Survey and environmental/health assessment of fluorinated substances in impregnated consumer products and impregnating agents
6 Environmental assessment of polyfluoroalkylated substances
- 6.1 Environmental chemistry, pathways and levels
- 6.2 Environmental fate and levels
- 6.2.1 Environmental release
- 6.2.2 Levels in the ambient air and precipitation
- 6.2.3 Levels indoors
- 6.2.4 Occurrence in remote area and long-range transportation
- 6.2.5 Levels in environmental waters, groundwater and drinking water
- 6.2.6 Levels in wastewater and sludge
- 6.2.7 Levels in soil and sediments
- 6.2.8 Levels in biota and wildlife
- 6.3 Ecotoxicity
- 6.4 Environmental risk assessment
6.1 Environmental chemistry, pathways and levels
6.1.1 Background
The family of about 1000 polyfluorinated chemical substances (PFCs) is very diverse with different chemical structures and properties determining their environmental pathways and fate in the biosphere. Some substances are simple perfluoroalkyl carboxylic acids (PFCAs like e.g. PFOA) or sulfonic acids (like e.g. PFOS) and their salts with various lengths of the perfluorinated alkyl groups and eventual branched chains. Other polyfluorinated substances - the substances mostly used in consumer products - are complex, high-molecular derivatives of the acids, e.g. bulky substituted sulfonamides), fluorotelomers (e.g. polyfluorinated alcohols (FTOH) and perfluorinated phosphates (PAPS) or polymers). These substances are mostly non-polar and more volatile but less persistent than the parent perfluoroalkanoic acids (PFAA), they are precursors of.
Polyfluorinated substances are used because of their surfactant properties. They have both hydrophobic and oleophobic properties and are chemically and thermally inert; especially the fluorocarbon chain is extremely resistant to heat and chemical attack, e.g. by acids and bases, and reducing and oxidizing agents.
The C-F binding is very strong. Thus the perfluoro chain is a stable identity, which in practice is non-degradable in nature. On the other hand a bulky functional end group will be more readily transformed in the environment and in organisms, and therefore the compounds will be degraded to the persistent sulfonates (e.g. PFOS) and carboxylates (PFOA) in the end. The sulfonates and carboxylates are polar species, which will not accumulate in fatty compartments but mainly in blood and liver, and these substances will often interact with polar sites in sediments.
The low biodegradability of PFCs is, together with their tendency to bioaccumulate, characteristics typical of persistent organic pollutants (POPs). After PFCs have been found to be distributed globally in all environmental compartments, the use and production of several perfluorinated compounds have been regulated by national and international agencies such as U.S. EPA (2006), Environment Canada (Renner, 2005) and European Union (2006). PFOS has recently been added to the OSPAR list of chemicals for priority action (OSPAR, 2003) and nominated for inclusion in the Stockholm Convention (2005) as a persistent organic pollutant.
6.1.2 Physical-chemical properties
Large differences exist between the water solubility and vapour pressure for the individual PFOS- and PFOA-related substances. Some data are shown in Table 6.1 and Table 6.2. It should be noted that commercial products are not pure substances but may be mixtures and can contain several percentages of branched isomers and traces of other PFCs.
The acids and their free salts are solids that are soluble and dissociated in water and insoluble in lipids and do not evaporate from the water phase. The aqueous solubility will decrease with increased chain length. In absence of being in solution 8:2 FTOH will rapidly sublime at ambient temperature (Kaiser et al. 2006).
Liu & Lee (2007) discovered that the fluorocarbon chain length was the dominant structural feature influencing water solubility and sorption to soil of fluorotelomer alcohols (polyfluorinated alcohols). The water solubility of fluorotelomer alcohols is low and decreases with increasing chain length. Each CF2 moiety decreased the aqueous solubility by about 0.78 log units and increased the sorption by surface soils with 0.87 log units.
Fluorotelomer alcohols have higher calculated vapour pressures than the parent alcohol; for example 10:2 FTOH is 1000 times more volatile than dodecanol, possibly because of the unique molecular geometry with possibility of formation of intramolecular hydrogen bonding (Stock et al. 2004b).
Table 6.1: Water solubility and vapour pressure for some perfluorinated sulfonates (Heckster et al. 2003; Shoeib et al. 2004a; Lei et al. 2004b).
| Substance name | Acronym | CAS no. | Solubility in water (g/L) | Vapour pressure (Pa) | Octanol-air partition coefficient (Log Koa) |
| Perfluorooctane sulfonyl fluoride | PFOSF | 307-35-7 | |||
| Perfluorooctane sulfonic acid | PFOS | 1763-23-1 | |||
| Potassium perfluorooctane sulfonate | PFOS-, K+ | 2795-39-3 | 0.519 0.57 |
3.31 x 10-4 | |
| Perfluorooctane sulfonamide | PFOSA; FOSA | 754-91-6 | |||
| N-Methyl perfluorooctane sulfonamide | MeFOSA | 31506-32-8 | |||
| N-Ethyl perfluorooctane sulfonamide | EtFOSA | 4151-50-2 | 6.9 (25°C) | ||
| N-Methyl perfluorooctane sulfonamidoethanol | MeFOSE | 24448-09-7 | 0.002 | 7.7 (20°C) 7.05 (25°C) |
|
| N-Ethyl perfluorooctane sulfonamidoethanol | EtFOSE | 1691-99-2 | 1.51 x 10-4 | 0.504 | 7.78 (20°C) 7.30 (25°C) |
| N-Methyl perfluorooctane sulfonamidoethyl acrylate | MeFOSEA | 25268-77-3 | |||
| N-Ethyl perfluorooctane sulfonamidoethyl acrylate | EtFOSEA | 423-82-5 | 8.9 x 10-4 | 0.002 | 7.87 (20°C) |
| Perfluorobutane sulfonic acid | PFBS | 29420-49-3 | 0.51 | ||
| Potassium perfluorobutane sulfonate | PFBS-, K+ | 29420-43-3 | |||
| Perfluorohexane sulfonic acid | PFHxS | 432-50-7 | |||
| Perfluorononane sulfonic acid | PFNS | 474511-07-4 | |||
| Perfluorodecane sulfonic acid/sulfonate | PFDS | 335-77-3 |
Table 6.2: Water solubility and vapour pressure for some perfluorinated carboxylates and fluorotelomer alcohols (Heckster et al. 2003; Shoeib et al. 2004a; Toxnet/HSDB; Liu & Lee 2007; Thuens et al. 2007; Kaiser et al. 2006; Stock et al. 2004b).
| Substance name | Acronym | CAS no. | Solubility in water (g/L) | Vapour pressure (Pa) | Octanol-air partition coefficient (Log Koa) |
| Perfluorobutanoic acid | PFBA | 375-22-4 | |||
| Perfluoropentanoic acid | PFPeA | 2706-90-3 | |||
| Perfluorohexanoic acid | PFHxA | 307-24-4 | |||
| Perfluoroheptanoic acid | PFHpA | 375-85-9 | 118 (24°C) | 128 | |
| Pentadecafluorooctanoic acid; Perfluorooctanoic acid | PFOA | 335-67-1 | 9.5 3.4 4.3 (24oC) |
70 20 (25 ºC) |
|
| Branched perfluorooctanoic acid | 90480-55-0 | ||||
| Ammonium perfluorooctanoate | PFOA-, NH4+ APFO | 3825-26-1 | >500 374 (24°C) |
<1.3-9.2 x 10-3 | |
| Sodium perfluorooctanoate | PFOA-, Na+ | 335-95-5 | 169 (24°C) | ||
| Perfluorononanoic acid | PFNA | 375-95-1 | |||
| Perfluorodecanoic acid | PFDA | 335-76-2 | 0.26 (24°C) | ||
| Perfluoroundecanoic acid | PFUnA | 2058-94-8 | 0.092 (24°C) | ||
| Perfluorododecanoic acid | PFDoA | 307-55-1 | |||
| Perfluorotridecanoic acid | PFTrA | 72629-94-8 | |||
| Perfluorotetradecanoic acid | PFTeA | 376-06-7 | |||
| 1H,1H,2H,2H-Perfluorohexanol | 4:2 FTOH | 2043-47-2 | 0.97 (22oC) | 992 | 3.3-4.8 (25oC) |
| 1H,1H,2H,2H-Perfluorooctanol | 6:2 FTOH | 647-42-7 | <1.2-1.7 x 10-2 1.9 x 10-2 (22oC) |
713 | 3.6 -5.3 (25oC) |
| 1H,1H,2H,2H-Perfluorodecanol | 8:2 FTOH | 865-86-1 | 1.40 x 10-4 1.1 x 10-5 (22oC) |
254 | 4.2-5.6 (25oC) |
| 1H,1H,2H,2H-Perfluorododecanol | 10:2 FTOH | 678-39-7 | 6,0 x 10-6 – 8,9 x 10-4 (22oC) |
144 | 4.8 -5-5 (25oC) |
| 1H,1H,2H,2H-Perfluorotetradecanol | 12:2 FTOH | 39239-77-5 | 5.3-5.6 (25oC) | ||
| 1H,1H,2H,2H-Perfluorohexadecanol | 14:2 FTOH | 60699-51-6 | 6.2 (25oC) |
For PFOA the octanol-water partition coefficient (Log Pow) is 5, but the special solubility profiles and surface-active properties of PFOA (and other perfluorinated acids) make environmental fate predictions based on octanol-water partition coefficients irrelevant for these chemicals. Water is believed to be the target compartment for perfluorinated acids in the abiotic environment.
Perfluorinated carboxylic acids (PFCA) are stronger acids than their non-fluorinated counterparts and have the corresponding lower pKa. For PFOA the pKa is 2.80 (Kissa 2001).
6.1.3 Degradation
Because of the very strong C-F binding, the perfluoroalkyl chain is extremely resistant to heat, UV-radiation, chemical attacks by acids and bases, or reducing and oxidizing agents. The functional groups (sulfonamides, esters, alcohol etc.) at the other end of the molecule will be more readily transformed in the environment and in organisms, and the more complex molecules will gradually be degraded to the ultimate, perfluorinated sulfonates and carboxylates, which under normal circumstances seem to persist in the environment for foreseeable time.
The 175 polyfluorinated substances on a list developed by Canadian authorities were studied with a computer program simulating microbial degradation. The prediction was that 109 substances might be precursors and be degraded to PFOS and 61 to PFOA (Dimitrov et al. 2004).
6.1.3.1 Abiotic degradation
Fluorinated organic polymers are very stable to hydrolysis resulting in half-lives from 1-5 years to 500 years. However, heating of fluoropolymers such as poly[tetrafluoroethylene] (PTFE) to more than 350oC, degradation in smaller molecules occurs, including a small (0.01%) formation of PFOA (Ellis et al. 2001).
Yamada et al. (2005) investigated the thermal degradation of a small polyester/cellulose fabric substrate treated with a fluorotelomer-based acrylic polymer under laboratory conditions conservatively representing typical combustion conditions of time, temperature, and excess air level in a municipal incinerator, with an average temperature of at least 1000 oC and 2 seconds residence time. The fabric was destroyed by this treatment, and no PFOA was detected, only SiF4. The authors concluded that under typical municipal waste incineration conditions no significant quantity of PFOA would be formed from the incineration of a textile or paper substrate treated with a fluorotelomer based acrylic polymer, even without consideration of post-combustion pollution control equipment for acid gas scrubbing in place at municipal incinerators. This conclusion is however questionable. Actual waste incineration is performed in another and larger scale and is inhomogeneous and less controlled.
Photodegradation of PFOS by UV irradiation in air is not yet confirmed experimentally but in both water and alkaline 2-propanol slow photodegradation occurred in the laboratory tests; mostly (ten times more) in the alkaline solvent (rate constant: 0.93 days-1). The resulting compounds are mixtures of shorter chain compounds by stepwise removal of CF2 (Yamamoto et al. 2007).
Perfluorocarboxylic acids including PFOA were effectively photodegraded after 4 hrs in water containing persulfate (S2O82-) (Hori et al. 2005).
PFOS and related fluorochemicals are almost completely decomposed in the laboratory using zerovalent iron in subcritical water for six hours forming a. o. CHF3 and F- (Hori et al. 2006).
A few minutes ultrasonic irradiation of PFOS and PFOA in aqueous solution in the laboratory degraded both chemicals to perfluoroalkyl substances with a shorter chain length (Moriwaki et al. 2005).
6.1.3.2 Degradation in air
A prevailing hypothesis on the origin of non-volatile perfluorinated compounds such as PFOS and PFCAs in remote places is that volatile precursors, among others, substituted sulfonamides for PFOS and fluorotelomer alcohols (FTOH) for PFCAs, undergo long-range transport and hereby reach remote areas (Ellis et al. 2004).
The volatile compounds are transport vehicle for the non-volatile fluorinated acids. In the air the precursors will by time degrade to the stable and water-soluble PFCAs, which are washed out of the air or deposited to the surface. Atmospheric lifetime of short chain FTOHs, as determined with its reaction with OH-radicals, was approximately 20 days making the molecule able to travel about 7000 km. A possible chemical reaction (many steps) in the atmosphere is:
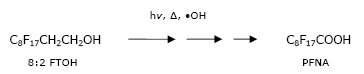
Smoke chamber experiments with fluorotelomer alcohols (4:2, 6:2 and 8:2 FTOH) exposed to chlorine atoms, as a surrogate for OH-radicals, indicated that these chemicals in the atmosphere can oxidise/degrade to series of perfluorinated carboxylic acids (Ellis et al. 2004). The yields from 8:2 FTOH were mainly PFNA (1.6%) and PFOA (1.5%) and to a lesser extend shorter chain acids.
The atmospheric chemistry of N-methyl perfluorobutane sulfonamidoethanol (MeFBSE), a short-chain alternative to PFOS derivatives, was studied by reaction with OH radicals (Martin et al. 2006; D’Eon et al. 2006). The atmospheric life time of this basic chemical was estimated to 2 days; however, it was only degraded to the sulfonamide MeFBSA, which again had an atmospheric life time of >20 days. The ultimate degradation products were perfluorobutane sulfonic acid and perfluorobutanoic acid, short-chain acids which are not considered very bioaccumulative.
6.1.3.3 Biodegradation
Dinglasan et al. (2004) examined the aerobic biodegradation of the 8:2 telomer alcohol (8:2 FTOH) using a mixed microbial system. The initial measured half-life of the 8:2 FTOH (purity 97%) was 0.2 days/mg of initial biomass protein. Volatile and non-volatile metabolites were identified and quantified. The telomer acids: CF3(CF2)7CH2COOH (8:2 FTCA) and CF3(CF2)6CF=CHCOOH (8:2 FTUCA) and PFOA were identified as major metabolites. The telomer acids may further degrade to PFNA and PFOA.
In a laboratory test using a microbial enrichment culture aerobic degradation of 8:2 FTOH was observed (Schröder 2003). Telomer acids and PFOA were identified as metabolites. 85% of the telomer alcohol was degraded after a week. The half-life was about one day. No aerobic degradation of either PFOS or PFOA derivatives was observed. However, microbiological degradation of PFOS and PFOA in contaminated sludge occurred under anaerobic conditions. PFOS was biodegraded more easily than PFOA. The former compound disappeared within 2 days and the later in 25 days. The degradation products were not identified (Schröder 2003).
In another test activated sludge from a domestic sewage plant was used as degradation medium for 8:2 FTOH (Wang et al. 2005a). After 28 days 35% was transformed to unsaturated (5%) and saturated C10 acids (27%) and PFOA (2.1%). This study was followed by a more comprehensive biodegradation study in mixed bacterial culture and activated sludge medium conducted up to 4 months (Wang et al., 2005b). The authors reported the presence of 3 new metabolites along with other five metabolites previously reported. It was found that strong adsorption to the activated sludge greatly reduced partitioning of 8:2 FTOH or any transformation product to air. It was also shown that replenishment of organic carbon enhanced microbial mineralization of multiple –CF2- groups. After 90 days a 12% total mineralization of 8:2 FTOH was observed. Fiebig et al. (2007) found that 8:2 fluorotelomer alcohol also was biodegraded under anaerobic conditions in digested sludge. The main degradation product was, however, an 8:2 fluorotelomer acid.
Researchers at the Clariant Company have found that 8:2 FTOH undergoes biotransformation in soil to oxidation products, including PFOA. The half-life was calculated to 28 days. Bound to a commercial acrylate type polymer this telomer was not released or degraded in an aerobic soil test for one year. Traces of PFOA detected originated from degradation of fluorotelomer residuals in the polymer (Koch et al. 2007).
Biodegradation of 8:2 FTOH in soil by two strains of Pseudomonas has been recently investigated by Liu et al. (2007). These authors observed that transformation of 8:2 FTOH to PFOA was highly dependent on the presence of other easily metabolized carbon sources. However, they concluded that other microbes directly able to utilize 8:2 FTOH as carbon source may exist in the environment.
6.1.3.4 Conclusion
PFOS, PFOA and other perfluoroalkylated acids/salts are very stable chemicals; however, under extreme laboratory conditions with addition of potent chemicals, high-energy radiation and high temperatures some degradation products are formed. However, these chemicals are not biodegradable and will persist in the environment.
The case is different with functional derivatives (e.g. substituted sulfonamides and esters) of these perfluorinated acids and with other polyfluorinated substances not fully fluorinated such as fluorotelomer alcohols. These polyfluorinated substances are somewhat degradable in the environment and will slowly but finally be transformed to the basic perfluorinated acids, which will persist.
A prevailing hypothesis on the origin of non-volatile perfluorinated compounds such as PFOS and PFCAs in remote places is that volatile precursors undergo long-range air transportation and hereby reach remote areas.
6.2 Environmental fate and levels
6.2.1 Environmental release
Emissions of polyfluorinated chemicals to the environment may happen either directly from production and processing plants, or during product use. PFOA is mainly emitted from production of fluoropolymers, such as PTFE (e.g. Teflon®), and from use of products containing PFOA as an impurity.
Production processes and use of fluorotelomers (impregnation, fire-fighting etc.) may emit polyfluorinated substances, which degrade into the persistent perfluorinated acids in the environment. Therefore, polyfluorinated substances are determined in the environment primarily in the form of the final stable degradation products PFOS, PFOA and higher PFCAs. Se also section 6.1 above.
Fluorinated polymeric materials used for textiles, carpets etc. may also release residual amounts of the telomer that failed to be covalently linked to the polymer during production, or the polymeric material may decomposes itself by heat or release polyfluorinated substances by wearing.
Armitage et al. (2006) estimated that between 2700 and 5900 tons of polyfluorinated compounds were emitted to the environment between the years 1950 and 2004. The largest single source contribution (~72% of the total) originated from fluoropolymer manufacturing, followed by ammonium perfluorooctanoate manufacturing (~12% of total) and fluoropolymer dispersion processing (~7% contribution). Direct sources were in this study estimated to be approximately an order of magnitude larger than indirect sources (emissions from product application of telomers etc.).
The historical industry-wide emissions of total PFCAs from direct- and indirect sources were estimated by Prevedouros et al. (2006) to be 3200-7300 tonnes in the period from 1950 to 2004.
The overall emissions from the global fluorotelomer industry have been estimated to contribute approx. 1-2% of the PFCAs in North American rainfall, considered consistent with previous global emissions estimates (Yarwood et al. 2007).
6.2.2 Levels in the ambient air and precipitation
6.2.2.1 Levels in North America
Six different polyfluorinated substances (EtFOSE, MeFOSE, EtFOSA, 4:2 FTOH, 6:2 FTOH, 8:2 FTOH and 10:2 FTOH) have been detected in the air at a highly urbanized site of Toronto, Canada, in 2001. Tropospheric concentrations typically range from 7 to 106 pg/m³ and from 14 to 393 pg/m³. The mean concentrations ranged from 14 pg/m³ for EtFOSA to 205 pg/m³ for EtFOSE, and totally 260 pg/m³. At a rural site (Long Point) in Canada the levels were 2-3 fold less (74 pg/m³) and only one substance (EtFOSE) was found (Martin et al. 2002). In samples from warm summer periods more PFAS was in the gas phase than in the colder seasons.
Stock et al. (2004a) measured in 2001 perfluoroalkyl sulfonamides and fluorotelomer alcohols in the ambient air in six North American cities (Reno (NV), Griffin (GA), Cleves (OH), Winnipeg (MB), Long Point (ON) and Toronto (ON)). Mean concentrations of total perfluoroalkyl sulfonamides (EtFOSA, MeFOSE and EtFOSE) ranged from 22 pg/m³ in Winnipeg to 403 pg/m³ in Griffin. Mean concentrations of total FTOHs (6:2, 8:2 and 10:2) ranged from 11 pg/m³ in Winnipeg to 165 pg/m³ in Toronto. Surface treatment products, previously manufactured by 3M Company for soil, stain and water protection of home furnishings – including carpets, were primarily MeFOSE-based polymers. It corresponded with that the highest mean and single levels of MeFOSE in the air (359 pg/m³ and 1549 pg/m³) were measured in Griffin, a location of carpet production.
A study of atmospheric levels of perfluoroalkyl substances in the Canadian Arctic (Cornwallis Island) in the summer of 2004 was published by Stock et al. (2007). Mean values of gas plus particle phase concentrations of FTOHs (observed in 20-50% of samples) ranged from 2.8 pg/m³ for 10:2 FTOH to 14 pg/m³ for 8:2 FTOH. Levels of sulfonamide derivatives were higher with a total mean concentration of 112 pg/m³. Mean concentrations of PFOSA and MeFOSE were 20 pg/m³ and 29 pg/m³, respectively. Mean concentration of other perfluorinated compounds (EtFOSE, EtFOSA, MeFBSE and EtFBSE) ranged from 11-23 pg/m³. The high concentration of the two PFBS precursors may be caused by the increasing use of perfluorobutane sulfonamide derivatives as substitutes for perfluorooctane sulfonamide derivatives. In the air particulates the non-volatile perfluorinated acids were also detected in this study. The highest observed concentration was that of PFOS (mean 5.9 pg/m³) followed by PFOA (mean 1.4 pg/m³); PFNA and PFDA had both mean concentrations 0.2 pg/m³.
Samples from the Global Atmospheric Passive Sampling (GAPS) study from 40 sites collected in December 2004 to March 2005 were screened for four PFAS (MeFOSE, EtFOSA, EtFOSE and MeFOSEA) (Lee et al. 2007). MeFOSEA was not detected in any sample. For 42% of the sites the other three substances were not detected. In 70% of the sites EtFOSE and EtFOSA were not detected. Most abundant was MeFOSE with the highest concentration of 280 pg/m³ in Athens, Georgia, a region, where carpet production occurred.
6.2.2.2 Levels in Japan
The content of PFOS in airborne dust collected along Japanese roads was up to 427 ng/g. The air concentrations in cities ranged 0.1-2.1 pg/m³ in a rural town and 2.3 -22 pg/m³ in an urban city (Sasaki et al. 2003). Higher concentrations were observed in summer than in winter.
PFOA and PFOS were measured in atmosphere particulate matter at two stations in the Kyoto area, Japan (Harada et al. 2005b). FOA concentrations were significantly higher than PFOS concentrations and ranged between 72 and 919 pg/m³. PFOS concentrations ranged between 2.5 and 9.8 pg/m³.
Shoeib et al. (2004ab) measured three perfluoroalkyl sulfonamides (MeFOSE, EtFOSE and MeFOSEA) used in surface treatment formulation for textile and paper products to impact oil and water resistance in outdoor air. Levels of MeFOSE and EtFOSE in outdoor air were 16-32 pg/m³ and 8.5-10 pg/m³, respectively. MeFOSEA was not detected.
6.2.2.3 Levels in Germany
Jahnke et al. (2007a) carried out a study on the occurrence of airborne PFAS in the gaseous and particulate phase of air samples taken in spring 2005 at a metropolitan site in Germany (Hamburg) as well as in a rural area (Waldhof). A wide distribution of fluorotelomer alcohols (4:2 FTOH, 6:2 FTOH, 8:2 FTOH, and 10:2 FTOH), fluorinated sulfonamides (MeFOSA and EtFOSA), and -sulfonamidoethanols (MeFOSE and EtFOSE) in air was found, and all of the detected compounds belonged to the group of volatile precursor compounds of PFOS and PFOA. The ?FTOHs concentrations ranged from 64-311 pg/m³ (mean 181 pg/m³) in the rural area to the higher levels 150-546 pg/m³ (mean: 288 pg/m³) in Hamburg. The most abundant compound was 8:2 FTOH. The sum of PFOS precursors was in the range 8-68 pg/m³ (mean 34 pg/m³) in Waldhof and in the range 13-171 pg/m³ (mean 68 pg/m³) in Hamburg. A significant correlation was found with the ambient temperature for the partitioning of airborne FOSEs between the gaseous and particulate phase, whereas FTOHs and FOSAs were almost exclusively found in the gaseous phase.
6.2.2.4 Measurements during ship cruises
In 2005 an icebreaker ship cruising in the Arctic from the North Atlantic to the Canadian Archipelago was equipped with a high volume air sampler, and each day during 6-27 July an air sample (particles and gas phase) was collected from the different places during the expedition and later analysed for 3 fluorotelomers and 3 perfluorooctane sulfonamide derivatives (Shoeib et al. 2006). In the twenty 24-h air samples one of the chemicals (MeFOSEA) was not detected at all (<0.001 pg/m³), and 6:2 FTOH was only found in the gas phase with levels ranging <1.1-5.98 pg/m3 and with an arithmetic mean of 2.65 pg/m³. Levels of the two fluorotelomers alcohols (8:2 FTOH and 10:2 FTOH) in the gas phase ranged 4.16-22.7 pg/m³ (mean 11.4 pg/m³) and 1.45-16.4 pg/m³ (mean 6.27 pg/m³), respectively, and in the particle phase levels were in the range 1.07-8.37 pg/m³ (mean 3.50 pg/m³) and 0.29-1.27 pg/m³ (mean 0.80 pg/m³), respectively; thus the levels were much higher in the gas phase. Levels of the two sulfonamides present (MeFOSE and EtFOSE) were also higher in the gas phase and were in the range <1.9-23.6 pg/m³ (mean 8.30 pg/m³) and 1.0-5.17 pg/m³ (mean 1.87 pg/m³), respectively. In the particle phase the levels were <1.7-15.0 pg/m³ (mean 3.53 pg/m³) and <0.001-5.5 pg/m³ (only detected in 25% of samples; mean 1.05 pg/m³), respectively. The results from the remote regions were compared to the results of three samples from Toronto. The results from Toronto samples for 6:2 FTOH, 8:2 FTOH, 10:2 FTOH, MeFOSE and EtFOSE in gas phase ranged between 12.4 and 7.2 pg/m³ (mean 17.7 pg/m³), 25.1-59.6 pg/m³ (mean 40.2 pg/m³), 12.0-36.1 pg/m³ (mean 21.1 pg/m³), 5.38-11.8 pg/m³ (mean 8.0 pg/m³) and 1.04-3.01 pg/m³ (mean 2.33 pg/m³), respectively. In the particle phase the levels were lower, and the results for 6:2 FTOH, 8:2 FTOH, 10:2 FTOH, MeFOSE and EtFOSE were in the range 0.20-0.42 pg/m³ (mean 0.31 pg/m³), 0.30-1.31 pg/m³ (mean 0.71 pg/m³), 0.42-1.82 pg/m³ (mean 1.09 pg/m³), 2.67-6.51 pg/m³ (mean 4.20 pg/m³) and 0.40-1.68 pg/m³ (mean 0.96 pg/m³), respectively. These findings confirmed model predictions of atmospheric transport of perfluorinated chemicals to the Arctic.
Polyfluorinated alkyl substances (6:2 FTOH, 8:2 FTOH, 10:2 FTOH, EtFOSA, MeFOSA, EtFOSE, and MeFOSE) in 8 air samples have been measured on board a German research ship during a cruise from Bremerhaven to Cape Town in South Africa (Jahnke et al. 2007b). The study showed that airborne PFAS are mainly restricted to the Northern Hemisphere with a maximum concentration of 190 pg/m³ of 8:2 FTOH in the first sample collected in the Channel. That was ten times higher than south of Equator. Ionic PFOA and PFOS were determined in the particulate phase of the first sample but at almost two orders of magnitude lower levels; the maximum observed levels were 2.0 and 2.5 pg/m³ for PFOA and PFOS, respectively.
Barber et al. (2007) analysed PFAS in air samples from 4 field sites in Europe (rural, semi-rural and urban). They found that the prevailing substances found were the fluorotelomers 8:2 FTOH and 6:2 FTOH. These volatile PFAS were ubiquitous in air samples, in the gas phase at 5–243 pg/m³ and 5–189 pg/m³, respectively. The concentrations were several orders of magnitude higher in indoor air than outdoor air, making homes a likely important diffuse source of PFAS to the atmosphere.
6.2.2.5 Levels in wet precipitation
Six fluorotelomer carboxylic acids, which are transformation/oxidation products of telomer alcohols, were analysed in rainwater collected in July 2004 in the city of Winnipeg, Canada (Loewen et al. 2005). Low ppt levels of C10- and C12-FTCA and –FTUCAs (oxidation products of 8:2 and 10:2 FTOH) were detected indicating that FTOH may be removed from the atmosphere by oxidation and wet deposition. The concentration of PFOS in rainwater was 0.59 ng/L but none PFCAs was detected.
Perfluoroalkyl carboxylates (PFCAs), fluorotelomer carboxylates (FTCAs) and fluorotelomer unsaturated carboxylates (FTUCAs) were determined in wet only precipitation samples from nine sites in North America (Scott et al. 2006). Significantly higher concentrations of PFOA were found at 4 northeastern United States and 2 southern urban Canadian sites (range: 0.6-89 ng/L). 8:2- and 10:2-FTUCA were detected at all 4 U.S. sites (<0.07-8.6 ng/L).
Barton et al. (2007) have shown that PFOA exists primarily in the particulate phase and is efficiently scavenged by rain droplets, making wet deposition an important removal mechanism from the atmosphere.
6.2.2.6 Conclusion
The polyfluorinated substances identified in air have mainly been the volatile PFOS precursors MeFOSE and EtFOSE, and fluorotelomer alcohols (FTOH) precursors of PFCAs. In urban areas levels were often 10 times higher than in rural areas and remote background levels further 10 times lower. In cities with carpet production the levels were highest (up to 1500 pg/m³). Concentrations observed in the summer were higher than in the winter. PFOS and PFCAs are not volatile and were only found in low concentrations in particulate matters.
6.2.3 Levels indoors
Indoor air levels of perfluorinated chemicals are especially high and may be up to 100 times higher than outdoors, and since the modern individual spends much time indoor, this exposure will be most important for the human body burden of PFCs (Renner 2004). Indoor air may also act as a key source to these chemicals to the outside environment (Shoeib et al. 2007). It is important to note that PFOS, PFOA and other acids and salts have a low volatility and will concentrate in the dust, whereas the volatile polyfluorinated chemicals such as sulfonamides and telomers will dominate in the particle phase in air. Some indoor levels determined in various parts of the World are presented in the following sections.
6.2.3.1 Indoor air levels
Shoeib et al. (2004a) measured in indoor air with passive sampling three perfluoroalkyl sulfonamides (MeFOSE, EtFOSE and MeFOSEA) used in surface treatment formulation for textile and paper products. Levels in indoor air from 4 houses and an old laboratory ranged 667-8315 pg for MeFOSE/m³ (geom. mean 2590 pg/m³) and 289-1917 pg for EtFOSE/m³ (geom. mean 770 pg/m³). Indoor levels of MeFOSEA in three of the houses were 5-283 pg/m³. Indoor air levels were about 25 times higher than outdoor values.
The high indoors air levels of perfluoroalkyl sulfonamides compared to outdoors levels were also confirmed in another study by the same research group. Shoeib et al. (2004b) found by using passive air samplers 25 times higher levels indoors of MeFOSE (geometric mean 1968 pg/m³) and 13 times higher levels of EtFOSE (geometric mean 1033 pg/m³). Results for EtFOSA (geometric mean 54 pg/m³) and MeFOSEA (geometric mean 38 pg/m³) were a magnitude lower. The mean levels indoors were 20-100 times higher than outdoor levels.
Updates of these studies of PFOS derivatives were published by Shoeib et al. (2005a, 2007). The results of 59 air samples are summarized in Table 6.3.
Table 6.3: PFOS derivatives in indoor air (Shoeib et al. 2005a, 2007).
| Indoor air (pg/m³) passive sampling | ||||
| Substance | No. samples with detectable levels | Range | Mean | Median |
| MeFOSE | 59 | 366 - 8190 | 1970 | 1490 |
| EtFOSE | 59 | 227 - 7740 | 1100 | 744 |
| EtFOSA | 52 | 6- 646 | 59 | 40 |
| MeFOSEA | 10 | 12 - 109 | 35 | 29 |
| 8:2 FTOH | 59 | 261-28600 | - | 2070 |
| 10:2 FTOH | 59 | 104-9210 | - | 891 |
Barber et al. (2007) analysed air samples from indoor locations in Norway. Perfluorooctanoate (PFOA) was often the predominant compound found in the particulate phase at concentrations ranging from 1–818 pg/m³.
6.2.3.2 Levels in window film
One of the major sources to fluorinated chemicals indoors is newly impregnated carpets. In Canada levels of perfluoroalkyl contaminants have been measured in inner window film before and after installation of a carpet. The total concentration of contaminants in the carpeted room increased 2.4-fold from 5.62 pg/cm² window before to 13.4 pg/cm² two months after. The major compounds were PFOA followed by PFOS, PFDS and PFTeA (Gewurtz et al. 2007).
6.2.3.3 Levels in house dust
Vacuum cleaner dust from Japanese homes contained between 11 and 2,500 ng PFOS/g dust (mean: 200 ng/g; median: 24.5 ng/g) and between 69 and 3,700 ng PFOA/g dust (mean: 380 ng/g; median: 165 ng/g), respectively. PFOA levels were in general higher than PFOS levels. The highest concentrations of both were in the same sample, and there was an association between PFOS and PFOA in all samples, which indicates a common source (Moriwaki et al. 2003).
Shoeib et al. (2005ab & 2007) analysed both PFOS derivatives and PFOA precursors (fluorotelomer alcohols) in house dust from homes in Ottawa, Canada, sampled in the winter 2002-2003. The results of 66 dust samples are summarized in Table 6.4.
Table 6.4: PFCs in indoor dust (Shoeib et al. 2005ab, 2007).
| House dust (ng/g) | ||||
| Substance | Samples with detectable levels | Range | Mean | Median |
| PFOS | 66 | <1 - 5063 | 443 | 59 |
| MeFOSE | 66 | 3 - 8860 | 412 | 113 |
| EtFOSE | 66 | 1.4 - 75440 | 2200 | 138 |
| EtFOSA | 0 | - | - | - |
| MeFOSEA | 16 | 0.7 - 44 | 14 | 8 |
| PFHxS | 66 | <1 - 4305 | 391 | 46 |
| PFOA | 66 | <1 - 1234 | 106 | 18 |
| 6:2 FTOH | 66 | 2-2500 | 156 | 33 |
| 8:2 FTOH | 66 | 3-16315 | 410 | 55 |
| 10:2 FTOH | 66 | 2-8176 | 233 | 35 |
Levels in paired air and dust samples from the same homes correlated indicating same source for air and dust contamination. Surprisingly EtFOSA was not detected in dust although it has been found in indoor air.
Older homes had lower levels of PFOA and PFOS in the dust, and houses with more carpeting on the floors had higher levels (Zhu et al. 2005).
A series of ionic PFCs was measured in dust from 67 Canadian homes (Kubwabo et al. 2005). The data revealed a correlation between the concentrations of PFCs and the percentage of carpeting in the house.
6.2.3.4 Conclusion
PFOS, PFOA and other acids and salts have a low volatility and will concentrate in the house dust, whereas the volatile polyfluorinated chemicals, such as sulfonamides and telomers, will concentrate in the indoor air, mainly in the particle phase.Indoor air levels of perfluorinated chemicals may be up to 100 times higher than outdoors, and PFC levels in house dust are also high. Levels up to 75 ppm have been determined. A main source seems to be impregnated carpets. Indoor exposure may sometimes be the most important human source of exposure and body burden of PFCs. Indoor air may also act as a key source to these chemicals to the outside environment.
6.2.4 Occurrence in remote area and long-range transportation
The transport of PFOS, PFOA, other perfluorinated acids and their precursors to remote regions, such as the Arctic, is a puzzle for the scientific community (Simcik 2005). Local use in remote regions of fluorosurfactants in fire-fighting foams (airports, fuel storage) and other products may constitute a source of PFOS and PFOA but cannot explain the high level of contamination. Neither can movement of contaminated wildlife. The binding to water and the low volatility make it less likely that PFOS and PFOA themselves will be transported through long distances by the "grass-hopping" and cold condensation mechanisms as persistent organic pollutants (POPs). Nevertheless, the occurence in the Arctic wildlife indicate that polyfluorinated chemicals are distributed widely in the environment and may be transported over very long distances.
6.2.4.1 Contribution from transport with ocean water
Perfluorinated compounds in waters seem to be most concentrated in the surface foam; whether alone in an aqueous solution or with another co-surfactant it is not known. That suggests that marine aerosol (foam) transport should be considered an important long-range transport mechanism (Kaiser et al. 2006).
PFOA directly emitted from polymerization processes, waste water plants, etc. is expected to be present in the environment almost entirely as the dissociated acid/salt. With its negligible vapor pressure, high water solubility and surfactant properties, accumulations in surface waters and to particulate matter herein are likely.
Based on actual concentrations measured in open ocean water, the annually flux of perfluorooctanoic acid (PFOA) to the Arctic was calculated to be between 2 and 12 tons (Prevedouros et al. 2006a; Stock et al. 2007). However, this flux was not considered by the authors to contribute significantly to the observed biota contamination, as evidence suggests that transport from the Atlantic into the Arctic Ocean will be at a depth greater than 200 m.
Using another model a net flux of approx. 8-23 tons per year of PFOA was estimated to flow into the Northern Polar zone in 2005, and that was 20-60 times greater than the amount estimated to be deposited from the global emission, distribution, and degradation of 8:2 FTOH (Armitage et al. 2006).
Transport of PFOA to the Arctic via the ocean takes on the order of decades (20-30 year) as a result of the time required for extremely persistent chemicals to redistribute throughout the oceans (Armitage et al. 2006). In addition, oceanic rates of exchange between northern temperate oceans and the Arctic are likely to be different for the Atlantic and Pacific Oceans which could lead to varying time lags depending on the major oceanic transport routes for PFOA to the Arctic. This means that a delayed response in the Arctic to emission reductions in industry countries will be observed. If concentrations in the primary exposure media (i.e., surface water) continue to increase due to direct sources, it follows that concentrations in wildlife will also continue to increase long after emissions have been drastically reduced or even eliminated.
6.2.4.2 Contribution from transport with air
It was estimated by Ellis et al. (2004), based on prevailing air concentrations, that atmospheric oxidation of FTOHs gives rise to a (C8-C10) PFCA flux in the range 1-100 t/yr in North America or a deposition of 40-4100 ng/m².
Newer results for the calculation of the globally distribution of PFOA using the IMPACT 3-D chemistry/transport model analysis, suggest that telomers and their degradation products are ubiquitous in the Northern Hemisphere (Wallington et al. 2006; Nielsen and Andersen 2007). The concentrations of 8:2 FTOH and its degradation products are typically a factor of five lower in remote oceans and Arctic locations in the Northern Hemisphere, and it is consistent with an atmospheric lifetime of 20-40 days for the group as a whole, which makes them able to travel more than 7000 km. Telomer species in the remote Northern Hemisphere in the model are one-third primary 8:2 FTOH, one-third long-lived fluorine-containing aldehydes, and one-third terminal reaction products. PFOA is also ubiquitous in the Northern Hemisphere. Using estimated emissions of FTOHs based on air concentrations and the three-dimensional model, 0.4 t/yr of perfluorooctanoic acid was calculated to be deposited at latitudes north of 65°N via atmospheric oxidation. The modeling results do not prove that the atmospheric oxidation of 8:2 fluorotelomer alcohol is the source of PFCAs observed in remote locations. However, the results show that with current estimates of chemistry and flux, the atmospheric oxidation of 8:2 FTOH can provide a quantitative explanation for the presence of PFCAs in remote regions.
Young et al. (2007) described the investigation of high Arctic ice caps to determine seasonal cycles, temporal trends, and atmospheric fluxes in order to illuminate the source of PFOA to the Arctic. They found high fluxes for total PFCAs (PFOA and PFNA dominated) averaging 313 kg in 2004 and 651 kg in 2005. These fluxes agree with those determined through modeling of FTOH degradation by Wallington et al. (2006). The flux for PFOS was a little above 30 kg/year in 2005. PFOS levels were 10 times higher, when they peaked in 1999. That is opposite to the levels of PFCAs, which increase at a constant rate.
6.2.4.3 Comparisons of atmospheric and aquatic input
Wania (2007) carried out a model simulation with the zonally averaged global fate and transport model Globo-POP, in combination with historical emission estimates for FTOHs and PFOA, in order to evaluate the relative efficiency and importance of the two transport pathways (air and sea) of direct and indirect PFOA sources. The author used a global model that considers and contrasts both transport hypotheses with the objective to evaluate their relative efficiency and importance, building on the model used by Armitage et al. (2006). It also needed to be confirmed that reductions of the emissions of volatile precursor compounds would indeed result in fast declines in Arctic seawater concentrations. Estimates of the emission-independent Arctic Contamination Potential reveal that the oceanic transport of directly emitted PFCAs is more than 10-fold more efficient than the atmospheric degradation of FTOHs in delivering PFCAs to the Arctic, mostly because of the low yield of the reaction. The cumulative historic emissions of FTOHs are lower than those estimated for PFOA alone by a factor of 2-3, further limiting the contribution from precursor oxidation.
A focused investigation of environmental media (water and soil) was performed by Davisa et al. (2007) in order to understand the pathways for transport of ammonium perfluorooctanoate (APFO) from a manufacturing plant. These results led to a conceptual model that indicates deposition of airborne material as a primary contributor to aquatic and terrestrial systems near the facility.
6.2.4.4 Conclusion
Simcik and Dorweiler (2005) proposed pathways for environmental distribution of perfluorochemicals and compared atmospheric and non-atmospheric sources to surface waters (see Figure 6.1).
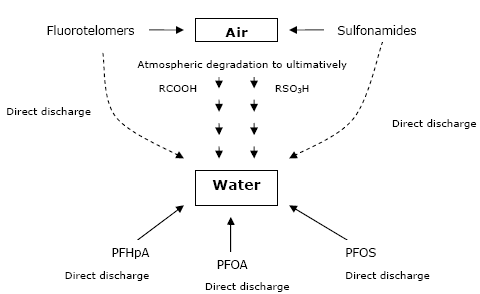
Figure 6.1: Pathways to environmental distribution of perfluorochemicals (modified after Simcik and Dorweiler 2005)
They concluded that “urban” waters, such as Lake Michigan, received most input from direct discharge from waste water treatment effluents, while remote surface waters did receive most perfluorinated chemicals from atmospheric deposition of degradation products. The ratio of PFHpA to PFOA increased with increasing distance from non-atmospheric sources and could be used as an indicator.
According to Ellis et al. (2004) and Wallington et al. (2006), atmospheric transport and degradation of 8:2 FTOH is the most plausible explanation for the fast response to changes in production and changing concentrations of PFOA in ice cores. The findings of the non-commercial PFDA and PFUnA with similar concentrations argue against the deposition of direct transported PFOA but for contamination by atmospheric oxidation. Additionally, these studies state that PFOA levels in the Arctic are too high to be explained by oceanic transport. This is, however, in disagreement with the studies by Armitage et al. (2006) and Wania (2007). Thus, presently no consensus exists in the scientific community regarding the most important transport pathways and environmental fate of PFOA.
6.2.5 Levels in environmental waters, groundwater and drinking water
Contamination sources of aquatic ecosystems from PFCs can mainly be identified as direct discharge from production of fluorochemicals, effluents from wastewater treatsment plants (WWTPs) and accidental discharge of e.g. fire-fightings foams containing fluorochemicals. Atmospheric transport also contributes to contamination of the aquatic environment. In the case of accidental discharge relatively large concentrations of PFCs may occur in surface water or groundwater (concentration level: µg/L). Otherwise, PFCs concentrations in oceanic water or surface waters are in the concentration range ng-pg/L. At these concentrations no toxicity of these compounds is observed. However, these data are important to identify the sources of PFCs in the environment and to understand the global circulation of these compounds in the aquatic environment.
A very sensitive analytical method allowed the detection of PFCs at sub-ppt levels in oceanic water collected during several international research cruises undertaken during 2002-2004 in the central to eastern Pacific Ocean (19 locations), north and mid Atlantic Ocean (12 locations), south China and Sulu Seas (5 locations) and the Labrador Sea (20 locations) (Yamashita et al. 2005). An additional 50 samples of coastal seawater from several Asian countries were analyzed. PFOA was the major contaminant detected in oceanic waters, followed by PFOS. The concentrations of PFOS and PFOA in coastal waters were about a factor 1000 higher than those from oceanic waters.
In a general screening of polar compounds in surface and drinking water from northern Italy, Loos et al. (2007) found PFCs concentrations between 0.2 and 8.1 ng/L. Concentrations in tap water were very close to those found in lake water. Since the tap water was obtained from processing of lake water, the contamination of drinking water from PFCs originates directly from the contamination of lake water. This indicates that perfluorinated surfactants are at present not successfully removed by water treatment steps. Much higher concentrations of PFCs were found in drinking water obtained by surface water treatment in the Rhine-Ruhr region in Germany (Skutlarek et al. 2006). These authors found up to 598 ng/L total PFCs concentration in drinking water and up to 4385 ng/L total PFCs in surface water.
Surface water contamination by PFCs was also found in the Cape Fear drainage basin in North Carolina, USA (Nakayama et al. 2007). One hundred samples from 80 different locations were collected and analysed for 10 target PFCs. Detectable levels of the target compounds were found in all samples with maximum concentration of perfluoroheptanoic acid (C7) of 329 ng/L.
A total of 14 PFCs were quantified in river water samples collected in the Pear River basin (south China) and the Yangtze River basin (central China) (So et al. 2007). PFOS was the dominant compound found in samples from the Pear River basin, while PFOA was the predominant compound found in the Yangtze River basin. Considerable amounts of perfluorobutane sulfonate (PFBS) or 22.9-26.1% of total PFCs analysed, were found at a specific location, indicating local sources of this particular compound. Different PFC profiles were observed in the two basins, indicating the presence of dissimilar sources in the two regions.
The discharge of C6-C9 perfluorinated carboxylates (PFCAs) was studied and employed to assess European emissions of these compounds (McLachlan et al. 2007). PFCAs were determined in water collected to the mouth of 14 major European rivers, including the Rhine, Elbe, Danube, Oder, Seine, Loire and Po. The highest PFOA discharge was found for river Po (Italy) and the authors suggested that these concentrations were due to industrial sources. PFOA concentrations in the lower ng/L range were found for the other rivers, indicating widely distributed sources as significant contributors to the PFCs emissions to waters in Europe.
In conclusion, the contamination of surface water from PFCs may pose an important intake source of PFCs for the consumer when the surface water is used for production of drinking water. Direct contamination of groundwater from accidental spill has not been reported recently. Particularly contaminated surface waters (e.g. some rivers in central Europe) significantly contribute to discharge of PFCs in seas and oceans.
6.2.6 Levels in wastewater and sludge
In the past two years there has been more focus on investigation of mass flow of PFCs in municipal and industrial wastewater plants, since these have been recognized as important point source to the diffuse pollution of the aquatic environment from PFCs.
The origin and amount of PFCs in six wastewater treatment plants (WWTP) and their transformation during treatment have been evaluated by measuring these compounds in influent, effluent, and river water at the point of WWTP discharge in Iowa City (USA) (Boulanger et al. 2005). PFOS and PFOA were quantified in effluent (26±2.0 and 22±2.1 ng/L, respectively) and river water (23±1.5 and 8.7±0.8 ng/L, respectively). The biotransformation of EtFOSE was also investigated in order to determine whether EtFOSE could be the precursor compound for the presence of PFOS-related compounds after treatment. The results suggested that transformation of precursors is not an important source for PFOS and PFOA compared to direct use and disposal of products containing the end products as residual amounts.
Higgins et al. (2005) developed an analytical method for the analysis of perfluoroalkyl sulfonates, perfluoroalkyl carboxylates, and PFOS precursors (MeFOSAA and EtFOSAA). The method was applied to a limited survey of these compounds in domestic sludge and sediments from the San Francisco Bay Area. The concentration of PFCs in domestic sludge ranged from 5 to 153 ng/g for total PFCAs and from 55 to 3370 ng/g for perfluoroalkyl sulfonates. PFOS precursors were also found in both sediment and domestic sludge at levels often exceeding those of PFOS.
A different method was developed by Schultz et al. (2006a) for the analysis of PFCs in municipal wastewater influents and effluents. The method included perfluoroalkyl sulfonates, fluorotelomer sulfonates, perfluorocarboxylates, and selected perfluoroalkylsulfonamides. The method was applied to analysis of raw influent and final effluent of ten wastewater treatment plants located nationwide in USA. PFCs were observed in wastewater at all treatment plants and each plant exhibited unique distributions of PFCs despite similar treatment processes. In nine out of ten plants, at least one class of PFCs showed increased concentrations in the effluent water. In some plants, decreased concentrations in effluent water were attributed to sorption to sludge.
In a following work, Schultz et al. (2006b) investigated the fate of PFCs in a municipal wastewater treatment facility in USA by analysing raw influent, primary and secondary effluent, and sewage sludge. Precursor of the acidic compounds, such as perfluoroalkyl sulfonamides and 6:2 fluorotelomer sulfonate were included in the analytical program. Mass flows of 6:2 fluorotelomer sulfonate and PFOA were unchanged after wastewater treatment, while a net increase in the mass flow of PFOS and perfluorodecane sulfonate (PFDS) occurred after treatment. Mass flows of perfluoroalkyl sulfonamides and perfluorononanoic acid (PFNA) also increase after activated sludge treatment. The authors concluded that conventional treatment in wastewater plants are ineffective in removing most of PFCs and more data are needed on the precursors such as perfluoroalkyl sulfonamides, perfluoroalkylethanols or fluorotelomer olefins, which could account for the observed increases in fluorochemical mass flow.
In a similar study Sinclair and Kannan (2006) observed an increase in mass flow of several PFCs measured in six wastewater treatment plants from New York State. The authors hypothesize that the observed increase may have resulted from degradation of precursor compounds such as fluorotelomer alcohols, which was supported by a significant correlation in the mass flow of PFOA/PFNA and PFDA/PFUnA. Dominance of the longer carbon chained PFCAs were found in sludge samples, suggesting a preferential partitioning of these compounds to sludge.
On the basis of the results of the studies performed on PFCs in municipal and industrial wastewater plants, it can be concluded that these plant are important point sources for discharge of PFCs into the aquatic environment. While domestic waste appears to be a consistent source of PFOS, commercial waste introduces higher and more variable levels of PFOA. Precursor, such as PFOS-based chemicals, fluorotelomer alcohols and sulfonamides are transformed to PFOS and PFCAs after activated sludge treatment. Several studies provide evidence that PFCs are not removed from wastewater by conventional treatment and therefore their dischare into receiving waters is of particular concern.
6.2.7 Levels in soil and sediments
There are few studies reporting the levels of PFCs in soil and sediment and investigations about the potential for bioaccumulation of PFCs from sediment or soil. Complicating the study of PFC fate in soil or sediment-water systems is the presence of precursors. It is not yet clear whether these precursors can contribute to PFOS bioaccumulation or they are a less bioavailable form of PFOS.
A newly developed analytical method for determination of perfluorocarboxylic acids was applied to a screening of these compounds in harbour sediments from Barcelona, Spain (Alzaga et al. 2005). PFCAs with carbon chain from C8 to C10 were detected in the concentration range 10.4-12.4 ng/g in 50% of the samples taken from the commercial harbour. Lower concentrations (<1.3-2.6 ng/g) were detected in sediments from the marina.
The sorption of three classes of PFCs (perfluorosulfonates, perfluorocarboxylates and perfluorooctyl sulfonamide acetic acids) has been reported by Higgins et al. (2006). Sediment content of organic carbon was the dominant parameter affecting sorption, indicating the importance of hydrophobic interactions. Both the length of the perfluorocarbon tail and the functional group were important to determine sorption. Each CF2 moiety contributed by approximately 0.5-0.6 log unit to the distribution coefficients. The sorption of perfluorosulfonates was on average 1.7 times stronger than the perfluorocarboxylate analogues.
In a follow up study Higgins et al. (2007) assessed the bioaccumulation of the same classes of PFAS from contaminated sediments using the freshwater oligochaete Lumbriculus variegatus. The results suggested that PFCs in sediments are readily bioavailable and that bioaccumulation from sediments does not increase with increasing perfluorocarbon chain length. PFOS and PFBA were the most bioaccumulative PFCs. EtFOSAA accumulated in the worm tissues and appeared to undergo biotransformation to PFOS and other PFOS precursors.
6.2.8 Levels in biota and wildlife
Most of the studies published until 2004 have been focused on concentration of PFCs in selected biota from a specific area/ecotype or on bioaccumulation through the food chain of these compounds. In the recent years several studies have been published on temporal trends of PFAS concentrations from 25-30 years ago to the present year. Several studies have extended their analysis program to new compounds such as fluorotelomer sulfonates and fluorotelomer saturated and unsaturated carboxylates (FTCAs, FTUCAs). The last ones are degradation products of fluorotelomer alcohols (FTOH).
6.2.8.1 Temporal trend studies
One of the first published temporal trend study of PFCs was performed using archived guillemot eggs from the Baltic Sea (Holmström et al. 2005). Samples were collected from Stora Karlsö (Sweden) between 1968 and 2003. PFOA was not detected in any sample (MDL 3 ng/g ww). A 30-fold increase was observed for PFOS concentrations, from 25 ng/g ww in 1968 to 614 ng/g ww in 2003, which corresponded to a 7-11% average increase per year (see Figure 6.1).
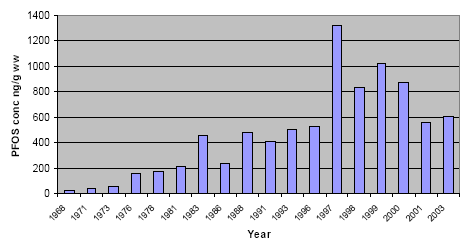
Figure 6.1: Trends of PFOS concentration in Guillemot eggs from Stora Karlsö at Gotland in Sweden (Holmström et al. 2005).
A time trend study of PFCs concentrations was performed in beluga (Delphinapterus leucas) from Baffin Island in the Canadian Arctic in the period 1982-2002 (Tomy et al. 2005). PFOS, PFOSA and PFCAs (C8-C12) were analyzed together with FTCAs, FTUCAs and EtFOSA. The last three groups of compounds were not detected in any sample. Exponential increase in both PFOA and PFDA concentrations were observed with respective doubling times of 6 and 11 years. The concentration profile for both PFOSA and PFOS were similar. PFOS and PFOSA concentrations were found to be increasing up to 2002 although there was a small decrease in concentration in 1995 for both compounds.
Spatial and temporal trends in the concentrations of selected PFCs were measured using archived liver samples of ringed seal (Phoca hispida) from East and West Greenland (Bossi et al. 2005a). The samples were collected in four different years at each location, between 1986 and 2003 in East Greenland and between 1982 and 2003 in West Greenland. PFOS was the major contributor to the burden of PFCs in samples, followed by perfluoroundecanoic acid (PFUnA). Perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA) were also detected in most samples. Regression analysis of logarithmic transformed PFOS, PFDA and PFUnA median concentrations indicated a significant temporal trend with increasing concentrations at both locations. A spatial trend in PFOS concentrations was observed between the two sampling locations, with significantly higher concentrations in seals from East Greenland.
Archived polar bears (Ursus maritimus) liver tissue samples from two locations in the North American Arctic were analyzed for PFCs (Smithwick et al. 2006). The study covered the period 1972-2002. The samples originated from an eastern location (Baffin Island, Canada) and a western location (Barrow, Alaska). Concentrations of PFOS and PFCAs with carbon chain length between C9 to C11 showed an exponential increase between 1072 and 2002 at both locations. The doubling time for PFOS was similar to the doubling time of production of perfluorooctylsulfonyl-fluoride-based products in the 1990s. The degradation products of FTOH, 8:2 and 10:2 fluorotelomer acids and their unsaturated acid counterparts, were also analyzed but they were not detected in any sample.
PFOS concentrations in eggs from two colonies of herring gulls (Larus argentatus) from Northern Norway showed a nearly 2-fold increase in the period 1983-1993, followed by a levelling off between 1993 and 2003 (Verreault et al. 2006). The SPFCAs (8 to 15 C) also showed a marked increase in 1983-2003 and only a weak decrease in the period 1993-2003. The accumulation profile of PFCAs was characterized by a higher proportion of odd carbon chain length compounds.
Temporal trends in PFCs concentrations were investigated in liver samples from two ringed seals (Phoca hispida) populations in the Canadian Arctic in the period 1992-2005 (Arviat, West Hudson Bay) and in the period 1972-2005 (Resolute bay, Lancaster Sound) (Butt et al. 2007a). PFAS analysed included C7-C15 perfluorinated carboxylates (PFCAs) and their suspected precursors, the 8:2 and 10:2 fluorotelomer saturated and unsaturated carboxylates (FTCAs, FTUCAs), C4, C6, C8, C10 sulfonates, and perfluorooctane sulfonamide (PFOSA). Perfluoroheptanoate (PFHpA), perfluorohexane sulfonate (PFHxS), perfluorodecane sulfonate (PFDS) were not detected in any sample. Fluorotelomer saturated and unsaturated acids were detected in some individuals, most of the time at concentrations close to the detection limit. The detection of fluorinated telomer acids supports the hypothesis of FTOH as a source of PFCs in the environment. Statistically significant decreasing PFOS concentrations were observed in both populations from 2000 to 2005. According to the authors, these results indicate that the ringed seals and their food web are rapidly responding to the phase out of perfluorooctane sulfonyl fluoride based compounds by 3M in 2001.
Another temporal trend study investigated the concentrations of PFAS in liver samples from two seabird species, thick-billed murre (Uria lomvia) and northern fulmars (Fulmaris glacialis) from the Canadian Arctic (Butt et al. 2007b). In contrast to most other wildlife samples, PFCs profiles were dominated by long-chained perfluorinated carboxylates (PFCAs), mainly in the range C11-C15 PFCAs. PFCs concentrations were found to increase significantly from 1975 to 2003/2004. Again, detection of the 8:2 and 10:2 FTUCAs suggested FTOH as a source of some PFCAs in arctic birds.
Many temporal trend studies on PFOS have shown increasing concentrations over time. Temporal trends studies are particularly useful not only to follow the historical use of PFCs, but also to follow in the future the effect of ceased production and use of PFOS-based products in Europe and North America. In the Arctic regions a different picture is obtained when looking at PFOS concentrations in ringed seals from the Canadian Arctic and Greenland. PFOS is still increasing in ringed seals from Greenland, while a statistically significant decrease was observed in ringed seals from the Canadian Arctic after 2000. This will suggest different sources and transport path to the Arctic regions.
6.2.8.2 Biomagnification studies
Several biomonitoring studies have been conducted in which simultaneous concentrations of PFCs in organisms, water and sediments have been measured, thus enabling to estimate field-based bioaccumulation factors (BAFs). The trophic magnification factor (TMF) has also been determined in several studies performed in different food webs.
Studies involving the biomagnification of PFAS through the food chain involving the analysis not only of biota but also of abiotic matrices from the same environment are reported in this paragraph.
A food web from Lake Ontario was investigated for the presence of PFCs (Martin et al. 2004). The investigation included a top predator fish, forage fish species, and two invertebrates. The highest mean concentration of each PFC was detected in the benthic macroinvertebrate Diporeia hoyi. The observed concentrations were often 10-fold higher than those found in Mysis relicta, a predominantly pelagic feeder. This suggested that the sediment and not the water was the major source for PFCs contamination. Bioaccumulation of PFCs was observed at the top of the food web, with the exception of PFOA.
Trophic transfer of PFCs was examined in Great Lakes benthic food web; in addition, PFCs were analyzed in predatory fish (Chinook salmon, carp and lake whitefish) and in eggs from brown trout from Michigan (Kannan et al., 2005a). PFCs were also analyzed in green frog livers, snapping turtle plasma, mink livers and bald eagle tissue. The calculated bioconcentration factor (BCF) for PFOS concentration in biota/concentration in water was 1000 in benthic invertebrates. Concentrations of PFOS in mink and bald eagles were 5- to 10-fold greater than those in salmon, carp or snapping turtle. Eggs of fish contained notable concentrations of PFOS, suggesting oviparous transfer of this compound. The calculated biomagnification potential of PFOA was lower than that of PFOS.
A preliminary screening of PFOS and related compounds has been performed in liver samples of fish, birds and marine mammals from Greenland and the Faeroe Islands in order to assess the presence and distribution of PFCs in the food chain from these two remote areas (Bossi et al. 2005b). PFOS was found at concentrations above LOQ (10 ng/g wet weight) in 13 out of 16 samples from Greenland and in all samples from the Faeroe Islands. The results from Greenland showed a biomagnification of PFOS along the marine food chain (shorthorn sculpin<ringed seal<polar bear). The greatest concentration of PFOS was found in liver of polar bear from East Greenland (mean: 1285 ng/g wet weight, n = 2).
Distribution of PFCs through the food chain has been investigated in biota from the Southern Ocean and the Antarctic (Tao et al. 2006). In this study, livers from albatrosses, blood from elephant seals, and blood and eggs from penguins and polar skua were analyzed for PFCs. The samples were collected in the period 1995-2005. PFOS was found in all liver samples from albatrosses from the Southern Ocean, while PFOA was found in 30% of the samples. PFOS was also found in the blood of elephant seals from Antarctica at concentrations ranging from <0.08 to 3.52 ng/mL. This study documents the global scale distribution of PFCs.
Occurrence of PFCs was found to be ubiquitous in a study from New York State (Sinclair et al. 2006). This study included the analysis of surface water from the main water bodies of NYS, liver from two species of popular sport fish, smallmouth (Micropterus dolomieu) and largemouth brass (Micropterus salmoides) and liver from both migratory and resident bird species, the totally piscivorous common merganser (Margus merganser) and the highly herbivorous mallard (Anas platyrhyncos). PFOS was the most abundant compound found in all biota, whereas high concentrations of PFOA were found in Hudson River. Significantly higher concentrations of PFOS were found in piscivorous birds than in non-piscivorous birds. An average BCF of 8850 was estimated for PFOS accumulation in fish relatively to ambient water. An average BMF of 8.9 was estimated for the accumulation of PFOS in the fish common merganser.
The fate of PFCs in shallow water marine organisms and a food web represented by sediment-associated tidal flat organisms was investigated in Ariake Sea, Japan (Nakata et al. 2006). In shallow water species, PFOS was the dominant compound, while PFOA was the most abundant compound in tidal flat organisms and sediments, indicating differences in exposure between the two ecosystems.
The environmental distribution and the biomagnification of a series of PFCs were investigated in the food web of the bottlenose dolphin (Tursiops truncatus) near Florida (USA) by Houde et al. (2006). PFCs were analyzed in WWTP effluents, seawater, sediment, zooplankton, whole fish, and plasma from dolphins. The fact that PFCs were found at high concentrations in WWTP effluents suggested that these facilities were sources for PFCs contamination of the food web. Biomagnification factors (BMFs) ranged from <1 to 156. Trophic magnification factors (TMFs) of PFOS were in the range of those predicted for Arctic food webs, indicating that the rate of accumulation of PFOS is not dependent on differences in the food web and climatic conditions.
Concentrations and biomagnification potential of PFCs was investigated in species from the Barents Sea food web, including amphibians, fish and birds (Haukås et al. 2007). PFOS displayed the highest concentrations, with values up to 225 ng/g in glaucous gull liver. Liver based magnification factors displayed values >1 for PFHxS, PFOS, PFNA and SPFCs in the majority of predator-pray relationship. The authors found that the degree of trophic transfer of PFCs was similar to that of PCB, DDT and PBDE.
Based on the empirical laboratory and field data, PFCs with seven or fewer fluorinated carbons are not bioaccumulative and do not possess significant biomagnification potential in food webs. In studies measuring the accumulation of PFCs in aquatic-based food webs, biomagnification of perfluorinated acids has been noted to be several orders of magnitude lower than that of most persistent lipophilic compounds, with PFOS being identified as the only perfluorinated acid exhibiting potential for biomagnification. More research is needed to charachterize the bioaccumulation potential of PFCAs with longer fluorinated carbon chains (>8).
6.2.8.3 Levels in fish and mussels
A study performed in ten north-central Portuguese estuaries evaluated the tissue burden of PFOS in mussel (Mytilus galloprovincialis) and the potential relationship between PFOS content and age/size and sex of the organisms (Cunha et al. 2005). PFOS was observed in all mussels collected with whole body burden concentrations in the range 36.8 – 129 ng/g. Significant different PFOS concentrations were observed in the different body tissues with higher concentrations in haemolimph and digestive gland tissues. Significant differences were also found between mature and non-mature animals, the latter showing significantly lower PFOS levels.
The PFOS concentration in fish liver from freshwater locations in Flanders (Belgium) was correlated to biological endpoints (serum ALT enzyme activity, serum protein content, hematocrit value, serum electrolyte levels, condition and growth rate) (Hoff et al. 2005a). Although some biological endpoints were suggested to be altered by PFOS exposure, no indications were obtained for a decrease in fish condition or reduce growth capacity.
A limited number of mussels and oyster samples from South China and Japan were analyzed in order to test a new developed analytical method for PFCs determination in biota (So et al. 2006). Concentrations ranged from 0.114 to 0.586 ng/g for PFOS, 0.063 to 0.512 ng/g for PFHxS, <0.012 to 0.030 ng/g for PFBS, and 0.038 to 2.957 ng/g for PFOSA. Oyster samples from Tokyo Bay had the greatest concentrations of PFOS and PFOSA.
Perfluorinated carboxylates (PFCAs), perfluorinated sulfonates and unsaturated fluorotelomer carboxylates (8:2 and 10:2 FTUCAs) were measured in lake trout (Salvelinus namaycush) samples from the Great Lakes in Canada (Furdui et al. 2007). The major perfluoroalkyl contaminant observed was PFOS with the highest concentration found in samples from Lake Erie (121±14 ng/g). Perfluorodecane sulfonate (PFDS) was detected in 89% of the samples. The 8:2 FTUCA was detected at concentrations ranging between 0.1 and 0.2 ng/g; with the highest level in samples showing also elevated concentrations of PFOA. The presence of 8:2 FTUCA and PFOA may originate from metabolization of 8:2 fluorotelomer alcohol (8:2 FTOH) as observed in rat metabolism by Martin et al. (2005). The 10:2 FTUCA was detected only in 9% of the samples.
6.2.8.4 Levels in birds
PFCs have been detected worldwide in seabirds and terrestrial birds. The highest PFCs concentrations are found in birds from the industrialized areas. PFCs concentrations in fish-eating birds are usually lower than concentrations measured in fish-eating mammals. This could be due toa different trophic position in the respective foodweb or a shorter elimination half-life in birds.
PFCs levels were investigated in plasma, liver, brain and eggs from adult glaucous gull (Larus hyperboreus) from the Norwegian Arctic (Verreault et al. 2005). PFOS was highest in plasma (48.1-349 ng/g wet weight), followed by liver tissue and egg. PFCAs with 8-15 carbon atoms were found, with the highest concentrations in plasma (sum PFCAs: 41.8-262 ng/g ww). PFBS, PFOSA and the unsaturated fluorotelomer carboxylic acids (FTUCA) were not detected in any sample. The accumulation profiles of PFCAs were characterized by high proportions of the long and odd-numbered carbon-chained-length compounds (C11 and C13).
PFOS was determined in liver and blood of a small song bird, the great tit (Parus major) in the vicinity of a large fluorochemical plant in Belgium (Dauwe et al. 2007). The concentrations of PFOS ranged from 553 to 11,359 ng/g in liver and from 24 to 1625 ng/g in blood. The hepatic concentrations exceeded in most birds the hepatic benchmark concentrations for the protection of avian species (Beach et al. 2006). Near the same fluorochemical plant, Hoff et al. (2005b) found that PFOS significantly affected alanine aminotranferase, triglycerides and cholesterol in plasma of nestling great tits.
6.2.8.5 Levels in aquatic mammals
A greater number of studies have been conducted on marine mammals compared to terrestrial mammals. The studies are often conducted on naturally dead animals (e.g. dolphins and whales), where the concentrations may not be representative for free-ranging animals.
Occurrence and trends of PFCs levels have been investigated in harbour porpoises (Phocoena phocoena) from coastal waters around Iceland, Norway and Denmark, and in the German Baltic Sea (Van de Vijver et al. 2004). Liver samples were collected for a total of 49 individuals. PFOS concentrations ranged from 26 to 1149 ng/g ww. A geographical difference was observed with a decreasing trend in PFCs contamination from south to north, with the highest concentrations in porpoises from the Baltic Sea.
PFCs levels in polar bears (Ursus maritimus) from East Greenland were reported for the first time by Smithwick et al. (2005a). Liver tissue from a total of 29 individuals collected from 1999 to 2001 was analyzed. The mean PFOS concentration (2470 ± 1320 ng/g ww) was similar to Hudson Bay, Canada, and both populations had significantly greater concentrations than those reported for polar bears from Alaska, indicating a spatial trend.
A circumpolar study of perfluoroalkyl contaminants was carried out by analyzing these compounds in liver tissue and blood from polar bears (Ursus maritimus) from five locations in the North American Arctic and two locations in the European Arctic (Smithwick et al. 2005b). PFOS concentrations were significantly correlated with age at four of seven sampling locations, while gender was not correlated to concentration for any compound. Populations in South Hudson Bay (2000-2730 ng/g ww), East Greenland (911-2140 ng/g ww), and Svalbard (756-1290 ng/g ww) had significantly higher PFOS concentrations than western populations (435-729 ng/g ww). Common sources for PFCAs at the same location were suggested by correlating PFCAs with adjacent chain length.
The concentrations of PFCs were compared to those of chlorinated and brominated organic pollutants in two subpopulations of polar bears (Ursus maritimus) from Alaska, the Beaufort Sea and the Chukchi Sea populations (Kannan et al. 2005b). The results of the study indicated differences in concentrations and profiles of organohalogen compounds between the two subpopulations. It was hypothesized that the difference could be due to different exposures from different transport paths of contaminants (from Japan and Southeast Asia or from Russia and Atlantic Ocean). The source of difference could also be due to different feeding habits of the two subpopulations.
The isomer profile of PFCAs was identified in liver tissue from 15 polar bears (Ursus maritimus) from Canadian Arctic and Eastern Greenland (De Silva et al. 2004). The PFOA isomer pattern in Greenland showed a prevalence of branched isomers, while only the linear isomers were found in bears from Canada. The presence of branched isomers suggested some contribution from compounds obtained with electrofluorination (EF), while prevalence of linear isomers suggested the contribution from compounds obtained with telomerisation.
The tissue distribution of PFCs was investigated in harbour seals (Phoca vitulina) from the Dutch Wadden Sea (Van de Vijver et al. 2005). PFCs were analyzed in liver, kidney, blubber, muscle, and spleen tissue. PFOS was the predominant compound with concentrations ranging from 89 to 2724 ng/g wet weight. Perfluorobutane sulfonate (PFBS) was found at concentrations of 2.7 ± 0.7 ng/g ww and only in spleen tissue. Increasing PFOS concentration was observed in the order kidney> liver> blubber> skeletal muscle.
Concentrations of perfluorinated carboxylic acids (PFCAs) and sulfonic acids were determined in plasma of bottlenosed dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean (Houde et al. 2005). PFOS was the predominant compound found at concentrations between 49 ng/g wet weight (dolphins from Bermuda) and 1171 ng/g wet weight (dolphins from Charleston, SC). Fluorotelomer 8:2 and 10:2 unsaturated acids (FTUCAs) were detected for the first time in the concentrations range 0.5-1.4 ng/g.
PFCs were determined in plasma, milk, and urine of free-ranging bottlenosed dolphins (Tursiops truncatus) from Sarasota Bay, Florida (USA) (Houde et al. 2006ab). Samples were taken in the period 2002-2005. Mean seasonal sum of PFCs detected in plasma ranged from 530 to 927 ng/g ww. No significant differences were observed between seasons, indicating a constant exposure to PFCs. Sexually immature calves (age, <10 years) had significantly higher PFC concentrations than their mothers. Analysis of PFCs in milk confirmed the hypothesis that PFCs are transferred through lactation in dolphins. PFCs were also detected in urine samples, indicating that the urinary system is an important path of depuration from PFCs in dolphins.
PFOS and PFOA were measured in liver tissue from 80 female sea otters collected from the California coast during 1992-2002 (Kannan et al. 2006). Concentrations of PFOS and PFOA were in the range <1-884 and <5-147 ng/g wet weight, respectively. PFCs concentrations were compared among the otters that died from infectious diseases, non-infectious causes, and from apparent emaciation. Concentrations of PFOS and PFOA were significantly higher in sea otters with the infectious disease category than in the non-infectious category. Moreover, PFOA concentrations were found to increase from 1992 to 2002, whereas PFOS concentrations increased from 1992 to 1998 and then decreased after 2000.
In a recent paper Van de Vijver et al. (2007) investigated the distribution of PFCs in liver, kidney, muscle, brain, and bubble in harbour porpoises (Phocoena phocoena) from the Black Sea. PFOS concentrations were highest in liver (327 ± 351 ng/g ww) and kidney (147 ± 262 ng/g ww) tissues. Concentrations in muscle, brain and blubber were about a factor 10 lower. Shorter carbon-chained perfluorinated carboxylic acids, such as perfluorobutanoic acid (PFBA) and PFOS were not detected. PFOS concentrations were comparable to those found in porpoises from the German Baltic Sea.
Polar bears and bottlenose dolphins from the United States have the highest PFCs concentrations. Generally, the concentrations of PFCs in top predators can reach a level which may be close to some measurable effects. Moreover, these species are already exposed to other persistent compounds (e.g. PCBs). The presence of PFCs at high concentrations may constitute an additional treathen to these species through additive and synergistic effects with the other contaminants.
6.2.8.6 Levels in terrestrial animals
Few monitoring studies have been conducted on terrestrial mammals. Those species living close to contaminated areas can reach extremely high PFCs concentrations, as observed by Hoff et al. (2004). In this study a concentration of 180 µg/g ww of PFOS was measured in mice inhabiting the area close to a perfluorochemical plant.
PFCs were measured in blood of captive red panda and giant panda from eight cities from six different provinces in China (Dai et al. 2006). PFOS was the predominant compound in all samples with concentrations ranging from 0.80 to 73.80 ng/ml for red panda and from 0.76 to 8.2 ng/ml for giant panda. Concentrations of PFOS and PFOA were positively correlated. No age- or sex-related differences were observed in PFC concentrations. The serum of those individual living close to urbanized areas had the highest PFC concentrations.
With the exception of those species living close to fluorochemicals production plant, the levels of PFCs observed in terrestrial mammals are in the same range as those measured in marine mammals.
6.3 Ecotoxicity
Most ecotoxicity studies with PFCs have used aquatic organisms and have published only in internal reports from the laboratories of 3M and DuPont. Based hereupon it was concluded in the previous assessment that PFOS was moderate acute toxic and slightly chronic toxic to aquatic organisms, and that PFOA was practically non-toxic (Poulsen et al. 2005).
A fish species, fatheaded minnow (Pimephales promelas) was exposed for 39 days to varying concentrations of perfluorooctanoic acid (PFOA) under microcosm conditions (Oakes et al. 2004). The PFOA concentrations used in this study were much higher than those normally found in the aquatic environment. However, no mortality was associated to exposure to these concentrations. Significant declines in circulating plasma steroids were observed, but these were accompanied by only limited increases in time to first oviposition and decreases in overall egg production. Effects such as peroxisome proliferation and oxidative stress were also observed but at low levels. The authors concluded that PFOA is relatively non toxic at environmentally relevant concentrations, but that biochemical and reproductive endpoints might be affected.
The toxicity of PFOS and PFOA was evaluated for the aquatic midge, Chironomus tentans (MacDonald et al. 2004). The median lethal concentration (LC50) for PFOS calculated during a 10 days laboratory test was 45.2 µg/l, while the median effective concentration (EC50) was 27.4 µg/l. A parallel test performed with PFOA in the same concentration range (0.1 to 100,000 µg/l) did not produce any effect. A chronic life test was performed for PFOS in the concentration range 1-100 µg/l. Three of the four endpoints measured - survival, growth, and emergence - were significantly affected with EC50 values of 92.2, 93.8, and 94.5 µg/l, respectively. Reproduction was not affected.
The marine mussel Mytilus californianus was used as a test organism to determine the toxicity of PFCs with respect to the multixenobiotic resistance (MXR) mechanism (Stevenson et al. 2006). This mechanism acts as a first cellular defence against different xenobiotics. The most studied transporter involved in the MXR mechanism is the p-glycoprotein. The function of the MXR mechanism can be compromised by the presence of some xenobiotics. This effect is called chemosensitization. Four of the nine PFCs studied showed chemosensitizing behaviour, with PFNA and PFDA having the highest values.
Recently, the toxicity of precursor compounds of PFCAs (saturated and unsaturated fluorotelomer carboxylic acids, FTCA and FTUCA) has been tested to Daphnia magna, Chironomus tentans, and Lemna Gibba (Phillips et al. 2007). It was observed that the toxicity increased with increasing chain length. In addition FTCA were more toxic than FTUCA. The acute E50 ranged from 0.025 mg/l for D. magna to 63 mg/l for C. tentans. The toxicity threshold found for these compounds is a factor 10.000 lower than those calculated for PFCAs.
One of the few avian toxicology studies revealed for bobwhites a reproductive lowest observable adverse effect level (LOAEL) of 10 ppm in the diet or 0.77 mg PFOS/kg bw/day for male and female birds (Giesy and Jones 2004). This corresponded to 8.7 mg PFOS/mL and 4.9 mg PFOS/g in female serum and liver, respectively, and to 141 mg PFOS/mL and 86 mg PFOS/g in male serum and liver. In mallards the LOAEL was the same (10 ppm) for males but 50 ppm for females.
At the concentrations usually found in the environment, PFCs will not induce acute toxic effects. Generally, the toxicity of PFCs is species dependent and sometimes gender-dependent for the same species. It is therefore difficult to perform risk assessment for these compounds on the basis of the few published studies. The toxicity of the precursors to the perfluorinated acids has been found to be much higher than the toxicity of the acids selves. Further research is needed in order to clarify this aspect, as well as the occurrence of the precursor compounds in the aquatic environment.
6.4 Environmental risk assessment
An environmental risk assessment for PFOS has been performed for the Environmental Protection Agency in England (Brooke et al. 2004). The aim of the study was to review the risks arising from current uses of PFOS-related substances. Risk evaluation was performed for the following compartments: aquatic (surface water and sediments), terrestrial compartment, atmospheric compartment, and marine environment. Moreover, non compartment-specific effects relevant to the food chain (secondary poisoning) were examined. The predicted no-effect-concentration (PNEC) for the aquatic and terrestrial environments is 25 µg/l. The measured levels of PFOS in surface waters and the water concentrations calculated after discharge from a wastewater treatment plant (WWTP) were below the calculated PNEC. The calculated emission to waste water which would give rise to a risk for the terrestrial compartment is 7.4 g/day. This is for applications of sludge over 10 years; the emission which would give rise to a risk following a single application is 0.67 kg/day. No data were available for risk assessment for the atmospheric compartment. For non- compartment-specific effects relevant to the food chain (secondary poisoning) the PNEC for secondary poisoning was 0.0167 mg/kg ww in food. An alternative PNEC of 0.067 mg/kg was also used. These values were applied for risk assessment of both aquatic and terrestrial food chains. A comparison of the PNEC value with measured concentrations for freshwater fish showed that the highest measured concentrations reported exceed the PNEC of 0.0167 mg/kg. A number of values also exceed the alternative PNEC value of 0.067 mg/kg. The emissions to waste water giving rise to a risk for the terrestrial food chain were estimated to be in the range 1.65 – 3.30 g/day. These are for 10 years of application; the emissions giving a risk from a single application of sludge were estimated to be 15 – 30 g/day. The authors concluded that the major area of concern was the secondary poisoning in the aquatic environment, for both freshwater and marine environments.
Version 1.0 October 2008, © Danish Environmental Protection Agency