Survey and environmental/health assessment of fluorinated substances in impregnated consumer products and impregnating agents
10 Animal bioassays and in vitro tests
- 10.1 Toxicokinetics and metabolism
- 10.2 Toxicology
- 10.2.1 Acute toxicity
- 10.2.2 Effects on the liver
- 10.2.3 Toxicological mechanism
- 10.2.4 Toxicogenomics
- 10.2.5 Effects on cell membranes
- 10.2.6 Effect on mitochondrial bioenergetics
- 10.2.7 Effect on intercellular communication
- 10.2.8 Developmental toxicity
- 10.2.9 Endocrine disruption
- 10.2.10 Mutagenicityt
- 10.2.11 Cancer
- 10.2.12 Cocktail effects
- 10.3 Structure-activity relations
10.1 Toxicokinetics and metabolism
10.1.1 Absorption
PFOS and PFOA (as ammonium salt) are readily and rapidly absorbed in the gastro-intestinal tract and the lungs of rats. Toxic concentrations of PFOA are absorbed through the skin of rabbits (Lau et al 2004; Kennedy et al 2004; Hinderliter et al. 2006). About 93% of an oral dose of PFOA given to rats was absorbed, and peak blood levels were attained 1-2 h after dosing (Hundley et al. 2006). Also an oral dose of 8:2 FTOH is readily absorbed in rats but the skin absorption was neglible. The maximum concentration in the blood occurred by 1 hour after oral dosing, and it cleared rapidly with a half-life of less than 5 hours (Fasano et al. 2006).
10.1.2 Biotransformation
PFOA and PFOS are both considered being metabolically inert (Clark et al. 1973). Other perfluoroalkyl acids with shorter or longer alkyl chain do have similar properties. Their precursors and functional derivatives will ultimately be transformed to their basic acids. For example, the fluorotelomer alcohol 8:2 FTOH and its phosphates are rapidly transformed to PFOA, PFNA and other metabolites in mice and rats (Hagen et al. 1981; Kudo et al. 2005; Fasano et al. 2006; Henderson and Smith 2007; D’Eon and Mabury 2007). The transformation in rat hepatocytes is catalyzed by cytochrom P-450 (Martin et al. 2005).
About 20% of an oral dose of 100 ppm EtFOSE is in male rat metabolised to PFOS (Thomford et al. 2002). Rat liver microsomal fractions also degrade EtFOSE by de-alkylation to FOSE and further to FOSA and finally to PFOS (Xu et al. 2004).
10.1.3 Accumulation
PFOA and PFOS bind to proteins and accumulate - more or less depending on animal species and sex - in various body tissues, mainly in blood, liver, and kidneys of exposed organisms (in total 88% in male rats) but also in lungs, heart, skin, testes (3% in male rats) and brain (Vanden Heuvel et al 1992; Austin et al. 2003; Hundley et al. 2006). The accumulation in fat tissues and muscles is, however, minimal.
Kudo and co-workers (2001) studied PFCAs with different chain length (C7 - C10) in male liver. The result showed that the longer the chain the more of the compound was accumulated in the liver.
10.1.4 Elimination
Once absorbed in the body PFOA is eliminated as the free carboxylic acid mainly with urine and to a less extent in faeces. Thus the renal elimination is critical for detoxification (Vanden Heuvel et al. 1991).
The biological half-life of PFOA in male rats after i.v. administration was 5.6 days or 70 times longer than that in female rats for which it was 2 hrs. The difference was mainly due to the difference in renal clearance, which was significantly reduced by probenezid, suggesting that PFOA is excreted by organic anion transporters. Castration of male rats caused a 14-fold increase in renal clearance, comparable with female rats. The increase was reduced again by treatment with testosterone. Treatment with estradiol also increased the renal clearance, and in female rats ovariectomy increased the renal clearance (Kudo et al. 2002). Elimination of PFOA increases with age in female rats but not in male rats (Hinderliter et al. 2006).
Perfluorocarboxylic acids (PFCA) with a shorter carbon chain length than PFOA are more rapidly eliminated in urine and PFHpA (0.10/0.05) > PFOA (5.6/0.08) >PFNA (30/2.4) >PFDA (40/59 days) for male/female rats (Kudo et al. 2001; Ohmori et al. 2003). The sex differences were most marked for PFOA.
The sex-related clearance of PFOA differs between animal species (Hundley et al. 2006). In hamsters it is opposite of in rats. Male and female hamsters excrete respectively 99% and 58% of a dose in 5 days. In mice and rabbits there was no sex difference, and mice had a slow excretion as male rats, and rabbits had a fast excretion as female rats. In dogs the plasma half-life of PFOA was about 20 days in males and the half in females.
The elimination half-lives in male and female Cynomolgus monkeys for PFOA were 33 days and 21 days, respectively, and the urine was the major excretion route (Butenhoff et al. 2004a). PFOS has a slower elimination rate, and the half-life of PFOS in the Cynomolgus monkey was about 200 days (Seacat et al. 2002a; Andersen et al. 2006).
After an oral dosing of 8:2 FTOH, the majority of the 14C-labelled substance was excreted with feces and 37-55% occurred there as 8:2 FTOH. A small part (1%) was excreted as PFOA in urine (Fasano et al. 2006).
10.2 Toxicology
10.2.1 Acute toxicity
The acute lethal toxicity is moderate corresponding to a classification as harmful. PFOS is more toxic than PFOA, The oral rat LD50 for PFOS is 250 mg/kg bw (3 M 1999), and for PFOA the oral rat LD50 is between 430 and 1800 mg/kg (Kennedy et al. 2004). For PFOA and PFDA the rat intraperitoneal LD50’s are 189 and 41 mg/kg, respectively. Thus PFDA is much more toxic and have delayed effects (Olson and Anderson 1983). Generally, the toxicity of perfluorinated chemicals tested increases with the length of the alkyl chain.
10.2.2 Effects on the liver
Toxicological studies have demonstrated that the liver is the primary target organ for PFOS and PFOA, and body weight loss, increased liver weight, liver cell hypertrophy and changed lipid metabolism with reduction in serum cholesterol are early responses in experimental animals (Kennedy et al. 2004; Seacat et al. 2003).
The no-observed-adverse effect level (NOAEL) for liver effects in male rats exposed for PFOS during 14 weeks feeding was 5 mg/kg/d (Seacat et al. 2003). NOAEL and LOEAL for liver effects by PFOA in male rats exposed by feeding in 13 weeks were 0.06 and 0.64 mg/kg/d, respectively (Perkins et al. 2004). Another repeated-dose study found a LOAEL of 0.3-1 mg/kg for PFOA in male rats, and branched forms of PFOA had lower toxicity than linear forms (Loveless et al. 2006).
PFDA having a longer chain than PFOA was more toxic in rat, hamster, mouse and Guinea pig (van Rafelgheim et al. 1987; Kawashima et al. 1995).
Rats seem to tolerate somewhat higher liver concentrations of PFOS than monkeys, because the NOAEL in a 6-month monkey study was 0.15 mg/kg/d (Seacat et al. 2002a). PFOA with a LOAEL of 3 to 10 mg/kg/d is considerably less liver toxic in monkeys than PFOS, and the target is different (Butenhoff, 2002a). The only site of change in the monkey was liver enlargement at the doses cited.
10.2.3 Toxicological mechanism
PFOA and PFOS induce hepatomegaly characterized by the subcellular proliferation of organelles such as smooth endoplasmic reticulum, mitochondria, but most notably peroxisomes. The changes observed in the liver could be the result of peroxisome proliferation, a well-known toxicological mechanism in rodents. It may cause lipid accumulation in the liver, uncoupling of the mitochondrial oxidative phosphorylation, and reduction of thyroid hormone in circulation. Induction of peroxisome proliferation and benign liver tumors are associated with activation of the nuclear hormone receptor - peroxisome proliferator-activated receptor-a (PPARa). This is one of the three isoforms of PPAR encoded by separate genes and differentially expressed in various tissues, and are found in all mammalian species examined to date. Ligands for PPARs have been widely developed for the treatment of various diseases including dyslipidemias and diabetes. Some hypolipidemic drugs, solvents and environmental chemicals are ligands for PPARa and can induce peroxisome proliferation, for example, clofibrate, phthalates, chloroform, perchloroethylene, trichloroethylene, HFC-123, and MTBE. Humans do not exhibit the liver toxicities by these chemicals found in rodent models (Peraza et al. 2006).
This PPAR receptor is the most likely target of PFOA and PFOS both in rodents and humans (Vanden Heuvel et al. 2006). Polyfluorinated acids are analogue ligands to natural long-chain fatty acids and may displace them in biochemical processes and at receptors such as PPARa. The activation by PFOA and PFOS is more selective but less potent. PFOA is more capable than PFOS in activating PPARa, and mouse is more responsive than human in test systems (Takacs and Abbott 2007).
PFOA is an immunosuppressant through induction of PPARs and enhance the IgE-mediated hypersensitivity response to ovalbumin, and in this way it may provoke asthma (Fairley et al. 2007).
Perfluorocarboxylates (PFCA), particularly PFOA, PFNA and PFDA, are highly potent peroxisome proliferators in rodent livers and affect mitochondrial, microsomal, and cytosolic enzymes and proteins involved in lipid metabolism (Ikeda et al. 1985; Vanden Heuvel 1996; Upham et. al. 1998; Kudo et al. 2000).
PFOS is less reactive as peroxisome proliferator in rodents, and EtFOSE has no effect (Berthiaume and Wallace 2002). PFBA has a slighter effect on indicators of peroxisome proliferation (Ikeda et al. 1985).
The differences between animal species are large for PFDA. Peroxisome proliferation was greatest in mice and almost absent in Guinea pigs. However, accumulation of lipid droplets in liver cells was more pronounced in hamsters and guinea pigs than in rats and mice exposed to PFDA (van Rafelgheim et al. 1987).
Increase in hepatic fatty acid b-oxidation activity (acyl-CoA oxidase) is a biochemical measure of peroxisome proliferation. Kudo and co-workers (2001) studied PFCAs with different chain length (C7 - C10) in male liver. The result indicated that the liver concentration and not the chain length was decisive, but the longer the chain the more of the compound was accumulated in the liver. In vitamin A deficient mice, PFOA had a stronger effect and caused a 3-6 times increase in the ß-oxidation of fatty acids (Sohlenius et al. 1995).
In an in vitro test various perfluorinated chemicals were tested for interference with the liver-fatty acid binding protein (L-FABP). The most potent chemical was PFOS followed by EtFOSA, EtFOSE, and PFOA (Luebker et al. 2002). This interference may contribute to the toxicity of these chemicals.
10.2.4 Toxicogenomics
Toxicogenomic analysis is able to predict toxicity and pathological responses, categorize chemicals, and elucidate mechanism of toxicity. Such analysis showed that PFOA and PFOS exhibited peroxisome proliferator-activated receptor a (PPARa) agonist-like effects on genes (enzymes etc.) associated with lipid metabolism and fatty acid homeostasis (Shipley et al. 2004; Martin et al. 2007). It results e.g. in
- down-regulation of cholesterol biosynthesis genes, matching an in vivo decrease in serum cholesterol, and
- perturbation of thyroid hormone metabolism genes matched by serum thyroid hormone depletion in vivo.
Guruge et al. (2006) used a microarray technique to study gene regulation in livers from male rats treated with hyper doses of PFOA. Over 500 gene expressions were significantly altered. The largest categories of induced up-regulated genes were those involved in transport and metabolism of lipids, particularly fatty acids. Other induced genes were involved in cell communication, adhesion, growth, apoptosis, hormone regulatory pathways, proteolysis and peptidolysis and signal transduction. The gene expressions suppressed (down-regulated) were related to transport of lipids, inflammation and immunity and especially cell adhesion. Genes involved in apoptosis, regulation of hormones, metabolism were also suppressed.
In the lung and liver of PFOA-exposed mouse fetuses the expressions of genes related to fatty acid catabolism were changed (Rosen et al. 2007). That was especially robust in the fetal liver and also genes associated with lipid transport, ketogenesis, glucose metabolism, lipoprotein metabolism, cholesterol biosynthesis, steroid metabolism, bile acid synthesis, phospholipid metabolism, retinol metabolism, proteosome activation and inflammation. Most changes were associated with the PPARa receptor.
10.2.5 Effects on cell membranes
Despite of its lipophobic character PFOS and to a lesser extend PFOA can partition into model bilayers and cell membranes, where it causes changes in membrane structure and function and increased fluidity. Interaction with pulmonary surfactants such as dipalmitoylphoshatidyl choline (DPPC) may be a mechanism by which PFOS causes perinatal mortality in animal studies (Xie et al. 2007). The orientation in the membrane of the PFAS molecules is with the functional group end combined to membrane proteins with the fluorocarbon tails sticking out like hairs (Roon et al. 2006).
Some of the observed effects of perfluorinated compounds may be due to alterations in cell membrane fluidity, which is a measure of the relative mobility of the phospholipid bilayer of the cell membrane. This selectively permeable cell membrane forms the first barrier that separates the cell from exogenous exposures. Effects on the permeability status of the cell membrane could play an important role in mediating the adverse effects of a number of environmental contaminants. In some in vitro assay systems PFOS - but not the shorter chain PFBS and PFHxS – significantly increased in a dose-dependent manner membrane fluidity of fish leukocytes, and decreased mitochondrial membrane potential determined by flow cytometry (Hu et al. 2003).
PFOA, PFOS and EtFOSE caused a slight increase in the intrinsic proton leak of the mitochondrial inner membrane, which resembled a surfactant-like change in membrane fluidity (Starkov and Wallace 2002).
10.2.6 Effect on mitochondrial bioenergetics
PFOS, PFOA, FOSA, FOSAA, EtFOSA, EtFOSE and EtFOSAA had the capacity in vitro to interfere with mitochondrial respiration. FOSA was the most potent uncoupler of the oxidative phosphorylation (Schnellmann and Manning 1990; Starkov and Wallace 2002).
In laboratory tests N-acetyl perfluorooctane sulfonamides (FOSAA, EtFOSAA) disrupt mitochondrial bioenergetics by inducing “the mitochondrial permeability transition”. PFOA had a slight effect but PFOS and EtFOSE had no effect (O’Brien and Wallace 2004).
10.2.7 Effect on intercellular communication
Gap junction intercellular communication (GJIC) is the major pathway of intracellular signal transduction, and it is thus important for normal cell growth and function. Defects in this communication may lead to teratogenesis, neuropathy, infertility, diabetes, autoimmune disorders, cancer, and other diseases (Trosko et al. 1998).
Upham and co-workers (1998) showed that perfluorinated carboxylic acids with carbon chain length of 7-10 can rapidly and reversibly inhibit gap junction intercellular communication in a dose-dependent manner in vitro and with PFDA inhibiting more than PFOA.
In various test systems (in vitro and in vivo) both PFOS, PFOSA, and PFHxS – but not PFBS - inhibit gap junction intercellular communication in a dose-dependent fashion, and this inhibition occurred rapidly and was reversible (Hu et al. 2002).
Cellular effects such as cell membrane fragility and gap junction communication are two of the hypothetical explanations for the effects of these molecules. These are not considered the hallmark effects, such as interference with liver function or lipid metabolism.
10.2.8 Developmental toxicity
PFOA and PFOS can cross the placenta barrier in rodents and be found in placenta, amniotic fluid, embryo and fetus. The concentration of PFOA in fetal plasma at day 21 was approximately half of the concentration in maternal plasma. Lactational transfer is also occurring but the concentration in milk is ten times lower than in blood (Hinderliter et al. 2005).
PFOA and PFOS in rats and mice have showed developmental toxicity and other adverse effects in vivo. The developmental toxicity of PFOS is higher than that of PFOA. These effects included reduction of fetal weight, cleft palate, anasarca, delayed ossification of bones and cardiac abnormalities, as well as decreased neonatal survival following in utero exposure, reduction in mean post natal body weight, altered mammary gland development, decrease in gestation length, not fully matured lungs with laboured breathing, and significant delay in sexual maturation. The structural abnormalities were only found in teratological studies at the highest dose groups (³ 30 mg/kg/d), where significant reductions of weight gain and food consumption and increase of mortality in the pups were also observed in the pregnant dams (Case et al. 2001; Butenhoff et al. 2004b; Kennedy et al. 2004; Grasty et al. 2005; Luebker et al. 2005b; Lau et al. 2003, 2004, 2006; Thibodeaux et al. 2003; White at al. 2007). Concurrent exposure to PFOS and restraint stress enhance effects (Fuentes et al. 2007).
In a two-generation reproduction study the NOAEL value in rats for PFOS was 0.1 mg/kg/d (Luebker et al. 2005a). However, at this low maternal dose level the longer chain PFDA significantly reduced fetal body weight in mice (Harris and Birnbaum 1989).
High doses of EtFOSE also caused reduced maternal body weight and foetal weight in rodents and had effects quite similar to its metabolite PFOS. Both PFBS and PFHxS have been assessed for developmental and reproductive effects. Maternal exposure to PFBS potassium salt did not produce any adverse effect on embryo/foetal development, and no significant alterations were noted in a two-generation study in rats at doses as high as 1 g/kg. PFHxS is only examined in a screening system at lower doses without any effect observed (Lau et al. 2004).
Similar effects, to what is mentioned for rodents, happen in rabbits exposed to PFOS and EtFOSE during gestation. The no-observed-effect-level (NOEL) for PFOS in rabbits was 0.1 mg/kg/d (Case et al. 2001).
Interaction with pulmonary surfactants, such as dipalmitoylphoshatidyl choline (DPPC), may be a mechanism by which pfos causes perinatal mortality in animal studies (Xie et al. 2007).
10.2.9 Endocrine disruption
Some polyfluorinated chemicals may act as endocrine disruptors. Exposure of adult rats to PFOA (25 mg/kg bw by gavage) decreased the testosterone level in testicular interstitial fluid, increasing the serum estradiol level and decreased relative accessory sex organ weights (Cook et al. 1992; Biegel et al. 1995; Biegel 1997; Shi et al. 2007). It could be explained by induction of a hepatic aromatise by PFOA, which converts testosterone to estradiol. Some other studies show no such effects, for instance in a 6-months oral study in monkeys, daily doses of PFOA up to 20 mg/kg/day did not produce any changes in hormone levels, including oestrogen (Butenhoff 2002a).
Some polyfluorinated chemicals have estrogenic effects in cell cultures (”E-screen assay”; Soto et al. 1995). For example, the fluorotelomer alcohols 6:2 FTOH and 8:2 FTOH induce MCF-7 breast cancer cell proliferation and up-regulates the estrogen receptor, but PFOS, PFOA and PFNA have no estrogenic effect in that test (Maras et al. 2006; Vanparys et al. 2006).
The effects on hormone levels in rodents are reflected in changes in the testis where exposure to perfluorooctanoate results in Leydig cell hyperplasia and eventually development of Leydig cell adenomas (Biegel et al. 1995). A study of testis effects in adult rats exposed to perfluorododecanoic acid (PFDoA) also showed a reduced gene expression of many genes involved in cholesterol transport and steroidogenesis and a reduced serum testosterone level (Shi et al. 2007). Thus, it seems that exposure to some PFCs can severely affect proliferation and function of Leydig cells in the adult rat. Leydig cells in the testis are the main sites for testosterone biosynthesis.
This is of considerable concern, because Leydig cell hyperplasia is common among infertile men (Holm et al. 2003) who, as a group, also shows lower testosterone levels than comparable normal controls (Andersson et al. 2004). Reduced testis function has been linked to the testicular dysgenesis syndrome (TDS) (Skakkebaek et al. 2001). The TDS hypothesis states that in utero exposure to endocrine disruptors can damage testis development and lead to reduced testis function in the adult, with symptoms ranging from a moderately reduced semen quality to testis cancer. The best animal model for TDS consists of rats exposed to long-chain phthalates in a critical time window during development, which results in testis dysgenesis with Leydig cell hyperplasia and clustering of the Leydig cells in the centre of the testis, resulting in reduced testosterone levels and compromised fertility in the adults (Sharpe 2006; Hallmark et al. 2007).
The compromised Leydig cell function is reflected in a reduced expression of genes involved in cholesterol transport and steroidogenesis (Liu et al. 2005). This has striking similarities to the reported effect of PFAS exposure; however, it seems as if PFAS compounds, in contrast to phthalates, can induce the effects in the adult.
PFOS did affect the neuroendocrine system in rats, when female rats were injected intraperitoneal with 0, 1 and 10 mg PFOS/kg bw for two weeks (Austin et al. 2003). The oestrous cyclicity was affected, serum corticosterone level was increased, and serum leptin concentration and norepinephrine concentration in the paraventricular nucleus of the hypothalamus were decreased.
10.2.10 Mutagenicity
PFOA was non-mutagenic in the Ames test using five strains of Salmonella typhimurium and in a single strain of Saccharomyces cerevisiae (Griffith and Long 1980). Several other mutagenicity studies of PFOA published by contract laboratories support the inactivity of PFOA (Kennedy et al. 2004). PFDA is also negative in the Ames-Test and various other test systems. However, PFDA was active in a chromosome aberration assay in the presence of S-9 mix and in S-phase DNA synthesis assay (Godin et al. 1992).
Polyfluorinated chemicals may increase the carcinogenicity of other chemicals since the genotoxicity of cyclophosphamide in the micronucleus assay with hamster lung V79 cells increased many fold by simultaneous exposure to PFOS (Jernbro et al. 2007). See Figure 10.1:
Figure 10.1: Genotoxicity of cyclophosphamide (CPP) + PFOS in micronucleus test (After Jernbro et al. 2007)
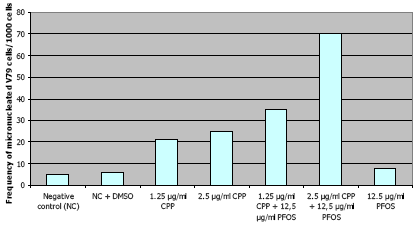
No results of mutagenicity testing with other polyfluorinated chemicals were found in the open literature.
10.2.11 Cancer
In animal studies with CD rats, a strain that has a low spontaneous incidence of these tumours, PFOA produced a dose-dependent increase in Leydig cell adenomas (Biegel et al. 1995; Liu et al. 1996). The tumours may be a result of endocrine changes, because a reduced aromatase activity and a sustained increase in serum estradiol were observed (Biegel et al. 2001). Nevertheless, US Environmental Protection Agency classifies nevertheless PFOA as a carcinogen in animals (US EPA 2002).
A finished two-years rat feeding study with PFOS has only been briefly reported (Seacat et al. 2002b). A modest liver tumour response was observed in the high dose group of 20 ppm PFOS as potassium salt.
A dietary dose of 100 ppm EtFOSE caused an increase of hepatocellular adenomas in females and thyroid follicular cell adenomas in males rats (Thomford et al. 2002). About 20% of an oral dose of EtFOSE is metabolised to PFOS.
10.2.12 Cocktail effects
Since exposure to polyfluorinated compounds is ubiquitous to humans this exposure is added to all other exposures humans may experience. This raises the question of possible mixture effects, which could increase effects from other exposures.
Mixture effects have already been shown in vitro and in vivo for estrogenic compounds (Silva et al. 2002; Tinwell and Ashby 2004) and for anti-androgens (Birkhoj et al. 2004; Metzdorff et al. 2007), but there are very few studies of mixture effects of compounds with different modes of action.
Kudo and Kawashima (1997) found that fish oil-feeding prevents PFOA induced fatty liver in mice.
For polyfluorinated substances there are more reports of such effects. It was just mentioned that the genotoxicity of cyclophosphamide in the micronucleus assay with hamster lung V79 cells increased many fold by simultaneous exposure to PFOS (Jernbro et al. 2007). Therefore, polyfluorinated chemicals may increase the carcinogenicity of other chemicals.
Co-administration of PFOS and dioxin (TCDD) resulted in an increased p450 A1A expression as compared to TCDD alone (Hu et al. 2003).
10.3 Structure-activity relations
The major pile of toxicological information is about PFOS and PFOA, thus the knowledge about other polyfluorinated chemicals is scarce. Qualitatively we know that PFOS and PFOS derivates seem to be more toxic than PFOA and derivatives. Furthermore, the persistence and toxicity of perfluorinated acids increases in general with the chain length and substances with branched chain are less toxic than linear substances. There seems presently to be insufficient background data to make quantitative structure-activity relationships (QSAR) for these homologues but it may be possible at a later stage.
It is even more difficult making theoretical predictions of the properties of the more complex telomers and other complex polyfluorinated compounds used in consumer products. They belong to various chemical classes and may ultimately be degraded or metabolised to their parent perfluorinated acids. Qualitatively, it will be possible with some certainty to predict, which perfluorinated acid the chemical compound is precursor for, and OECD has made an attempt in their substances lists to indicate the potential lengths (or range of lengths) of the fluorinated carbon chain as a result of degradation.
Version 1.0 October 2008, © Danish Environmental Protection Agency