Toxicity testing with the collembolans Folsomia fimetaria and Folsomia candida and the results of a ringtest
4 Ringtest results
- 4.1 Test guideline
- 4.2 Participants
- 4.3 Model chemicals
- 4.4 Range finding
- 4.5 Statistical analysis
- 4.6 Experimental design
- 4.7 Test conditions
- 4.8 Control mortality
- 4.9 Control reproduction
- 4.10 Variability of testing results
- 4.11 Conclusion
4.1 Test guideline
A draft OECD test guideline was developed in the prevalidation phase of this project (OECD, 2006b) by Scott-Fordsmand and Krogh (2005) and was changed according to input from ringtest participants and further refined during the final reporting phase (Annex 6). Existing OECD guidelines were used as templates to ensure consistency and to ensure that the content was sufficient to perform the test.
F. candida and F. fimetaria is reared in lab cultures in closed containers with a bottom layer of a mixture of plaster of Paris and activated charcoal in a ratio of 9:1 by weight. The charcoal absorbs waste products that may be harmful to the optimal productivity of the cultures. The black colour of the bottom layer eases the visibility of the white collembolans. Wholes and furrows in the plaster may help stimulating oviposition (Fountain and Hopkin, 2005), although this was not needed for our cultures to thrive. The substrate is kept moist but not waterlogged to ensure saturated air humidity. The collembolans are watered and fed granulated dry Baker’s yeast weekly; during this operation they are aerated.
Breeding of synchronous cultures is induced by transferring adults to fresh containers and collecting the eggs after three days, recommendable over a week-end. Alternatively the adults may be removed from the substrate and the eggs left behind. In the first case eggs are collected, in the second case adults are removed. After approx. 10 days the eggs hatch and at the age of 9-12 days the juveniles of F. candida or the 23-26 days old adults of F. fimetaria are ready for testing. Allowing for an age range span of 3 days in the test has important practical consequences, as it now provides for a working schedule that does not involve working with the test during the weekend spanning over 3 days.
The test exposes the collembolans to chemicals through the test soil, which is the artificial OECD soil based on a recipe originating from the earthworm acute test (OECD, 1984). On the day of preparing the mixture of moist soil and chemical collembolans, 10 F. candida or 10 male and 10 female F. fimetaria are added.
Test and breeding conditions are 20 oC and a light:dark cycle of 12:12 hours and light intensity of 400–800 lux.
At test termination after 3 weeks for F. fimetaria and 4 weeks for F. candida the collembolans are removed from the soil by flotation or heat extraction. While flotation immediately terminates the test, heat extraction runs for 2 days where the collembolans actively have to move out of the soil.
The practicability of performing the tests with the two species is identical with the exception of the need to discriminate the F. fimetaria males from females.
4.2 Participants
Participants spanned a broad range of laboratories from highly experienced professional contract laboratories to research laboratories at universities. This has aided in exposing the guideline procedure to diverse situations exposing weak or yet unresolved issues even for the existing ISO standard test for F. candida. A list of the 14 participating laboratories is presented in Annex 1; they have been given a code to enable linking the data to a certain laboratory in Annex 2. A total of 51 tests were performed in the ringtest exercise (Table 4).
4.3 Model chemicals
The three model compounds chosen for the ringtest are evaluated for use as positive controls and reference chemicals for the guideline. Boric acid is the preferred candidate because it is easily accessible, while dimethoate is less accessible and the commercial production may cease, and CuCl2 is more difficult to handle in the test due to the need to compensate for a pH effect changing with the CuCl2 concentration. While boric acid and copper chloride are generally available, dimethoate was kindly delivered to the participants from Cheminova.
The model chemicals boric (H3BO4), copper chloride (CuCl2), and the insecticide dimethoate, were chosen to cover 3 different modes of action, i.e. effects caused by: acidity, heavy metal inhibition of fecundity and inhibition of choline esterase. The benefit of boric acid is its accessibility and it has been suggested as a positive control for tests with plants, mites and collembolans (Environment-Canada, 2005b, 2007; OECD, 2007). Boric acid was applied in the concentrations corresponding to 0, 25, 50, 100, 200, 400, 800 mg kg-1; anhydrous copper chloride in the concentrations: 0, 200, 400, 800, 1200, 1600, 2000; and dimethoate in the concentrations 0, 0.25, 0.5, 1, 2, 3, 4 mg kg-1.
4.4 Range finding
In many cases range-finding tests were not performed or did not contribute to an appropriate final concentration series. A general problem of range-finding is that it is usually performed as a lethal test, but is used to guide the selection of concentrations for reproduction tests. Obviously this would give faulty guidance for chemicals with sublethal effects.
4.5 Statistical analysis
Statistical analyses for the estimation of control mortality and reproduction and concentrations causing a decrease of 10% and 50% in reproduction or survival (i.e., LC10, LC50, EC50 and EC50) and their 95% confidence limits were performed using SAS/STAT® version 9.1.3 procedures NLIN and NLMIXED (SAS-Institute-Inc., 2004b). Non-linear modelling was used to estimate concentration-response relationships by fitting the binomially distributed mortality data to the mortality rate (m) formula (probit analysis):
m = c + (1 - c) Φ (a+bd)
where c is control mortality rate, Φ (phi) is the cumulative normal probability function, a slides the curve along the x-axis, b determines the slope, and d is the mg kg-1 concentration of the testing compound in soil. Other models were employed when it was more appropriate to fit the actual mortality data: asymptotic growth, c+(1-c)(1-ead), and exponential growth, c×ead. The reproduction data was fit to the sigmoid model:
![]()
and to exponential decay, c×ead, and a convex decrease, (k/(1-b))×(1-b×e(ad)).
Often a concentration-response curve does not contain sufficient information to estimate parameters for a non-linear curve such as the logistic or exponential because the curve is simply linear, the variability is too high[6], or the fitting procedure cannot attain reasonable parameters, i.e., it cannot converge. In such cases, there still may be a clear and significant decrease of the response with increasing concentration, and therefore, a linear section of the data can be selected by choosing a lower and an upper concentration limit within the decreasing section. Responses outside and on these borders were then added together and a new linear dataset created containing the sum of data for the upper and lower limit and the original data between these concentrations.
95% confidence limits are written in brackets [ ] throughout. Tests for normality were performed with the distribution analysis tool of SAS/INSIGHT (SAS-Institute-Inc., 2004a). The Coefficient of Variation (CV) is calculated as ![]() .
.
4.6 Experimental design
A spacing factor of 1.8 has been recommended for other tests such as the H. aculeifer and the enchytraeid test (OECD, 2004a, 2007), while the guideline on plant growth states that “the number and spacing of the concentrations or rates should be sufficient to generate a reliable concentration-response relationship and regression equation and give an estimate of the ECx or ERx.” (OECD, 2006c). The ringtest does not per se support the spacing factor approach as an inspection of the concentration-response figures reveal (Fig. 4 to Fig. 7). As the purpose of using the spacing factor is to evenly cover the whole response curve, the actual result of the factor is to lump together many low concentrations at the expense of covering the higher concentrations.
4.7 Test conditions
The draft guideline (Annex 6), prescribes a soil humidity content of approximately 50% of the soil’s WHC, but it should be ensured that the soil will maintain a crumbled structure. Hence, the water content is not regulated according to the usual 50% of the WHC. Generally the loss of water is controlled during the test and should not impose any stress on the collembolans.
4.8 Control mortality
The highest mortality was observed in tests with F. fimetaria (Table 2).For F. candida control mortality was less than 20% for 79% of the tests and F. fimetaria had a mortality of less than 20% for 44% of the tests (Table 2). These proportions were significantly different. The failure of some tests to meet the mortality validity criterion is indeed expected to happen even for highly experienced laboratories but at a much lower rate as observed here for F. fimetaria.
Table 2 Summary of control performance evaluation criteria for the two collembolan tests for all tests, including tests not fulfilling the validity criteria, and detection of number of outliers for the boric acid tests. Percentages are the % of tests fulfilling the criteria. CV: Coefficient of Variation for the reproduction. Juv.: Reproductive output of the test in number of juveniles. Raw data presented in Annex 4.
| F. fimetaria | F. candida | |
| Mean reproduction | 132 [67-197] | 399 [310-488] |
| Mean mortality | 35% [22-48] | 14% [8.7-20] |
| Mean control mortality <20% | 44% | 79% |
| Mean control reproduction > 100 juv. | 50% | 97% |
| CV < 30% | 44% | 76% |
| Mean CV | 59.5 | 25.5 |
| Mean CV when reproduction>100 juv. | 28.8 | 24.8 |
| Outliers: Inter-laboratory variability of LC50, h (P<1%) | 0 | 1 |
| Outliers: Inter-laboratory variability of EC50, h (P<1%) | 0 | 0 |
4.9 Control reproduction
The validity criterion for the F. candida, F. fimetaria and O. folsomi control reproduction is an average minimum of 100 juveniles (ISO, 1999; Environment-Canada, 2007). The coefficient of variability (CV) of the reproduction has been set to a maximum of 30% (ISO, 1999), identical to the earthworm and draft mite reproduction tests (ISO, 1998; OECD, 2004b, 2007). For the ringtest, it was suggested to adopt the validity criteria of 100 juveniles and a CV of <30% for both species; therefore, these values are used for the evaluation of the ringtest results (Table 2). For comparison it should be noted that experience from the ringtest paving the way for the F. candida ISO 11267 standard has shown that variability in terms of the CV was greater than 30% for 30% of the tests (BBA, 1995) and 10% of the tests had a mean number of juveniles in the controls less than 100. Intrinsically F. candida has a reproduction rate twice the reproduction rate of F. fimetaria.
One of the F. candida tests had a reproductive output below the suggested validity criteria of 100 juveniles and 24% produced less than 200 juveniles. F. fimetaria produced less than 100 juveniles in 43% of the tests. The mean reproductive CV for F. fimetaria was significantly larger than the CV for F. candida (ANOVA F-test P<0.1%) (Table 2). But when excluding the data sets not meeting the mean minimum 100 juvenile reproduction criterion, the mean CV of the F. candida control reproduction was 23.6 [19-28] (n=33) and 28.7 [17-40] (n=8) for F. fimetaria, which did not differ significantly from each other (one-way ANOVA, P>5%) for the mean or the variance. Thus, this demonstrates that if a sufficient reproduction is obtained, a F. fimetaria test would have a normally accepted CV. In other words it can be concluded that the precision of the control reproduction is potentially identical for the two species. The reproduction was particularly high in three F. candida tests (ref. no. 4, 48, 49), and this may be explained by the appearance of a third clutch (see section 2.6).
4.10 Variability of testing results
The inter-laboratory variability is evaluated by calculating h, the standardized difference of a toxicity test result observed for one laboratory from the mean toxicity values as given in Table 2 (Weyers et al., 2002). The test statistic (x-m)/STD is t-distributed and if x, the individual toxicity estimate from one laboratory, deviates considerably from the mean, it is considered an outlier. The criterion for outliers consists of toxicity estimates that differ from the mean at the 1% level of significance (Weyers et al., 2002). For mortality, only the LC50 of 815 mg kg-1 for boric acid (ref. no. 43) qualified as an outlier, which was the outcome of an otherwise fully valid F. candida test. For the boric acid reproduction tests, none of the EC50 values were detected as outliers.
Graphical presentations of the EC50’s and the LC50’s are presented in Fig. 8 and Fig. 9 for all three testing compounds. However, as boric acid testing results were most numerous only those have been used for evaluation of the endpoint variability.
Boric acid has a pronounced sublethal effect for both species (Table 3). The variances of the two identical mean EC50‘s for F. candida and F. fimetaria with boric acid were not significantly different (P>10%) and both proved to conform to a normal distribution (P>15% for Kolmogorov’s D). The precision of the EC50 estimates in terms of width of the 95% confidence limits (Table 4), were roughly spread up to ±50% around the EC50 for both species, and they were statistically identical. This precision depends on a proper model choice and it would not reflect the true precision if the model has a poor fit.
Table 3 Mean LC50 and EC50 for the two species and the three model compounds. Numbers in brackets: 95% confidence limits.
| Species | Compound | N | LC50 | EC50 | ||
| F. candida | Boric acid | 16 | 259 | [154-364] | 90.8 | [61.8-120] |
| Copper | 11 | 1541 | [442-2639] | 1251 | [423-2080] | |
| Dimethoate | 8 | 2.1 | [0.74-3.4] | 1.65 | [0.4-2.9] | |
| F. fimetaria | Boric acid | 9 | 560 | [271-849] | 107 | [67.9-146] |
| Copper | 2 | 1260 | [-3551-6070] | |||
| Dimethoate | 3 | 1.0 | [-1.7-3.7] | 0.81 | [-1.9-3.5] |
The LC50 for F. fimetaria were significantly higher than the LC50 of F. candida (ANOVA, F-test) (Table 3), but the apparent higher variability of F. fimetaria LC50’ies is related to the mean and vanishes by transformation to obtain variance-homogeneity. The precision in terms of the width of the 95% confidence limits of the LC50-estimates were seemingly better for F. candida ranging up to ±50% of the LC50, but the wider range of F. fimetaria, ±100%, did not differ significantly (ANOVA, P>5%) (Table 4). When excluding the two F. fimetaria boric acid tests with a control mortality >50%, the LC50‘ies of F. fimetaria tests still varied within a factor of 2.6 (n=7, CV=38%) and the F. candida tests varied within a factor of 3.1 (n=16, CV=70%) in relation to the mean LC50. This alternative to the outlier analysis way of describing variability (Table 2) gives the same result, and it is concluded that variability of the LC50 and EC50 toxicity outcome does not differ for the two species.
4.11 Conclusion
The reliability and performance of the test with the new standard species F. fimetaria were assessed by comparing its performance with the F. candida test, which then acted as a reference test method currently accepted by regulatory agencies, while still being a candidate species of the new draft guideline. The range of criteria used for this assessment was largely fulfilled, but the control reproduction and survival performed badly in some tests. In spite of this the toxicity was accurately and precisely estimated, in particular when invalid test results were omitted.
Table 4 Control mortality and reproduction and toxicity endpoints of the ringtest in terms of LC10, LC50, EC10 and EC50 estimated from the complete concentration-response data of each test. Missing cells is due to no effects detected or 50% effect levels outside the concentration range. C.V.: Coefficicient of variability of the control reproduction.
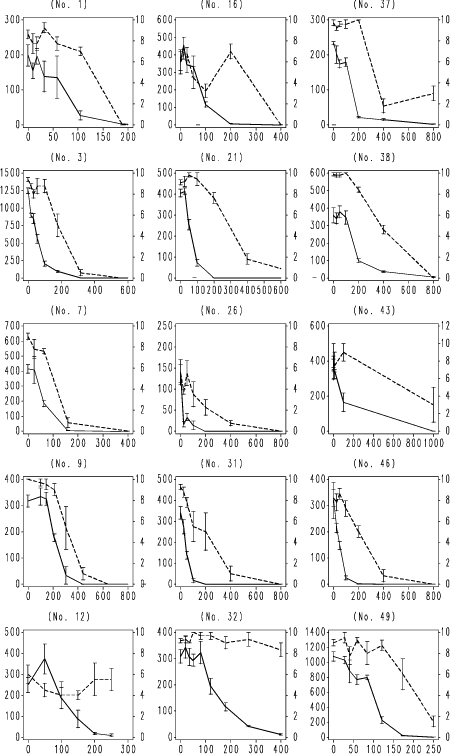
Fig. 4. F. candida testing results with boric acid. Horizontal axis: Nominal concentration of boric acid, mg kg-1 soil; Left vertical axis: number of juveniles produced per replicate; Right vertical axis surviving adults per replicate. Vertical bars: standard error of the mean. Numbers in brackets: ringtest ref. no. used to anonymize the laboratory. Broken line: adult survival per replicate; unbroken line reproduction, number of juveniles per replicate produced by initially 10 adults.
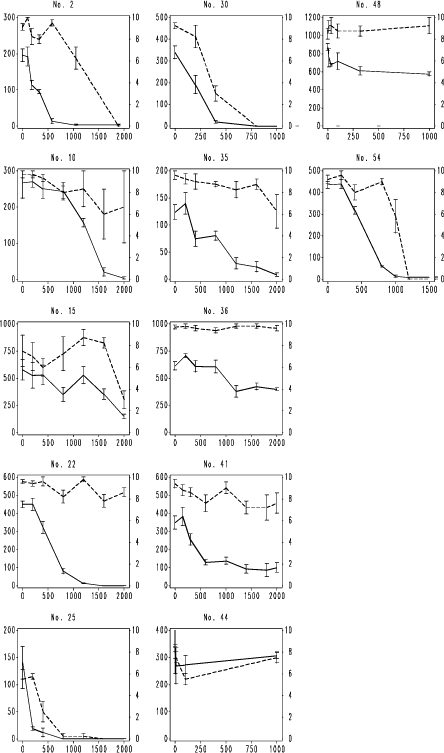
Fig. 5. F. candida testing results with nominal CuCl2 concentration. Legend as Fig. 4.
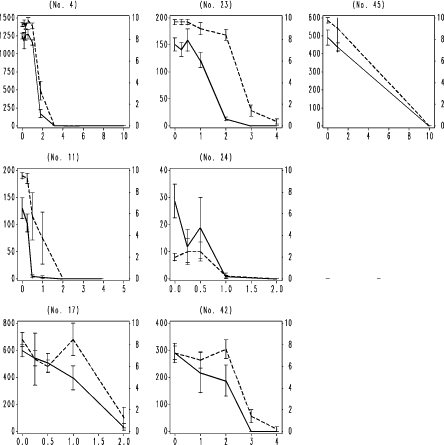
Fig. 6. F. candida testing results with nominal dimethoate concentration. Legend as Fig. 4
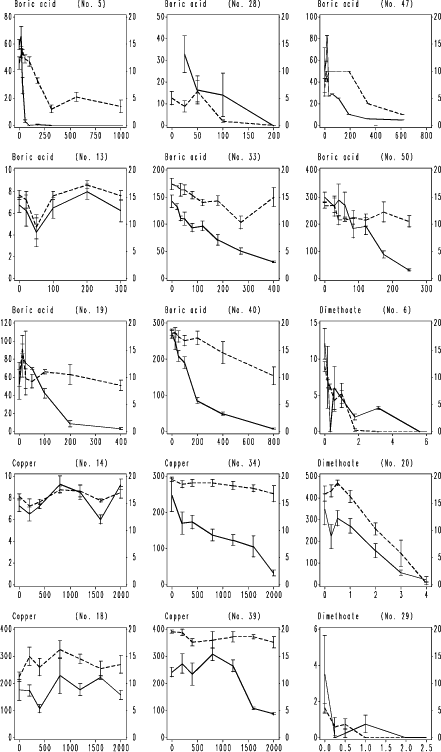
Fig. 7. F. fimetaria testing results with the three model compounds. Legend as Fig. 4, except unbroken line is the reproduction of juveniles per replicate produced by initially 20 adults.
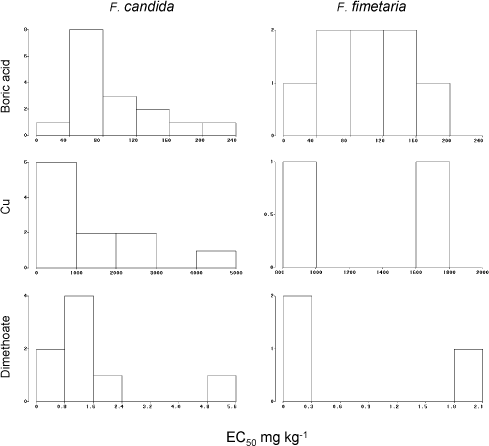
Fig. 8. Frequency distribution of chronic reproduction EC50‘ies from the ringtest. Y-axis number of occurrences of EC50.
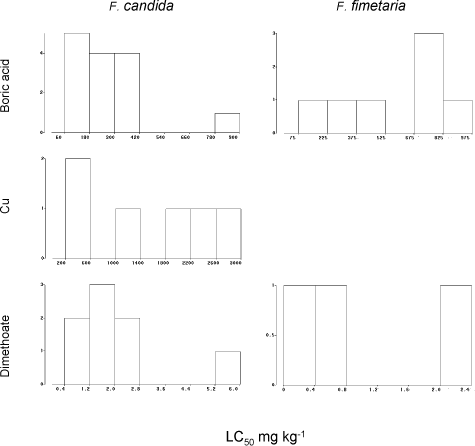
Fig. 9. Frequency distribution of chronic LC50‘ies from the ringtest. Y-axis number of occurrences of LC50.
[6] Presently no OECD guideline has validity criteria for the power of a test and the confidence limits of ECX-estimates, and high variability will lead to lower power and undesirable wide confidence intervals. A maximum of 50% width of the confidence interval would be a reasonable validity criterion. To implement such a criterion guidance should be followed concerning modelling as provided by Environment Canada and OECD (Environment-Canada, 2005a; OECD, 2006a).
Version 1.0 December 2008, © Danish Environmental Protection Agency