Environmental Project No. 1272, 2009
Development of an analysis method for quantification of colophonium components in cosmetic products
Contents
2 Analytical method for colophonium
- 2.1 Phase 1: Literature study of analytical methods
- 2.2 Phase 2: Development of an analytical method
- 2.3 Phase 3: Validation of the developed method
Appendix B: A method for quantification of resin acids in cosmetics
Preface
The project has been performed by Göteborg University (GU), Stockholm University (SU) and National Environmental Research Institute (NERI) at Aarhus University. NERI has been responsible for project organization and reporting.
The literature survey of published methods for analysis of colophonium was performed by Ann-Theresa Karlberg, GU, and Ulrika Nilsson, SU.
The development of an analytical method for the analysis of colophonium in cosmetic products was performed by Ulrika Nilsson and Naghmeh Berglund, SU.
The method validation was performed by Pia Lassen and Gitte Hellerup Jensen, NERI.
The project has been performed for the Danish Environmental Protection Agency (the Danish EPA) under the scheme of Development of analytical methods for cosmetics.
The contact person at the Danish EPA was Dorrit Skals.
The project started in January 2007 and was finalized in December 2007.
Summary and conclusions
Colophonium (rosin) is a common cause of contact allergy and allergic contact dermatitis. According to the EU legislation on dangerous substances – Annex 1 (Directive 67/548 /EEC) a content of >1% colophonium in a product must be declared and the product must be labelled with risk phrase R 43 (“May cause sensitisation by skin contact”). Colophonium is listed under the following CAS numbers: 8050-09-7, 8052-10-6 and 73138-82-6. According to the Cosmetics Directive (76/768/EØF) the component is listed on the INCI-list (Index on Cosmetic Ingredients) under the name ”Colophonium”, (EF. nr. 232-475-7) and there is no restriction for its application.
However, colophonium is a mixture of many compounds. Quantifying a mixture used as an ingredient of another mixture cannot be achieved by any analytical means. This applies to any natural extracts used as ingredient of a compounded consumer product. To overcome the impossibility of quantifying a complex ingredient in a product, the quantification of tracers (= defined substances) specific of the complex natural sources is feasible. Therefore, quantification must be based on chemical analyses of specific major compounds. The objective of the present study was to develop a suitable method to be used for screening of cosmetics with regard to content of colophonium. We have chosen to base our quantification on its two major resin acids, abietic acid (CAS no. 514-10-3) and dehydroabietic acid (CAS no. 1740-19-8) together with a tracer, 7-oxodehydroabietic acid (CAS. 18684-55-4), for air oxidation which readily takes place at storage and handling of colophonium.
Earlier published analytical methods for identification and quantification of the major colophonium components have been thoroughly scrutinized from different aspects e. g. possibility to be used outside very sophisticated analytical laboratories, robustness, and possibility to detect compounds without derivatisation. Based on this, it was concluded that a new method was needed for the purpose of the present study. We have thus developed a reversed phase HPLC method with UV detection using diod-array-detector. Pure non-oxidized resin acids and a stable major oxidation product as a marker for a possible autoxidation and the formation of allergenic oxidation products are used as reference substances. The analytical separation obtained is good and has never been obtained before using HPLC methods.
1 Background
Colophonium (rosin) is a common cause of contact allergy and allergic contact dermatitis (1). According to the EU legislation a content of >1% colophonium in a product must be declared and the product must be labelled with warning according to R 43 (“May cause sensitisation by skin contact”). Colophonium is listed under the following CAS numbers: 8050-09-7, 8052-10-6 and 73138-82-6. According to the Cosmetics Directive (76/768/EØF) the component is listed on the INCI-list (Index on Cosmetic Ingredients) under the name ”Colophonium”, (EF. nr. 232-475-7) and there is no restriction for its application.
In clinical studies, significant concomitant positive patch test reactions have been observed involving colophonium and reactions to fragrance markers (fragrance mix and balsam of Peru) among consecutive patients (2-4). The concomitant test reactions described are best explained as the result of concomitant sensitization caused by simultaneous exposure to a wide variety of fragrance chemicals and colophonium components in products in everyday life (5). However, no extensive investigations regarding the content of colophonium in cosmetics have been preformed since no applicable analytical method exists that also consider the allergenic oxidized resin acids.
Colophonium is a resin obtained from different species of coniferous trees. It contains a complex mixture of resin acids (about 90%) and neutral substances (10%) which varies depending on species, recovery process and storage condition. The major acids are abietic acid (AbA) and dehydroabietic acid (DeA) (Figure 1) (1). Oxidized material is present in colophonium due to air exposure at normal handling and storage. Extensive studies have identified that oxidized compounds formed at air exposure originate mainly from AbA (6-9). While AbA itself is a weak allergen or non-allergenic, some of the oxidation products formed are allergens. One of the most prominent oxidation products is 15-hydroperoxyabietic acid, which is a potent allergen (10).
Colophonium is often modified or derivatized to obtain better technical properties. However, the modifications are usually interrupted when the desired technical properties are obtained leaving unmodified colophonium in the product. Both modified and unmodified colophonium can cause contact allergy. However, modified colophonium has been described to cause contact allergy due to content of unmodified colophonium (11).
Colophonium in unmodified and modified form is used in a range of different products and materials e.g. soldering fluxes, paper (paper size), paints, glues, adhesives, and also in cosmetics. The amount of unmodified colophonium in different products varies from 20% or more in some adhesives, paints and soldering fluxes for electronic assemblies to small traces in products containing mainly modified colophonium (12).
As colophonium is non-toxic, non-irritating and very sticky it is a perfect material to be used for application of cosmetics on the skin. Allergic contact dermatitis caused by colophonium in various cosmetic products such as eye shadows, rouge, lip preparations and mascaras is reported in literature. Colophonium has been detected also in mascaras marketed as “hypoallergenic” and the content of colophonium in the products investigated was enough to cause elicitations in sensitized individuals (13). Extensive reviews on the usage of colophonium have been presented during the years (6, 11, 14, 15).
Figure 1. The major resin acids in unmodified colophonium
2 Analytical method for colophonium
- 2.1 Phase 1: Literature study of analytical methods
- 2.2 Phase 2: Development of an analytical method
- 2.3 Phase 3: Validation of the developed method
The objective of the present study was to develop a suitable method to be used for screening of cosmetics with regard to content of colophonium, based on its major components AbA and DeA and including a marker for oxidized material. The project is divided into three phases. Phase 1: A literature study of published analytical methods; Phase 2: Development of an analytical method; and Phase 3: Validation of the developed method.
2.1 Phase 1: Literature study of analytical methods
Published methods for identification and quantification of colophonium components have been scrutinized and evaluated to find out if any method was suitable for further development into a new method.
2.1.1 Results
Published methods for identification and quantification of colophonium components are presented in Appendix A. Brief descriptions and evaluations of the methods are given. From the literature search two methods could be suggested. Method I is a GC-FID method which requires derivatisation. Method II is an HPLC-fluorescence method which is unspecific and therefore requires development to be applied on complex samples. Thus, it was concluded that a new method was needed for the purpose of the present study.
2.2 Phase 2: Development of an analytical method
In this phase a method for detection and quantification of the major colophonium components AbA and DeA, including a marker for oxidized resin acids, was developed. An HPLC – UV method with diode array detection (DAD) was chosen in order to gain high specificity for the target compounds and without need of derivatisation.
Traditionally, the major colophonium components are quantified using gas chromatography (GC) (16), but also HPLC methods have been developed (12). However, analyses based only on the non-oxidized resin acids do not tell anything about the amount of oxidized acids. A small amount of AbA detected might be due to a small amount of colophonium present, but could also be due to an extensive oxidation. As the oxidation products in colophonium are the major allergens, it is necessary to develop a method for quantification that takes the presence of oxidized material into consideration. However, as the method should be used for screening purposes it is important that it is a robust, inexpensive and simple method, not involving highly dangerous materials. Use of the highly allergenic 15-hydroperoxyabietic acid as a marker for oxidation products is not suitable, since it is very unstable and difficult to handle. Instead, the stable oxidation product 7-oxodehydroabietic acid (7-O-DeA) was selected as a marker for oxidized colophonium (Figure 2). This oxidation product is stable enough to be used as reference compound and not as allergenic to handle as the hydroperoxides.
Figure 2: One of the oxidation products identified in air-exposed colophonium chosen as a marker compound for the oxidative degradation of AbA.
For AbA, the content may vary between 30 – 50% for gum rosin and
35 – 40% for tall oil rosin. Assuming that the resin acid content is 90%, a detection limit of 10 microgram/g AbA thus corresponds to a detection limit of approximately 30 microgram/g (ppm) of colophonium. This could be considered acceptable compared to the reactivity in allergic patients. Patients sensitized to colophonium of the gum rosin type were tested with serial dilutions of gum rosin 20 - 0.001% in petrolatum. Of the subjects tested, 50% reacted down to a concentration of 0.1% (1000 ppm), while the most sensitive patient reacted to a level as low as 0.001% (10 ppm) (18). In other patients tested with tall oil rosin (20 – 0.001% in petrolatum), no reactions were found to concentrations lower than 1% (10 000 ppm) (7).
2.2.1 Materials and Methods
2.2.1.1 Chemicals
Standard compounds
AbA, DeA and pimaric acid (all >95% purity) were obtained from Helix Biothech, Vancouver, Canada. cis-5,8,11,14,17-Eicosapentaenoic acid (EPA, >98.5%), used as internal standard, was purchased from Fluka, Sigma-Aldrich ChemieGmbH, Steinheim, Germany. 7-O-DeA was synthesized as described below.
Standard stock solutions of each compound were prepared in 100% of acetonitrile at a concentration of 1mg/ml. They were stored in freezer throughout the study.
Standard solutions for HPLC were made in acetonitrile:MilliQ water 9:1 with 0.2% formic acid. They were stored in refrigerator and were stable for at least 2 weeks (not tested for longer periods).
2.2.1.2 Solvents and samples
Methanol of 99.8% purity was purchased from Ltd BDH, formic acid, 98-100%, from Scharlau and acetonitrile of Chromasolv quality from Sigma-Aldrich.
Gum rosin (colophonium) samples (unmodified and maleic acid anhydride-modified from Soccer, Portugal) and unmodified colophonium (from Fluka) were dissolved in 100% acetonitrile to approximately 1.5 mg/ml.
Cosmetic samples, (1 g foundation from Face Stockholm) were spiked with standard compounds at different concentration levels, from 1 microgram /g up to 500 microgram/g of each compound.
2.2.1.3 Synthesis of 7-O-DeA
7-O-DeA was synthesized in two steps according to the procedure described in literature (17). A mixture of AbA (5.2 g, 17.19 mmol) and 5 % Pd/C (260 mg, 5 % w/w) was heated to 245 °C for 1 hr under nitrogen in a two necked round bottom flask. After cooling, the solid was dissolved with ethyl acetate (4 x 50 ml, or until all was dissolved) and filtered through celite. The organic layer was then extracted with 0.25 M sodium hydroxide (2 x 100 ml) followed by acidifying the aqueous layer with 1 M hydrochloric acid. The crude product was obtained by washing the aqueous layer with ethyl acetate (3 x 50 ml), dried with magnesium sulphate and concentrated under reduced pressure. Purification by flash chromatography (ethyl acetate/hexane/ trifluoroacetic acid: 14/85/1) afforded 2.5 g (46 %) of DeA. This was in agreement with previous reported data.
To a mixture of DeA (2.1 g, 6.99 mmol) and potassium hydroxide (0.4 g) in water (40 ml), was added a solution of potassium permanganate (2.8 g, 17.72 mmol) in water (60 ml) drop-wise at room temperature. The resulting mixture was stirred for 2.5 hr followed by saturating with sulphur dioxide. A white precipitate was formed and filtered off, washed with water and air dried over night. Purification by flash chromatography (ethyl acetate/hexane/ trifluoroacetic acid: 14/85/1) afforded 1.15 g (52 %) of 7-O-DeA in 95% purity determined by Nuclear Magnetic Resonance (NMR). This was in agreement with previous reported data
2.2.1.4 Equipment
An Agilent 1100 gradient system was used for analyses, equipped with an injection loop of 20 microliter, a DAD and a column heater set at 25°C. The HPLC column was a Prism RP-12 from Thermo (4.6mm i.d., length 150mm, particle size 3 micrometer). The stationary phase in the column contains both C12-chains and urea functions. For SPE, Oasis MAX columns (6cc, 500mg) from Waters were used.
2.2.2 Analytical Procedure
2.2.2.1 Analysis of cosmetic samples
Spiked 1 g samples were ultrasonicated in 20 ml of acetonitrile during 30 min. An aliquot of 500 microliter EPA from a 928 microgram/ml solution, was added as internal standard (IS) prior to extraction.
Each extract was transferred to plastic tubes and centrifuged during 15 min at 4000 rpm. The supernatant was transferred to a flask, while the pellet was redissolved in acetonitrile, ultrasonicated and centrifuged using the same procedure. The supernatants were pooled in the flask and evaporated in a rotavapor to a volume of approximately 5 ml. Adjustment of the volume to 7 ml was made by adding acetonitrile. MilliQ water was then added to a final volume of 10 ml containing acetonitrile:water 7:3. Addition of water made some of the solutions opaque. In those cases, filtration through a syringe filter (0.45 micrometer polypropylene membrane) was performed prior to the solid-phase extraction (SPE).
2.2.2.2 Solid phase extraction
Conditioning was performed by percolating 1) 3 ml of methanol and 2) 3 ml of MilliQ water through the SPE column. The sample was loaded and the column was then washed with 1) 3 ml of 50 mM sodium acetate in MilliQ water to eliminate very polar neutrals or bases, 2) 4 ml of methanol to eliminate lipophilic neutrals and finally 3) 1 ml of 2% formic acid in methanol. The sample was then eluted with 2 ml of 2% formic acid in methanol. An additional volume of 1 ml 2% formic acid in methanol was gathered in case of breakthrough. Fresh formic acid solution was made daily.
2.2.2.3 Analysis of gum rosin
Internal standard, 200 microliter (186 microgram) was added to 2 ml of 1.5 mg/ml gum rosin solutions in acetonitrile. The gum rosin samples were analyzed directly by HPLC after syringe filtration (without SPE clean-up).
2.2.2.4 HPLC method
Methanol:water 8:2 with 0.05% formic acid was used as mobile phase at a flow rate of 1 ml/min (yielding a pressure of approx 240 bar). An isocratic elution was performed. Four different wavelengths were used 210, 220, 240 and 250 nm. The different absorption ratios were used for identification of the compounds. Isocratic elution has to be performed to avoid increasing baseline, which would lead to higher LODs. The amount of formic acid was found to be critical for both retention and noise level.
A calibration curve was made from injected concentrations in the interval 0.8 ng/µl to 500 ng/microliter of each compound. For quantification single-point calibration was performed by a daily triplicate injection of a 250ng/microliter standard. Quantification was performed by using relative response factors analyte/IS.
Specific absorption ratios (CV below 10%) were used for identification of the different compounds:
AbA: 240nm/250nm 1.36
DeA: 220nm/210nm ratio 0.55
7-O-DeA: 220nm/210nm ratio 0.53; 240nm/210nm ratio 0.26; 250nm/210nm ratio 0.45.
EPA (is): 220nm/210nm 0.40
Quantification of DeA and EPA was performed by integration of peaks at 210 nm. For both AbA and 7-O-DeA the wavelength at 240 was used.
2.2.3 Results
2.2.3.1 Selectivity
The method developed is able to separate and identify the three reference colophonium compounds selected (AbA, DeA and 7-O-DeA) (Figure 3). Detection of the acids is performed at the wavelengths given above. Baseline separation of the peaks corresponding to AbA and DeA was obtained. One problem with LC-separation based on UV detection is the co-elution of pimaric acid with AbA. The new method with an LC column, which makes the stationary phase more selective to molecular shape, allows for a separation of pimaric acid and AbA (even though not fully baseline-separated). With the chosen mobile phase (MeOH:H2O 8:2 with 0.05% formic acid), and flow rate (1ml/min) the selected resin acids in colophonium can be separated within 22 min. It seems to be important to control the amount of acid thoroughly. To low a concentration (0.02%) affected the separation in a bad way and to a high level (0.1%) increased the noise level severely. There was used isocratic elution, since gradient elution increased the noise.
2.2.3.2 Separation of the target compounds in colophonium samples
Baseline separation of peaks corresponding to other resin acids was also obtained as analysis of unmodified colophonium shows (See Figure 4).
The chromatograms are measured at 220, 240, 250 and 210 nm, respectively
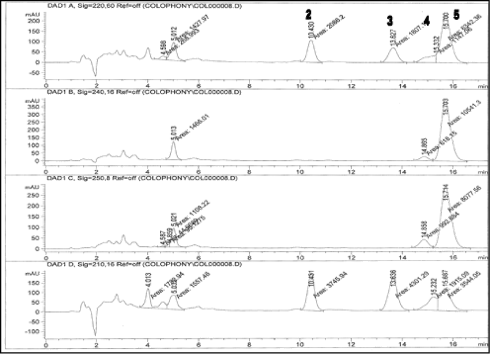
Figure 4: Colophonium sample, unmodified rosin from Soccer (1.5 mg/ml) analyzed with the developed method. 2 = DeA, 3 = EPA, 4 = pimaric acid, 5 = AbA. The oxidized resin acid 7-O-DeA could not be detected. The chromatograms are measured at 220, 240, 250 and 210 nm, respectively
2.2.3.3 Quantification of the target compounds in colophonium samples
The different analytes were quantified in unmodified and modified colophonium samples and the results are shown in Table 1.
Table 1. Concentrations of the target compounds in different samples unmodified and modified colophonium (mg/g)
| AbA | DeA | 7-O-DeA | |
| Unmodified colophonium of the gum rosin type (Soccer) |
255 | 62 | Not det |
| Unmodified colophonium of the gum rosin type (Fluka) |
691 | 40 | Not det |
| Maleic anhydride-modified gum rosin (Soccer) |
74 | 23 | Not det. |
2.2.3.4 Linearity
The peak areas for AbA, DeA and 7-O-DeA varied linearly with resin acid concentrations within the concentration range of 10 microgram/g and 500 microgram/g.
2.2.3.5 Limit of detection
Using the procedure described, the instrumental limit of detection (LOD) was 4 microgram/ml for AbA, 31 microgram/ml for DeA, and 3 microgram/ml for 7-O-DeA.
2.2.3.6 Recovery
The recoveries were investigated at three different levels of spiked foundation. The different spiked levels for each analyte were 100, 200 and 500 microgram/g. The found recovery for AbA was 78% (CV 1%), 88% (CV 5%) for DeA, 76% (CV 2%) for 7-O-DeA and 97% (CV 5%) for EPA.
2.2.3.7 Precision
The precision of the HPLC method in terms of both repeatability (within day variation, n=6) and reproducibility (n=3) of the relative response factor analyte/IS was in both cases below 10% (CV). The precision was tested with a 250 microgram/g standard.
2.3 Phase 3: Validation of the developed method
The method developed in Phase 2 was validated for quantification limit, repeatability and recovery of colophonium and oxidized colophonium marker at two concentration levels by standard addition using cosmetic products. Analysis of colophonium and oxidized colophonium in a series of products was considered, but due to limited resources available for this project, it is proposed that the investigation of colophonium content in the marketed cosmetic products should be performed in a separate project. However, selected cosmetic products were analyzed for the content of colophonium.
2.3.1.1 Separation of the target compounds in cosmetics
The analytes were quantified in different cosmetic samples according to the method developed. The separation was sufficient for identification and quantification in the tested cosmetics samples. This is illustrated by the chromatogram of a wax strip product for hair removal (Figure 5). The chromatogram measured at 210 nm is shown as an example.
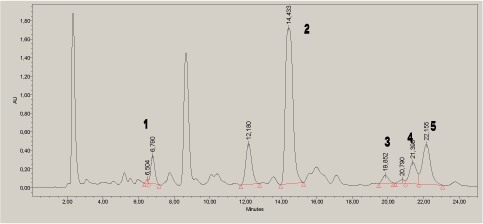
Figure 5. Chromatogram of a wax strip product at 210 nm. 1=7-O-DeA, 2=DeA, 3=EPA, 4=pimaric acid, 5=AbA: .
2.3.1.2 Precision
The repeatability in terms of CV was determined at two levels based on standard addition. For all compounds, the repeatability at low levels (50 – 90 microgram/g for each compound) was in good agreement with the results from phase two (See table 2). At high levels (135 - 250 microgram/g) the precision was in the range of 5-7% (See Table 3).
Table 2. Analysis of cosmetic product at low level (50 – 90 microgram/g) by standard addition
| component | precision | % precision | % recovery | LOD | LOQ |
| AbA | 2,4 | 2,4 | 85 | 7,2 | 21,6 |
| DeA | 6,4 | 13 | 84 | 19,2 | 57,6 |
| 7-O-DeA | 4,3 | 11 | 91 | 12,9 | 38,7 |
Table 3. Analysis of cosmetic products at high level (135 – 250 microgram/g) by standard addition
| component | precision | % precision | % recovery |
| AbA | 13,1 | 6,5 | 94 |
| DeA | 11,2 | 4,8 | 92 |
| 7-O-DeA | 11,8 | 7,5 | 94 |
2.3.1.3 Linearity and Repeatability of HPLC
The linearity and repeatability were tested with regard to the developed HPLC method by multiple injections (n=10). The method was linear in the concentration range of approximately 1 to 400 microgram/g (Correlation, r²: 0.999 – 1.000). The repeatability of the instrument was good in the range of 5 to 185 microgram/ml. AbA: 2-4.5%, DeA: 0.3-2%, 7-O-DeA: 0.3-1% and the internal standard, EPA: 2.5% at a concentration of 125 microgram/ml.
2.3.1.4 Limit of detection
Limit of detection was calculated as 3 x SD based on 6 analyzed samples spiked at low level and ranged from 7-20 microgram/g, (See Table 2).
2.3.1.5 Limit of quantification
The method limit of quantification was calculated based on the spiked cosmetic samples using the equation: LOQ = blank + 3 x LOD. This was equal to LOQ = 3 x LOD as there was no detectable blank values.
2.3.1.6 Recovery
The recoveries based in standard addition were in accordance with those obtained in phase 2. See table 2 and 3. CV of the recoveries was 4-6%.
2.3.1.7 Identification of the analytes
The specific absorption ratios for identification were controlled as different detectors were used in Phase 2 and Phase 3. There are some differences, especially for DeA and EPA. It is therefore recommended that the ratios were verified by standards before analysis. The CV was less than 10% in accordance with phase 2 (see Table 4).
Table 4: Absorption ratios for identification
| AbA | DeA | 7-O-DeA | EPA | |||||
| nm ratio | Phase 2 |
Phase 3 |
Phase 2 |
Phase 3 |
Phase 2 |
Phase 3 |
Phase 2 |
Phase 3 |
| 220/210 | 0,55 | 0,83 | 0,53 | 0,53 | 0,4 | 0,06 | ||
| 240/210 | 0,26 | 0,24 | ||||||
| 250/210 | 0,45 | 0,44 | ||||||
| 240/250 | 1,36 | 1,54 | ||||||
2.3.1.8 Quantification in products
Different common cosmetics products were tested with respect to content of the target resin acids. Based on the obtained data the content of colophonium was estimated (See Table 5). Only one wax strip had significant content corresponding to approximately 7 mg/g colophonium. For the other products AbA and DeA could be detected (0.5 – 4.5 microgram/gram), however, the concentrations were below detection limits.
Table 5: Quantification in products (microgram/g)
| Component | Foundation | Lip gloss | Wax strip, normal |
Wax strip, face |
| AbA | <7 | <7 | 2302 | <7 |
| DeA | <19 | <19 | 2517 | <19 |
| 7-O-DeA | <13 | <13 | 27,8 | <13 |
3 Discussion
A new method for detection and quantification of the major components, AbA and DeA, in unmodified colophonium (rosin) was developed. The method also includes the possibility of detection and quantification of one of the major oxidation products, 7-O-DeA, obtained at the oxidative degradation of colophonium in contact with air. The final method involves an HPLC method with UV-DAD able to separate the resin acids. The method is fast, robust, simple, without using toxic chemicals and has a high specificity compared to ordinary UV methods. High specificity is needed due to the complexity of the cosmetics to be analyzed. This is obtained by a DAD which allows specificity without using mass spectrometry. Compared to GC methods used no derivatisation is needed.
According to the EU legislation on dangerous substances – Annex 1 (Directive 67/548 /EEC) a content of >1% colophonium in a product must be declared and the product must be labeled with risk phrase R 43 (“May cause sensitisation by skin contact”). However colophonium should always be listed on the list of ingredients if it is used in a cosmetic product according to the Cosmetic Directive (76/768/EØF) .
However, colophonium is a mixture of many compounds. Quantifying a mixture used as an ingredient of a product cannot be achieved by any analytical means. This applies to any natural extracts used as ingredient of a compounded consumer product. To overcome the impossibility of quantifying a complex ingredient in a product, the quantification of tracers (= defined substances) specific of the complex natural sources is feasible. Therefore, quantification must be based on chemical analyses of specific major compounds. For colophonium we have chosen to base our quantification on its two major resin acids. However, it is not possible to give the exact content of colophonium in a compounded product based on analysis of the acids, since there is a difference in the acid content in different types of colophonium due to variations in extraction, handling, storage and manufacturing. This is especially true for the content of DeA. For AbA the content can vary between 30-50% for gum rosin and 35-40 % for tall oil rosin. For DeA the figures can be 5-10% in gum rosin and around 30 % in tall oil rosin. Furthermore, at the wavelength used for detection of DeA (220 nm) the risk for interference with constituents from the sample matrixes is high. Thus, the detected amount of AbA should be used for quantification of colophonium in most cases.
A low level of AbA can indicate a low level of colophonium. However, it is important to check that the low level is not due to an oxidative decomposition of AbA resulting in highly allergenic compounds. To avoid such a misinterpretation, we also analyzed for DeA, a much more stable compound than AbA, and for 7-O-DeA as a tracer for oxidation compounds of AbA. High values of these tracers together with a low level of AbA indicate that AbA has decomposed to a great extent and that the content of colophonium is higher than estimated based on the AbA analysis and further that the presence of allergenic oxidation product might be high.
In general, two major types of analyses for colophonium components are described in literature. Traditionally, GC-FID methods with derivatisation of the resin acids to allow a good separation have been used e. g. in the pulp industry. Later on, LC methods with UV, fluorescence, and mass spectroscopic methods for detection have been developed. Most published methods have been developed for the investigation of specific products that might contain colophonium (See Appendix A). The developed method is not restricted to a specific group of consumer products in. e. cosmetics. Rather, it can be used universally. However, there could be some limitations of the methods of sample preparations for certain products, where the components are stronger bound in the material compared to cosmetic products..
In the present investigation the SPE phase used for sample preparation is a mixture of lipophilic and weak anion exchanging sites, which seems to suite the resin acids perfectly. Other lipophilic compounds can be washed out from the column by using methanol, while our compounds are retained until acid is added. The method used gives very clean extracts according to the LC analyses.
Samples from different products were analyzed. Only one wax strip for hair removal had significant concentrations of colophonium. For the other products (lip gloss, foundation, wax strips for face) there were traces of AbA and DeA present, however, below detection limits. According to the declaration of the products only the two types of wax strips were declared containing colophonium.
4 Conclusions
Earlier published analytical methods for identification and quantification of the major colophonium components have been thoroughly scrutinized from different aspects e. g. possibility to be used outside very sophisticated analytical laboratories, robustness, and possibility to detect compounds without derivatisation. Based on this, it was concluded that a new method was needed for the purpose of the present study. We have thus developed a reversed phase HPLC method with UV detection using diode-array-detector. The method is fast, robust, simple, without using toxic chemicals and has a high specificity compared to ordinary UV methods. Pure non-oxidized resin acids and a stable major oxidation product as a marker for a possible autoxidation and the formation of allergenic oxidation products are used as reference substances. The analytical separation obtained is good and has never been obtained before using HPLC methods.
5 References
- Karlberg A-T. Colophony. In: Handbook of Occupational Dermatology. Eds. L Kanerva, P Elsner, J E Wahlberg, H I Maibach. Springer-Verlag, Heidelberg. 2000 pp 509-516.
- Färm G. Contact allergy to colophony and hand eczema. Contact Dermatitis 1996: 34: 93-100.
- Wohrl S, et al. The significance of fragrance mix, balsam of Peru, colophony and propolis as screening tools in the detection of fragrance allergy. Br J Dermatol 2001: 145: 268-273.
- Matura M, et al. Selected oxidized fragrance terpenes are common contact allergens. Contact Dermatitis 2005: 52: 320-328.
- Bråred-Christensson J, et al. Hydroperoxides form specific antigens in contact allergy. Contact Dermatitis 2006: 55: 230-237.
- Karlberg A-T. Contact allergy to colophony. Chemical identifications of allergens, sensitization experiments and clinical experiences. Acta Derm-Venereol 1988; Suppl. 139, 1-43.
- Karlberg A-T. Air oxidation increases the allergenic potential of tall oil rosin. Gum rosin contact allergens also identified in tall oil rosin. Am J Contact Dermatitis, 1991; 2: 43-49.
- Hausen BM, et al. Contact allergy due to colophony (IX). Contact Dermatitis 1993: 29: 234-240.
- Gäfvert E, et al. Contact allergy to resin acid hydroperoxides. Hapten binding via free radicals and epoxides. Chem Res Toxicol 1994; 7: 260-266.
- Karlberg A-T, et al. Identification of 15-hydroperoxyabietic acid as a contact allergen in Portuguese colophony. J Pharm Pharmacol 1988; 40: 42-47.
- Gäfvert E. Allergenic components in modified and unmodified rosin. Acta Derm Venereol (Stockholm)1994: Suppl. 184: 1-36.
- Ehrin E, Karlberg A-T. Detection of rosin (colophony) components in technical products using an HPLC technique. Contact Dermatitis 1990; 23: 359-366.
- Karlberg A-T, Lidén C, Ehrin E. Colophony in mascara as a cause of eyelid dermatitis. Acta Derm-Venereol 1991; 71: 445-447.
- Färm G. Contact allergy to colophony. Clinical and experimental studies with emphasis on clinical relevance. Acta Derm Venereol (Stockholm)1997: Suppl. 201: 1-42.
- Downs AMR, Sansom JE. Colophony allergy: a review. Contact Dermatitis 1999: 41: 305-310.
- Holmbom B, et al. Capillary gas chromatography-mass spectrometry of resin acids in tall oil rosin. J Am Oil Chem Soc 1974: 51: 397-400.
- Ayer, W. A. and Migaj, B. S. Acids from blue-stain diseased lodgepole pine. Can J Bot. 1989: 67: 1426-1428
- Karlberg A-T and Lidén C. Comparison of colophony patch test preparations. Contact Dermatitis 1988: 18: 158-165.
Appendix A: Literature study on the detection of the main components of unmodified colophonium in commodities - regarding contact allergy
1 Introduction
1.1 Analytes
The two main components of colophonium are the diterpenoids abietic acid (AbA), CAS no.. 514-10-3 and dehydroabietic acid (DeA), CAS no.. 1740-19-8 [1]. Consequently, these two acids are the most commonly examined analytes when studying the presence of unmodified colophonium in commercial products [2-11]. An additional large number of other terpene species, often oxidised, have also been identified in unmodified colophonium as such [8, 12-17]. Some important allergens are generated through auto-oxidation of AbA and DeA, while the amounts of original acids present in the sample decrease. Therefore, it is important to include one or more oxidised species in the analysis as well as AbA and DeA when determining the presence of colophonium in a sample [8, 12, 14-16]. To be suitable as a marker for unmodified colophonium, an oxidation product should be stable enough for the analysis and preferably also be one of the major constituents of the oxidation mixture as to be present in maximum amounts. 7-oxo-dehydroabietic acid (7-O-DeA), CAS no. 18684-55-4, was chosen for the purpose [8, 12, 14, 15].
1.2 Investigated samples
Commodities investigated for colophonium content include disposable diapers [2], sanitary pads [6], herbal oils and ointments [4], sulphate soaps [9], mascaras [11, 18], adhesives [3, 5, 7], various forms of cardboard and paper [3, 8, 10, 19, 20], floor polish [8] and cutting fluid, soldering flux, and paint products [3]. As the heating of rosin flux during soldering causes a colophonium-containing aerosol, such fumes have been analysed [21]. Dust [8], wood [9] and papermaking process water [22] have also been examined. Furthermore, to study the exposure to colophonium, DeA has been used as a biomarker in urine of factory workers performing soldering [23].
1.3 Sample preparation
Prior to analysis, the samples are prepared by extraction of the analytes into a suitable solvent. Depending on the investigated sample different solvents have been utilized: acetone [2, 7, 8, 10, 19], methanol [3, 12, 19], ethanol [14], acetonitrile [4, 5], dichloromethane [3, 8, 15], ethyl acetate [3], methylene chloride [21], diethyl ether [4, 9, 23] and methyl tert-butyl ether (MTBE) [22]. If necessary, the following sample clean-up may involve filtration [3, 8, 12], centrifugation [4] and/or solid-phase extraction (SPE) [4, 5].
1.4 Analytical methods, separation and detection
To determine the presence of colophonium in the samples, the sample components are often derivatised and separated by gas chromatography (GC) [2, 6, 8-10, 13, 21-23] or separated by high-performance liquid chromatography (HPLC) in their unmodified form [3-5, 8, 12, 13]. Detection of the separated components by GC has been performed by flame ionization [2, 8-10, 22] or mass spectrometry (MS) [21, 23]. For detection of the HPLC separated samples, ultraviolet [3-5, 8, 12, 13], fluorescence detection [4, 5] and MS [12, 13] are normally used. Analytes which are sensitive to the high temperatures in GC have been introduced into the mass spectrometer through a direct insertion probe [13, 14]. An alternative method for such analytes is matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS), where a cellulose coated thin layer chromatography (TLC) plate serves as the sample probe [15].
2 Method description and evaluation
2.1 GC methods
2.1.1 GC-FID
Method [9]
- Description: Heptadecanoic acid as internal standard. Extraction with diethyl ether. AbA and DeA derivatised to methyl esters by diazomethane.
Advantages: Peak identification based on both retention time of known, pure analytes and mass spectra. - Disadvantages: Modification of analytes. Diazomethane is classified as carcinogenic. No oxidation products analysed. Not quantitative.
- Used for: wood extractives, sulphate soaps, crude and distilled tall oil
Method [22]
- Description: Heneicosanoic acid, betulinol, cholesteryl heptadecanoate and 1,3-dipalmitoyl-2-oleoyl glycerol as four internal standards. Extraction with MTBE. AbA and DeA silylated by bis(trimethylsilyl)-trifluoro-acetamide (BSTFA) and trimethylchlorosilane (TMCS).
- Advantages: Quantitative. Many other components also analysed.
- Disadvantages: Limit of detection unspecified. Modification of analytes. Silylated samples degrade and should be analyzed within 12 hours. No oxidation products analysed. Not optimised specifically for separating AbA and DeA. Method for peak identification unspecified.
- Used for: papermaking process waters and effluents
Method [2, 8, 10]
- Description: Methyl stearate as internal standard. Extraction with acetone. Analytes derivatised to methyl esters by diazomethane.
- Advantages: Detection of AbA, DeA, and 7-O-DeA. Quantitative.
- Disadvantages: Modification of analytes. Diazomethane is carcinogenic and explosive. Peak identification based on retention time of known, pure analytes; risk of interferences by and confusion with unknown peaks. Need to synthesise reference substance 7-O-DeA, since not commercially available. Detection limit unspecified.
- Used for: paper and linoleum floor covering, diapers
2.1.2 GC-MS
Method [21]
- Description: Heptadecanoic acid as internal standard. Extraction with methylene chloride. Detection of AbA and DeA; derivatised to methyl esters by potassium carbonate and methyl iodide.
- Advantages: Peak identification based on both mass spectra and retention time of known, pure analytes. Quantitative.
- Disadvantages: Modification of analytes. No oxidation products detected. Limit of detection unspecified.
- Used for: soldering fumes
Method [23]
- Description: Heptadecanoic acid as internal standard. Extraction with diethyl ether. DHA derivatised by dimethylformamide dimethylacetal. Limit of detection; 50nM DHA.
- Advantages: Peak identification based on both mass spectra and retention time of known, pure analytes. Quantitative.
- Disadvantages: No detection of AbA or oxidation products.
- Used for: biomarker in urine
2.2 HPLC methods
2.2.1 HPLC-UV
Method [3]
- Description: UV detection of AbA at 242nm and DeA at 265nm. Calibration with pure acids. Extraction with methanol, ethyl acetate or dichloromethane. Limits of detection; 10ppm AbA, 150ppm DeA.
- Advantages: No modification of analytes. Additional peak identification by UV-spectra at peak apex. Quantitative.
- Disadvantages: No oxidation product detected. Peak identification based on retention time of known, pure analytes; risk of interferences by and confusion with unknown peaks. DeA has low absorption at 267nm; at max absorption 215nm there is high matrix interference.
- Used for: adhesives, cutting fluid, soldering flux, and paint products
Method [13]
- Description: UV detection of 15-HPDA at 254nm. 15-HPDA thermally instable; for MS collect HPLC fraction; DIP.
- Advantages: No modification of analytes. Detection of the oxidation product 15-hydroperoxydehydroabietic acid (15-HPDA). Additional peak identification by MS.
- Disadvantages: AbA and DeA not detected. Not quantitative. Need to synthesise reference substance 15-HPDA, which is not commercially available and unstable. Peak identification based on retention time of known, pure analytes; risk of interferences by and confusion with unknown peaks.
- Used for: unmodified colophonium as such
Method [8]
- Description: Method according to [3]. Extraction with acetone or dichloromethane.
- Advantages: No modification of analytes. Detection of the oxidation product 7-O-DeA, also AbA and DeA.
- Disadvantages: Must synthesise reference substance 7-O-DeA, since it is not commercially available. Peak identification based on retention time of known, pure analytes; risk of interferences by and confusion with unknown peaks. DeA has low absorption at 267nm; at max absorption 215nm there is high matrix interference. Not quantitative.
- Used for: Paper, floor polish, dust
Method [12]
- Description: UV detection of AbA and DeA at 245nm. Extraction with methanol. Separation optimised to for isolation of max number of components.
- Advantages: Detection of the oxidation product 7-O-DeA, also AbA and DeA and a number of additional components. No modification of analytes. Identification of peaks by MS.
- Disadvantages: Peak identification based on retention time of known, pure analytes; risk of interferences by and confusion with unknown peaks. Need to synthesise reference substance 7-O-DeA, since it is not commercially available. DeA has low absorption at 245nm; at max absorption 215nm there is high matrix interference. Not quantitative.
- Used for: unmodified colophonium as such
2.2.2 HPLC-UV and fluorescence detection in combination
Method [5]
- Description: UV detection of AbA at 240nm and fluorescence detection of DeA excitation 225nm, emission 285nm. Calibration with pure acids. Extraction with acetonitrile. SPE prior to HPLC. Limits of detection; 1.25ng AbA, 0.5ng DeA.
- Advantages: No modification of analytes. SPE limits matrix interferences and enhances detection specificity. Detection of DeA by fluorescence is more than 100 times more sensitive than UV. Both detectors on the same column. HPLC separation time only approx. 10 minutes. Quantitative.
- Disadvantages: No oxidation products detected. Peak identification based on retention time of known, pure analytes; risk of interferences by and confusion with unknown peaks.
- Used for: makeup adhesive
Method [4]
- Description: UV detection of AbA at 200nm and fluorescence detection of DeA excitation 225nm, emission 285nm. Calibration with pure acids. Extraction with acetonitrile or diethyl ether. SPE prior to HPLC. Limit of detection: 0.4ppm or 1ng for AbA and DeA.
- Advantages: No modification of analytes. SPE limits matrix interferences and enhances detection specificity. Detection of DeA by fluorescence is more than 100 times more sensitive than UV. Both detectors on the same column. HPLC separation time only approx. 10 minutes. Quantitative.
- Disadvantages: No oxidation products detected. Peak identification based on retention time of known, pure analytes; risk of interferences by and confusion with unknown peaks.
- Used for: herbal oils and ointments
2.2.3 HPLC-MS
Method [13]
- Description: HPLC-UV fractionation (see above); fractions analysed by DIP-MS.
- Advantages: No modification of analyte. Mass spectra of the oxidation product 15-HPDA, which is thermally instable.
- Disadvantages: AbA and DeA not detected. Not quantitative. Need to synthesise reference substance 15-HPDA, since it is not commercially available.
- Used for: unmodified colophonium as such
Method [12]
- Description: HPLC-UV fractionation (see above); fractions analysed by chemical ionization-MS.
- Advantages: Detection of the oxidation product 7-O-DeA, also AbA and DeA and a number of additional components. No modification of analytes.
- Disadvantages: Not quantitative. For 7-O-DeA identification; IR and NMR.
- Used for: unmodified colophonium as such
2.2.4 Direct insertion probe MS method
Method [14]
- Description: Extraction with ethanol. Chemical ionization and electron impact ionization MS. Compound identification based on reference mass spectra.
- Advantages: Detection of the oxidation product 7-O-DeA, also AbA and DeA and a number of additional components, including oxidation products. No modification of analytes.
- Disadvantages: No separation; complex mass spectra. Not quantitative.
- Used for: unmodified colophonium as such
2.2.5 MALDI-MS method
Method [15]
- Description: Extraction with dichloromethane. TLC plate coated with cellulose as sample probe.
- Advantages: Detection of 7-O-DeA and other oxidation products. No modification of analytes.
- Disadvantages: No separation; complex mass spectra. Not quantitative. Requires mechanical modification of instrument sample probe.
- Used for: unmodified colophonium as such
2.3 Conclusions
The content of AbA may vary between 30 – 50% for gum rosin and 35 – 40% for tall oil rosin. Assuming that the resin acid content is 90%, a detection limit of 10 microgram/g AbA thus corresponds to a detection limit of approximately 30 microgram/g (ppm) of colophonium. This could be considered acceptable compared to the reactivity in allergic patients [3]. A detection limit of 10 ppm AbA may therefore serve as a guide to determine the order of magnitude for the desired limit of detection in the chosen analytical method.
Suggested method I, existing
The GC-FID-method [2, 8, 10] includes the quantitative analysis of AbA, DeA and 7-O-DeA. Amounts down to 1 ppm AbA [10], 1 ppm DeA [2] and 2 ppm 7-O-DeA [2] have been presented, however the limit of detection is unspecified. Note that the necessary derivatisation of the analytes includes the use of hazardous chemicals.
To avoid peak interferences and confusion with unknown peaks, a complementary CG-MS analysis could be developed and performed when needed [12].
Suggested method II, method development
The HPLC-UV-fluorescence method [4, 5] is quantitative for AbA and DeA down to 0.5-1.25 ng, corresponding to about 0.4 ppm. No sample derivatisation of the analytes is needed. Matrix interferences are limited and the detection specificity is enhanced by SPE.
The oxidation product 7-O-DeA may be included in this method. This analyte has been investigated by HPLC-UV method [8], however not quantitatively.
As the HPLC-UV-fluorescence method is non-destructive, fractionation and further analysis is an option, e.g. directly by MS to avoid confusion with potential unknown peaks [12-14].
2.4 References
- Hausen, B.M., et al., Contact Allergy Due to Colophony.3. Sensitizing Potency of Resin Acids and Some Related Products. Contact Dermatitis, 1989. 20(1): p. 41-50.
- Karlberg, A.T. and K. Magnusson, Rosin components identified in diapers. Contact Dermatitis, 1996. 34(3): p. 176-8O.
- Ehrin, E. and A.T. Karlberg, Detection of rosin (colophony) components in technical products using an HPLC technique. Contact Dermatitis, 1990. 23(5): p. 359-66.
- Lee, B.L., et al., High-performance liquid chromatographic determination of dehydroabietic and abietic acids in traditional Chinese medications. Journal of Chromatography A, 1997. 763: p. 221-226.
- Lee, B.L., et al., High-Performance Liquid-Chromatographic Method for Determination of Dehydroabietic and Abietic Acids, the Skin Sensitizers in Bindi Adhesive. Journal of Chromatography A, 1994. 685(2): p. 263-269.
- Kanerva, L., et al., Colophonium in sanitary pads. Contact Dermatitis, 2001. 44(1): p. 59-60.
- Gafvert, E. and G. Farm, Rosin (colophony) and zinc oxide in adhesive bandages - An appropriate combination for rosin-sensitive patients? Contact Dermatitis, 1995. 33(6): p. 396-400.
- Karlberg, A.T., et al., Airborne contact dermatitis from unexpected exposure to rosin (colophony) - Rosin sources revealed with chemical analyses. Contact Dermatitis, 1996. 35(5): p. 272-278.
- Holmbom, B., Improved Gas Chromatographic Analysis of Fatty and Resin Acid Mixtures with Special Reference to Tall Oil. J Am Oil Chem Soc, 1977. 54: p. 289-293.
- Karlberg, A.T., E. Gafvert, and C. Liden, Environmentally Friendly Paper May Increase Risk of Hand Eczema in Rosin-Sensitive Persons. Journal of the American Academy of Dermatology, 1995. 33(3): p. 427-432.
- Karlberg, A.T., C. Liden, and E. Ehrin, Colophony in Mascara as a Cause of Eyelid Dermatitis - Chemical-Analyses and Patch Testing. Acta Dermato-Venereologica, 1991. 71(5): p. 445-447.
- Sadhra, S., C.N. Gray, and I.S. Foulds, High-performance liquid chromatography of unmodified rosin and its applications in contact dermatology. J Chrom B, 1997. 700: p. 101-110.
- Shao, L.P., et al., 15-Hydroperoxydehydroabietic Acid - a Contact Allergen in Colophony from Pinus Species. Phytochemistry, 1995. 38(4): p. 853-857.
- Scalarone, D., et al., Direct-temperature mass spectrometric detection of volatile terpenoids and natural terpenoid polymers in fresh and artificially aged resins. Journal of Mass Spectrometry, 2003. 38(6): p. 607-617.
- Scalarone, D., et al., MALDI-TOF mass spectrometry on cellulosic surfaces of fresh and photo-aged di- and triterpenoid varnish resins. J Mass Spectrom, 2005. 40(12): p. 1527-35.
- Hausen, B.M., et al., Contact Allergy Due to Colophony.9. Sensitization Studies with Further Products Isolated after Oxidative-Degradation of Resin Acids and Colophony. Contact Dermatitis, 1993. 29(5): p. 234-240.
- Karlberg, A.T. and E. Gafvert, Isolated colophony allergens as screening substances for contact allergy. Contact Dermatitis, 1996. 35(4): p. 201-207.
- Sainio, E.L., M.L. HenriksEckerman, and L. Kanerva, Colophony, formaldehyde and mercury in mascaras. Contact Dermatitis, 1996. 34(5): p. 364-365.
- Karlberg, A.T. and C. Liden, Colophony (Rosin) in Newspapers May Contribute to Hand Eczema. British Journal of Dermatology, 1992. 126(2): p. 161-165.
- Bergh, M., T. Menne, and A.T. Karlberg, Colophony in Paper-Based Surgical Clothing. Contact Dermatitis, 1994. 31(5): p. 332-333.
- Smith, P.A., et al., Detection of resin acid compounds in airborne particulate generated from rosin used as a soldering flux. American Industrial Hygiene Association Journal, 1997. 58(12): p. 868-875.
- Örså, F. and B. Holmbom, A convenient method for the detemination of wood extractives in papermaking process waters and effluents. Journal of Pulp and Paper Science, 1994. 20(12): p. 361-365.
- Jones, K., et al., Identification of a possible biomarker for colophony exposure. Occup Med (Lond), 2001. 51(8): p. 507-509.
Appendix B: A method for quantification of resin acids in cosmetics
Chemicals
Cis-5,8,11,14,17-eicosapentaenoic acid (EPA, CAS no. 10417-94-4, >98.5%), and abietic acid (CAS no. 514-10-3, technical quality, 75%) were both purchased from Fluka.
Pimaric acid (CAS no. 510-39-4) and dehydroabietic acid (CAS no. 1740-19-8) were both obtained from Helix Biotech and 7-oxo-dehydroabietic (CAS no. 18684-55-4) was synthesized according to Ayer, and Migaj, (1989).
Standard solutions for HPLC were made in acetonitrile:MilliQ water 9:1 with 0.2% formic acid. They were stored in refrigerator and were stable for at least 2 weeks.
Colophonium samples were dissolved in 100% acetonitrile to approximately 1.5 mg/ml.
Samples
The method has been tested on Wax strips, foundation, rouge and mascara
Procedure
Extraction:
1 to 2 g samples (1 g for wax strips and mascara) were ultrasonicated in 20-100 ml (depending on sample type) of acetonitrile during 30 min. The wax strips were cut into pieces. The papers were separated to expose the wax to solvent. An aliquot of 500 ?l EPA, from a 928 micro?gram/ml solution, was added as internal standard (IS) prior to extraction.
For all cosmetic samples except wax strips: Each extract was transferred to plastic tubes and centrifuged during 15 min at 4000 rpm. The supernatant was transferred to a flask, while the pellet was redissolved in acetonitrile, ultrasonicated and centrifuged using the same procedure. The supernatants were pooled in the flask and evaporated in a rotavapor to a volume of approximately 5 ml. MilliQ water was then added to a final compostion of 7:3 acetonitrile:water and a total volume of 10 ml. Addition of water made the solution opaque. It was therefore filtrated through a syringe filter (0.45 micro?meter polypropylene membrane).
Note: Check that the syringe filter is resistant to acetonitrile.
For wax strips: After ultrasonic extraction, the sample was evaporated, using rotavapor, to about 5 ml. MilliQ water was added to a final composition of acetonitrile:water 7:3 and a total volume of 10 ml. The sample was transferred to plastic tubes. The flask was washed with a small volume of acetonitrile, which was also transferred to the tubes. The sample was then centrifuged at 4000 rpm during 15 min. The supernatant was loaded directly on the conditioned SPE, without syringe filtration.
Solid phase extraction (SPE):
MAX columns (6cc, 500mg) from Waters were used. Conditioning was performed by percolating 1) 3 ml of methanol and 2) 3 ml of MilliQ water through the SPE column. The sample was loaded and the column was then washed with 1) 3 ml of 50 mM NaOAc in MilliQ water, 2) 4 ml of methanol and finally 1 ml of 2% formic acid in methanol. The sample was then eluted with 2 ml of 2% formic acid in methanol. An additional volume of 1 ml 2% formic acid in methanol was gathered in case of breakthrough. 3 fractions were thus kept: 1) the last wash fraction (1ml), 2) the elution fraction (2 ml) and 3) an additional fraction (1ml). Only the elution fraction is to be analyzed.
Note 1: The composition of acetonitrile:water 7:3 is important for at good recovery from the SPE clean up.
Note 2: The formic acid solution has to be freshly made (daily). It is critical for good recoveries to collect exact volumes of the elution fractions.
For Colophonium samples: Internal standard, 200 ?microliter (186 ?microgram) was added to 2 ml of 1.5 mg/ml colophonium solutions. The colophonium samples were analyzed directly by HPLC after syringe filtration (without SPE clean-up).
HPLC:
HPLC system equipped with an injection loop of 20 ?l, a diode array detector (DAD) and a column heater 25ºC. The HPLC column was a RP-12 (4.6mm i.d., length 150mm, particle size 3 micrometer). The stationary phase contains both C12-chains and urea functions. Methanol:water 8:2 with 0.05% formic acid was used as mobile phase at a flow rate of 1 ml/min (yielding a pressure of approx 240 bar). An isocratic elution was performed. Four different wavelengths were used 210, 220, 240 and 250 nm. The different absorption ratios were used for identification of the compounds.
Note: Isocratic elution has to be performed to avoid increasing baseline, which would lead to higher LODs. The amount of formic acid is critical for both retention and noise level.
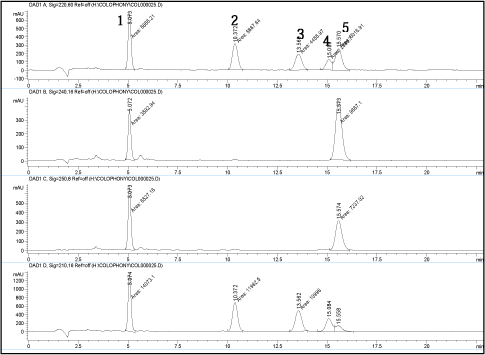
Chromatograms of a standard solution (200 ng/?l) at the different wavelengths are shown on next page.
The different absorption ratios used for identification (CV below 10%):
| 7-OxoDeA: | 220nm/210nm ratio 0.53 240nm/210nm ratio 0.26 250nm/210nm ratio 0.45 |
| DeA: | 220nm/210nm ratio 0.55 |
| EPA: | 220nm/210nm 0.40 |
| AbA | 240nm/250nm 1.36 |
It is recommended to control the specific absorption ratios on pure standards as these can vary depending on the specific detector.
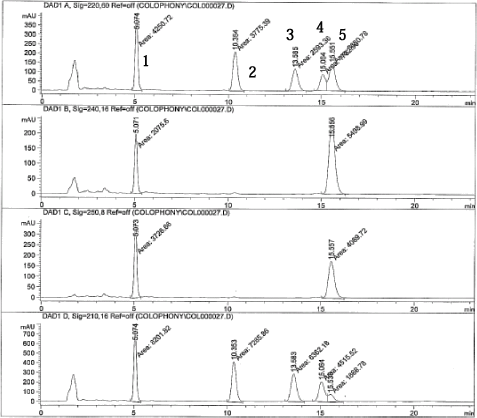
Peak 1 = 7-oxo-dehydroabietic acid
Peak 2 = Dehydroabietic acid
Peak 3 = EPA, internal standard
Peak 4 = Pimaric acid
Peak 5 = Abietic acid
The method was developed by Ulrika Nilsson and Nagmeh Berglund, Dept. of Analytical Chemistry, Stockholm University, Sweden
Reference
Ayer, W. A. and Migaj, B. S. Acids from blue-stain diseased lodgepole pine. Can J Bot. 1989: 67: 1426-1428
Version 1.0 Marts 2009, © Danish Environmental Protection Agency