|
Mapping and development of alternatives to chlorinated lubricants in the metal industry (KLORPARAFRI) 7 Health and environmental assessment of components in non-chlorinated lubricants
7.1 IntroductionChapter 7 describes the methods and results of the health and environmental assessments of components typically found in non-chlorinated lubricants for heavy-duty metal working. The assessments of the various substance groups are performed on the basis of available data. The strategy for the data search is described in chapter 4. The primary function of chlorinated paraffins in metal working lubricants is as extreme pressure additives. As described in chapter 2 it is not possible to substitute the chlorinated paraffins with a single component. A total reformulation of the lubricant normally takes place. It is estimated in this project that the most suitable frame of reference for comparison of the health and environmental properties of chlorinated and non-chlorinated lubricants, respectively, is a comparison of total lubricants. Thus, the health and environmental assessment of substance groups in non-chlorinated lubricants includes both compounds functioning mainly as lubricant bases, compounds functioning mainly as polar additives and compounds functioning mainly as extreme pressure additives (see also chapter 2). There is virtually no literature on the chemical composition of metal forming lubricants. The selection of substance groups for the health and environmental assessment is based on information retrieved from lubricant suppliers and raw material suppliers contacted in this project in addition to information retrieved through an internet search for home pages of raw material suppliers and by personal contact to industry experts. The chosen substance groups generally occur in non-chlorinated lubricants proposed in this project and are considered as representative of non–chlorinated lubricants. A health and environmental assessment of medium-chained chlorinated paraffins is included as a point of reference. Table 7.1 provides an overview of the substance groups occurring in non-chlorinated lubricants and for which a health and environmental assessment has been performed and described.
Table 7.1 Overview of substance groups in non-chlorinated lubricants for which a health and environmental assessment has been performed. Medium-chained chloroparaffins are included as a point of reference. Some of the substance groups assessed commonly occurring in non-chlorinated lubricants for heavy-duty metal working comprise a huge number of substances. The health and environmental assessments of the substance groups are performed at a screening level. The result of the health and environmental assessments of selected components should only be regarded as an indication of the health and environmental properties. As described in chapter 2, there is only to a certain degree a general concept of formulation of non-chlorinated lubricants. Lubricant formulation is also based on the specific knowledge of the individual supplier. Besides the substance groups, which are chosen for health and environmental assessment due to their common occurrence in lubricant alternatives, several of the non-chlorinated lubricants proposed as alternatives in this project contain specific substances. These substances only appear in the individual lubricant and cannot be discussed further due to confidentiality. However, they are included in the health and environmental screening and rating of the proposed alternative lubricants described and discussed in chapter 8. 7.2 Health and environmental assessments of substance groups in non-chlorinated lubricantsThe health and environmental assessment of substance groups in alternative lubricants focuses entirely on inherent properties. The use of lubricants for metal forming is not considered. The parameters that are included in the health and environmental assessment are described in chapter 3. Based on the assessment of the individual substance groups, each substance group is assigned a health score and an environmental score. The scores are assessed on the basis of a rating system developed by CETOX (Centre for Integrated Environment and Toxicology). The system was developed as a tool to facilitate the differentiation between chemical substances and between products with respect to health and environment. The rating system is described in further detail below in sections 7.3.1 and 7.3.2. 7.2.1 Assignment of health scoresTable 7.2 shows the rating system for the health assessment of chemical substances and products. The system was developed based on the EU classification system for chemical substances which focuses on the inherent properties of substances. The rating system for the health assessment is based on five health hazard groups. Group 1 comprises substances with no or few less severe health hazardous effects. The higher the group, the more serious the health effects are considered to be. Group 5 comprises substances that may cause the most severe health effects. Groups 4 and 5 include only substances classified according to the EU classification system while groups 1 through 3 include both substances classified according to the EU classification system and substances for which the documentation available is too weak to make a proper classification. The latter is true for group 3 – sensitizing which includes substances for which data is available that indicate sensitizing effects but not sufficient documentation for an actual EU classification. This is also the case for group 2 – sensitization which includes substances for which data is available on a few, isolated cases of allergy. Finally, substances exhibiting weak to no irritation to skin and eyes are included in group 1 – irritation. An additional group, ND, is included in the scoring system. This group comprises substances for which no data is available or for which the data available is insufficient to place the substance in a health hazard group. If a substance has more than one hazardous property, the most serious one determines the assignment of the score for that particular substance. The designations “health hazard group” (group) and “health score” (score) corresponds to each other.
7.2.2 Assignment of environmental scoresTable 7.3 shows the rating system for the environmental assessment of chemical substances and products. The system was developed based on the EU classification system for chemical substances, which focuses on the inherent properties of substances. The rating system for the environmental assessment is based on five health hazard groups. The following environmental data will typically be available: *ready biodegradability under aerobic conditions, *potential for bioaccumulation (determination of n-octanol/water partition coefficient, log KOW, or the bioconcentration factor, BCF), *toxicity towards algae, *toxicity towards crustaceans (Daphnia) and *toxicity towards fish. The properties marked with an asterisk (*) form part of the basis of the environmental hazard assessment of chemical substances according to EU Directive 67/548/EEC. The environmental assessment is based on the principles used in relation to the EU environmental hazard classification and the global classification system (OECD 2001). Using the OECD harmonized system for classification of chemical substances on the basis of their hazard towards the aquatic environment, the individual substances are given an environmental rating from 1 to 5 (groups 1 to 5), of which 1 is best. The rating is based on the biodegradability and potential for bioaccumulation of the chemical substances and their toxic effects on aquatic organisms. The environmental assessment is made for single substances, if at all possible, or for groups of substances when similarities in their chemical structure justify an analogous assessment.
Table 7.3 Environmental rating system developed by CETOX (Centre for Integrated Environment and Toxicology). 7.3 Mineral base oils7.3.1 FunctionMineral base oils enter non-chlorinated as wells as chlorinated lubricants as lubricant bases (13,14). 7.3.2 IdentificationThe base oils are high boiling fractions of crude oil extracted by distillation. Initially, the crude oil passes through a distillation at atmospheric pressure followed by a further distillation of the distillation rest in vacuum. A range of vacuum distillates are produced by this. The distillates are further treated by solvent extraction and /or hydro-fining in order to increase viscosity index, enhance the colour and to convert unwanted structures such as unsaturated hydrocarbons and aromatics to less chemically reactive species. Finally, solvent de-waxing is used to reduce the wax content of the base oils so as to prevent wax crystals forming within the normal working temperature range of the lubricant. High viscosity grades of lubricating oil base stocks are produced by solvent de-asphalting of the vacuum residue and subsequent solvent extraction and/or hydrogenation (15). All crude oils contain polycyclic aromatic hydrocarbons (PAH). Some of these are known carcinogens. The content of PAH in the mineral base oil is primarily dependent on the severity of the refining process, which the oil has passed through. Severe solvent extraction and/or hydro-treatment substantially reduces the total content of aromatics in the mineral oil including PAH (15). Mineral lubricating oils are described as paraffinic or naphthenic depending on the dominating kind of hydrocarbons. Besides hydrocarbons, the mineral lubricating oils also contain varying amounts of sulphur, nitrogen and traces of metals. In the end, the composition of the base oils depends on the origin of the crude oil and the refining process. The final base oil can be a blend of lubricant base stocks and additives can be added (15). Lubricating base oils found in non-chlorinated as wells as in chlorinated lubricants for metal forming are severely refined mineral oils. They include a large number of different CAS numbers. Table 7.4 states the CAS numbers which are included in the health and environmental assessment of mineral base oils.
Table 7.4 Chemical names and CAS Nos. of substances included in the health and environmental assessment of mineral base oils. 7.3.3 Physical/chemical dataMineral lubricating oils are complex mixtures of hydrocarbons. They are composed of varying amounts of paraffins, naphthenes and aromates. The number of carbon atoms in the single molecules varies from 15 to 30. The boiling point of the oils ranges from 300 to 600°C. Mineral base oils have low vapour pressures at room temperature and very low solubility in water (15,16). 7.3.4 Health assessmentAcute toxicity Highly refined mineral base oils have low acute toxicity by ingestion and skin contact. As an example, LD50 (oral, rat) > 5000 mg/kg bw for distillates (petroleum), hydro-treated heavy naphthenic (CAS No. 64742-52-5), and LD50 (skin, rabbit) > 2000 mg/kg bw for CAS No. 64742-52-5 (16). There are only few data regarding exposure by inhalation. These data indicate low acute toxicity also by this route. LC50 (rat, 4 hours) > 4 mg/l for residual oils (petroleum), solvent deasphalted (CAS No. 64741-95-3) (16). Irritation Based on animal tests and experience from the working environment, highly refined mineral base oils are in general not more than slightly irritating to skin and eyes (16). Repeated and prolonged exposure to mineral oils may degrease the skin and cause dermatitis and oil acne (17). Sensitization Highly refined mineral base oils exhibit a low potential for skin sensitization by standard test in guinea pigs and by experience from humans. No data is available on sensitization by respiration, but it not considered relevant considering absence of sensitizing potential by skin contact and general experience from the working environment (15,16). Repeated dose toxicity There are no data for the relevant mineral oils on toxicity by repeated oral exposure. Highly refined mineral base oils exhibit low toxicity by repeated exposure to skin. In a standard test, rabbits were exposed five days a week for four weeks of doses not exceeding 1000 mg/kg bw of five different paraffinic oils covered by CAS Nos. 64742-56-9, 101316-70-5, 101316-71-6, 101316-72-7 and 64742-62-7. Minor dermal irritation was observed following prolonged exposure but there were no treatment related effects observed by necropsy or clinical observations and no effects on body weight. NOAEL (No Observed Adverse Effect Level) for all five oils > 1000 mg/kg bw (16). In another study, rabbits were exposed to two highly refined naphthenic base oils three times a week for four weeks at doses of 200, 1000 and 2000 mg/kg bw distillates (petroleum), hydro-treated light naphthenic (CAS No. 64742-53-6) and distillates (petroleum), hydro-treated heavy naphthenic (CAS No. 64742-52-5). There were no deaths, and minimal to moderate irritation in dose groups. Erythema and flaky skin were observed in all dose groups, oedema in medium and high dose groups, and statistically significant lower overall body weight gain were observed in medium and high dose groups. NOAEL > 200 mg/kg bw (16). The dominating effect of repeated skin exposure to highly refined mineral oils in animal test is slight to moderate skin irritation (15). There exists only a few data on effects of highly refined mineral oils by repeated exposure by inhalation. These indicate low toxicity. In a study consistent with OECD Guideline 412, rats were exposes to aerosol concentrations of 50, 210 or 1000 mg/m³ of one of three different highly refined mineral oils (CAS No. 64742-70-7, 64742-54-7, 8042-47-5). The exposure period was 6 hours/day, 5 days/week for four weeks. Body weights and clinical signs were not affected by treatment. A dose dependent increase in wet lung weight and dry/wet lung weight ratio was observed. This was associated with accumulations of foamy alveolar macrophages in the lung tissue (16). In another study with rats consistent with OECD Guideline 412, a two week exposure, 6 hours per day for 10 days of 55, 507 or 1507 mg/m³, resulted in treatment-related clinical signs of central nervous system and dermal effects at >500 mg/m³ (16). Carcinogenicity All mineral base oils in the proposed non-chlorinated lubricants are highly refined mineral oils with a content of PAH measured by DMSO extraction (IP 346) less that 3 % by weight (Concawe, 1994, IP , 1993). Several skin painting studies in mice show that mineral oils with a PAH content measured by DMSO below 3 % are not carcinogenic by skin contact (15,16). There are very few data regarding the carcinogenicity of highly refined mineral oils by oral exposure and inhalation. These data indicate, that the mineral oils are not carcinogenic by these exposure routes (15). Genotoxicity Similar to carcinogenicity of mineral oils, a connection is observed between genotoxic effects of the oils and the content of PAH. Highly refined mineral base oils with a low content of PAH below 3 % (measured by DMSO-extraction) do not exhibit mutagenic effects in various standard test systems (15,16). Reproductive toxicity Very few data exist on effects of mineral base oils on reproduction and the developing foetus. In a study, rats were dermally exposed to one of three highly refined mineral oils from day 0-19 of gestation at levels up to 2000 mg/kg/day. No abnormal development was observed in the offspring (15). Health rating Mineral oils entering into proposed non-chlorinated lubricants are assigned health score 1. 7.3.5 Environmental assessmentAquatic toxicity The few data available show that mineral base oils have a low toxicity towards aquatic organisms. Available LC50 values are above 1000 mg/l. Distillates (petroleum), hydro-treated light naphthenic (CAS No. 64742-53-6) have an LC50(48h) towards Daphnia magna above 1000 mg/l (tested on the water accommodated fraction (WAF)). A WAF can be made by rapid stirring, ultra sound or other ways to ensure maximum concentration of test substance in the water fraction. The test period was 21 days with WAF replacement every 3 days. No effects were observed. The European Oil Industry Association (CONCAWE) refers in its report No. 01/54 similar results from short-term as well as long-term testing (15). Environmental fate All referred tests showed that base oils are not readily biodegradable, e.g., distillates (petroleum), hydro-treated heavy naphthenic (CAS No. 64742-52-5) with 6% degradation in 28 days (OECD 301 B) (16). Similar results were found for other base oils. Bioaccumulation All base oils contain compounds with log POW > 4 indicating that bioaccumulation may be a potential concern (16). Environmental rating Based on the sparse available data on this large group of substances, base oils are assigned environmental score 2. 7.4 Calcium sulphonates (“over-based”)7.4.1 FunctionThe function of sulphonates in metal working lubricants is primarily as extreme pressure additives. In addition, they act as metallic dispersants and corrosion inhibitors. Sulphonates may occur in the lubricant in such high amounts, that they also partly make up the lubricant base (13,14,18). 7.4.2 IdentificationSulphonates are produced by neutralization of a sulphonic acid group with a metallic base – a divalent metal oxide (MO) or a divalent metal hydroxide (MOH). By this process, a salt is formed. R-SO3H + MO or MOH → R-SO3M + H2O R represents an organic radical. The radical can be a straight-chained or branch-chained alkyl group or alkaryl group. There are two types of commercial sulphonates: petroleum sulphonates and synthetic sulphonates. Formerly, petroleum sulphonates were by-products of the sulphonic treatment of oil fractions in the manufacture of white oils. Currently, with the high demand for detergent oils, sulphonates rather than white oils have become the principal product. The structure of the organic portion of petroleum sulphonates are not completely known. Depending on the crude oil source, the structure can have varying aliphatic, naphthenic, and aromatic hydrocarbon groups. Synthetic sulphonates are metal salts of acids produced from the sulphonation of alkylated aromatics by reaction with sulphur trioxide. In many cases, synthetic sulphonates are derivates of benzene with long alkyl substitutes. The structure is illustrated below in Fig 7.1, where R and R' are aliphatic radicals with a combined number of carbon above 20. 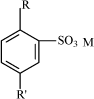
Fig. 7.1 Synthetic sulphonate. R and R' are aliphatic radicals with a combined number of carbon above 20. The molecular weight of the organic radical in commercial sulphonates is 350 or more. The radical is considered necessary for the oil solubility of the sulphonate. The metal ion in the sulphonates, which are used as extreme pressure additive in metal working lubricants, is most often calcium, however it can also be sodium or magnesium. Oil soluble sulphonates containing metal in excess of the stoichiometric amount are called over-based sulphonates. The advantage of over-based sulphonates is a greater ability to neutralize acidic bodies in addition to serving as dispersant for contaminants (18,19,20). Calcium sulphonates in the non-chlorinated lubricants for metal forming are mixtures of petroleum sulphonates and synthetic sulphonates. They are over-based. Calcium sulphonates include a large number of different CAS numbers. Comparable substances can be sulphurized detergents as long chained alkyl phenols and alkylbenzenes, which are included in the environmental assessment. Table 7.5 states the CAS numbers of substances which are included in the health and environmental assessment of the over-based calcium sulphonates.
Table 7.5 Chemical names and CAS Nos. of substances included in the health and environmental assessment of calcium sulphonates, over-based. 7.4.3 Physical/chemical dataOver-based calcium sulphonates are dark coloured viscous liquids with very high boiling points (> 500°C), high flash points (> 180°C), low vapour pressures (< 0.001 hPa at 20°C) and low solubility in water (20,21). 7.4.4 Health assessmentThere are very few data on sulphonates in common toxicological literature. The health assessment of over-based calcium sulphonates is thus based on information retrieved from suppliers and on the internet. Acute toxicity Calcium sulphonates exhibit low acute oral and dermal toxicity in animal studies. LD50 (rat, oral) > 5.000 mg/kg sulphonic acids, petroleum, calcium salts (CAS No. 61789-86-4), sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68783-96-0), C14-C24 alkaryl calcium salt, over-based derivative (CAS No. 115733-09-0) (20). LD50 (rabbit, dermal) > 2000 – 5000 mg/kg (16). Limited data indicate that calcium sulphonates (sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68786-96-0)) also exhibit low acute toxicity by inhalation (16,21). Irritation Over-based calcium sulphonates (sulphonic acids, petroleum, calcium salts, over-based (CAS-Nr. 68783-96-0)) do not exhibit irritating potential towards skin and eye in standard tests with rabbits (16). There are no data on irritation to respiratory organs. Sensitization An over-based calcium sulphonate (sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68783-96-0)) did not exhibit a sensitizing potential in the Guinea Pig Maximization test (16). There are no data available regarding sensitization towards respiratory organs. Repeated dose toxicity Data on effects by repeated exposure indicates low toxicity of calcium sulphonates. In a four weeks study (OECD 407), rats were orally exposed to a C20-24- alkaryl calcium sulphonate (Benzenesulphonic acid, mono-C16-24-alkyl derives, calcium salt (CAS No. 70024-69-0)) at dose levels of 100, 500 and 1000 mg/kg/day. Decreased serum cholesterol was observed at 1000 mg/kg/day. NOAEL was 500 mg/kg bw/day in this study (20). In another four weeks study (OECD 410), rats were dermally exposed to an over-based calcium sulphonate (sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68783-96-0)) at dose levels of 100, 300 and 1000 mg/kg/day 6 hours/day and under occlusion. A low incidence of erythema, desquamation and scabbing was sporadically observed in treated animals. The NOAEL was estimated at 1000 mg/kg/day in this study (20). In a four weeks inhalation study with rats (OECD 412), the animals were exposed to an over-based calcium sulphonate (CAS No. 68783-96-0) in concentrations of 49.5, 156 and 260 mg/m³ respectively. Signs of toxicity including adverse effects on lungs were observed at the two highest dose levels. NOAEL was 49.5 mg/m³ in this study (20). Data from standard tests in different cell systems for CAS No. 68783-96-0 and CAS No. 70024-69-0 indicate that calcium sulphonates do not exhibit genotoxic properties (20,21). Reproduction toxicity and carcinogenicity There are no data for the assessment of the reprotoxic and carcinogenic potential of calcium sulphonates. Health rating On behalf of the available data, over-based calcium sulphonates are assigned health score 1. 7.4.5 Environmental assessmentAquatic toxicity Only few data were available on ecotoxicity and they mainly indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. However, higher toxicity, EC/LC50 below 1000 mg/l, has been referred for the following substances:
Environmental fate Two tests are referred and demonstrate that sulphonated petroleum is not readily biodegradable. A test for ready biodegradability (OECD 301 B) with sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68783-96-0) and benzene, C9-13-alkyl deriv., distu. ,residues, sulphonated, calcium salts (CAS No 97675-24-6) showed 16% degradation in 28 days (16). The two tests refer the same result and it is not possible determine whether one or two tests have actually been made. Bioaccumulation No test data on sulphonated petroleum substances are referred. QSAR calculations of log POW show that sulphonated petroleum products contain compounds with log POW > 4 indicating that these substances are potentially bioaccumulative. Environmental rating Based on the sparse available data on this large group of substances, sulphonated petroleums and related substances are assigned environmental scores 2-4. In general, these substances are scored 2 as they are not readily biodegradable, they contain compounds with log POW > 4 and the EC/LC50 values are above 100 mg/l. Some sulphonated petroleum substances are, however, more toxic and will be scored 3 or 4. 7.5 Alkyl sulphides (polysulphides)7.5.1 FunctionAlkyl sulphides (also named polysulphides) are added to metal forming lubricants as extreme pressure additives (13,22). 7.5.2 IdentificationThe molecular structure of polysulphides are R-[S]x-R, where x 2, and R are organic radicals (23). In alkyl sulphides, the R is linear or branched alkyl groups. Alkyl sulphides entering into non-chlorinated lubricants for heavy-duty metal working are generally of the type di-tertiary alkyl polysulphides. Di-tertiary alkyl pentasulphides are dominating. This implies that the average number of sulphur atoms in the molecules is five, however the number varies between two and five (23,24). Dialkyl polysulphides are manufactured by reacting corresponding thiols with sulphur: 2 R-SH + S → R-Sx-R (22). Figure 7.2 gives an example of the chemical structure of a dialkyl polysulphide commonly entering non-chlorinated lubricants as an EP additive. 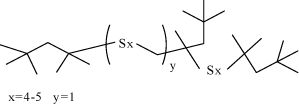
Fig. 7.2 The chemical structure of the polysulphide; sulphurized trimethyl pentane (CAS No. 68515-88-8). Table 7.6 states the CAS numbers of substances which are included in the health and environmental assessment of the substance group alkyl sulphides.
Table 7.6 Chemical names and CAS Nos. of substances included in the health and environmental assessment of alkyl sulphides. 7.5.3 Physical/chemical dataDi-tertiary alkyl polysulphides are clear, yellow liquids at room temperature. They have high boiling points (> 200°C) relatively high flash points (85°C – 150°C) and low vapour pressure (< 0.1 hPa at 20°C). They are virtually insoluble in water (16). 7.5.4 Health assessmentThere are few toxicological data available on alkyl sulphides in literature and data bases. In addition to these data, the health assessment of the alkyl sulphides is also based on information from suppliers of dialkyl polysulphides used as extreme pressure additive in metal forming lubricants. Acute toxicity Dialkyl polysulphides exhibit low acute toxicity by ingestion and skin contact. Acute oral toxicity data for di(tert-dodecyl) pentasulphide (CAS No. 31565-23-8) was: LD50 (mouse, oral) > 20000 mg/kg and LD0 (rat, dermal) > 2000 mg/kg (16). LD50 (rat, oral) > 5000 mg/kg for di-tert-butyl polysulphides (CAS No. 68937-96-2) (16). LD50 (rat, oral) was 19500 mg/kg and LD50 (rabbit, dermal) > 2000 mg/kg for di-tert-nonyl polysulphides (CAS No. 68425-16-1) (16). Toxicity by inhalation is considered less relevant for the alkyl sulphides in focus due to very low vapour pressures. However, data on acute toxicity by inhalation for three di-tertio-alkyl polysulphides indicate low to moderate toxicity by this exposure route. LC50 (rat, 4 hours aerosol exposure) > 15.5 mg/l for di-tert-nonyl polysulphides (CAS-No., 68425-16-1) (25). LC50 (rat, exposure period not stated, aerosol exposure) > 5,6 mg/l for male rats and equal to 2.17 mg/l for female rats for sulphurized 2,4,4-trimethyl-pentene (CAS No. 68515-88-8). No treatment-related gross lesions were observed in surviving rats (26). LC50 (rat, 4 hours vapour exposure) > 0.39 mg/l for sulphurized 2-methyl-1-propene (CAS No. 68511-50-2). No significant clinical signs were observed after initial post-exposure observations at any dose level. No treatment-related macroscopic or microscopic findings were observed (26). Irritation Di-tertiary alkyl polysulphides (CAS Nos. 31565-23-8, 68937-96-2, 68425-15-0, 68425-16-1 and 68515-88-8) cause in general not more that slight irritation to skin and eyes in standard tests (16). However a single compound (CAS no. 68937-96-2) is stated to be irritating to skin in a study with rabbits (27). The study is not specified in further details. There are no data on the irritating potential of ditertiary alkyl polysulphides towards respiratory organs. Sensitization There are varying data regarding the sensitizing potential of dialkyl polysulphides by skin contact. The skin sensitizing potential of di-tert-butyl polysulphides (CAS No. 68937-96-2) was tested in the Guinea pig maximization. The result of the test was ambiguous, as the material (5 % solution in mineral oil) appeared to be a sensitizer at challenge, but not at re-challenge (16). A supplier states that the same CAS No. provoked sensitization in another test with Guinea pigs. There are no further details regarding this study (27). The supplier classifies the polysulphide as sensitizing by skin contact (Xi;R43) in accordance with EU regulations on classification and labelling of dangerous substances and preparations (27). Di-tert-nonyl polysulphides, (CAS No. 68425-16-1) is also stated to be sensitizing by skin contact by a supplier. Test data are not further specified (25). Di-tert-dodecyl polysulphides (CAS No, 31565-23-8) was examined in a standard test with Guinea pigs performed in accordance with the maximization method established by Magnusson and Kligman, the OECD 406 and GLP. After challenge the substance caused an a-specific, weak skin irritation in 4 of 20 animals. It was concluded in the study, that the substance may have a weak skin sensitizing potential (16). Repeated dose toxicity B: In a 28-day study, rats were daily exposed to di-tert-dodecyl penta-sulphides, (CAS No. 31565-23-8) by gavage at dose levels of 0, 50, 250 or 1000 mg/kg bw/day. There was a post observation period of two weeks. No treatment related deaths occurred. Salivation was observed in all animals in the highest dose group only during the treatment period. No treatment-related effects were observed on the mean food consumption, the body weight gain, the haematological and blood biochemical parameters, the urine analysis, the organ weights, and the macro- and microscopic examinations. NOAEL was defined as 1000 mg/kg/day in this study (28). In another 28-day study with rats orally exposed to 1-(tert-dodecylthio) propan-2-ol (CAS No. 67124-09-8) at dose levels of 100, 300 or 1000 mg/kg/day, gross and microscopic observations at study termination showed alterations in kidneys and liver. The effects in liver are considered an adaptive response and the effects on kidney are not considered relevant to humans (26). A: Four sub-chronic dermal toxicity studies (in rats or rabbits) have been conducted with either sulphurized 2-methyl-1-propene (CAS No. 68511-50-2) or sulphurized 2,4,4-trimethyl pentene (CAS No. 68515-88-8). The predominant effect observed was dermal irritation at the site of the test material administration. The lowest reported NOAEL was 50 mg/kg bw/day for a 13-week rat dermal study with sulphurized 2-methyl 1-propene (CAS No. 68511-50-2) at dose levels of 10, 50, 100, 250, 500 or 2000 mg/kg bw/day. In this study, a decrease in red blood cell number, an increase in neutrophils and an increase in spleen size and pigments in spleen were observed at 250 mg/kg bw/day and above, in addition to a decreased body weight gain in males. At 100 mg/kg/day and above, an increased production of white blood cells in spleen and bone marrow was observed. There is no information on whether these observed effects were reversible at cessation of exposure (26). C: A 28-day inhalation study in rats has been conducted with sulphurized 2,4,4-trimethyl pentene (CAS No. 68515-88-8) at dose levels of 15, 50 or 150 mg/m³. At dose levels of 15 mg/m³ and above there was a trend toward lower body weight gain (all males and two highest doses in females); increased kidney weights (males only); globular casts and hyaline droplets in the proximal tubule cells of the kidney (all males and recovery high dose males); increased liver weight (high dose males and females and mid-dose males). At 150 mg/m³ a decrease in hemoglobin concentration was observed. The NOAEL was < 15 mg/m³. Effects on the liver are considered to be adaptive responses and the effects on rat kidneys are not considered relevant to humans (26). Thus the available data on alkyl sulphides indicate moderate toxicity by repeated exposure. Genotoxicity There are data on genotoxic potential for several dialkyl polysulphides. CAS No. 68425-15-0, 68425-16-1, 31565-23-8 and 68937-96-2 did not exhibit a genotoxic potential in a standard bacterial gene mutagenicity test (Ames test), with and without metabolic activation or standard chromosomal aberration test in human lymphocytes (16,25,28). In another study of gene mutations of sulphides and polysulphides in the mouse lymphoma assay, di-tert-nonyl polysulphides (CAS No. 68425-16-1) produced a clear, dose-dependent mutagenic response while its homologue, di-tert-dodecyl polysulphides (CAS No. 68425-15-0) was inactive. Only the non-activated portions of the test was performed. The results of the total study suggest that the mutagenic species may be the hydrosulphide ion. Thus, the relatively stable alkyl mono- and disulphides are inactive, while the more easily hydrolized sodium sulphide and di-tertiary-nonyl polysulphide are mutagenic (29). No further data is available, including tests for chromosome aberrations. The mutagenicity of dialkyl polysulphides has not been fully investigated. Together, there are discrepancy regarding mutagenicity data for di-tert-nonyl polysulphides (CAS No. 68425-16-1), while data for the remaining polysulphides indicate non-genotoxicity. Reproduction toxicity There are test data on effects on prenatal development for a single compound di-tert-dodecyl polysulphides (CAS No. 31565-23-8). In a standard study (OECD 414), rats were exposed by oral route from day 6 to day 15 of pregnancy to dose levels of 0, 50, 250 and 1000 mg/kg/day of the polysulphide. On day 20, dams were sacrificed, litter values determined and foetuses subsequently examined for visceral or skeletal anomalies. No treatment-related effects were observed on the clinical signs, mortality, abortions or total re-sorption, food consumption, body weight gain, macroscopic findings, post-implantation loss, number of live foetuses per animal, foetal body weight, external anomalies or malformations, soft tissue malformations or anomalies, skeletal malformations, anomalies and variations. The maternal and foetal NOAEL was 1000 mg/kg/day in this study (30). Carcinogenicity There are no data on the carcinogenic potential of polysulphides. Health rating Di-tert-dodecyl polysulphides and di-tert-dodecyl pentasulphides (CAS Nos. 68425-15-0 and 31565-23-8) are assigned health score 1 based on the available data. Sulphurized 2,4,4-tri-methyl pentene (CAS No. 68515-88-8) is assigned health score 2 due to acute toxicity by inhalation (LC50 for female rats was 2.17 mg/l). Di-tert-nonyl polysulphides (CAS No. 68425-16-1) is assigned health score 4 due to a skin sensitizing potential. Di-tert-butyl polysulphides (CAS No. 68937-96-2) is assigned health score 4 due to a skin irritating and sensitizing potential. Sulphurized 2-methyl 1-propene (CAS No. 68511-50-2) is assigned health score 1 –2 due to data which may indicate a health hazard by repeated exposure to skin. In conclusion, the health score of the polysulphides in focus varies from 1 to 4 based on sparse data. 7.5.5 Environmental assessmentPolysulphides are a large group of chemicals and only few environmental data are available. Aquatic toxicity Of the few available data, most indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. However, EC/LC50 values below 100 mg/L are not unusual either. Toxicity tests with EC/LC50 values below 100 mg/l are referred for di-tert-butyl polysulphides (CAS No. 68937-96-2) with an EC50(96h) towards Selenastrum capricornutum (now Pseudokirchneriella subcapitata) of 29-39 mg/l and di(tert-dodecyl) pentasulphide (CAS No. 31565-23-8) with an LC50(24h) towards Daphnia magna of 0.449 mg/l (16). Environmental fate Only one test concerning biodegradation is available for di(tert-dodecyl) pentasulphide (CAS No. 31565-23-8) demonstrating 0% biodegradation after 28 days (Closed Bottle test) (16). Bioaccumulation No experimental data are available. QSAR calculations with the online estimation program KowWin (100) state log POW values above 4 for pentasulphides, e.g.:
Based on calculated log POW values, it is assessed that pentasulphides bioaccumulate in aquatic organisms. Environmental rating Based on the sparse available data on this large group of substances and on additional secondary data from material safety data sheets, in which some products are classified N;R50/53 or N;R51/53, polysulphides are assigned environmental scores 3, 4 or 5. 7.6 Vegetable and animal oils7.6.1 FunctionNatural oils of animal and vegetable origin enter lubricants for metal working as lubricity improving additives (polar additives) and as lubricant bases (14). 7.6.2 IdentificationRape oil and lard oil are the most frequent used oils in metal working lubricants. Also used are, however, soybean oil, palm oil, palm kernel oil and castor oil. Pure fatty acids extracted from the natural oils may also enter metal working lubricants. In addition, different types of modified natural oils including hydrogenated, polymerized and epoxidized oils are used. 7.6.3 Physical/chemical dataThe oils are liquids or solids at room temperature (16). In general, they have high boiling points (> 200°C), high flash points (> 200°C), low vapour pressures and very low solubility in water (16,31,32). Table 7.7 states the CAS numbers of substances which are included in the health and environmental assessment of the substance group vegetable and animal oils.
Table 7.7 Chemical names and CAS Nos. of substances included in the health and environmental assessment of vegetable and animal oils. 7.6.4 Health assessmentRape oil and lard oil are assessed here in a health context as representatives of the vegetable and animal oils group. 7.6.4.1 Rape oilRape oil (CAS No. 8002-13-9) is obtained from several species of the cruciferous genus Brassica. The oil is separated either by solvent extraction or by cold or hot pressing. Cold-pressed rape oil is used for edible purposes whereas refined oil is used as a lubricant. (33) Rape oil consists primarily of triglyerides of the fatty acids linoleic, oleic and erucic acid (34). Physical/chemical data Rape oil is a yellow, oily liquid with a high boiling point (> 350°C), a high flash point (> 300°C), a low vapour pressure (< 1 mbar (20°C) and it is insoluble in water (35). Health assessment It is well-known that high-erucic acid rape oil causes necrosis in the heart muscle by repeated ingestion (36) and today high-erucic acid rapeseed oil is produced only in small quantities for industrial non-food use. Rape oil for human consumption usually contains less that 2 % erucic acid (CAS No. 112-86-7) in the EU countries (37). Due to this effect of erucic acid, cultivation of rape variants containing a low content of erucic acid now prevails. Rape oil for human consumption may not contain more than 2 % erucic acid. Industrial oils may have higher contents (37). Acute toxicity There are no animal test data for rape oil in standard toxicological literature and databases. Fatty acids of rape oil exhibits very low acute toxicity by ingestion and skin contact. For oleic acid (CAS No. 112-80-1), LD50 (rat, oral) is 64000 – 74000 mg/kg bw and LD50 (Guinea pig, dermal) > 3000 mg/kg (16). Rape-oil fatty acids (CAS No. 85711-54-2) typically contains 9 – 25 % of oleic acid. Toxicity by inhalation is considered of minor relevance due to the very low vapour pressure. Irritation There are several studies with rabbits and humans of the skin irritating potential of rape oil fatty acids (CAS No. 85711-54-2). In general, oleic acid (CAS No. 112-80-1), linoleic acid (CAS No. 60-33-3) and erucic acid (CAS No. 112-86-7) caused no or slight to moderate irritation (16). However, there is no indication that the fatty acids occurring as triglycerides in rape oil causes irritation (35). Rape oil is used as a skin conditioning agent in cosmetic (37). Fatty acids of rape oil (oleic acid CAS No. 112-80-1, linoleic acid CAS No. 60-33-3 and erucic acid CAS No. 112-86-7) caused slight irritation in rabbit eyes (16). Sensitization Oleic acid (CAS No. 112-80-1) did not provoke sensitization in a standard sensitization test with Guinea pigs. Rape-oil fatty acids (CAS No. 85711-54-2) typically contain 9 – 25 % of oleic acid (16). Repeated dose toxicity As mentioned above, it is well-documented in animal studies that rape oil with a high content of erucic acid causes necrosis in the heart muscle (36). The NOAEL value for this effect has been estimated at 95 mg/kg bw in a two-year feeding study with rats given rape oil fatty acids (CAS No. 85711-54-2) (16). In a 24-week study, rats were exposed to oleic acid (CAS No. 112-80-1) in the diet at a dose level of approximately 7500 mg/kg bw/day. The content of erucic acid was not stated. Normal growth and general good health was reported. The NOAEL was estimated at > 7500 mg/kg bw/day. Rape-oil fatty acids (CAS No. 85711-54-2) typically contain 9 – 25 % of oleic acid (16). Genotoxicity The mutagenicity of oleic acid (CAS No. 112-80-1) has been tested in several laboratory mutagenicity tests, some with and without metabolic activation, with negative result. Thus, there is no indication of genotoxic effects. Rape oil fatty acids (CAS No. 85711-54-2) typically contain 9 – 25 % oleic acid (16). Carcinogenicity The carcinogenic potential of oleic acid by oral and dermal exposure has been studied in several studies in rats and mice exposed to rape oil fatty acids. The results of these studies indicate that rape oil fatty acids including oleic acid (CAS No. 112-80-1) do not posses a carcinogenic potential (16). Reproduction toxicity In a 16-week study, rats were orally fed to 7500 mg/kg bw/day of oleic acid (CAS No. 112-80-1) in the diet. Rape-oil fatty acids typically contain 9-25 % oleic acid. There are no further details on test conditions. No adverse effects on fertility were observed in male rats, however the exposure appeared to impair the reproductive capacity in female rats by interfering with parturition and mammary gland development. Mortality in the offspring was increased (16). There was no evidence of maternal or foetal toxicity and no teratogenicity was observed in an older study with rats dermally exposed to 2000 mg/kg of a hair dye formulation containing 15 % oleic acid (CAS No. 112-80-1) every third day during pregnancy until day 19 of gestation. Rape oil fatty acids (CAS No. 85711-54-2) typically contains 9 - 25 % oleic acid (16). In an older study, rats were fed 10 or 30 % erucic acid (CAS No. 112-86-7) in the diet between 9-45 weeks for male and 9-28 weeks for female rats in a pre-mating exposure period. Rape oil fatty acids (CAS No. 85711-54-2) may contain 30 - 60 % erucic acid. Male rats fed 10 % became completely sterile after 5 months due to degeneration of testes tissue and failure of spermatogenesis. The female fertility was also affected. There was impairment of parturition, a high mortality rate in offspring and surviving pups were underweight due to deficient mammary gland development and lactation. Recovery of fertility of rats returned to the stock diet proceeded slowly and may be limited. The rats were otherwise healthy and vigorous and there were no lesions in organs other than those of reproduction. Rats fed 30 % erucic acid in the diet for 5 months suffered only a temporary retardation in growth rate and thereafter remained healthy and continued to grow at a normal rate (16). Health rating – rape oil Rape oil and the fatty acids oleic and linoleic acid occur naturally in human diets in substantial amounts. Low-erucic acid rape oil is based on animal test data for rape oil acids and general human experience assigned health score 1. High-erucic acid rape oil is assigned health score 5 due to the effects on the heart muscle and fertility by repeated exposure. 7.6.4.2 Lard oilLard oil (CAS No. 8016-28-2) is of animal origin. It is extracted by fractional crystallization and cold pressing of lard. The main constituent in lard oil is the triglyceride olein (CAS No. 122-32-7). Minor constituents are glycerides of solid fatty acids, e.g. stearin (29),(40). There are very few data on lard oil in toxicological standard literature and databases. Therefore, the health assessment of lard oil is thus based on supplier data, long term experience and data for the main ingredient olein (CAS No. 122-32-7) and tallow (CAS No. 61789-97-7) resembling the composition of lard oil to a high degree (40). Acute toxicity The acute toxicity of lard oil by ingestion is low. LD50 (rat) > 2000 mg bw/kg (41). The corresponding LD50 for tallow is > 18.000 mg/kg (16). LD50 (oral, rat) for the fatty acid of olein, oleic acid (CAS No. 112-80-1) is 64000 – 74000 mg/kg bw and LD50 (Guinea pig, dermal) > 3000 mg/kg (16). There is no further animal test data available on lard oil. Genotoxicity A standard mutagenicity test (Ames test) with tallow oil (CAS No. 61789-97-7) indicates that tallow oil does not possess genotoxic properties (16). Carcinogenicity Carcinogenicity studies in rats, mice and hamsters of minor reliability indicate that a life time high fat intake or an intake of heated fat may have a tumour promoting effect in the experimental animals (16). The studies are considered of less relevance in this context due to very high doses of exposure. Reproduction toxicity No repro-toxic effects were observed in a study with pigs which were daily fed with 8 % tallow (CAS No. 61789-97-7) in the diet from day 90 of gestation (16). Health rating – lard oil Lard oil is extracted from lard, which has been a natural part of human food for several thousand years and which may occur in the food in substantial amounts. Olein, which is the main constituent of lard, is a constituent of many fluid vegetable and animal oils which are part of the human diet (40). Olein is used as an ingredient in cosmetics and pharmaceuticals (40,42). Lard oil is based on the available data and experience assigned health score 1. 7.6.5 Environmental assessmentVegetable and animal lipids are a large group of chemicals. Environmental data are available on oils, e.g. rape oil, soybean oil, castor oil, tallow fat and their fatty acids. This group also includes hydro-treated (hydrogenated) and epoxidized oils. Aquatic toxicity The most of few available data indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. However, EC/LC50 values below 100 mg/l are not unusual either. Toxicity tests with EC/LC50 values below 100 mg/l are referred for the following oils:
Environmental fate Several tests concerning biodegradation were available. All tests showed that fatty acids and lipids are readily biodegradable (16). Bioaccumulation No experimental bioaccumulation data were available but IUCLID refers log POW data (21). All data show log POW higher than 4, which indicates that fatty acids and natural lipids have a potential for bioaccumulating e in aquatic organisms. Environmental rating Based on the available data, this large group of substances is assigned environmental score 1 but some epoxidized lipids can be assigned environmental score 3 or 4. 7.7 Phosphorous compounds7.7.1 FunctionOrganic phosphorous compounds enter lubricants for metal working as extreme pressure additives. The compounds may have other functions, such as corrosion inhibition and anti-wear (13,14,18,19). 7.7.2 IdentificationThe phosphorous extreme pressure additives is a broad group of substances, - including phosphate esters (mono-, di- and tri-ester compounds), salts and amines of phosphate esters, complex phosphate esters, mono- and diester compounds of phosphite, diphosphoric acid esters and trialkyl phosphines. The organic radicals (R) in the phosphorous additives can be aliphatic as well as aromatic groups. The aliphatic phosphate esters comprises C4 – C10-compounds as well as polyethylene- and polypropoxylene oxide derivates (18,43). Fig. 7.3 illustrates the chemical structure of some of the groups of phosphorous compounds entering non-chlorinated lubricants as extreme pressure additives. 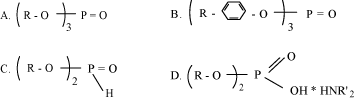
Fig. 7.3 Molecular structures for some of the groups of phosphorous compounds used as extreme pressure additives in metal forming lubricants. A. Trialkyl phosphate B. Triaryl phosphate C. Dialkyl phosphate D. Amine phosphate Another type of phosphorous compounds – the phosphorous-sulphur compounds, also enters lubricants as extreme pressure additives. Dialkyldithiophosphates – including zinc dialkyldithiophosphates, - are used as extreme pressure additives especially in motor oils, but also in lubricants for metal working (18). Fig. 7.4 illustrates the molecular structure of zinc dialkyldithiophosphates. 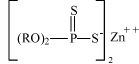
Fig. 7.4 Molecular structure of zinc di-alkyldithiophosphates Phosphate esters are produced by reacting phenols or alcohols with anhydrides or chlorides of phosphoric acid (18). Zinc dialkyldithiophosphates are produced by reacting phosphorous pentasulphide with the corresponding alcohols at 80 –150°C. The resulting dialkyldithiophosphoric acid is neutralized with zinc oxide at 25- 75°C (18). Table 7.8 states the CAS numbers of substances which are included in the health and environmental assessment of the phosphorous compounds.
Table 7.8 Chemical names and CAS Nos. of substances included in the health and environmental assessment of phosphorous compounds. 7.7.3 Physical/chemical dataThe assessed phosphorus extreme pressure additives are liquids at room temperature. The substances have boiling points varying from about 50°C to more than 200°C, flash points > 100 °C and low vapour pressures. The solubility in water is varying (16). 7.7.4 Health and environmental assessmentHealth and environmental effects of phosphate esters and other phosphorous compounds used as extreme pressure additives in metal working lubricants show a great variation. Therefore, it is not possible to consider the phosphorus additives as a uniform group. The health effects of selected CAS Nos. known to occur in metal working lubricants and representing some of the different groups of phosphorous extreme pressure additives are assessed below. 7.7.4.1 Triaryl phosphates and aryl phosphitesHealth assessment Acute toxicity A triaryl phosphate – phenol, isopropylated , phosphate 3:1 (ITAP) (CAS No. 68937-41-7) exhibits low acute toxicity by ingestion, skin contact and inhalation in animal studies. LD50 (rat, oral) > 5000 mg/kg) (16). LD50 (rabbit, dermal) > 5000 mg/kg (16). LC50 (rat/4 hours) > 6350 mg/m³) (43). Irritation ITAP does not exhibit irritating properties to skin or eye in standard tests (OECD 404 and 405) (16). Sensitization ITAP has been tested in Guinea Pigs in accordance with OECD Guideline 406. The test substance was not sensitizing in this model (16). Repeated dose toxicity A: A standard study with rats indicates moderate toxicity of ITAP by repeated dermal exposure. In accordance with OECD Guideline 410, rats were dermally exposed to ITAP (Kronitex 50) 6 hours/day, 5 days/week for four weeks at doses of 0, 100, 500 and 2000 mg bw/kg/day. There was a slight inhibition of plasma cholinesterase activity in females receiving 500 mg/kg bw/day as well as in both sexes of the 2000 mg/kg bw/day group. The erythrocyte cholinesterase activity was significantly inhibited in the males treated with 2000 mg/kg bw/day. Adrenal weights were increased in males receiving 500 and 2000 mg/kg bw/day. Microscopic examination of tissues showed a slight fatty change in the adrenal cortex of 2/5 males receiving 500 mg/kg bw/day and in 3/5 males receiving 2000 mg/kg bw/day. The NOAEL was 100 mg/kg bw/day in males and 500 mg/kg bw/day in females (16). B: In an analogous study, rats were dermally exposed to ITAP (Reolube HYD 46) at doses of 0, 40, 200 and 1000 mg/kg bw/day. A slight inhibition of the plasma cholinesterase activity was observed in the females receiving 1000 mg/kg bw/day. A decrease in absolute and relative testicular weight was observed in the males receiving 1000 mg/kg bw/day. Microscopic examination of the testes showed slight tubular atrophy in both controls and treated groups. Slightly increased absolute and relative adrenal weights were observed in the treated group but no microscopic findings were observed. There are no further details for this study, however it was concluded that dermal application over a period of four weeks at a dosage of 200 mg/kg bw/day did not produce observable adverse effects. NOAEL was 200 mg/kg/day (16). Neurotoxicity C: Several studies with hens exposed to ITAP (Reofos 50, Reofos 65 and Reofos 95) by ingestion have been performed. A number of these studies indicate delayed neurotoxic effects including signs of ataxia and axonal degeneration. The lowest observed dose level for neurotoxic effects in hens by ingestion was 90 mg/kg/day in a 13 weeks study. However other neurotoxicity studies with hens exposed to ITAP by ingestion at higher dose levels (up to 12000 mg/kg) did not result in neurotoxic effects (16). In a study with rats exposed to ITAP in a single oral dose of 2 g/kg, a decrease in serum cholinesterase in addition to significant inhibition of brain cholinesterase was observed in treated animals. However no clinical signs were observed (16). D: Hens were exposed by inhalation to ITAP in aerosol form (Reofos 50) in a single eight hour period and the hens were examined for the next 21 day period for signs of neurotoxicity. Actual does levels were 620, 2400, 2540 and 3090 mg/m³. Mild or modest ataxia was seen in 2/10 hens exposed to 2400 mg/m³ and in 4/10 hens exposed to 3090 mg/m³. Histologic examination of nervous tissues confirmed that degenerative changes were observed at these two dosage levels. No effects were observed at the 620 mg/m³ exposure level. NOAEC was 620 mg/m³ (16). A couple of epidemiological examinations of possible neurotoxic effects of ITAP in the working environment are available. The results of these studies are inconclusive (16). Genotoxicity Several studies of effects of ITAP on genes indicate, that the substance is not genotoxic (16). Reproduction toxicity and carcinogenicity There are no data on effects of ITAP on reproduction, foetal development or carcinogenicity. Health rating Some data indicate that ITAP may have neurotoxic effects by repeated exposure at high doses. However, in total the available data are incomplete to make an assessment regarding this effect. Thus, ITAP is assigned health score 1. ITAP has been selected for the health assessment of aryl phosphates and aryl phosphites. However, the toxicity of the group varies considerably implying that the health score of individual substances in the group may be between 1 – 5. Environmental assessment Aquatic toxicity Tri-p-tolyl phosphate (CAS No. 78-32-0) and triphenyl phosphite (CAS No. 101-02-0) are classified as dangerous to the environment; N;R51/53 and N;R50/53, respectively (4). Toxicity tests with EC/LC50 values are referred for the following compounds:
Environmental fate Several tests concerning biodegradation are available. The referred tests indicate both ready biodegradability and not ready biodegradability. It is not possible to find any relation between structure and biodegradability (16). Bioaccumulation All phosphate esters have log POW values above 4 and one test showed a bio-concentration factor above 1000. E.g., 2-ethylhexyl diphenyl phosphate (CAS No. 1241-94-7) had a BCF of 1241 (16). Many tests showed BCF values of 10-250. Based on the available data, phosphate esters are assessed to have a potential for bioaccumulation in aquatic organisms. Environmental rating Phosphate esters in general are assigned environmental scores 4 or 5. However, it is expected that certain phosphate esters fulfil criteria for environmental scores 3, 2 and even 1. 7.7.4.2 Trialkyl phosphatesHealth assessment Acute toxicity A trialkyl phosphate – tributyl phosphate (TBP) (CAS No. 126-73-8) is classified as harmful by ingestion (Xn;R22) (4). LD50 (rat, oral) is 1500 - 3000 mg/kg (16,46). TBP exhibits low acute toxicity by skin contact and inhalation (16). Irritation TBP is slightly irritating to skin and eyes in standard studies (OECD 404 and 405). However, other studies indicate moderate to strong skin irritation and moderate irritation to mucous membranes (16,46). Sensitization There is no evidence of a sensitizing potential of TBP (16,46). Repeated dose toxicity TBP shows moderate toxicity by repeated exposure by inhalation. A: Rats exposed to 5.1 or 13.6 mg/m³ TBP 5 hours/day, 5 days/week for 4 months showed decreased cholinesterase activity after 3 months in addition to effects on physiological and biochemical parameters, especially in the liver, in the high dose group. The effect retained to normal during a one-month post exposure period. The NOAEC was 5.1 mg/m³ (16). Several studies in rats, mice and rabbits of TBP effects by repeated oral exposure have been performed and indicate moderate toxicity (16,46). In a 13-week study rats were daily exposed to TBP by gavage at doses of 32, 100 and 325 mg/kg bw/day. Mortality, salivation and muzzle staining were observed in the 325 mg group and less severe in the 100 mg group. In addition, reduced body weight, body weight gain, reduced food intake and initial weight loss were observed in the highest dose group. Motor activity test results were not significantly different between the groups. There were no abnormal gross pathology findings and the neuropathological assessment revealed no effects of treatment (16). B: In a 2-weeks study with rats exposed to 270 or 400 mg/kg bw/day by gavage, no overt signs of toxicity were observed. However, reduction in conduction velocity of the caudal nerve was observed in high dose males. Electron microscopic examination revealed morphological changes of the nerve. The NOAEL was 270 mg/kg bw/day (16). C: In another 2-weeks study with rats exposed to TBP by gavage at dose levels of 136 or 400 mg/kg bw/day, no overt signs of toxicity were observed. However in the high dose group microscopic degenerative changes were observed in the seminiferous tubules of one of four male rats, in addition to some changes in clinical chemistry, an increase in relative and absolute liver weight and a decrease in spleen weight (female rats). The NOAEL was 136 mg/kg bw/day (16). There are no data regarding repeated exposure of TBP by skin contact. Genotoxicity In mutagenicity studies, equivocal results have been obtained by using the Ames test in the presence and absence of metabolic activation. However, all other types of mutagenicity tests indicate that TBP is non-mutagenic (16,46). Carcinogenicity In a 18-week study, TBP was administered by gavage once a day, 5 days/week to rats. Low dose animals received 200 mg/kg/day throughout the study, high dose animals received 300 mg/kg/day for the first 6 weeks and 350 mg/kg/day for the remaining 12 weeks. Histopathological examination revealed that all treated animals developed diffuse hyperplasia of the urinary bladder epithelium (47). D: In another study, groups of 50 male and female rats each obtained TBP with their diet (up to 140 and 180 mg/kg bw/day respectively) for 2 years. In this experiment hyperplasia and also neoplastic lesions dependent on the dose (papilloma and especially for the males also carcinoma) were found in the urine bladder. The male and female NOAEL was 9 and 12 mg/kg bw/day respectively, assuming that TBP is a non genotoxic oncogene (48). On the EU provisional list Existing substances for the 29th ATP Rev. 18, TBP is classified as a carcinogen in category 3 with risk phrase R40: “Limited evidence of a carcinogenic effect” (49). Reproduction toxicity E: In a single two-generation reproduction study, rats were daily exposed to TBP in food at dose levels of 200, 700 and 3000 mg/kg diet (approximately 15, 53 and 225 mg/kg bw/day). Reduction in body weight, weight gain and food consumption were observed during F0 and F1 pre-bred dosing periods in the two highest dose groups. In the 200 mg/kg dose groups, transient effects on body weight and food consumption were observed. No signs of toxicity and no treatment related mortality were observed in any dose group. There was no evidence of reproductive organ histopathology at any dose level and no effect on pre- and postnatal mortality. A NOAEL for reproductive toxicity could not be established. LOAEL (reduced pup weight) was < 15 mg/kg bw/day (16). Two studies in rats and two studies in rabbits do not indicate embryotoxic or fetotoxic effects of TBP (16). Other effects TBP is a weak plasma cholinesterase inhibitor in rats and causes toxic effects to the peripheral nervous system at high dose levels (1000 mg/kg bw) (16). TBP is readily absorbed through the skin (16,4046). Health rating Based on the available data, TBP is assigned health score 5 due to the potential carcinogenic effect. TBP has been selected for the health assessment of trialkyl phosphates. However, the toxicity of the group varies considerably implying that the health score of individual substances in the group may be between 1 – 5. Environmental assessment Environmental rating Based on available data on triaryl phosphates (section 7.7.4.1), phosphate esters in general are assigned environmental scores 4 or 5. However, it is expected that certain phosphate esters fulfil criteria for environmental scores 3, 2 and even 1. 7.7.4.3 Dialkyl phosphates, dialkyl phosphates, monoalkyl phosphates and complex phosphate estersHealth assessment Health rating - dialkyl phosphates The dialkyl phosphate – bis(2-ethylhexyl) hydrogen phosphate (CAS No. 298-07-7) -exhibits low acute toxicity by ingestion (40). The substance is harmful by skin contact (LD50, rabbit, dermal > 1250 mg/kg) and corrosive to skin and mucous membranes. The substance is assigned health score 3. Health rating - dialkyl phosphites The dialkyl phosphite – didodecyl phosphite (CAS No. 21302-09-0) - exhibits low acute toxicity by ingestion, inhalation and skin contact (43). Based on the limited data, the substance is assigned health score 2 due to a skin irritating potential. Dimethyl hydrogen phosphite (CAS-No. 868-85-9) exhibits low acute toxicity by ingestion and inhalation (43). The substance should be classified as harmful by skin contact (LD50 (rabbit, dermal) = 681 mg/kg) (43). Dimethyl phosphite is mildly to moderately irritating to skin and eyes (43). Limited evidence from animal studies indicates that the substance may have a carcinogenic potential. IARC classifies the substance as carcinogenic in category 3 (40,44). The compound is assigned health score 5 due to a carcinogenic potential. Health rating – monoalkyl phosphate The monoalkyl phosphate – 2-ethylhexyl hydrogen phosphate (CAS No. 1070-03-7) exhibits low acute toxicity by ingestion and skin contact. The substance is strongly irritating to eyes (43). A supplier classifies the mono alkylphosphate as corrosive (C;R34) (50). The substance is assigned health score 3. Health rating – complex phosphate esters There are virtually no toxicological data available on complex phosphate esters, including polypropylene and polyethylene oxide derivates. Polyethoxy oleyletherphosphate (CAS No. 39464-69-2), which is used as an extreme pressure additive for metal working lubricants, should not be classified as dangerous to the health based on the available supplier information (51,52). This corresponds to health score 1. The compound is used in cosmetics (42). Environmental assessment Environmental rating - dialkylphosphates, dialkylphosphites and monoalkylphosphates Based on available data (section 7.7.4.1), phosphate esters in general are assigned environmental scores 4 or 5. However, it is expected that certain phosphate esters fulfil criteria for environmental scores 3, 2 and even 1. 7.7.4.4 Zinc dialkyldithiophosphatesZinc alkyl thiophosphates are a large group of chemicals. The database IUCLID (16) contains 10 zinc alkyl thiophosphates that are used as lubricant additives. However, only little data is available for these substances especially concerning biodegradation and bioaccumulation. Zinc dialkyldithiophosphates are manufactured and commercially distributed in highly refined lubricant base oil (IP 346 DMSO extractables < 3%). The zinc dialkyldithiophosphates are never isolated from base oil at any time during their life cycle. Hence all testing for environmental and health effects are performed on zinc dialkyldithiophosphates in highly refined lubricant base oils. Health assessment Acute toxicity In general, commercial zinc dialkyldithiophosphates exhibit low acute toxicity. Acute oral LD50 in rats ranges from 2000 - 3500 mg/kg. Acute dermal LD50 in rabbits > 2000 mg/kg (53). Irritation Zinc dialkyldithiophosphates (CAS Nos. 4259-15-8, 28629-66-5, 68457-79-4) are moderately to strongly irritating to skin and eyes implying a risk of serious eye damage (16). Sensitization There is no indication of a sensitizing potential (53). Genotoxicity Several standard mutagenicity tests have been performed for a number of zinc dialkyldithiophosphates. Findings indicate that commercial zinc dialkyldithiophosphates have a low potential for inducing genetic toxicity (53). Repeated dose toxicity Data from several repeated-dose toxicity studies of commercial zinc dialkyldithiophosphates have been reviewed. Repeated dermal exposure of experimental animals results in moderate-to-severe dermal irritation, behavioural distress, body weight loss and emaciation, reduction in haematological parameters and adverse effects on male reproductive organs. Oral administration causes significant gastric irritation and related gastrointestinal disturbances, signs of distress but no evidence of adverse effects on male reproductive organs (53). Reproduction toxicity Data from a study on zinc bis[0,0-bis(2-ethylhexyl)] bis(dithiophosphate) (CAS No. 4295-15-8) indicates a low concern for reproduction/ developmental toxicity. Furthermore, an epidemiological study on workers exposed to oil-based zinc dialkyldithiophosphates (range C4-C8) in an additive manufacturing plant revealed no adverse effects on worker reproductive health (53). Carcinogenicity There are no data regarding the carcinogenic potential of zinc dialkyldithiophosphates. Health rating Based on available data, zinc dialkyldithiophosphates are irritating to skin. The eye irritating potential of zinc dialkyldithiophosphates ranges from moderately irritating to risk of serious damage to eyes. This classification implies that zinc dialkyldithiophosphates are assigned health score 2 – 3. Environmental assessment Aquatic toxicity Few data indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. Most of the data indicate EC/LC50 values below 100 mg/l. Toxicity tests of zinc dialkyldithiophosphates showed the following EC/LC50 values:
Environmental fate No test results for biodegradation were available. In a MSDS, the used zinc additive 2-ethylhexyl zinc dithiophosphate was referred to as not readily biodegradable according to an OECD 301 D test (5% degraded). Bioaccumulation No experimental data were available. The information on the structure of the zinc salts was considered insufficient for QSAR calculations to be made. Environmental rating Based on the sparse available data on this large group of substances and on additional secondary data from one MSDS, in which the zinc additive is classified R51/53, zinc additives are assigned environmental scores 4 or 5. 7.8 Sulphurized fatty compounds7.8.1 FunctionSulphurized fatty compounds are added to lubricants for metal working as extreme pressure additives (13,14). 7.8.2 IdentificationSulphurized fatty compounds are obtained by reaction of unsaturated compounds such as triglycerides from soy, rape seed or lard oil or synthetic esters produced from natural fatty acids, e.g. rapeseed methyl ester, with elemental sulphur at high temperatures or with hydrogen sulphide. By this, sulphur is added to double bonds forming sulphur links or ring structures. Fig. 7.5 shows an example of the chemical structure of sulphurized compounds used as extreme pressure additives in non-chlorinated lubricants. 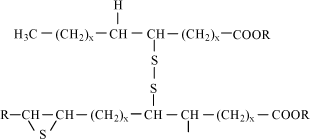
Fig. 7.5 Sulphurised fatty acid The function as extreme pressure additives depends on the degree of sulphurization. There are compounds with active and inactive sulphur on the market. The more active sulphur the compound contains the lower is the response temperature for the extreme pressure function (18,54). 7.8.3 Physical/chemical dataSulphurized fatty compounds are liquids at room temperature with high flash points (> 110°C to 200°C), negligible vapour pressures and very low solubility in water (55,56,57). 7.8.4 Health assessmentThere are virtually no health data in literature and databases on sulphurized fatty compounds. Thus, the health assessment of the group is based on sparse supplier information for a mixture of sulphurized rape seed oil and vegetable fatty acid methyl esters and a mixture of sulphurized animal oils and vegetable fatty acid methyl esters, in addition to conclusions by analogy from health data on starting materials for sulphurized fatty compounds. Acute toxicity Supplier data are available from studies in rats of acute oral toxicity. These data indicate low acute toxicity. LD50-values range from > 2000 mg/kg - > 5000 mg/kg (55,56,57). There are no data on acute dermal toxicity or toxicity by inhalation. Hydrogen sulphide may be formed by decomposition due to heating above 150° to 180°C. Hydrogen sulphide is very toxic by inhalation. Irritation Standard irritation tests (OECD Guideline 404 and 405) in rabbits of the mentioned mixtures of sulphurized vegetable or animal fatty oils and vegetable methyl esters indicate that the compounds are not irritating to skin and eyes (55,56,57). There are no data regarding inhalation. Sensitization Standard sensitization tests with Guinea pigs (OECD Guideline 406) have been performed for the compounds. The sulphurized fatty compounds do not possess a sensitizing potential based on the results of the tests (55,56,57). Repeated dose toxicity There are no further toxicological data on the sulphurized fatty compounds. Health rating There are very sparse data regarding the health effects of sulphurized fatty compounds. However, there exists considerable experience and knowledge of health effects of the starting materials, fatty vegetable and animal oils, esters synthesized from natural fatty acids and elemental sulphur. See also section 7.6.4. This knowledge indicates that health effects of sulphurized fatty compounds are limited. Sulphur dust is irritating to skin and mucous membranes (40,48). Thus based on the available supplier data and conclusion by analogy, sulphurized fatty compounds are assigned health score 1. 7.8.5 Environmental assessmentAquatic toxicity Only few data are available. According to a few material safety data sheets (MSDS), sulphurized oils and esters show EC/LC50 values above 100 mg/l. Environmental fate Very few data are available. According to a few material safety data sheets (MSDS), sulphurized esters and oils are readily biodegradable. Bioaccumulation No experimental bioconcentration factors were available. Based on the general structure of sulphurized oils, it is expected that log POW is above 4 for many compounds in this group. Environmental rating Based on these very sparse data, sulphurized natural and synthetic oils and esters are assigned environmental score 1. 7.9 Synthetic ester oils7.9.1 FunctionSynthetic ester oils, predominantly aliphatic ester oils, enter into lubricants for metal forming as lubricity improving additives (polar additives – see also section 2.2) and as lubricant bases (13,14). 7.9.2 IdentificationAs mentioned above, aliphatic ester oils are the most frequently used type of synthetic ester oils in metal forming lubricants. Thus, the health and environmental assessment of synthetic ester oils will focus on this group of esters (13,14). An ester is the condensation product of an acid, most often an organic acid, and an alcohol. Aliphatic ester oils are produced in a condensation process at 120-240°C under water cleavage (14). Aliphatic ester oils are a large and varying group of substances. They are synthesized from relatively pure and simple starting materials as natural fatty acids and alcohols (monoalcohols, glycols and polyols). There is an incredible versatility in the design of ester molecules due to the large number of commercially available acids and alcohols, from which to choose. A large number of esters can be designed with different chemical structures selected for specific desired properties. Performance properties, which can be varied in the ester design include viscosity, viscosity index, volatility, biodegradability, lubricity, hydrolytic stability, additive solubility, high temperature coking tendency and seal comparability (58). Synthetic ester oils for metal working lubricants can be divided into five groups:
Monoesters are used primarily as lubricant base oils. The function of diesters, polyglycol esters, polyol esters and complex esters in lubricants for metal working are mainly as lubricity improving additives (polar additives). However they can also enter as lubricant base oils. The polyglycol esters also have emulsifying properties which are used in water based lubricants (14,18). 7.9.3 Monoesters7.9.3.1IdentificationMonoesters are synthesized from natural or synthetic carboxylic acids and monoalcohols. Typical starting materials for monoesters in lubricants are oleic acid, palmitic acid, cocos fatty acids and methanol, isopropanol, isobutanol, 2 –ethylhexanol, n-octanol/n-decanol and isotridecanol (14). Fig. 7.6 illustrates the chemical structure of an aliphatic organic monoester. Fig. 7.6 The chemical structure of an organic monoester. R and R' are aliphatic radicals. Table 7.9 states the name and CAS number of substances which are included in the health and environmental assessment of monoesters.
Table 7.9 Chemical names and CAS Nos. of substances included in the health and environmental assessment of monoesters in metal working lubricants. 7.9.3.2 Physical/chemical dataThe monoesters are liquids at room temperature. They have high boiling points (< 200°C), high flash points (> 100°C) and low vapour pressure. The solubility in water is generally low (16,59,60). 7.9.3.3 Health assessmentAcute toxicity Monoesters of higher fatty acids (C12 or more) in general exhibit low toxicity and a number are cleared for use in the food industry (59,60). In addition, the majority of the monoester compounds included in this health assessment is used as skin conditioning agents in cosmetics (38,42). Data for acute toxicity by ingestion (LD50, rat, oral) for the specified CAS Nos. are in the range of more than 2000 mg/kg to 27000 mg/kg. Data for acute toxicity by skin contact (LD50, rabbit or rat, dermal) are in the range of 3000 mg/kg to more than 5000 mg/kg (16,44,61,62). Inhalation exposure at toxicological significant levels is not expected due to the low volatility of the mentioned monoesters. Irritation The monoesters have varying irritating properties. Methyl oleate (CAS No. 112-62-9) is moderately irritating to rabbit skin. Methyl laurate (CAS No. 111-82-0) is stated as slightly irritating to rabbit skin in an unspecified test. However the substance is stated as highly irritating to rabbit skin when tested in accordance with EU Directive 84/449/EEC, B.4. The same substance is stated as non-irritating to human skin in the Epicutan test according to Burckhardt (16). The remaining monoesters exhibit slightly irritating properties on rabbit skin (16,44). Methyl laurate (CAS No. 111-82-0) and 2-ethylhexyl laurate (CAS No. 20292-08-4) are non-irritating in rabbit eyes, while 2-ethylhexyl-2-ethyl hexanoate (CAS No. 7425-14-1) is moderately to strongly irritating in rabbit eyes (16). Sensitization Data for sensitization potential of the monoesters indicate that they do not possess this property. Methyl laurate (CAS No. 111-82-0) did not provoke an allergic response when tested in Guinea pig (test method not specified), while 2-ethylhexyl laurate (CAS No. 20292-08-4) and decyl oleate (CAS No. 3687-46-5) were non-sensitizing in the Guinea pig maximization test (16,61). Methyl oleate (CAS No. 112-62-9) was not sensitizing by skin contact in a test with volunteers, exposed to the substance in 10 % petrolatum solution (16,61). Repeated dose toxicity A 28-day oral gavage study in rats with decyl oleate (CAS No. 3687-46-5) at doses of 100, 500 and 1000 mg/kg showed no toxicity as observed with respect to clinical symptoms, biochemistry, hematology, gross lesions or tissue/organ histopathology (16). The NOAEL was 1000 mg/kg. In a corresponding 28-day gavage study of 2-ethylhexyl laurate (CAS No. 20292-08-4) in rats, the NOAEL was 1000 mg/kg (16). The findings support a low order of toxicity of monoesters by repeated exposure. Genotoxicity Genotoxicity has been studied in bacterial cells for three of the mentioned monoesters (decyl oleate (CAS No. 3687-46-5), methyl laurate (CAS No 111-82-0) and 2-ethylhexyl laurate (CAS No. 20292-08-4)). The test results were negative in all three cases. In addition, 2-ethylhexyl laurate has been tested in vivo in mice in the micronucleus assay with negative result (16). The findings indicate that the monoesters do not posses a genotoxic potential. Reproduction toxicity Fertility, litter size and survival of offspring were normal in rats fed diets containing 6,25 % (approx. 3125 mg/kg bw/day) of butyl stearate (CAS No. 123-95-5) for 10 weeks. However, growth was reduced in offspring during the pre-weaning and post-weaning periods. No gross lesions were observed among the offspring killed at the end of the 21-day post-weaning periods. These results indicate that long-chain fatty acid esters do not cause reproductive toxicity to rats (59). Assessment of developmental effects for long chain fatty acid monoesters is primarily based on data for C16-18, 2-ethylhexyl ester (CAS No. 91031-48-0). In oral gavage studies in rats administered doses of 100, 300 and 1000 mg/kg during gestation, the maternal NOAEL was 1000 mg/kg and the NOAEL for foetal effects was 1000 mg/kg (59). The findings indicate that long chain fatty acid monoesters do not posses a foetotoxic potential. Carcinogenicity There is data available from carcinogenicity studies of a single monoester, methyl oleate CAS No. 112-62-9. Studies in mice exposed to the substance by oral ingestion, dermal application or injection under the skin for a life long period indicate, that methyl oleate may have a weak tumour promoting activity (16). Health rating Monoesters used in metal working lubricants are based on the available data, assigned score 1. However, some monoesters as methyl oleate (CAS No. 112-62-9) and 2-ethylhexyl-2-ethylhexanoat (CAS No. 7425-14-1) exhibit an eye irritating potential and are thus assigned health score 2. 7.9.3.4 Environmental assessmentEnvironmental data are available for methylesters of different fatty acids. Aquatic toxicity Few data are available for this group of chemicals. The available data indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. Two toxicity tests with EC/LC50 values below 100 mg/l were referred, i.e. fatty acid C6-C10 methyl esters (CAS No. 68937-83-7) with LC50(48h) towards Leuciscus idus of 95 mg/l and 88 mg/l, respectively (16). Environmental fate Several biodegradation tests are available. All tests indicate that fatty acids methyl esters are readily biodegradable (16). Bioaccumulation No experimental bioconcentration data are available but many log POW data are referred in IUCLID (16). All data showed log POW values above 4, which indicates that fatty acid methyl esters have a potential for bioaccumulating in aquatic organisms Environmental rating Based on the available data, fatty acid monoesters are assigned environmental score 1. 7.9.4 Diesters7.9.4.1 IdentificationDiesters are ester compounds of diacids and monoalcohols. Starting materials for diesters in metal working lubricants are fatty acids as adipic acid, sebacic acid and dimer acid and monoalcohols as methanol, isopropanol, isobutanol, 2-ethylhexanol and isotridecanol (14). Adipates are the most widely used diesters in lubricants (58). Fig. 7.7 illustrates an example of the chemical structure of an aliphatic organic diester. 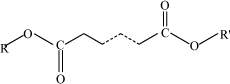
Fig. 7.7 The chemical structure of an organic diester. R1 and R' are aliphatic organic radicals. Table 7.10 states the name and CAS number of substances which are included in the health and environmental assessment of diesters.
Table 7.10 Chemical names and CAS Nos. of substances included in the health and environmental assessment of diesters in metal working lubricants. 7.9.4.2 Physical/chemical dataShort-chain alkyl (e.g. methyl, isopropyl and butyl) diesters are generally more water soluble and more volatile than the corresponding long-chain alkyl (C7-C13 alcohol) diesters. The diesters included in the assessment are liquids at room temperature with high boiling points (> 200°C), high flash points (> 100°C), low vapour pressures and moderate to very low solubility in water (16,59). 7.9.4.3 Health assessmentAcute toxicity The diesters in general demonstrate a low order of acute toxicity. Oral rat LD50 values range from > 2000 mg/kg to > 45.000 mg/kg (16,59). Dermal rabbit LD50 values for diisodecyl adipate (CAS No. 27178-16-1) > 5000 mg/kg and for bis(2-ethylhexyl) adipate (CAS No. 103-23-1) > 8.000 mg/kg (16). Inhalation exposure at toxicological significant levels is not expected due to the low volatility of the diesters in focus. Irritation Standard skin and eye irritation test with rabbits are available for several of the diesters in focus. The results mainly indicate slightly irritating properties. (16). Sensitization Sensitization data are available for bis(2-ethylhexyl) adipate (CAS No. 103-23-1) and diisodecyl adipate (CAS No. 27178-16-1). Bis(2-ethylhexyl) adipate was not sensitising in Guinea pig tested by the Draize test and two unspecified tests, and when tested in rabbits in a Patch test. Diisodecyl adipate was not sensitising in the Guinea pig maximization test (16). Thus there is no dating indicating a sensitising potential of diesters in metal working lubricants. Repeated dose toxicity There are a number of animal studies of repeated exposure to bis(2-ethylhexyl) adipate (CAS No. 103-23-1). In 90-day sub-chronic dietary studies, the NOAEL was approximately 300 mg/kg /day in rats and 230 mg/kg/day in mice. The LOAEL was approximately 600 mg/kg/day in rats and 460 mg/kg/day in mice. Hepatic hypertrophy and increased peroxisomal enzyme activity occurred in rats and mice (16,59). Data on repeated dose toxicity have also been reported for diisononyl adipate (CAS No. 33703-08-1) and bis(tridecyl) adipate (CAS No. 16958-92-2). In 90-day toxicity study, rats were administered diisononyl adipate in the diet at levels equivalent to 0, 50, 150 and 500 mg/kg/day. The NOAEL was 500 mg/kg/day. Feeding studies were also carried out in beagle dogs for 13 weeks at dietary concentrations of 0, 0.3, 1 and 3 % of diisononyl adipate (increased to 6 % at week 9). The NOAEL was determined to be 1 % in the diet or approximately 274 mg/kg /day. In another 13-week study, bis(tridecyl) adipate was well tolerated in rats given dermal doses of 800 and 2000 mg/kg/day (59). Overall the findings indicate a low order of toxicity by repeated exposure to the diesters in focus. Several of the adipates and sebacates have Indirect Food Additive Status for use in food wrapping materials (63). In addition, several of the adipates and sebacates are used as ingredients in cosmetic (38,42). Genotoxicity Bis(2-ethylhexyl) sebacate (CAS No. 122-62-3), bis(tridecyl) adipate (CAS No. 16958-92-2) and diisononyl adipate (CAS No. 33703-08-1) were shown to be negative in the Ames assay. Bis(2-ethylhexyl) adipate (CAS No. 103-23-1) has also been evaluated for mutagenicity and was found to be negative in both the Ames and mouse lymphoma assay (59). Regarding chromosomal aberration, bis(tridecyl) adipate (CAS No. 16958-92-2) has been tested in the micronucleus assay, and bis(2-ethylhexyl) adipate (CAS No. 103-23-1) has been tested in the Chinese hamster ovary cell with negative results (16,59). Thus the available findings do not indicate genotoxic properties of the diesters in focus. Carcinogenicity Bis(2-ethylhexyl) adipate (CAS No. 103-23-1) was tested for carcinogenicity by oral administration in one experiment in mice and one experiment in rats. In mice, liver adenomas and carcinomas were produced in both males and females. No treatment-related tumours were observed in rats. The International Agency for Cancer Research (IARC) evaluates that there is limited evidence in experimental animals for the carcinogenicity of bis(2-ethylhexyl) adipate. According to the IARC evaluation criteria, the substance is not classifiable as to its carcinogenicity in humans (Group 3) (64). There are no further data on carcinogenicity of the diesters in focus. Reproduction toxicity Bis(2-ethylhexyl) adipate (CAS No. 103-23-1) has been evaluated for reproductive effects in a one-generation study. Male and female rats were administered the substance in their diets at same levels (0, 28, 170 or 1080 mg/kg/day). After 10 weeks on the diet, the animals were mated to produce one generation of offspring. Test diets were administered continuously throughout the study (18 –19 weeks of exposure). No effects were seen on male or female fertility. However, at the highest dose, there was a reduction in body weight in the dams, and reduction in offspring body weight, total litter weight and litter size. The NOAEL in this study was 170 mg/kg/day. The LOAEL was 1080 mg/kg/day (59). A developmental toxicity/teratogenicity study in rats is available for bis(2-ethylhexyl) adipate (CAS No. 103-23-1). In accordance with OECD Guideline 414, rats were exposed to the substance in the diet during the gestation period at dose levels of 28, 170 and 1080 mg/kg/day. A significant reduction in the maternal body weight gain and feed intake was observed in the highest dose group, however no maternal toxicity was observed at 28 and 170 mg/kg/day. The foetal weight, litter weight and number of external and internal abnormalities were not influenced by the treatment. However, a dose dependent fetotoxicity was observed as minor skeletal defects in the groups receiving 170 and 1080 mg/kg/day. It was concluded that bis(2-ethylhexyl) adipate caused a dose-related foetotoxic effect, however no teratogenic effect (16). A newer study support the observation of foetotoxicity of bis(2-ethylhexyl) adipate (CAS No. 103-23-1). In rats orally exposed to pre- and postnatal doses of 0, 200 , 400 or 800 mg/kg bw/day of bis(2-ethylhexyl) adipate, a prolonged gestation period was observed at a dose level of 800 mg/kg bw/day, and an increased frequency of pre- and postnatal death of pups was observed at dose levels of 400 and 800 mg/kg bw/day. Bis(2-ethylhexyl) adipate at a dose level of 800 mg/kg bw/day also decreased the pup weight and the decrease in body weight persisted until adulthood. The NOAEL was 200 mg/kg bw/day in this study (65). In 13-week dermal studies with bis(tridecyl) adipate (CAS No. 16958-92-2), no sperm morphological changes were observed in rats treated at levels of 2000 mg/kg. Increase in organ weight of the epididymides and uterus was observed at dermal exposure to 2000 mg/kg but not at 800 mg/kg (59). In a 19-week oral feeding study with bis(2-ethylhexyl) sebacate (CAS No. 122-62-3), no adverse reproductive effects were reported for this substance (59). There are no data on developmental toxicity/ teratogenicity for the remainder diesters in focus. Health rating Bis(2-ethylhexyl) adipate (CAS No. 103-23-1) is assigned health score 5 due to an indication of a foetoxic effect. Based on the available data, the remaining diesters in focus are assigned health score 1. 7.9.4.4 Environmental assessmentAquatic toxicity Few environmental test data are available on adipates. Some of these data indicate EC/LC50 values below 1 mg/kg. Toxicity tests of diesters showed the following EC/LC50 values:
Environmental fate Several biodegradation tests are available. All the tests show that adipates are readily biodegradable except one test result for diisotridecyl adipate (CAS No. 26401-35-4) (16). Bioaccumulation Only few test data were available. Test results were found for the following compounds:
On this basis, adipates are assessed to have a potential for bioaccumulating in aquatic organisms. Environmental rating Based on the available data on this large group of substances, adipates are in general assigned environmental scores 4 or 5. This assessment is based on the few available data on bioaccumulation (diethylhexyl adipate and diisononyl adipate). 7.9.5 Polyglycol esters7.9.5.1IdentificationPolyglycol esters are ester compounds of mono- or dicarboxylic acids and polyglycols. Typical starting materials for production of polyglycol esters for lubricants are co-polymers of ethylene oxide, propylene oxide and butylene oxide, eventually with glycerine, trimethylol propane (TMP) or pentaerythritol (PENTA) as starters, and carboxylic acids as oleic acid, palmitic acid, cocos fatty acids, adipic acid, sebacic acids and dimer acid (14). Fig. 7.8 shows an example of the chemical structure of an aliphatic polyglycol ester. 
Fig. 7.8 The chemical structure of an aliphatic polyglycol ester. R and R' are aliphatic radicals. Short chain aliphatic glycol esters are included in the health and environmental assessment of polyglycol esters. Table 7.11 states the name and CAS number of substances which are included in the health and environmental assessment of polyglycol esters.
Table 7.11 Chemical names and CAS Nos. of substances included in the health and environmental assessment of polyglycol esters in metal working lubricants. 7.9.5.2 Physical/chemical dataThe polyglycol esters and short chain aliphatic glycol esters in focus are liquids at room temperature with high boiling points (> 250°C), high flash points (> 200°C) and very low vapour pressures. The solubility in water varies from completely soluble to very limited solubility (16,59,66,67). 7.9.5.3 Health assessmentAcute toxicity Polyethyleneglycol monooleate (CAS No. 9004-96-0) exhibits low acute toxicity by oral exposure (LD50 rat > 2000 mg/kg) (68). Data from rat studies with shorter chain aliphatic glycol esters also indicate a low order of toxicity (LD50 values ranging from 2000 mg/kg to 34600 mg/kg) (59). Irritation Polyethyleneglycol monooleate (CAS No. 9004-96-0) exhibits mild skin and eye irritating potential in standard tests with rabbits (43). A polyoxyalkylene glycol (56 % solution in water) (CAS No. not specified) is stated to be non-irritating to rabbit skin and eyes by the supplier (67). A short chain aliphatic glycol ester, triethyleneglycol diheptanoate (CAS No. 7434-40-4) is non-irritating to rabbit skin and eyes in standard tests (16). Polyethyleneglycol monooleate (CAS No. 9004-96-0) and a number of the short chain aliphatic glycol esters in focus are used as ingredients in cosmetics (16). Sensitization There is no data available regarding the sensitizing potential of polyglycolesters or short chain aliphatic glycol esters. Repeated dose toxicity There are no data available on repeated exposure for polyglycol esters. However, sub-chronic studies have been carried out with short chain glycol esters. In a 28–day oral gavage studies in rats exposed to PEG-4 heptanoate (CAS No. 70729-68-9), the NOAEL was determined to be 1000 mg/kg. No clinical signs of toxicity were observed and no treatment-related changes in hematology or clinical chemistry were reported (59). Propylene glycol monostearate (CAS No. 1323-39-3), which was administered for 13 weeks at dietary concentrations up to 7.52% (approx. 3760 mg/kg bw/day), showed no signs of toxicity in rats (59). Similarly, in 6-month oral studies, no signs of toxicity, gross or histological pathology were observed in rats and dogs fed diets containing up to 10% propylene glycol monostearate (CAS No. 1323-39-3) (59). Doses up to 1000 mg/kg/day (approx. 5000 mg/kg bw/day) of 2,2,4-trimethyl-1,3-pentanediol heptanoate (CAS No. 71839-38-8) were well tolerated in rats that were orally (gavage) administered the test material for 28 days (59). The findings indicate a low toxicity by repeated exposure of short chain aliphatic glycol esters and polyglycol esters as the toxicity of the last-mentioned are expected to be lower than that of the short chain aliphatic glycol esters. Genotoxicity There are no data on the genotoxic potential of polyglycol esters. Four short chain aliphatic glycol esters, PEG-4 diheptanoate (CAS No. 70729-68-9), 2,2,4-trimethyl-1,3-pentanediol heptanoate (CAS No. 71839-38-8), triethyleneglycol diheptanoate (CAS No. 7434-40-4) and propyleneglycol monostearate (CAS No. 1323-39-3) have shown to be negative in the Ames assay (59). PEG-4 diheptanoate (CAS No. 70729-68-9) has been tested in the Chinese hamster ovary cell assay and did not cause chromosomal aberrations. 2,2,4-Trimethyl-1,3-pentanediol heptanoate (CAS No. 71839-38-8) has also been evaluated in the in-vitro cytogenetics test using human peripheral lymphocytes with negative result (59). Together, these findings indicate that glycol esters do not cause gene mutations or chromosomal aberrations. Carcinogenicity and reproductive toxicity There are no data on the carcinogenic, reprotoxic or teratogenic potential of polyglycol esters or shorter chain aliphatic glycol esters. Health rating CESIO (The European trade organisation for manufacturers of surfactants and their intermediates) recently concluded in a report that ethoxylated fatty acids should not be classified with respect to health hazards according to the Dangerous Substances EU Directive (69). Polyethylenglycol esters are based on the available data on polyglycol esters and short chain aliphatic glycol esters, assigned health score 1. 7.9.5.4 Environmental assessmentAquatic toxicity The few available data showed that polyglycol esters have a low toxicity towards water-living organisms. All available EC/LC50 values are above 100 mg/l, e.g. (60):
Environmental fate All referred tests showed that polyglycol esters are readily biodegradable, e.g. (60):
Bioaccumulation All assessed polyglycolesters have log POW below 3. Polyglycol esters made by long-chained acids may have log POW above 4 (60). Environmental rating Based on the sparse available data on this large group of substances, polyglycol esters are assigned environmental score 1. 7.9.6 Polyol esters and complex esters7.9.6.1 IdentificationPolyol esters are ester compounds of poly-functional alcohols and monocarboxylic acids. Starting materials for manufacture of polyol esters are neopentylglycol (NPG), trimethylolpropane (TMP) and pentaerythritol (PENTA) in addition to oleic acid, palmitic acid, cocos fatty acids and isononanoic acid (14). Complex esters are ester compounds of poly-functional alcohols and mono- and dicarboxylic acids. Starting materials for manufacture of complex esters are the same as for polyol esters in addition to dicarboxylic acids, such as adipic acid, sebacic acid and dimer acids (14). Fig. 7.9 gives an example of the chemical structure of an aliphatic polyol ester using pentaerythritol as a starting materials. 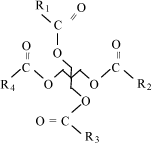
Fig. 7.9 The chemical structure of an aliphatic polyolester using pentaerythritol as one of the starting materials. The R's are aliphatic radicals. Complex esters can be considered a subgroup of polyolesters. As both ester groups to a great extent are manufactured from the same raw materials, they are considered as one substance group in the health and environmental assessment. There are very few toxicological data on longer chain (C16 and more) fatty acid polyol esters, and consequently data on shorter chain (< C11) will be included in the assessment. Table 7.12 states the name and CAS number of substances which are included in the health and environmental assessment of polyol esters including complex esters.
Table 7.12 Chemical names and CAS Nos. of substances included in the health and environmental assessment of polyol esters and complex esters in metal working lubricants. 7.9.6.2 Physical/chemical dataPolyolesters and complex esters are large molecules with molecular weights above 400. They are liquids at room temperature with high boiling points (> 200°C), high flash points (> 200°C), very low vapour pressure and very low solubility in water (59,70). 7.9.6.3 Health assessmentAcute toxicity Oral LD50-values from studies with rats indicate a low order of toxicity (LD50 > 2000 mg/kg) for a number of polyol esters (CAS Nos. not stated) (59). Irritation A single polyol ester (CAS No. not stated) was not irritating to rabbit skin and eyes (70). A polyol ester, (PENTA mixed esters, C6,7,8,10 acids (CAS No. 68130-55-2)) applied to the skin of rats five days a week for four weeks at dose levels of 0, 125, 500 and 2000 mg/kg/day caused no visible signs of irritation. However, microscopically treated skin of rats in the two highest dose groups exhibited a dose-related increased incidence and severity of hyperplasia and hyperkeratosis of the epidermis and sebaceous gland hyperplasia. The effects were reversible (59). Sensitization There are no data available on the sensitizing potential of polyol esters or complex esters. Repeated dose toxicity Data from five 28-day oral toxicity studies in rats and one 28-day dermal toxicity study in rats are available for polyolesters designated a) TMP esters of heptanoic and octanoic acid, b) heptanoic acid, ester with 2,2,4-trimethyl-1,3-pentanediol, c) hexanedioic acid, mixed esters with C10-rich, C9-11 isoalcohols and TMP, d) fatty acid, C6-10, tetraesters with PENTA, e) hexanedioic acid mixed esters with decanoic acid, heptanoic acid, octanoic acid and PENTA. No CAS Nos. are stated. The polyol esters a) through d) were well tolerated by rats in the 28-day oral studies. The NOAEL for these studies was 1000 mg/kg/day in rats. The polyol ester a) did not produce signs of overt systemic toxicity at any dose level tested (100, 300, and 1000 mg/kg/day). There were no treatment-related changes in clinical symptoms, functional observation battery or gross post mortem findings. There were no treatment-related mortality, no adverse effects on body weight, food consumption, clinical laboratory parameters, or organ weights. However, an increased number of hyaline droplets in kidneys of the 300 and 1000 mg/kg/day in male rats was observed. Based on these findings, the NOAEL was established at 100 mg/kg/day for male rats. The observed hyaline droplet formation is considered to be of little relevance to humans (59). The polyol ester e) was applied to the skin on groups of 10 (male and female) rats for five days a week for four weeks at dose levels of 0, 125, 500 and 2000 mg/kg/day. Treated animals exhibited no signs indicative of systemic toxicity. No visible signs of irritation were observed at treatment sites. Microscopically, treated skin (500 mg/kg/day or more) exhibited a dose related increased incidence and severity of hyperplasia and hyperkeratosis of epidermis and sebaceous gland hyperplasia. These effects were reversible. None of the minor changes in hematology and serum chemistry parameters were considered biologically significant. High dose females (2000 mg/kg/day) exhibited a significant increase in relative adrenal and brain weight in the female animals. The NOAEL in this study was 500 mg/kg/day for systemic toxicity and 125 mg/kg/day for skin irritation (59). A TMP ester (C8, C10 acid) (CAS No. 11138-60-6), was evaluated for repeated dose toxicity in a 28-day dermal study. Dose levels in the study are not stated. The effects observed as a result of treatment (decrease in body weight and serum protein values) were insignificant and of little toxicological concern. No evidence of microscopic changes was observed in the histopathological evaluation. Thus, the NOAEL for the TMP ester (C8, C10 acid) was 2000 mg/kg bw (59). Together, the findings of the repeated dose toxicity studies suggest that polyol esters exhibit a low order of toxicity following repeated application. Genotoxicity Standard bacterial cell mutagenicity tests (Ames test) and chromosomal aberration tests have been carried out for a number of polyolesters (TMP esters, C7 and C8 acids (CAS No. not stated), TMP esters, C7 acids (CAS No. 71839-38-8), TMP and other alcohols mixed, C6 dioic acids (CAS No. 180788-27-6), TMP ester, C8, C10 acid (CAS No. 11138-60-6), PENTA tetraester, C5-9 acids (CAS No. 67762-53-2), PENTA mixed esters, C6,7,8,10 acids (CAS No. 68130-55-2) (59). The results are in all studies negative, indicating that polyol esters do not possess a genotoxic potential. Reproduction toxicity and carcinogenicity There are no data available on the toxicity of polyol esters towards reproduction and foetal development or the carcinogenic potential. Health rating Based on the available data, polyol esters are assigned health score 1. 7.9.6.4 Environmental assessmentAquatic toxicity The few available data indicate that polyol esters have a low toxicity towards water-living organisms. All available EC/LC50 values are above 100 mg/l. Toxicity tests of polyol esters showed the following EC/LC50 values (16,60):
Environmental fate Referred tests of ready biodegradability showed variable results, e.g. (60):
Bioaccumulation All assessed polyol esters have log POW above 4 (60). Environmental rating Based on the sparse available data on this large group of substances, polyol esters are assigned environmental scores 1 or 2. 7.10 Soaps7.10.1 FunctionThe function of soaps in lubricants for metal working is as lubricant improving additives (polar additives) and corrosion inhibitors (14). 7.10.2 IdentificationSoaps are metal salts of fatty acids. They are produced by reaction of fatty acid esters from animal or vegetable fats heated with aqueous alkali as potassium or sodium hydroxide. Glycerol is produced as a by-product. The reaction is called a saponification. An example of a saponification is stated below (71). Ex.: (C17H35COO)3C3H5 + 3 NaOH → 3C17H35COONa + C3H5(OH)3 The health assessment of soaps focuses on potassium and calcium soaps of long-chain fatty acids (C 16). Experiments have shown that the behaviour of an acid and its salt is very similar in a physical, chemical and toxicological context (72). Thus, the health assessment of the soaps is based on animal and human test both on soaps and on the pure fatty acids of the soaps in addition to experience. Table 7.13 states the name and CAS number of substances which are included in the health and environmental assessment of soaps.
Table 7.13 Chemical names and CAS Nos. of substances included in the health and environmental assessment of soaps in metal working lubricants. 7.10.3 Physical chemical dataSoaps seen in lubricants for heavy-duty metal forming are typically potassium and calcium soaps of longer-chain fatty acids (C ≥ 16). These soaps are solids with melting point above 100°C (16). Sodium soaps are so-called hard soaps, while potassium softs are soft soaps. Both types of soaps are water soluble. The so-called metallic soaps (aluminium, calcium, cobalt, lead and zinc) are not water soluble. (71,73). 7.10.4 Health assessmentAcute toxicity Test data with rats indicate a low acute toxicity of longer-chain fatty acid salts of calcium and potassium. Oral LD50-values range from > 2000 mg/kg for C18 and C18-unsaturated fatty acid salts of potassium (CAS No. 68647-90-5) to > 10.000 mg/kg for C16-18 fatty acid calcium salts (CAS No. 85251-71-4) (16). Dermal LD50-values in rats, rabbits or guinea pigs for long-chain fatty acids and their salts and a single inhalation study in rats for decanoic acid (C10) (CAS No. 334-48-5) also indicate low acute toxicity by these exposure routes (72). Irritation Tests in animals and humans show that the skin irritating potential of fatty acids and their salts decrease with increasing chain length, to the effect that medium chain lengths (C10) are irritants, whereas C12 is minimally irritant and the longer chain lengths, C14 and above, are not irritant (72). To some extent, soaps degrease the skin and make the surface of the skin alkaline. This may increase the skin permeability of substances, which are irritating (74). As with skin irritation, animal test data show that the eye irritation potential of fatty acids and their salts decrease with increasing chain lengths, to the effect that chain lengths C10 and C12 are irritant, whereas the longer chain lengths, C14 and above, are not irritant (72). There are very few reports on eye damage in humans caused by soaps. The effects have been transient (75). Soaps in commercial detergents with free alkali and high alkalinity may cause severe and irreversible eye damage (76). Sensitization Soaps are based on test data for fatty acids and their salts notoriously not sensitizing by skin contact. However soaps degrease the skin and make it more permeable for substances as perfumes and preservatives, which may provoke an allergic response (72,74). Repeated dose toxicity Available tests demonstrate a low toxicity of fatty acids and their salts by repeated exposure. This is consistent with the long history of safe use in foods for both fatty acids and glycerides (72). In a 24-week oral study, rats were fed doses of 15 % oleic acid (C18) (CAS No. 112-80-1) (approximately 7500 mg/kg bw/day). Normal growth and general good health were reported in the rats and the NOAEL was reported to be 7.500 mg/kg bw/day (16). A formulation ”bath soap and detergent” containing 10-25% sodium stearate (C18) was used to conduct a dermal toxicity study in rabbits. Formulations at a dose of 2000 mg/kg bw were applied daily for three months to the skin by syringe, five days a week. No untoward reactions were observed (72). Together the available data indicate low toxicity of fatty acids and their salts by repeated exposure. Genotoxicity Potassium salts of fatty acids, C18 and C18-unsaturated (CAS No. 68647-90-5) has been tested in a standard bacterial mutagenicity assay (the Ames test), with and without metabolic activation, with negative result (16). The fatty acids, capric acid (C10), lauric acid (C12), stearic acid (C18), oleic acid (C18) and fatty acids (C18-22) have produced negative results in the Ames test (72). There is no data available regarding the potential to cause chromosome aberration. Based on available data, there is no indications that fatty acids and their salts possess a genotoxic potential. Carcinogenicity Numerous studies of mechanisms for the role of dietary fat in tumorigenesis have been studied. In a two-year study, groups of male and female rats, initially 7 weeks old, were given sodium oleate (C18) (CAS No. 143-19-1) for 108 weeks at concentrations of 2,5 and 5,0 % in the drinking water. The conclusion of this study was that sodium oleate did not cause cancer in rats exposed to the substance through the diet (72). Reproduction toxicity 15 % oleic acid (C18) (CAS No. 112-80-1) in the diet (approximately 7.500 mg/kg bw/day) for 10 to 19 weeks did not affect the fertility of male rats but appeared to impair reproductive capacity in the female rats by interfering with parturition and mammary gland development. Increased mortality in the offspring was observed. No further information is available (16). In a three-generation study in mice, which were reared on semi-purified diets containing 8.6 % (approx. 12900 mg/kg bw/day) Cuphea oil (contained 76 % capric acid (C10)), no adverse effects on reproductive parameters or any tissue pathology were observed (72). Soap (not specified further) was examined for foetotoxic potential following percutaneous administration. Groups of rats and mice were treated with concentrations of 0.3, 3 and 30 % (corresponding to 50, 500 and 5000 mg/kg/day in mice) of a standard soap solution. The formulated solutions were applied to the skin at the rate of 0.5 ml/rat or mouse per day with rats being dosed on days 2 –15 and mice on days 2 –13 of gestation. There was no evidence of reproductive or foetotoxic effects (72). Health rating Fatty acid esters in the form of triglycerides are occurring in substantial amounts as a natural part of the human diet. Several of the fatty acids are normal degradation products of fats in the human metabolism. Their degradation pathway and fate in the organism are well-known. Potassium, calcium and sodium are all essential nutrients in the human diet. Several of the fatty acids are approved as direct and as indirect food additives. Long experience with safe use of fatty acids and their salts in foods and available test data demonstrate that soaps do not posses carcinogenic, reprotoxic or teratogenic potential (72). Long chain fatty acid soaps of potassium and calcium are assigned health score 1. 7.10.5 Environmental assessmentAquatic toxicity Most of the available data indicated low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. However, toxicity tests with EC/LC50 values below 100 mg/l and even below 1 mg/L were referred (soap with chain length C12-C14) with a LC50(96h) towards Oncorhynchus mykiss of 0.6 mg/L (the geometric mean of five tests) (16). Environmental fate Several biodegradation tests are available. All tests show that soaps are readily biodegradable (16). Bioaccumulation No experimental bioaccumulation data are available. QSAR calculations show log POW values from 0.3 (C7) to 4.7 (C17) (95). Environmental rating Based on the available data, soaps are assigned environmental scores 1 or 2. 7.11 Reference point – medium-chained chlorinated paraffins7.11.1 FunctionThe function of chlorinated paraffins in lubricants for metal working is mainly as extreme pressure additives. In addition, the chlorinated paraffins may constitute a substantial part of the lubricant implying that the compounds also function as a lubricant base (1). 7.11.2 IdentificationChlorinated paraffins are produced by adding chlorine gas to n-alkane fractions (straight-chained saturated hydrocarbons). The general structure of chlorinated paraffins is CxH(2x-y+2)Cly., The chain length of commercial chlorinated paraffins is normally between 10 and 30. Chlorine content varies between 40 –70 %. The chlorinated paraffins are divided into three groups: Short-chained chlorinated paraffins (C10 – C13), medium-chained chlorinated paraffins (C14 – C17) and long-chained chlorinated paraffins (C18 – C30). There may be a further classification of the chloroparaffins depending on their chlorination degree. Low chlorinated paraffins have a chlorination degree < 50 %, whereas high chlorinated paraffins have a chlorination degree > 50 % (2). There exist more than 200 different grades of commercial chloroparaffins. They are all complex mixtures of n-alkanes characterized by an average chain length and chlorination degree (2,3). Table 7.14 states the names and CAS numbers of substances which are included in the health and environmental assessment of medium-chained chlorinated paraffins.
Table 7.14 Chemical names and CAS numbers of substances included in the health and environmental assessment of medium-chained chloronated paraffins. 7.11.3 Physical/chemical dataChloroparaffins are viscous, colourless or yellow liquids. Chloroparaffins with a chain of more than 20 carbon atoms and high chlorination degree (70 %) are solids. Chloroparaffins are chemically very stable, but decompose above 300°C forming hydrogen chloride. The substance group have very low vapour pressures at room temperature, high flash points and are virtually insoluble in water (2). Due to risk reduction measures in the EU for short-chained chlorinated paraffins (SCCPs), medium-chained chlorinated paraffins (MCCPs) are the dominating type of chloroparaffins in lubricants for metal working (3). 7.11.4 Health assessmentThe health assessment of MCCPs are based on data for MCCPs (CAS No. 85535-85-9 (alkanes, C14-17, chloro)) and the similar SCCPs (CAS No. 85535-84-8 (alkanes, C10-13, chloro). Acute toxicity MCCPs have very low oral acute toxicity when ingested. LD50 (rat) > 15.000 mg/kg (16). Animal test data for SCCPs indicate low acute toxicity by skin contact and inhalation. Based on data for SCCP, which is structurally very similar to MCCP, MCCP may also have a very low acute toxicity by skin contact and inhalation (3). Irritation Studies in rats and rabbits have demonstrated slight irritation at most to the skin caused by MCCPs. However, there was some potential for cracking of the skin following repeated dermal application of liquid MCCPs, probably caused by de-fatting properties (3). Studies in rabbits indicate that MCCPs have low eye irritation potential (3). There are no data on respiratory irritation of MCCPs. As there are no reports relating to this endpoint and due to the widespread use of chloroparaffins, this suggests a lacking potential to cause such an effect. The low skin and eye irritation potential and the generally unreactive nature of the substance group lends further support to this (3). Sensitization There was no evidence of skin sensitization in standard tests in Guinea pigs. These data and the generally un-reactive nature of MCCPs indicate that they do not possess a sensitizing potential (3). Repeated exposure A number of studies in rats, mice and Guinea pigs in addition to a single study in dogs examine the effects of MCCPs by repeated oral exposure. These studies demonstrate that liver, thyroid gland and kidneys are the target organs. Only some of the observed effects are considered to be of toxicological significance to humans. However, in rats, single cell necrosis was observed in the liver at 360 mg/kg/day. In addition, in the same study minor effects were observed in kidney tissue at dose levels of 4 mg/kg bw/day and 10 mg/kg bw/day and considered of toxicological significance to humans (3). Genotoxicity Studies of genotoxic effects of MCCPs and the structurally similar SCCPs in bacterial and mammal cell systems and rats and mice indicate that MCCPs do not possess a genotoxic potential (3). Carcinogenicity There are no data on the carcinogenic potential of MCCPs. Rodent carcinogenicity studies using a SCCP (60 % chlorination) produced toxicologically significant dose-related increases in the incidence of several tumour types. Due to the underlying mechanisms of these tumours, they were considered of little or no relevance to human health. However, kidney tubular call adenomas were seen in male rats as a result of exposure to the SCCPs. The underlying mechanism for the kidney tumours has no yet been fully elucidated. The carcinogenic potential to humans cannot be entirely excluded based on the available data. However, recent mechanistic evidence strongly suggests that the mechanism of the kidney tumours are probably not of toxicological significance to humans. This is supported by the general un-reactive nature of chloroparaffins and the fact that MCCPs do not exhibit a mutagenic potential (3). Reproduction toxicity There is no data available in humans regarding effects of MCCPs on fertility and foetal development. One limited animal study is available in relation to the effects of MCCPs on fertility. The administration of a MCCP (52 % chlorination) to rats in a 2-generation reproduction study at up to 384 mg/kg/day for females and 463 mg/kg/day for males in the diet had no apparent effect on fertility (3). No adverse effects during gestation were produced in two reproductive studies with rats and rabbits orally exposed to a MCCP in doses up to 5000 and 100 mg/kg/day respectively (3). In contrast, exposure of rats (dams) to a MCCP (52 % chlorination) at approximately 400 mg/kg/day in the diet produced internal haemorrhaging and deaths in the pups. A mechanistic study has indicated interference of the MCCP to the vitamin-K-dependent neonatal blood clotting system. It is considered that either direct exposure to the MCCP via transferral to the pups in the breast milk disrupted the vitamin-K-dependent clotting system or the pups received less vitamin K in the breast milk due to treatment-related effects upon their mothers. This would appear to be a phenomenon specific for neonates as there is no indication of haematological effects in adult animals in conventional repeated-exposure studies. From the studies available, a NOAEL of 8 mg/kg/day as a maternal dose can be identified for this effect (3). In summary, based on limited animal data, MCCPs do not exhibit an effect on fertility or foetal development when administered to rats up to approximately 400 mg/kg/day in the diet. However, MCCPs may present a hazard to the neonatal offspring via the lactating mother (3). Health rating MCCPs are based on the available data proposed a classification as reproduction toxic in category 3 with risk phrase R63 ”Possible risk of the unborn child”, risk phrase R64 ”May cause harm to breastfed babies” and risk phrase R66 ”Repeated exposure may cause skin dryness or cracking” (Rep3;R63 R64 R66) (3). The substance group is assigned health score 5. 7.11.5 Environmental assessmentAquatic toxicity The medium-chained chlorinated paraffins (MCCPs) seem to have low acute toxicity towards. Data refers LC50(96h) towards fish above 5,000 mg/l. Long-term tests also showed little effect on fish (1). Studies show, however, that MCCPs are very toxic to daphnids. In a short-term toxicity test, LC50(48h) was 6-8 µg/l. Results from long-term tests with NOEC of 10 µg/l are, however, conflicting with the results from the acute test. Other short-term studies show LC50 values of approx. 1 mg/l (1). Environmental fate More tests indicate that biodegradation of MCCPs is limited. BOD5 values of <10 and 20 mg O2/g have been reported for a MCCP (41% Cl) and MCCP (49% Cl), respectively. Other experiments show a decrease in biodegradability with increasing degree of chlorination (1). Bioaccumulation A large number of studies concerning bioconcentration from water are reported in the EU risk assessment of MCCPs (CAS No. 85535-85-9). However, in a number of these studies, calculated test concentrations exceeded the solubility, and it could be suspected that not all of the MCCPs were truly dissolved. A BCF(fish) of 1087 measured for Oncorhynchus mykiss is referred as a reliable result (1). Environmental rating In the draft for EU's environmental risk assessment of MCCPs (CAS No. 85535-89-5), it is proposed that MCCPs are classified as dangerous to the environment: very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment (N;R50/53) (1). Based on this proposal, MCCPs are assigned environmental score 5. 7.12 Conclusion7.12.1 ResultsThe chosen representative substance groups of non-chlorinated lubricants for metal forming are assigned a health and an environmental score. The scores are based on a health and environmental rating system developed by CETOX (Centre for Integrated Environment and Toxicology). The system is developed on the basis of the EU classification system for chemical substances which focus on the inherent properties of substances. The higher the score, the more adverse health or environmental properties of a compound. Thus, score 5 implies the most serious health or environmental effects while score 1 implies the least adverse effects. The rating system is described in further detail in section 7.2. Table 7.14 provides an overview of the result of the health and environmental assessment of representative components in non-chlorinated lubricants.
Table 7.14 The result of the health and environmental assessment of representative substance groups in non-chlorinated lubricants for metal forming. Subgroups are assigned a health and environmental score respectively. Assignment of health or environmental score 5 implies the most serious health or environmental effects, while health or environmental score 1 implies the least adverse effects. For further details see section 7.2. 7.12.2 ConclusionIn the EU human risk assessment draft of medium-chained chloroparaffins (MCCPs) it is proposed, that MCCPs should be classified as toxic to reproduction in category 3 (Rep3) with risk phrases R63 ”Possible risk of harm to the unborn child” and R64 ”May cause harm to breastfed babies” in addition to R66 “Repeated exposure may cause skin dryness or cracking” (3). This classification implies that MCCPs are assigned health score 5. The substance groups mineral base oils, over-based calcium petroleum sulphonates, vegetable and animal oils, sulphurized fatty compounds, synthetic ester oils and soaps enter non-chlorinated lubricants for metal forming lubricants as lubricant bases, lubricity enhancing additives (polar additives) or extreme pressure additives. The health assessment of these substance groups has resulted in an assignment of health score 1 or 2 (except for synthtetic diesters). This indicates that these compounds do not possess properties which may cause serious adverse health effects. The substance groups alkyl sulphides (polysulphides) and phosphorous compounds are added to non-chlorinated lubricants primarily as extreme pressure additives, while synthetic diesters are added as lubricity improving additives and lubricant bases. The three substance groups are assigned health score 1 – 4, 1 – 5 and 1 -5, respectively. This implies that there is a considerable variety in the toxicity of individual substances in the groups. The substance groups includes substances which posses properties which may cause adverse health effects. Some of these substances have the potential to cause adverse health effects as serious as assessed for MCCPs. In the EU environmental risk assessment draft of MCCPs it is proposed that MCCPs should be classified as dangerous to the environment (N) with the risk phrase R50/53 ”Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment” (1). This implies that the substance group is assigned environmental score 5. The environmental assessment of mineral base oils, over-based calcium petroleum sulphonates, vegetable and animal oils, sulphurized fatty compounds, synthetic ester oils (except for some diesters) and soaps result in assignment of environmental score 1 or 2. This indicates that these substance groups possess properties which may cause less severe effects in the environment. Similar to the health assessment of the selected substance groups in non-chlorinated lubricants, the environmental assessment indicates that alkyl sulphides (polysulphides) and phosphorous compounds possess properties which may cause the most severe environmental effects. These two substance groups are assigned environmental score 3 – 5 and 1 – 5. Thus the eco-toxicity of especially phosphorous compounds may vary considerably. Both substance groups include substances which may cause severe environmental effects at a rating level as assessed for MCCPs. Finally, the health and environmental assessment of selected substance groups in non-chlorinated lubricants demonstrates that the health and especially the environmental data platform is poor for a number of the substance groups generally occurring in non-chlorinated lubricants. This includes alkyl sulphides, some of the phosphorous compounds, sulphurized fatty acids and some of the synthetic ester oils. Thus, substitution of chlorinated lubricants to the currently available non-chlorinated lubricant alternatives imply a movement from a reasonable health and environmental data platform to a substantially poorer data platform.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||