Development and use of screening methods to determine chromium (VI) and brominated flame retardants in electrical and electronic equipment
3 Presence of hexavalent chromium in electronic equipment
- 3.1 What is hexavalent chromium?
- 3.2 Use of hexavalent chromium for anticorrosive coatings
- 3.3 Uses of metallic chromium
- 3.4 Chromium(VI) in plastic and paint/enamel pigments
- 3.5 Chromium in glass
- 3.6 Exemptions from the RoHS Directive
- 3.7 Decision tree
3.1 What is hexavalent chromium?
Chromium occurs in both metallic form and as ions (chemical building blocks) in solutions or solid salts. Chromium ions may occur in three different forms known as oxidation states, which determine how the chromium ions react with other substances. The two most common oxidation states for chromium ions are 3 and 6, but occasionally, chromium ions may also be present in oxidation state 2. Chromium in oxidation state 6 is also called hexavalent chromium, because of the atom's ability to bond with six other atoms. It is also called chromium(VI) or Cr(VI). Correspondingly, the two other forms are called trivalent chromium, Cr(III), and divalent chromium, Cr(II), while metallic chromium in oxidation state 0 is often called Cr(0).
Hexavalent chromium is highly oxidising and the form that is most hazardous to the environment and to health. This is why the RoHS Directive only comprises hexavalent chromium. Normally, hexavalent chromium is not present in nature, and salts with hexavalent chromium are almost always man-made. Hexavalent chromium released from a product will relatively quickly react with other substances (e.g. iron(II)), thereby reducing the chromium to a lower oxidation state. When sampling, it should therefore be noted that a chemical reaction by which the hexavalent chromium is changed to the trivalent form is not allowed.
Hexavalent chromium will not be present in electrical and electronic products as a natural contamination element, so it is only necessary to check for hexavalent chromium where it can be deliberately used in the products.
Traditionally, the most common applications of hexavalent chromium in electrical and electronic equipment have been:
- Anticorrosive coatings on metal parts;
- Pigments in plastic parts and paint/enamel;
- Emerald green glass.
3.2 Use of hexavalent chromium for anticorrosive coatings
Passivation (also called chromating) with hexavalent chromium is used to protect metal surfaces against corrosion. The corrosion resistance of a large number of metals, including zinc, aluminium, cadmium, copper and stainless steel, can be strengthened by treatment with chromate-based solutions. The treatment generates a very thin surface consisting of chromium salts that make the metal part especially corrosion-resistant.
Passivation with hexavalent chromium is used in electrical and electronic equipment, particularly for coating of electro-galvanised steel and aluminium.
Metal parts coated with hexavalent chromium can be placed anywhere in appliances and individual components, e.g.:
- Screws, rivets, bolts;
- Frames and chassis;
- Electric switches, etc.;
- Plugs and terminators;
- Spacers;
- Antennae and accessories;
- Bars, etc.
Chromating also ensures better paint and enamel adhesion, and steel, aluminium and zinc parts are therefore often chromated before painting.
Electro-galvanised steel is coated with a thin layer of zinc (5-20 µm), traditionally topped by a thin layer of hexavalent chromium. Galvanised steel parts treated with hexavalent chromium have four typical appearances:
- Clear metallic with a slightly bluish tinge (thin layer; from 0.025 µm)
- Yellowish with a pearlescent sheen (medium-thick layer: 0.3-0.6 µm)
- Olive/dark colour (thickest layer; up to 1.5 µm)
- Black colour (< 1.5 µm)
The colours are familiar from DIY centres where the clear metallic screws are for indoor purposes and the coloured ones for outdoor purposes.
In Denmark, the use of hexavalent chromium for blue passivation of zinc has been largely discontinued for the last 10 years. Blue passivation can be attained with a chemical solution containing chromium(III) salts, and the passivation layer will therefore not contain chromium(VI). This probably applies to most Western European countries and the USA. On the other hand, it must be assumed that to a large extent chromium(VI)-containing chemicals are still used for blue passivation in Eastern Europe, Asia and Africa.
In Denmark and Western Europe, the trend is towards yellow and olive passivation without the use of chromium(VI). This trend was mainly a consequence of the RoHS Directive, and as a result, chromium(VI)-free zinc passivation is now also used in areas other than electrical and electronic components. Until recently, chromium(VI) was primarily used for yellow and olive passivation in Eastern Europe, Asia and Africa.
Typical contents of hexavalent chromium in coatings appear from the table below. In addition to hexavalent chromium, these coatings typically contain 70-90% trivalent chromium. In electrical and electronic equipment for indoor use, thin, clear coatings have been most commonly used. To some extent, yellow and olive coatings have been used in equipment for outdoor use or for use in corrosive environments. Black-chromated surfaces are primarily used for decorative purposes.
Table 1 Layer thickness, weight and chromium content of passivation layers on zinc and aluminium
| Passivation | Layer thickness µm |
Layer weight mg/m² |
% chromium in passivation layer |
% Cr(VI) of total chromium |
| Zinc, clear | < 0.2 | < 100 | 30 | 0 - 20 |
| Zinc, blue | < 0.2 | 50 - 500 | 35 | 0 - 20 |
| Zinc, yellow | 0.3 - 0.6 | 500 - 1500 | 35 - 40 | 20 - 30 |
| Zinc, olive | < 1.5 | > 2000 | 40 - 50 | 20 - 30 |
| Zinc, black | 2000 | 30 - 50 | no data | |
| Aluminium, clear | 50-100 | 30 | 20 - 25 | |
| Aluminium, yellow | 250 - 500 | 40 - 45 | 20 - 30 |
Largely all aluminium parts in electrical and electronic equipment have traditionally been coated with hexavalent chromium to avoid surface oxidation. This is often referred to as the Alodine process (Henkel) with regard to both yellow and bright passivation. Chromated aluminium surfaces are used inside electronic products. The corrosion resistance of bright passivated aluminium is more limited than that of yellow chromated aluminium.
Yellow chromating of aluminium is also used for pre-treatment of aluminium products before painting and enamel painting. This ensures good paint adhesion and extra strong corrosion resistance of the painted surface. A number of other passivation products without chromium have been introduced over the last 5-10 years and have gradually gained a foothold in the market because of the RoHS Directive and a general desire to minimise the use of chromium(VI).
Traditionally, hexavalent chromium has also been used for passivation of electrolytically deposited copper foils used in printed circuit boards, Li-ion batteries and plasma screens. The layer is typically less than 15 nm (0.015 µm) and the chromium content amounts to 0.02-0.03 mg/m².
Screening methods
In new RoHS-compliant equipment, hexavalent chromium is often replaced by a layer consisting exclusively of trivalent chromium, Cr(III), or the equipment is completely chromium-free. This means that an XRF instrument that is only capable of indicating whether chromium is present or not would not be able to give a good indication of whether the surface contains hexavalent chromium. In addition, a handheld XRF instrument readout would indicate a very small chromium content because of the thinness of the surface layer.
The screening method developed (section 4) can be used to examine whether hexavalent chromium is present in the surface of a metal part, and in principle the method can be used on all metal parts with metallic surfaces. For metal parts that have also been painted or enamel painted, it will be necessary to remove the paint/enamel first, thus rendering the screening method fairly uncertain.
None of the screening methods are suited for examining whether hexavalent chromium has been used to passivate the surface of electrolytically deposited copper foils.
3.3 Uses of metallic chromium
Chromium is widely used as an alloying element in steel. In these cases the chromium is always in the metallic form, Cr(0). Stainless steel typically contains 12-18% chromium, while other types of steel contain 0.1-2% chromium. Corrosion of the steel may generate chromium compounds, but they will not consist of hexavalent chromium.
The familiar bright, chromium-plated surfaces of steel, copper and brass consist of metallic chromium. In most cases, hexavalent chromium continues to be used to produce such chromium layers, but hexavalent chromium will not be present in the finished pieces, because the chromium(VI) salts are rinsed off after the electrolytic process. For several years it has been possible to produce these chromium coatings using chromium(III) salt electrolysis, and now the method finally seems to be gaining a foothold in Denmark. The traditional chromium-plating process using hexavalent chromium continues to be widely used, however.
3.4 Chromium(VI) in plastic and paint/enamel pigments
Hexavalent chromium is used in a number of pigments.
Together with lead and molybdenum, hexavalent chromium generates a number of pigments in clear red, orange, yellow and green colours. Lead chromates and lead-molybdenum chromates have traditionally been important pigment types in plastic and paint.
In electrical and electronic equipment these pigments will primarily occur in plastic of clear red, orange, yellow and green colours that may be found in:
- Cabinets;
- Plastic parts on individual components, including switches, fuses, etc.;
- Wire/cable insulation.
Together with strontium, barium or zinc, hexavalent chromium generates a number of pigments that have traditionally been used for anticorrosive paints. Such paints have typically not been used in electrical and electronic equipment, but the possibility that they may be used in equipment for outdoor use or for use in corrosive environments cannot be excluded.
Pigments with trivalent chromium yield olive-green colours, but such pigments are mainly used in other product areas, including cosmetics, soaps, detergents and paint.
Screening methods
As the clear red, yellow and green pigments are based on lead chromates and lead-molybdenum chromates and also contain lead, an XRF screening showing a lead content of more than 0.1% will be sufficient to determine whether a plastic part complies with the RoHS requirements. It should be noted that when measuring chromium in thin plastic parts, the XRF instrument readout may well indicate a chromium content of less than 0.1%, even though the actual content is higher.
The presence of strontium or barium together with chromium, determined with an XRF instrument, may indicate the presence of anticorrosive paints based on hexavalent chromium, but it has not been examined whether similar results would be obtained if the elements are present as alloying elements in steel.
The screening method developed (section 4) cannot be used to determine hexavalent chromium in plastic and paint pigments.
If any plastic parts contain chromium, but not lead, a laboratory analysis is necessary to determine whether the chromium is present in the hexavalent form.
3.5 Chromium in glass
Hexavalent chromium has traditionally been used to manufacture a particular type of green glass: clear emerald green crystal. It is stated that the chromium content may be up to 2%. This type of glass seems to have been used mainly for decorative purposes, and in relation to electrical and electronic products it is primarily mentioned in connection with crystal lamps and watches/clocks with electronic parts. An XRF scanning of green crystal showing chromium content would indicate the presence of hexavalent chromium, but a laboratory analysis is necessary to determine with certainty whether the chromium is present in the hexavalent form.
3.6 Exemptions from the RoHS Directive
The list of exemptions from the RoHS Directive includes two exemptions relating to hexavalent chromium. Both exemptions concern the use of hexavalent chromium for passivation in certain product groups.
The two exemptions concern:
- Hexavalent chromium used as an anti-corrosion of the carbon steel cooling system in absorption refrigerators;
- Hexavalent chromium used for corrosion preventive coatings of unpainted metal sheets and fasteners and used for corrosion protection and electromagnetic interference shielding IT and telecommunications equipment (the exemption expired on 1 July 2007).
3.7 Decision tree
The decision tree below illustrates the procedure of the proposed method in relation to hexavalent chromium used for coatings. Obviously, it is also possible to proceed straight to laboratory analyses without prior screenings. The decision tree shows two possible options in case of a positive screening. If the screening clearly indicates the presence of hexavalent chromium, it will be possible to contact the producer on this basis alone, but verification of the positive result by an accredited laboratory test is also an option. In any case, if the result is more doubtful, it is relevant to verify the result by a laboratory analysis.
Chapter 5 includes a particular decision tree for the screening stage.
For documentation from producers, see the corresponding section under PBDEs and PBBs.
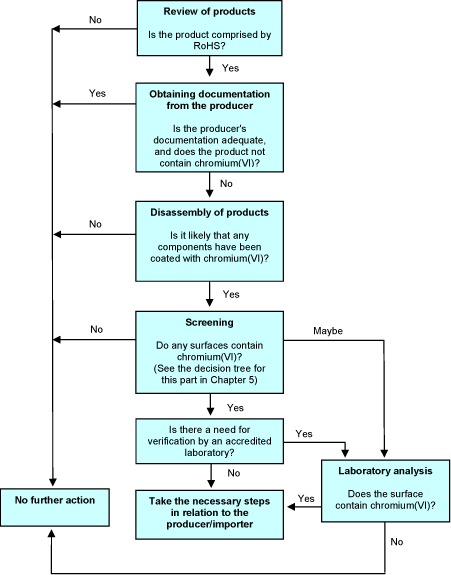
Figure 2 Decision tree in relation to metal surfaces containing hexavalent chromium
Version 1.0 May 2009, © Danish Environmental Protection Agency