate has so far not been used in products sold in Scandinavia. The price of the alternative is approximately 35 - 40 DKK/kg while cyclopentasiloxane is more expensive, approximately 55 - 65 DKK/kg. The use of the diethylhexyl carbonate will not result in changes in the production equipment, but will of course involve new packaging due to new declarations of the product. 5.1.6 Hydrogenated polydecenHydrogenated polydecen is an alternative to different basis mineral oil or paraffin oils. If a product contains both paraffin oils and cyclomethicone, hydrogenated polydecen can, however, usually substitute both substances, as hydrogenated polydecen can give some of the soft feeling on the skin and can easily be absorbed in the skin without greasing. Hydrogenated polydecen will therefore to some extent match the properties of cyclomethicone. It can, however, not give the extra soft feeling that the siloxanes can add to a cosmetic leave-on product. The product has been sold in Denmark for the last two years and can be found in several products in Denmark. The price of hydrogenated polydecen cannot be compared to cyclomethicone due to fact that it is not a direct alternative to cyclomethicone. 5.1.7 Summary and general experienceThe investigation of alternatives to siloxanes in cosmetic products has shown that some alternatives have been developed and are used in Denmark, but the alternatives can at present typically not substitute the properties of siloxanes 100 %. The producers of cosmetic products must therefore often dispense with some of the properties of the siloxanes. Cyclomethicone and dimethicone in emulsions and creams are especially difficult to substitute, as the alternatives typically do not have a foam-reducing effect in the products. One alternative used in a cream has, however, been tested to have the same qualities as a cream with cyclopentasiloxane. The suppliers of alternatives are experiencing great interest in alternatives to siloxanes, partly because of the environmental and health aspects, partly due to the options for evading different patents. Many international producers use the Internet to advertise for hair and skin care products that are "silicone-free", but it seems that this is so far not a competition parameter for Danish producers. Most of the alternatives are competitive with the siloxanes as regards price, however for some of the alternatives the price is twice the price of the siloxanes. A few of the alternatives are more expensive than the siloxanes. It is the general opinion that the use of alternatives to siloxanes does not cause changes in the production equipment, and the costs of substitution will therefore primarily be the extra costs for buying an alternative substance. Costs of new packaging are not regarded to be significant, as the substitution in most cases can be implemented when it suits into the production. As earlier mentioned the Danish industrial organization for cosmetics and toiletries, SPT, assesses the consumption of cyclomethicone used in Danish production to be approximately 5-6 tonnes per year. If the price of the most expensive alternative is used to predict an estimate of the costs of substitution of this amount of cyclomethicone, the price of the substitution will be approximately 200,000-300,000 DKK for the 5-6 tonnes. The 5-6 tonnes are however only a small part of the total consumption of siloxanes with cosmetics in Denmark (approximately 240 tons/year), as cyclomethicone is only one out of several siloxanes, and most cosmetic products are imported and not produced in Denmark. 5.2 Alternatives to siloxanes in cleaning agents and polishesThe siloxanes used in cleaning agents, waxes and polishes are in general not the same as the siloxanes used in cosmetics, although some of the wanted properties are the same, for example shine, spreadability and antifoaming. The identified alternatives are therefore also quite different from the alternatives developed for cosmetic products. As for alternatives to siloxanes in cosmetic products it is the general opinion in the cleaning agent trade that silicones have some special properties that cannot easily be found in alternatives. These properties are especially as solvent, emulsifier and anti-soiling agent. Especially the use of the siloxane as defoaming agent is in many products difficult to substitute. Siloxanes are used for polish, primarily because they give extremely high shine and strong adherence to the materials, especially glass, metal and plastic. The siloxane content in cleaning agents is for most products less than 1% of the product which is further diluted in use. For polish products the siloxane content can be up to 5%, perhaps 10 % in some products. The use of siloxanes and alternatives to siloxanes in cleaning agents and polishes has been investigated through contact with manufacturers and suppliers. The 6 main suppliers of siloxanes and chemicals to the cleaning agent trade and 10 of the Danish main manufacturers of cleaning agents and polishes have been contacted. It has however been difficult to identify alternatives to siloxanes, because substitution of siloxanes has not had special focus in the cleaning agent trade. Some alternatives have been used for several years, but have not been developed in the aim of substituting siloxanes, and it is a question to what extent they actually have similar properties. Substances that are insoluble in water and with long carbon chains are in general suitable as alternatives to siloxanes in cleaning and maintenance agents. The longer carbon chains the substance have the more effective defoaming effect is obtained. It is the general impression that there are fewer different types of alternatives to silicones in cleaning and maintenance products than in cosmetic products. If a search is made on the Internet for 'silicone-free' antifoaming agents, wax or polishes several products can be found, indicating that the use of alternatives is quite widespread. The reason for using the 'silicone-free' products is, however, most often technical. The alternatives have been developed both due to the general interest in environment-friendly products in the trade for cleaning and maintenance agents, but also because the use of siloxanes can be problematic in some products. Silicones are for example often avoided in car waxes and other polish products for cars, because the use of silicones is disadvantageous if the car should later be re-painted. Table 5.2 shows the types of alternatives to siloxanes in cleaning and maintenance agents identified through contact to Danish suppliers and manufacturers. Table 5.2 Identified alternatives to siloxanes from Danish producers and suppliers
5.2.1 Mineral oils (tensides)Many of the suppliers and manufacturers of maintenance products point at nonionic mineral oils or tensides as the main alternative to polydimethylsiloxanes in antifoaming agents. The mineral oils can be used in both polish products and antifoaming agents. Odourless kerosene is mentioned as a possible alternative to antifoaming. The mineral oils typically do not have all the properties of polydimethylsiloxan. It can especially be difficult to obtain the same smooth surface for polishes. As antifoaming agents, the mineral oils are not always suitable for cleaning agents used in cold water, for example in floor cleaning machines. The price level of the mineral oils is approximately the same as that of siloxanes, and in some cases they are cheaper, but as the siloxanes or alternatives only constitute few per cent of the product or even less than one per cent, the costs for using an alternative instead of a siloxane is regarded to be minimal. 5.2.2 Paraffin oils and vegetable oilsParaffin oils and vegetable oils may like mineral oils be used as alternatives to polydimethylsiloxanes. Paraffin oils and vegetable oils are not very frequently used as alternatives to siloxanes in cleaning agents and polish products, but these types of oils can be used, as they have the defoaming properties. The price of the paraffin oils and vegetable oil is in general lower than that of siloxanes. 5.2.3 Lipophilic tensidesLipophilic tensides can be used as alternative to aminofunctional polydimethylsiloxanes used in polishes. The attractive shine and easy-cleaning surface that is obtained by using siloxanes can however not always be obtained to the same degree by using lipophilic tensides instead of siloxanes. 5.2.4 Block polymersBlock polymers consisting of polyethylenglycol and polypropylenglycol are a very applicable alternative to siloxanes in cleaning agents and polish products. The block polymer can be supplied with an extra alkyl chain by which the substance gets a lower surface tension that makes it possible to use the block polymer as antifoaming agent. Block polymers are some of the most used alternatives to siloxanes in cleaning and maintenance agents, but are still less widespread used than the siloxanes. Block polymers are in general more expensive than the siloxanes that can be used in this type of products. 5.2.5 SummaryIt has been difficult to identify alternatives to siloxanes, because substitution of siloxanes has not had particular focus in the cleaning agent trade. Some alternatives have been used for several years, but have not been developed in the aim of substituting siloxanes, and it is a question to what extent they actually have similar properties. Siloxanes are mainly used as antifoaming agent and to provide shine and spreadability to polishes. The antifoaming properties can be provided by a number of alternatives at approximately the same price as the siloxanes, but often the properties of the alternatives cannot fully match the properties of the siloxanes. ReferencesAllan, R.B., Kochs, P. & Chandra, G. 1997 Industrial organosilicon materials, their environmental entry and predicted fate. In: Chandra, H.G (Ed.). The handbook of environmental chemistry. Vol 3. part: Organosilicon Materials. Springer-Verlag, Berlin. Andersen M.E., Sarangapani, R., Reitz, R.H., Gallavan, R. H., Dobrev, I.D. & Plotzke, K.P. 2001. Physiological Modeling Reveals Novel Pharmacokinetic Behavior For Inhaled Octamethylcyclotetrasiloxane in Rats. Toxicological Sciences, 60, 214-231. Bek. 439. BEK nr 439 af 03/06/2002: Bekendtgørelse om listen over farlige stoffer [Statutory order on the list of dangerous substances] Berthiaume, M.D. 1995. Silicones in hair fixatives & finishing products. A brief review. GE Silicones, Waterford, N.Y
http://www.gesilicones.com/silicones/americas/business/ Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA). 2001. AGS Begründungen zur Bewertung von Stoffen als krebserzeugend, erbgutverändernd oder fortpflanzungsgefährdend. Burns, L.A., Mast, R.W., Meeks, R.G., Mann, P.C. & Thevenaz, P. 1998. Inhalation Toxicology of Decamethylcyclopentasiloxane (D5) Following a 3-Month Nose-Only Exposure in Fischer 344 Rats. Toxicological Sciences 43:230-240. Cassidy, S.L., Dotti, A., Kolesar, G. B., Dochterman, L. W., Meeks, R. G., Chevalier, H. J. (in print). Hexamethyldisiloxane: A 13-Week Subchronic Whole-Body Vapor Inhalation Toxicity Study in F344 Rats. International Journal of Tox. Centre Europeen des Silicones, 1999). (Comments to Swedish classification proposal for D4). http://ecb.jrc.it/classlab/3798a8.doc DEPA (Miljøstyrelsen). 2003. Liste over potentielle PBT og vPvB stoffer (List of potential PBT and vPvB substances). EPA DCN 84000000002. 1999. D4 Rat Uterotrophic Assay. Siloxane Research Program, Reston. EPA DCN 86010000003. 2000. Percutaneous Absorption Studies of Neat and Formulated Octamethylcyclotetrasiloxane (D4) in Human Skin/Nude Mouse Model. Siloxane Research Program, Reston. EPA DCN 86010000004. 2000. In-Vitro Evaluation of Estrogencity of Octamethylcyclotetrasiloxane (D4) Using the Human MCF-7 Cell Line. Siloxane Research Program, Reston. EPA DCN 86010000007. 2000. Absorption, Distribution and Elimination of 13C D4 in Humans after Dermal Administration. Siloxane Research Program, Reston. EPA DCN 86010000008. 2000. Evaluation of Decamethylcyclopentasiloxane (D5) as an Inhibitor of Human and Rat Cytochrome P450 Enzymes. Siloxane Research Program, Reston. EPA DCN 86010000009. 2000. In Vivo Dermal ADME of C14-D4 in Fischer 344 Rats (to clarify expired air values). Siloxane Research Program, Reston. EPA DCN 86010000010. 2000. Determination of both Parent Octamethylcyclotetrasiloxane (D4) and 14C-D4 in Female Sprague Dawley and Fischer 344 Rats Following a Single Nose-Only Vapor Inhalation Exposure to 700 ppm D4. Siloxane Research Program, Reston. EPA DCN 86950000153. 1995. Three-Month Repeated Dose Inhalation Toxicity Study with Octamethylcyclotetrasiloxane in Rats. Siloxane Research Program, Reston. EPA DCN 86950000154. 1995. Three-Month Repeated Dose Inhalation Toxicity Study with Decamethylcyclopentasiloxane in Rats. Siloxane Research Program, Reston. EPA DCN 86950000155. 1995. One-Month Repeated Dose Inhalation Toxicity Study with Octamethylcyclotetrasiloxane in Rats. Siloxane Research Program, Reston. EPA DCN 86950000174. 1995. One-Month Repeated Dose Inhalation Toxicity Study with Decamethylcyclopentasiloxane in Rats. Siloxane Research Program, Reston. EPA DCN 86960000398. 1996. An Inhalation Range-Finding Reproductive Toxicity Study of Octamethylcyclotetrasiloxane (D4) in Rats. Siloxane Research Program, Reston. EPA DCN 86960000517. 1996. A Pilot Study for the Determination of 14C-Octamethylcyclotetrasiloxane (D4) Pharmacokinetics in Fischer 344 Rats Following a single Nose-Only Vapor Inhalation Exposure to 700 PPM 14C-D4. Siloxane Research Program, Reston. EPA DCN 86960000593. 1996. In Vitro Percutaneous Absorption of 14C-D5 in Rat Skin. Siloxane Research Program, Reston. EPA DCN 86970000006. 1996. An Inhalation Range-Finding Reproductive Toxicity Study of Decamethylcyclopentasiloxane (D5) in the Rat. Siloxane Research Program, Reston. EPA DCN 86970000009. 1996. In Vivo Percutaneous Absorption of 14C-Decamethylcyclopentasiloxane (D5) in Rat Skin. Siloxane Research Program, Reston. EPA DCN 86970000023. 1996. An Inhalation Range-Finding Reproductive Toxicity Study of Octamethylcyclotetrasiloxane (D4) in Rats. Siloxane Research Program, Reston. EPA DCN 86970000024. 1996. Pharmacokinetics of 14C-Octamethylcyclotetrasiloxane (D4) in the Rat Following Single Nose-Only Vapor Inhalation Exposure. Siloxane Research Program, Reston. EPA DCN 86970000385. 1996. A 28-Day Inhalation Toxicity and Splenic Antibody Formation Study of Decamethylcyclopentasiloxane (D5) in Rats. Siloxane Research Program, Reston. EPA DCN 86970000723. 1996. Effects of Octamethylcyclotetrasiloxane on liver size and enzyme induction: A Pilot Feasibility Study. Siloxane Research Program, Reston. EPA DCN 86970000724. 1997. An Acute Whole Body Inhalation Study of Hexamethyldisiloxane in Albino Rats. Siloxane Research Program, Reston. EPA DCN 86970000725. 1997. Effects of D4 on Liver Enlargement and Enzyme Induction: A Pilot Feasibility Study II. Siloxane Research Program, Reston. EPA DCN 86970000847. 1997. Female Rat Inhalation Reproductive Study of Octamethylcyclotetrasiloxane (D4). Siloxane Research Program, Reston. EPA DCN 86970000875. 1997. Pharmacokinetics of 14C-Octamethylcyclotetrasiloxane in the Rat Following 14 Repeat Daily Nose-Only Vapor Inhalation Exposures to Unlabelled D4 and a Single (Day 15) Exposure to 14C-D4 at Two Dose Levels. Siloxane Research Program, Reston. EPA DCN 86980000017. 1997. Clinical Studies on the Respiratory Effects of Octamethylcyclotetrasiloxane (D4): Mouthpiece and Nasal Exposures. Siloxane Research Program, Reston. EPA DCN 86980000020. 1997. Effects of Decamethylcyclopentasiloxane on Hepatic Cytochrom P450 in the Female Fischer 344 Rat. Siloxane Research Program, Reston. EPA DCN 86980000032. 1997. Non-Regulated Study: Identification of Major Metabolites of Octamethylcyclotetrasiloxane (D4) in Rat Urine. Siloxane Research Program, Reston. EPA DCN 86980000037. 1997. A Pilot Study to Determine if Classical Inducing Agents Alter the Metabolic Profile of a Single Dose of 14C-Octamethylcyclotetrasiloxane (D4) in Rats. Siloxane Research Program, Reston. EPA DCN 86980000040. 1997. A Subchronic Toxicological Evaluation and Splenic Antibody Forming Cell Response in Sheep Erthrocytes Following a 28-day whole body inhalation Exposure with D4 in the Rat. Siloxane Research Program, Reston. EPA DCN 86980000041. 1997. One-Month Repeated Dose Inhalation Toxicity Study with Hexamethyldisiloxane in Rats. Siloxane Research Program, Reston. EPA DCN 86980000048. 1997. Three-Month Repeated Dose Inhalation Toxicity Study with Hexamethyldisiloxane in Rats. Siloxane Research Program, Reston. EPA DCN 86980000049. 1997. An Inhalation Range-Finding Reproductive Toxicity Study of Octamethylcyclotetrasiloxane (D4) in Male Rats. Siloxane Research Program, Reston. EPA DCN 86980000061. 1997. An Inhalation Range-Finding Reproductive Toxicity Study of Octamethylcyclotetrasiloxane (D4) in Male Rats. Siloxane Research Program, Reston. EPA DCN 86980000072. 1997. Immunological Evaluation of D4 Using 28-Day Exposure in Male and Female Rats. Siloxane Research Program, Reston. EPA DCN 86980000153. 1998. An Inhalation Reproductive Toxicity Study of Octamethylcyclotetrasiloxane (D4) in Female Rats Using Multiple Exposure Regimems. Siloxane Research Program, Reston. EPA DCN 86980000163. 1998. Absorption of C14-Octamethylcyclotetrasiloxane Using the Flow-Through Diffusion Cell System for In-vitro Dermal Absorption in Human Skin. Siloxane Research Program, Reston. EPA DCN 86980000182. 1998. Three-Month Repeated Dose Whole Body Inhalation Toxicity Study with Hexamethyldisiloxane in Rats. Siloxane Research Program, Reston. EPA DCN 86980000184. 1998. An Oral Gavage Study to Compare the Absorption Potential of 14C-Octamethylcyclotetrasiloxane (D4) in Fischer 344 Rats When Delivered in Various Carriers. Siloxane Research Program, Reston. EPA DCN 86990000015. 1998. Clinical Studies on the Immune Effects of Gastrointestinal Exposure to Octamethylcyclotetrasiloxane (D4). Siloxane Research Program, Reston. EPA DCN 86990000017. 1998. Evaluation of Octamethylcyclotetrasiloxane (D4) as an Inhibitor of Human Cytochrome P450 Enzymes. Siloxane Research Program, Reston. EPA DCN 86990000029. 1999. Effects of Repeated Whole Body Inhalation Exposure to Octamethylcyclotetrasiloxane (D4) Vapors on Hepatic Microsomal CYP2B1/2 Induction in Female Fischer 344 Rats: A Dose-Response Study. Siloxane Research Program, Reston. EPA DCN 86990000032. 1999. A Two-Generation Inhalation reproductive Toxicity and Developmental Neurotoxicity Study of Decamethylcyclopentasiloxane (D5) in Rats. Siloxane Research Program, Reston. EPA DCN 86990000058. 1999. An Inhalation Reproductive Toxicity Study of Octamethylcyclotetrasiloxane (D4) in Female Rats Using Multiple and Single-Day Exposure Regimens. Siloxane Research Program, Reston. EPA DCN 86990000059. 1999. Interim Risk Assessment of D4 Reproductive Effects. Siloxane Research Program, Reston. EPA, August 2003. Siloxane D5 in Drycleaning Applications. Fact Sheet. EPA/744-F-03-004. European Commission, Joint Research Centre (2000): International Uniform Chemical Information Database. IUCLID CD-ROM, Existing Chemicals – Year 2000 edition. http://ecb.jrc.it/IUCLID-Data-Sheet/556672.pdf European Commission, Joint Research Centre (2000): International Uniform Chemical Information Database. IUCLID CD-ROM, Existing Chemicals – Year 2000 edition. http://ecb.jrc.it/IUCLID-Data-Sheet/107460.pdf Fendinger, N.J., R.G. Lehmann & Mihaich. E.M. 1997. Polydimethylsiloxane. In: Chandra, H.G (Ed.). The handbook of environmental chemistry. Vol 3. part: Organosilicon Materials. Springer-Verlag, Berlin. Hobson, J.F., Atkinson, R and Carter, W.P.L. 1997. Volatile methylsiloxanes. In: Chandra, H.G (Ed.). The handbook of environmental chemistry. Vol 3. part: Organosilicon Materials. Springer-Verlag, Berlin. Huppmann, R., Lohoff, H.W. & Schröder, H. Fr. 1996. Cyclic siloxanes in the biological wastewater treatment process - determination, quantification and possibilities of elimination. Fresenius' J. Anal. Chem. 354: 66-71. IARC, 1999. Surgical implants and other foreign bodies. Internationl Agency for Research on Cancer (IARC) – Summaries & Evaluation. Vol: 74 INCI. 2000. Inventory of ingredients used in cosmetic products, published in Section I of the Annex to Commission Decision 96/335/EC. 1st Update EU Inventory 09/06/2000. The International Nomenclature of Cosmetic Ingredients. http://europa.eu.int/comm/food/fs/sc/sccp/out123cm_en.pdf IRG. 1998. Silicone gel breast implants. The report of the independent review group. http://www.silicone-review.gov. Kala S.V., Lykissa, E.D., Neely M.W. & Lieberman M.W. 1998. Low Molecular Weight Silicones are Widely Distributed after a Single Subcutaneous Injection in Mice. Short Communication. American J of Pathology 152:645-649. http://womnhlth.home.mindspring.com/studies/american_j_of_pathology.htm Kirk Othmer. 1997. Kirk Othmer Encyclopedia of Industrial Chemistry. 4th edition. Wiley Interscience. Krogh, H. 1999. Problematiske stoffer i byggevarer. SBI-Meddelelse 122. Statens Byggeforskningsinstitut, Hørsholm. Looney,R.J., Frampton, M.W., Byam, J., Kenaga,C., Speers, D.M., Cox, C., Mast, R.W., Klykken, P.C., Morrow, P.E., & Utell, M.J. 1998. Acute respiratory exposure of human volunteers to octamethylcyclotetrasiloxane (D4): absence of immunological effects. Toxicological Sciences 44: 214-220. Mathisen, A., D. 2003: Telephone communication with Anne Dorthe Mathisen, SPT, the 2. of September 2003. McKim, J.M., Jr., Wilga, P.C., Kolesar, G.B., Choudhuri, S., Madan, A., Dochterman, L.W., Breen,J.G., Parkinson, A., Mast, R.W. & Meeks, R.G. 1998. Evaluation of Octamethylcyclotetrasiloxane (D4) as an Inducer of Rat Hepatic Microsomal Cytochrome P450, UDP-glucuronyl transferase, and Epoxide Hydrolase: A 28-Day Inhalation Study. Toxicological Sciences 41: 29-41. Meeks R. G. 1999. The Dow Corning Siloxane Research Program: An Overview and Update. Medical Device &Diagnostic Industry Magazine, May 1999 Column, http://www.devicelink.com/mddi/archive/99/05/007.html Pedersen H. 2004. Kortlægningen af forekomsten af nogle potentielle eller mistænkte PBT/vPvB-stoffer i forbrugerprodukter. Praktikrapport, Roskilde Universitetscenter. Poulsen, P., A. A. Jensen & L. Hoffmann. 2002. Kortlægning af kemiske stoffer i hårstylingsprodukter. Kortlægning no. 18/2002. Danish EPA, Copenhagen. http://www.mst.dk/kemi/02052600.htm Sarangapani, R. , Teeeguarden, J., Andersen, M.E., Reitz, R.H.& Plotzke, K.P. 2003. Route-specific differences in distribution characteristics of octamethylcyclotetrasiloxane in rats: analysis using PBPK models. Toxicological Sciences 71: 41-52. Statistics Denmark. 1998-2001. 'Varestatistik for Industrien' and 'Udenrigshandelen Fordelt på Varer og Lande' for 1998, 1999, 2000, 2001. Statistics Denmark, Copenhagen. Thomas, D.E., (Centre Europeen des Silicones) 1999. Re : Swedish comments to the proposed classification and labelling of octamethylcyclotetrasiloxane (D4) (Y009; CAS no: 556-67-2). http://ecb.jrc.it/classlab/3798a8.doc U.S. EPA. 2003. PBT Profiler. Office of Pollution Prevention and Toxics, U.S. Environmental Protection Agency. May 2003. http://www.pbtprofiler.net/default.asp Ullmann. 2003. Ullmann's Encyclopedia of Industrial Chemistry. Release 2003, 7th Edition. Wiley Interscience. Utell, M.J., Gelein, R., Yu, C.P., Kenaga, C., Torres, A. Chalupa, D., Gibb, F.R., Speer, D.M., Mast, R.W. & Morrow, P.E. 1998. Quantitative exposure of humans to octamethylcyclotetrasiloxane (D4) vapor. Toxicological Sciences 44: 206-213. Varaprath, S., McMahon, J.M. & Plotzke, K.P. 2003. Metabolites of hexamethyldisiloxane and decamethylpentasiloxane in Fisher 344 rate urine - a comparison of a linear and a cyclic siloxane. Drug metabolism and disposition: The biological effect of chemicals 31:206-14. Vergnes J.S., Jung, R., Thakur, A.K., Barfknecht, T.R. & Reynolds, V.L. 2000. Genetic Toxicity Evaluation of Octamethylcyclotetrasiloxane. Environmental and Molecular Mutagenesis 36:13-21. Will, R; Schlag, S. & Yoneyama, M. 2003. Silicones. CEH Marketing Research Report, SRI International. Annex 1
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Decamethyl cyclopentasiloxane | |
CAS number: 541-02-6 Data compilation, environmental and health screening |
|
Summary |
|
| Health and Environment: No relevant information on health was found. The substance decamethyl cyclopentasiloxane is only slightly soluble in water and has a high affinity for organic phases (log Pow 5.2). Based on the physical/chemical properties Decamethyl cyclopentasiloxane is estimated to bioaccumulate, and biodegradation is not expected. Data on environmental toxicity were not found. |
|
Identification of the substance |
|
| CAS No. | 541-02-6 (CambridgeSoft Corp 2003) |
| EINECS | No. 208-764-9 (Spin2000.net 2003) |
| EINECS Name | Decamethylcyclopentasiloxane (HSDB 2003) |
| Synonyms | Dimethylsiloxane pentamer (HSDB 2003), Cyclopentasiloxane, decamethyl- (Spin2000.net 2003) |
| Molecular Formula | C10-H30-O5-Si5 (HSDB 2003) |
| Structural Formula | |
| Known Uses | Paint, laquers and varnishing. Fuel additives. Surface treatment (Spin2000.net 2003) |
| IUCLID | No data found |
| EU | |
Physico-chemical Characteristics |
|
| Physical Form | Oily Liquid (HSDB 2003) |
| Molecular Weight (g/mole) | 378.80 (HSDB 2003) |
| Melting Point/range (°C) | - 38°C (HSDB 2003) |
| Boiling Point/range (°C) | 210°C (HSDB 2003) |
| Decomposition Temperature (°C) | No relevant data found |
| Vapour Pressure (mm Hg at °C) | 0.2 mm Hg at 25°C (HSDB 2003) |
| Density | 0.9593 at 20°C (HSDB 2003) |
| Vapour Density (air=1) | No relevant data found |
| Solubility (water) | 0.017 mg/l at 25°C (PhysProp 2003) |
| Partition Coefficient (log Pow): | 5.20 (HSDB 2003) |
| pKa | No relevant data found |
| Flammability | Flashpoint 77°C (CambridgeSoft 2003) |
| Explosivity | No relevant data found |
| Oxidising Properties | No relevant data found |
Toxicological Data |
|
| Observation in Humans | None Found |
Acute Toxicity |
|
| Oral | No relevant data found |
| Dermal | No relevant data found |
| Inhalation | No relevant data found |
| Other Routes | No relevant data found |
| Skin Irritation | No relevant data found |
| Eye Irritation | No relevant data found |
| Irritation of Respiratory Tract | No relevant data found |
| Skin Sensitization | No relevant data found |
| Sensitization by Inhalation | No relevant data found |
Subchronic and Chronic Toxicity |
|
| Observation in humans | No relevant data found |
| Oral | No relevant data found |
| Inhalation | No relevant data found |
| Dermal | No relevant data found |
Genotoxicity and Carcinogenicity |
|
| Mutagenicity | No relevant data found |
| Gene Mutation | No relevant data found |
| Chromosome Abnormalities | No relevant data found |
| Other Genotoxic Effects | No relevant data found |
| Cancer Review | No relevant data found |
Reproductive Toxicity, Embryotoxicity and Teratogenicity |
|
| Reproductive Toxicity | No relevant data found |
| Teratogenicity | No relevant data found |
| Other Toxicity Studies | No relevant data found |
| Toxicokinetics | No relevant data found |
Ecotoxicity Data |
|
| Algae | No relevant data found |
| Crustacean | No relevant data found |
| Fish | No relevant data found |
| Bacteria | No relevant data found |
Environmental Fate |
|
| BCF | Estimated 5300 (HSDB 2003) |
| Aerobic biodegradation | Expected to be persistent (HSDB 2003) |
| Anaerobic biodegradation | Expected to be persistent (HSDB 2003) |
| Metabolic pathway | No relevant data found |
| Mobility | Estimated immobile (HSDB 2003) |
Conclusion |
|
| Health and Environment | No relevant information on health was found and no conclusion can be drawn. Based on the physical/chemical properties Decamethyl cyclopentasiloxane is estimated to bioaccumulate and biodegradation is not expected. Data on environmental toxicity were not found. The substance has to be further investigated e.g. on substance group level before a conclusion can be made. |
List of references:
CambridgeSoft 2003. CambridgeSoft Corporation database (available at www.chemfinder.com)
HSDB 2003. HSDB database (available through toxnet.nlm.nih.gov)
PhysProp 2003. PHYSPROP DEMO-database (available at esc.syrres.com/interkow/physdemo)
Spin2000.net 2003. SPIN database (available at www.spin2000.net)
References used for screening:
- 1. Aquire (available at www.epa.gov/ecotox)
- 2. CambridgeSoft Corporation database (available at www.chemfinder.com)
- 3. HSDB database (available through toxnet.nlm.nih.gov)
- 4. IUCLID-CD (1996)
- 5. PHYSPROP DEMO-database (available at esc.syrres.com/interkow/physdemo)
- 6. SPIN database (available at www.spin2000.net)
- 7. DART Special (RTECS) (available through toxnet.nlm.nih.gov)
- 8. ToxLine (available through toxnet.nlm.nih.gov)
Annex 8
Human toxicity test results for siloxanes
Abbreviations are described in the notes to the table. References are listed in the main report.
Version 1.0 September 2005, © Danish Environmental Protection Agency