Survey and Health Assessment of Products for Interior Car Care
8 Health assessment
- 8.1 Assessment of the evaporation of substances in relation to the limit values of the Danish Working Environment Authority
- 8.2 Selection of substances for health assessment
- 8.3 Health assessment of petroleum distillates
- 8.4 Health assessment of butane
- 8.5 Health assessment of ethyl acetate
- 8.6 Health assessment of 1-methoxy-2-propanol (PGME)
- 8.7 Health assessment of benzyl chloride
In this and the next chapter, health assessments were carried out on the compounds in the analysed products for interior car care. At first, it was assessed if the concentrations measured in the emission tests exceed the occupational threshold limit values that are used in the working environment. The limit values are applicable for the working environment and are put in relation to a whole working day. According to the list of limit values of the Danish Working Environment Authority (DWEA), 2007) the limit values are only advisory when assessing if health hazardous conditions exist and therefore it is in general recommended to keep air pollution as far below the limit values as possible. Therefore, the comparison with the limit values should be regarded as sort of a preliminary screening.
In addition, a ”traditional” health assessment is carried out, i.a. the inhaled amount of substance and the amount of substance absorbed through the skin are put in relation to the critical values. The critical values are the concentrations of substances where literature shows that the substances can result in health effects. The calculations are carried out in chapter 9.
8.1 Assessment of the evaporation of substances in relation to the limit values of the Danish Working Environment Authority
As described in chapter 6 ”Quantitative analyses and exposure” measurements of exposure via inhalation were carried out when using product no. 1, 5, 10 and 24, and subsequent ventilation for five hours. The results are presented in section 6.1.2.
In this section, the measured concentrations of the found substances are put in relation to the limit values of the substances to assess if a person who is in a car during or after application of the interior car care products is exposed to a health risk.
The occupational threshold limit values are determined in the light of the irritative effects of the substances or because of the special harmful effects of the substances (DWEA, 2008). The limit values were determined to protect people who daily work with the substances. A technical/economic evaluation of the limit value level can also form part of the determination of the level. The limit values of the substances have been revised several times through the years. In the working environment the concept “unnecessary exposure” is dealt with. Unnecessary exposure from dangerous substances and material must be avoided. That means, that even though a limit value is observed then additional measures have to be established if exposure is unnecessary (DWEA, 2007). Therefore, a comparison with a working environment limit value can only be advisory.
The definition of limit value and description of how the time-weighted concentration is calculated according to the limit value list of the DWEA is described in box 8.1.
Box 8.1 Definition and description of limit value. Source: The Danish Working Environment Authority, 2007
The limit values in the list of the limit values of the Danish Working Environment Authority (DWEA, 2007) indicate the time-weighted average concentration of a substance in the air breathed at the workplace over an eight-hour working day.
However, even if the time-weighted average concentration does not exceed the limit value, the short-term concentration (15-minute period at the most) must never exceed twice the limit value.
Calculation of time-weighted average
The time-weighted average concentration considers that different concentrations can be measured in different periods. That means that a concentration can exceed the limit value for a shorter period of time, but for a longer of period of time the concentration has to be below the limit value.
The time-weighted average concentration is calculated according to the following formula where tn is the different periods of time during which the different concentrations cn are measured. The product of the time and the concentration of the different periods of time are divided by the total period of time (typically an eight-hour working day):
Time weighted concentration =![]()
Regarding substances that can be absorbed through the skin (marked with an H in the limit value list) the qualification to use the stated limit value as basis of assessment is that absorption through the skin does not take place simultaneously.
Sum formula
When several substances appear at the same time, they can have an intensifying or weakening effect. If no specific information is available about the coordination of the substances, then a sum (additive) effect must be anticipated.
The following formula is used to calculate the total effect:
![]()
where C is the air concentration of the respective substances and GV the corresponding limit values. A fraction sum of 1 corresponds to the limit value of the total effect.
Units
The limit values for gases and vapours are normally expressed in ppm, corresponding to the number of cubic metres of pollutant per cubic metre of air. The concentration can also be expressed in mg/m³. The concentration specifications ppm and mg/m³ can be mutually converted by using the following formula:
Concentration in mg/m3 = ![]() concentration in ppm
concentration in ppm
where M is the molecular weight of the substance.
Evaporation of chemical substances from interior car care products were measured at different time intervals during a total time interval of app. 5 hours. (max. measured for 320 minutes). In general, very low substance concentrations were measured in the course of the 5 hours (often values below the detection limit). Therefore it is anticipated that when measurements have stopped evaporation can be set to zero, meaning all evaporation takes place within the 5 hours.
In order to calculate the concentration and the time-weighted concentration it is necessary to know the concentration at all time intervals during the entire measuring period 0-300 minutes (5 hours). For the periods when the concentration was not measured, the concentration is estimated by using the average concentration of the previous period of time and the concentration of the following period of time. In that way, it is possible to calculate a concentration at different times as stated in Figure 8.1Figure 8.1.
The average concentrations measured at a given time are calculated as a sum of the product of the concentration and the time interval divided by the total period of time according to the following formula:
Average concentration at the time n =
![]()
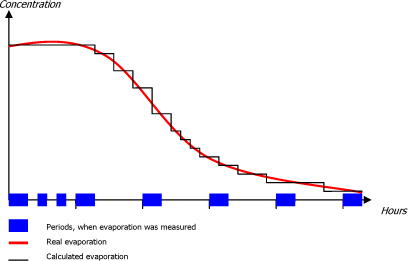
Figure 8.1 Course of evaporation and diffusion of substances after application
The time-weighted concentration is calculated for several periods, around 15, 30, 75, 195 and 315 minutes, respectively, (depending on how it fits with the measurements) to illustrate several situations, i.e.:
- The car is polished inside (duration 15 min.) at home. The car is not used again the same day.
- The car is filled with petrol, washed and polished on the outside at the local service station, and finally the car is polished inside (duration 15 minutes) and then the car is driven directly home. A total exposure of 30 minutes (15 minutes application and 15 minute drive home). The car is not used again the same day.
- Afterwards, a shorter or longer trip is immediately driven in the car. That means exposure during application and stay in the car varies (60, 180 and 300 minute drive, respectively, in the car after application).
That means that an exposure of between 15 and 315 minutes is anticipated. That exposure has to be put into relation with the limit value which is a limit value of a time-weighted concentration over a period of 8 hours. In the calculations it is anticipated that the person in the different situations is exposed between 15 and 315 minutes, after which exposure is set to zero during the remaining time interval up to 480 minutes (= 8 hours), as the scenario is that the person is exposed x minutes successively during/after application and is not exposed to the substances again. In the same way, it is anticipated that exposure is zero after the 315 minutes as most of the substances has evaporated after that time or as evaporation at that time max. is a few percentage of the initial evaporation.
The measured concentrations refer to the applied amount in relation to the volume of the climate chamber (0.42 m³). Therefore, it is necessary to correct the measured values of the actual volume of a car (”Factor volume”) and the actually used amount in a car (”Factor used amount”). The volume of a standard car is app. 3.5 m³ as previously mentioned. Therefore, the measured values have to be divided by a factor 8.3, as the volume of a car is 8.3 times larger than the volume of the chamber.
Correspondingly, the amount of car care product believed to be sufficient to coat a certain plate area was used during the tests. So much car care product was used so it ran down the plate (see detailed description in section 6.1.2). The area of car windows, panels and seats was measured for a standard car. The measured concentrations have therefore been multiplied by a factor 12.2, 8.1 or 17.0 (relation between the car surface area and the sample plate area) depending on the objective of the car care agent. In practice, a greater amount of car care agent is used in a car than during the tests and therefore the actual concentration will also be correspondingly higher.
The time-weighted concentrations of substances with a working environment limit value are shown in Table 8.1Table 8.1 below according to an example of the calculation of stated values in the table.
Example of calculation
The aliphatic hydrocarbons in product 1, vinyl make-up, are used as example.
The average concentration of the hydrocarbons during the first 15 minutes is calculated as described below. Data from Table 6.15Table 6.15 is used from the row ”Sum of hydrocarbons**”. All significant figures are used in the calculations and rounded up values are stated in Table 6.15Table 6.15.
Average concentration at the time n =
![]()
Example: Average concentration for the first 15 minutes =
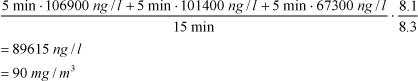
Correspondingly, the time-weighted concentration at different times was calculated as described in the example below. In this case, it is divided by the total time 480 minutes (= 8 hours) to calculate the time-weighted concentration at an arbitrary time n. If the time-weighted concentration at the time n = 15 minutes has to be calculated, then the concentration is set to zero from minute 15 and up to the 480 minutes. In that way, the time-weighted concentration for 15 minutes of exposure is obtained – but time-weighted against a period of 8 hours so the value can be compared to the limit value of the DWEA.
Time-weighted concentration at the time n over an 8 hour day =
![]()
Once again, the aliphatic hydrocarbons in product 1,vinyl make-up, were used as example and the time used was t = 15 minutes. In the time-weighted concentration it is considered that the concentration is zero from 15 minutes and up to the 8 hours which is the time the time-weighted concentration is calculated, The figures originate from ”Sum of hydrocarbons**” in Table 6.15Table 6.15.
Time-weighted concentration at the time 15 minutes =

The evaporated amount of the substances was measured every 5th minute at the beginning, and then every half hour. A calculation example of the time-weighted concentration of e.g. the time 315 minutes would be too comprehensive and therefore only a calculation example for the time n = 15 minutes was carried out.
Click here to see: Table 8.1 Time-weighted concentrations of compounds from products
| Product 1 Vinyl make-up |
Product 5 Fabric waterproofing |
Product 10 Vinyl cleaner |
Product 24 Glass cleaner |
|
|---|---|---|---|---|
| Sum formula limit value (must not exceed 1) | 0.0009 | 0.021 | 0.004 | 0.012 |
It appears from Table 8.1Table 8.1 that none of the evaporated substances have problems regarding the limit values of the substances. In addition, the limit values are not exceeded when regard is given to the additive effect of the substances (Table 8.2Table 8.2), i.e. if for instance fabric waterproofing as well as vinyl cleaner is used when the same substance forms part of both products. Finally, there are no problems when the measured concentration of the substances during the first 15 minutes exceeds 2 x limit value of the substances (rule concerning the limit values, see page 11 in “Arbejdstilsynet”, 2007).
The four investigated products for interior car care contain some of the same substances. Even if the car care products are applied simultaneously the limit limits will not be exceeded in the scenarios that are set up.
8.1.1 Which amount should be used in order to reach the limit value of the Danish Working Environment Authority?
During the climate chamber tests, the amount was applied that is anticipated to be used during normal use of the products. If it is anticipated that twice the applied amount would give the corresponding double measured concentration of each substance, it can be calculated which amount of the products has to be used to reach the limit value of the working environment. In the calculations, regard is not given to additive effects. The amount is calculated on the basis of the relation between the limit value and the measured average concentration. That factor is multiplied by the amount that is estimated to be used for a car.
The results are calculated for each compound with a limit value for 5 hours and 30 minutes. See the results in Table 8.3Table 8.3. The 5 hours and 30 minutes, respectively, correspond to the two scenarios where the person applies the products in a closed car and then remains in the car, so the total time spent in the car becomes 5 hours or 30 minutes, respectively. The latter scenario corresponds to a situation where the car care product is applied, e.g. at a service station and the car is then driven directly home.
It appears from the table that in connection with most of the substances a total product amount exceeding 1 kg by far has to be used before the limit value of the individual substance is reached if the person as worst-case scenario remains in the car 5 hours after application of the product. A substantially smaller amount only has to be used in connection with hydrocarbons in the vinyl make-up to reach the limit value. The 434 g that have to be used corresponds to a consumption of 1.7 cans (estimated for the volume and density of the product) or 21 times the amount anticipated to be used for one car in this project. When staying 30 minutes in the car, an even larger amount has to be used to reach the limit value (3.2 cans of the product).
In the light of the measured values of the measured products, observing the limit values of the compounds of the products is not assessed to be a problem. Even if several products are used simultaneously (e.g. vinyl make-up, fabric waterproofing and glass cleaner), there will be no problems related to observing the limit values of the compounds of the investigated products. Furthermore, it must be anticipated that there will be some kind of air change in the car when driving in it, unless the fan is switched off and the recirculation of air is switched on.
8.1.1.1 Theory of calculations: Product 1 – Vinyl make-up
According to the safety data sheet, product 1 contains a number of petroleum products:
- Distillates (crude oil), hydrogen treated light
- Naphtha (crude oil), hydro desulphurized light, dearomatized (benzene content < 0.1%)
- Crude oil gases, condensed, sweetened (does not contain 1.3-butadien).
In the climate chamber tests, these products were identified as C4-C7 and C10-C14 aliphatic hydrocarbons. The Danish Working Environment Authority has a limit value of 180 mg/m³ for petroleum distillates with the chain length C9-C14 (< 5% aromatic compounds). In the calculations it was assumed that the petroleum products described in the safety data sheets have this limit value (which also is stated by the manufacturer on the safety data sheet). That assumption was chosen as a precautionary approach as it is the lowest of the different petroleum fractions of the Danish Working Environment Authority. Calculations on the basis of the limit values of each compound in C4-C7 (pentane, hexane or heptane) were not carried out in this project.
The calculations show that 434 and 817 g, respectively, of vinyl make-up has to be used to reach the limit value of the petroleum distillates for staying in the car for a total of 5 hours and 30 minutes, respectively (incl. application time). As this vinyl make-up has a density of 0.636 kg/l and 400 ml of the product is sold each time that corresponds to having to use app. 1.7 cans and 3.2 cans before the limit value is reached.
8.1.1.2 Theory of calculations: Product 5 – Fabric waterproofing
According to the safety data sheet, product 5 contains:
- Propane/butane 20-40%
- Heptane 30-40%
- Butyl acetate
- Ethyl acetate.
The climate chamber tests i.a. identified butane and C5-C8 aliphatic hydrocarbons which in this case are anticipated to cover propane as well as heptane. Heptane has the limit value 820 mg/m³ and propane has the limit value 1800 mg/m³. During the first 15 minutes, the measured concentration did not exceed 2 x the limit value, if the C5-C8 aliphatic hydrocarbons are regarded as heptanes as stated in the safety data sheet.
The quantitative analyses (section 6.2.1) show that heptane is not necessarily pure but in the calculations it has been anticipated that pure heptane is in question and the limit value of heptane has been used.
8.1.1.3 Theory of calculations: Product 10 – Vinyl cleaner
The substance 1-propanol can be absorbed through the skin which means that exposure increases if the product is applied without using gloves. Exposure to 1-propanol that takes place through the skin has not been taken into account in this case, but, of course, it has to be added to exposure by inhalation. Exposure through the skin is anticipated to be min., as the product is not applied with the bare hands but a cloth is used.
8.1.1.4 Theory of calculations: Product 24 – Glass cleaner
It should be noted that during the first 15 minutes of the analysis it was not possible to distinguish 1-butoxy-2-propanol and 2-butoxy ethanol from each other with the chosen analysis method. Therefore, the amount measured during the first 15 minutes is the sum of the two substances. Therefore, it was in the calculations anticipated that the condition found between the two substances after the 15 minutes also exists between the two substances during the first 15 minutes.
The substance 2-butoxy ethanol can be absorbed through the skin which means that exposure increases if the product is applied without using gloves. Exposure to 2-butoxy ethanol that takes place through the skin has not been taken into account in this case, but it has to be added to exposure by inhalation. Exposure through the skin is anticipated to be min., as the product is applied with a cloth.
8.1.2 Calculated total concentration of hydrocarbons
The emission tests in the box did in a few cases give problems with overexposure of the pipes used to collect the substances for analysis, and therefore especially the measured hydrocarbons are uncertain and must be regarded as minimum values. However, the same concentrations of hydrocarbons were not measured during the first measuring periods (the first few minutes), which indicates that the problem with overexposure was not necessarily very big.
Therefore, a theoretical calculation of the concentration of hydrocarbons was also carried out for product no. 1 (the product closest to the limit value). According to Table 6.4Table 6.4, hydrocarbons in product 1 were quantified in a total amount of 250 mg/g sample (110 + 140 mg/g sample). According to Table 6.2Table 6.2, 20 g of product 1 is typically used for application in a car. That will result in an amount of 5000 mg hydrocarbons in a car volume of 3.5 m³, i.e. a concentration of 1429 mg/m³ if it is anticipated that all hydrocarbons evaporate immediately when used.
If it is anticipated that the hydrocarbon concentration is kept constant, i.e. no decay and no ventilation in the car, then a person can stay 60.5 minutes in the car before the working environmental limit value of petroleum distillates is exceeded.
![]()
If for instance twice the amount is used, then the concentration becomes twice as large and the time before the limit value is reached is halved.
In that way, it is theoretically possible to exceed the limit value, but it requires the use of a large amount of vinyl make-up and that the person remains seated in a completely closed car (with the application cloth) for a longer period of time before the limit value is exceeded. Finally, it should be stressed that a theoretical maximum value is in question as it is anticipated that evaporation takes place spontaneously and that the concentration is kept constant (unrealistic as a car is not tight and as the substances are decomposed in air).
The theoretical maximum calculated concentration of hydrocarbons in the car cabin will exceed 2 x theoretical value and therefore it is recommended to apply vinyl make-up with doors open.
A person can remain in the car without the limit value being exceeded (based on the theoretical maximum concentration) for a longer period of time, than the time the pipes were overexposed in the emission tests, and therefore it is assessed that the conclusions made on the basis of the emission tests are still valid as long as the largest concentration/evaporation takes place within the first half hour. There is no great difference between exposure during the first half hour and exposure during all 5 hours, as the concentration declines substantially after the first half hour.
8.2 Selection of substances for health assessment
In the light of the conversations with the staff in the shops, which formed part of the survey, and the information from the product labels, relevant exposure scenarios were drawn up for using products for interior car care (see chapter 4). Based on these exposure scenarios, actual application tests were carried out in climate chambers for four of the selected products for interior car care. In climate chambers it was measured which substances evaporate in the chamber when using realistic amounts of the selected products for interior car care (see detailed description of climate chamber tests in section 6.1.2).
The chemical substances that evaporate the most in the climate chambers and that have a relevant health classification (i.e. effects in relation to inhalation or skin contact, etc.), are the substances on which it is most obvious to carry out detailed health assessments.
In addition, quantitative analyses were carried out of the total content of chemical substances in 15 selected products for interior car care. In connection with these products it is relevant to assess the risk related to absorption through the skin if e.g. gloves are not used when applying the car care products. The products are typically applied with a cloth and therefore there is no direct skin contact, however, a smaller amount of the car care products must be expected to penetrate the cloth and could be absorbed through the skin. Therefore, it is also relevant to have a closer look at absorption through the skin of the danger classified substances that appear in the highest concentrations in the investigated products.
The substances that evaporate from the four investigated products for interior car care in the highest concentrations and at the same time have a relevant health classification/and or limit values are:
- Petroleum distillates (Xn; R65 (and CARC2; R45[9]
- where the content of benzene is ≥ 0.1%))
- Butyl acetate (R10, R66, R67)
- 2-butoxy ethanol (Xn; R20/21/22; Xi; R36/38)
- Limonene (R10, Xi; R38, R43, N; R50/53)
- Butane (E.g.: R12 (and CARC1; R45 and Mut2; R46 at content of 1.3-butadien > 0.1%[10]))
- Ethyl acetate (F; R11, Xi; R36, R66, R67)
- 2-propanol (F; R11, Xi; R36, R67)
- 1-butoxy-2-propanol (Xi; R36/38)
- 1-methoxy-2-propanol (R10).
The substances are listed chronologically, so the substances from which the largest amounts evaporate are stated first. Among those substances, health assessments on butyl acetate, 2-butoxy ethanol, limonene, 2-propanol and 1-butoxy-2-propanol were carried out in previous analysis projects of the Danish EPA.
In order to assess absorption through the skin, focus is placed on substances with a concentration exceeding 10 mg/g (i.e. >1% of the product) and substances that simultaneously have a health classification. However, benzyl chloride is also included although the max. concentration in one product was measured to 0.37 mg/g (i.e. 0.037%) as it is classified as carcinogenic (Carc 2). The substances are:
- Petroleum distillates (Xn; R65 (and Carc2; R45[11]if content of benzene is ≥ 0.1%))
- 1-methoxy-2-propanol (R10)
- 2-butoxy ethanol (XN; R20/21/22; Xi; R36/38)
- Xylenes (R10, Xn; R20/21, Xi R38)
- 1-butoxy-2-propanol (Xi; R36/38)
- Benzyl chloride (Carc2 R45, Xn; R22-48/22, T; R23, Xi R37/38-41).
The substances are listed chronologically, so the substances with the largest quantitative amounts are stated first. Among the substances, health assessments on 2-butoxy ethanol, xylenes and 1-butoxy-2-propanol were carried out in previous analysis projects of the Danish EPA. The NOEL values (No Observed Effect Level) of these previously assessed substances are stated in Table 9.1Table 9.1.
The five substances selected for health assessment were:
- Petroleum distillates (Xn; R65 (and Carc2; R45 10 if content of benzene is ≥ 0.1%))
- Butane (E.g.: R12 (and Carc1; R45 at content of 1.3-butadien >0.1%[12])
- Ethyl acetate (F; R11, Xi; R36, R66, R67)
- 1-methoxy-2-propanol (R10)
- Benzyl chloride (Carc2 R45, Xn; R22-48/22, T; R23, Xi R37/38-41).
8.2.1 Objective of health assessment
The objective of the health assessment is to describe the health effects of the assessed substances and to describe the critical effect. The critical effect of a substance is the effect that appears when exposed to the lowest dose where an effect is observed. That dose is also called NO(A)EL – No Observed (Adverse) Effect Level. The NOEL value is stated in mg/kg body weight.
On the basis of the safety factors, NOEL is converted to a TDI value (Tolerable Daily Intake). The calculated values for consumption (based on actual emissions or worst-case considerations on absorption through the skin) divided by the TDI value must not exceed 1 – if that is the case, and then there is a health related risk.
A safety factor of 100 is often used for conversion between NOEL value and TDI value. A factor 10 is used for species differences (between animals and humans) and a factor 10 is used to take particularly sensitive individuals into consideration. In some cases, a higher safety factor is used, as e.g. consideration can be given to experiments on animals not being long-term tests (chronic), but merely sub-chronic studies which is why yet another safety factor is added depending on the conditions.
In connection with the calculations concerning absorption through the skin, absorption is generally estimated due to lacking data. If no other information is available, a dermal absorption of 100% is used, however, a dermal absorption of 10% is used for substances with a molar weight larger than 500 g/mol that at the same time has a log KOW less than -1 or larger than 4 (as stated in TGD, 2003). That is because large molecules in general have greater difficulties in permeating the skin just as very lipophilic substances do.
8.3 Health assessment of petroleum distillates
The term petroleum distillates, covers a wide range of organic compounds that are very similar - actually so similar, so it has not been possible to distinguish between the different petroleum distillates during the quantitative analyses. The petroleum distillates found during the quantitative analyses are therefore stated as C6-C8, C8-C10 and C10-C12/C10-C14, respectively.
In connection with products, in which petroleum distillates were identified, we have to put our trust in the information stated on the safety data sheets of the products. According to the safety data sheets the following types of petroleum distillates form part of the products. Table 8.4 also states what the petroleum distillates have been identified as through the quantitative analyses and the box analyses.
| Product no. | CAS no. | Name | Classification according to LODS* and safety data sheet | Conc. according to MSDS | Identified as |
|---|---|---|---|---|---|
| Product 1 Vinyl make-up |
64742-47-8 | Distillates (crude oil), hydrogen treated light | LOFS: Xn; R65 MSDS: Xn; R65 |
25-50% | C4-C7/C6-C8* and C10-C14 |
| 92045-53-9 | Naphtha (crude oil), hydro desulphurized light, dearomatized (benzene content < 0.1%) | LOFS: Xn; R65 MSDS: Xn; R65, N; R51/53 |
25-50% | ||
| 68476-86-8 | Crude oil gases, condensed, sweetened (does not contain1.3-butadiene) | LOFS: Fx; R12 MSDS: Fx; R12 |
25-50% | ||
| Product 2 Vinyl make-up |
64742-49-0 | Naphtha (crude oil), hydrogen treated light | LOFS: Carc2; R451 Xn; R65 MSDS: Xn; R38 R65, R67; F; R11 |
12.5-35% | C6-C8 and C10-C12 |
| 64742-48-9 | Naphtha (crude oil), hydrogen treated heavy | LOFS: Carc2; R451 Xn; R65 MSDS: Xn; R65 |
0.1-1% | ||
| Product 37 Glass cleaner |
64742-48-9 | Naphtha (crude oil), hydrogen treated heavy | LOFS: Carc2; R451 Xn; R65 MSDS: Xn; R65, R67; Xi R66, R10 |
30-50% | C8-C10 |
| Product 38 Synthetic materials sealant |
64742-47-8 | Distillates (crude oil), hydrogen treated light | LOFS: Xn; R65 MSDS: Xn; R65, R66 |
No information | C10-C20 |
* For analyses carried out in the box, a solvent was not used in connection with the analyses and therefore it is possible to identify compounds way down to C4. Therefore, this difference between quantitative analyses and analyses in the box appears.
LODS = List of Dangerous Substances.
1 In this case it is not stated that the benzene content is below 0.1% and if that is not stated the labelling of the product should in principle be Carc2, R45. It is anticipated that the content of benzene is below 0.1%.
In English, petroleum distillates are also called TPH – Total Petroleum Hydrocarbons and the term covers a large family of several hundred chemicals that originate from crude oil (ATSDR, 1999a).
Petroleum products are complex mixtures of hundreds of different hydrocarbon compounds ranging from light, volatile, short chained organic compounds to heavy, long chained branched compounds. The exact composition depends on the source the crude oil comes from and the refining method (ATSDR, 1999a).
In the following, the health properties of petroleum products are described as a group because the data available for each compound as stated on the safety data sheet is extremely limited.
The identified petroleum products have all been identified as aliphatic hydrocarbons (i.e. no cyclic or ring-shaped organic compounds, and in addition it is anticipated that the aromatic content is insignificant or that the benzene content is below 0.1%).
In the following, the definition EC (Equivalent Carbon Number) Index is used. The EC Index represents the corresponding boiling points of hydrocarbons and is based on the equivalent retention times of a boiling point in a gas chromatographic column normalized in relation to n-alkane. In other words, the EC number of a compound represents the number of carbon atoms that an imaginary n-alkane would have in order to have exactly the same boiling point as the mentioned compound (Baars et al., 2001).
In connection with the short chained hydrocarbons, the health effects of n-hexane are quite unique compared to petroleum products and petroleum mixtures. Therefore, n-hexane specifically appears in the following text as some places refer to n-hexane in the selected studies.
Occurrence and application
Almost all crude oil that is produced is prepared in refineries through distillation and pressure distillation for different fuel or non-fuel fractions (WHO, 1982).
Petroleum products are used for a wide range of applications such as heating, lighting, industrial solvents and detergents (Wikipedia, 2007a).
Limit value in working environment
The occupational threshold limit value of petroleum distillates with the chain length C9-C14 (< 5% aromatic compounds) is 180 mg/m³ (25 ppm) (The Danish Working Environment Authority, 2007). The limit values of hydrocarbons with shorter chain lengths, C4 –C8, can be found under either solvent naphtha (tentative) or for the actual hydrocarbons (pentane, hexane, heptane, octane, etc.).
| Chemical name | Distillates (crude oil), hydrogen treated light | Naphtha (crude oil), hydrogen treated heavy | Naphtha (crude oil), hydrogen treated light | Crude oil gases, condensed, sweetened | Naphtha (crude oil), desulphur-ized light, dearoma-tized |
|---|---|---|---|---|---|
| Synonyms | Exxsol Kerosine Petroleum distillates |
Exxsol Naphtha White spirit (type 3) |
Exxsol Naphtha |
Fuel gas Petroleum product |
Exxsol heptane |
| CAS No. | 64742-47-8 | 64742-48-9 | 64742-49-0 | 68476-86-8 | 92045-53-9 |
| EINECS No. | 265-149-8 | 265-150-3 | 265-151-9 | 270-705-8 | 295-434-2 |
| Gross formula | - | - | - | - | - |
| Molecule structure | Chemical mixture | Chemical mixture | Chemical mixture | Chemical mixture | Chemical mixture |
| Legislation: List of Dangerous Substances (Executive Order 923, 2005) |
Xn; R65 | Xn; R65 (and Carc2; R45, if content of benzene > 0.1%) | Xn; R65 (and Carc2; R45, if content of benzene > 0.1%) | Fx; R12 (and Carc1; R45 Mut2; R46, if content of 1.3-butadien > 0.1%) |
Xn; R65 (Carc2; R45, if content of benzene > 0.1%) |
| List of undesirable substances (Information from the Danish EPA no. 8, 2004) |
No, not the specific CAS number, but other oil diverted substances | ||||
| MST Self classification (Danish EPA project no. 635, 2001) |
No | ||||
R12 Very inflammable
R45 Can be carcinogenic
R46 Can cause hereditary genetic damage
R65 Dangerous: Can give pulmonary damage when consumed
| CAS No. | 64742-47-8 | 64742-48-9 | 64742-49-0 | 68476-86-8 | 92045-53-9 |
|---|---|---|---|---|---|
| State of matter | Liquid | Liquid | Liquid | Gas | Liquid |
| Molar weight (g/mol) | - | - | - | - | - |
| Melting point | < 0 °C | < 0 °C | < -60 °C | -183- -20°C | < -60 °C |
| Boiling point | 150-290°C (1013 hPa) | 155-217°C (1013 hPa) | 55-140°C (1013 hPa) | -162- -0.5 °C (1013 hPa) |
94-99°C (1013 hPa) |
| Vapour pressure | 0.01-0.6 hPa (20°C) | 0.35-145 hPa (20°C) | App. 26-246 hPa (20°C) | 600-39000 hPa (20°C) | App. 53.7 hPa (20°C) |
| Octanol/water Distribution coefficient (log POW) | App. 3.4-8.7 (calculated) | App. 2.1-6 (calculated) | App. 2.1-6 (calculated) | < 2.8 (calculated) | App. 4.4 (calculated) |
| Solubility in water | 15 mg/l at 20°C | < 1000 mg/l at 20°C | < 1 mg/l at 20°C | 24-61 mg/l at 20°C | < 0.1 vol% at 20 °C |
| Reference | IUCLID, 2000a | IUCLID, 2000b | IUCLID, 2000c | IUCLID, 2000d | IUCLID, 2000e |
Absorption
Studies on humans who inhale n-hexane vapours indicate that 20-25% of the inhaled amount is absorbed and remains in the body (ATSDR, 1999b). In ATSDR (1999a) it is concluded that the value prevails for aliphatic EC5-EC8-fractions. On the other hand, aliphatic EC>8-EC16-fractions can easily be absorbed in the body when inhaled (ATSDR, 1999a). Therefore, 100% absorption is anticipated for these fractions and that is also consistent with log Pow typically being between -1 and 4 for hydrocarbons (meaning 100% absorption). In case of fractions with higher EC index, absorption during inhalation declines considerably.
Studies with rats show, that oral intake of aliphatic hydrocarbons declines when the molecular weight increases. There is complete absorption at low molecular weight, 60% absorption for C14-hydrocarbons, 5% for C28-hydrocarbons and hardly any absorption for C>32-hydrocarbons (Albro and Fishbein (1970); Miller et al. (1996) in ATSDR (1999a)). However, that does not comply with information in Baars et al. (2001), who write that petroleum distillates with low molecular weight are absorbed poorly from the gastrointestinal tract.
Limited studies exist on the absorption of aliphatic hydrocarbons through the skin. The aliphatic EC5-EC8 fractions and EC16-EC35 fractions seem to have very low potential for skin absorption (ATSDR, 1999a). According to an article on skin absorption of jet fuel[13] skin absorption is also very limited and it is not expected that absorption through the skin is considerable enough for this hydrocarbon fraction to give systematic effects (McDougal et al., 2000). No existing sources indicate percentage absorption – only that it is small. However, descriptions do exist of the hydrocarbons being able to be absorbed through the skin and in connection with the exposure calculations a 10% value is used for worst-case dermal absorption.
Distribution
When aliphatic hydrocarbons in the fraction EC5-EC8 have been absorbed, they are to a large degree distributed to tissue (especially fatty tissue) and organs in the body. Aliphatic EC>8-EC16 fractions are also distributed to tissue and organs and can accumulate in fat (ATSDR, 1999a). Compared to aromatic hydrocarbons, aliphatic hydrocarbons tend to result in lower concentrations in the blood, higher concentrations in the brain and other organs when inhaled and they have a high potential for accumulating in fat. The hydrocarbon concentration in tissue ((blood, brain, kidneys, liver and fat) normally increases when the carbon number increases (Zahlsen et al. (1992) in ATSDR (1999a)).
Studies on humans and animals show that the low aliphatic hydrocarbon fractions EC5-EC8 (tests with n-hexane) mainly are liberated through urine and only a small part is liberated through the expiratory air. When exposed to larger concentrations, the importance of the liberation through the expiratory air increases (ATSDR, 1999b). The higher aliphatic hydrocarbon fractions EC>8-EC16 are only liberated slowly from the body (Pedersen et al. (1984) in ATSDR (1999a)).
Acute toxicity
Inhalation of vapours from petroleum products can result in central nervous system (CNS) depression (e.g. dizziness, intoxication, headache and tiredness) and cardiac arrhythmias (Baars et al., 2001).
Tests with 19 different petroleum products on rats resulted in oral LD50-values from 4700 mg/kg bw to 17500 mg/kg bw, but six petroleum products did not result in death at concentrations of 23000 mg/kg bw (Baars et al., 2001).
Local irritation
Hydrocarbon mixtures (EC5-EC8 and EC>8-EC16) are irritating for skin as well as eyes (ATSDR, 1999a). An old study (Klauder & Brille (1947) in WHO (1982)) demonstrated that irritation from hydrocarbon mixtures declines when the boiling point increases. Mainly hydrocarbon mixtures with boiling points below 230 ºC are irritating. Hydrocarbon mixtures with aromatic content are more irritating than aliphatic hydrocarbons (WHO, 1982).
Skin tests with petroleum in mineral oil carried out on 34 persons showed that all test persons reacted (with irritated skin) at an 80% solution and nobody reacted at 40% solution (Tagami & Ogino (1973) in WHO (1982)). Petroleum distillates have a degreasing effect on the skin and repeated or long-term exposure can lead to dry and cracked skin (WHO, 1982).
According to the IUCLID documents of the mentioned petroleum distillates as stated on the safety data sheet of the products, the petroleum distillates are moderately irritating to irritating to the skin and not irritating to slightly irritating to the eyes (IUCLID, 2000 – a, b, c, d and e).
Allergy
According to the IUCLID documents of the mentioned petroleum distillates stated on the safety data sheet of the products, the petroleum distillates are non-sensitising (IUCLID, 2000 – a, b and c).
During tests on rats with 19 different types of petroleum products, only one single type of petroleum (heavy fuel oil with 0.8% sulphur content) demonstrated sensitising properties (Baars et al., 2001).
Long-term, repeated exposure and mutagenic effects
Consumption or long-term inhalation of petroleum can result in chemically conditioned pneumonia (ATSDR, 1999a).
Inhalation studies with rats show that petroleum products can result in kidney and lung effects. A 90-day study with rats and mice that constantly were exposed to marine diesel vapour in concentrations of 150-750 mg/m³ resulted in dose dependent nephropathy (kidney disease) but only in male rats. Other corresponding or long-term tests show the same effect (Baars et al., 2001). According to ATSDR (1999a) it is mainly n-hexane that seems to result in nephropathy whereas other compounds in the EC5-EC6 fraction do not seem to result in nephropathy by inhalation. Correspondingly it is stated in ATSDR (1999a) that exposure to the higher hydrocarbons EC>8-EC16 also resulted in nephropathy in male rates but the effect is regarded to be of doubtful relevance to humans.
Petroleum products (heavy fuel oils) have demonstrated effects of hereditary genetic damage in rats, in the dam as well as foetus at doses of 8 and 30 mg/kg bw/day (LOAEL) through exposure of the skin. In another study with rats that was to demonstrate effects of hereditary genetic damage a NOAEL (No Observed Adverse Effect Level) of > 250 mg/kg bw/day was demonstrated by exposure on the skin (for both sexes) (Baars et al., 2001). According to ATSDR (1999a) commercial hexane (i.e. mixture of n-hexane, 3-methyl pentane, methyl cyclopentane, 2-methyl pentane, cyclo hexane, 2,3-dimethyl butane etc.) demonstrated effects of hereditary genetic damage in chronic studies with mice. In addition, liver tumours were developed in the female mice, which indicates carcinogenic potential.
In 1997, TPHCWG (Total Petroleum Hydrocarbon Criteria Working Group) determined specific reference doses (RfD) for petroleum products. RfD stands for Reference Dose and is the maximum acceptable dose of a chemical. Normally, RfD (or TDI) appears by dividing the NOAEL value with a safety factor of 1000, 100 or 10, respectively, depending on the quality of the data from the NOAEL value.
The reference dose for C5-C8 aliphatic hydrocarbons was determined in the light of n-heptane and commercial hexane. According to TPHCWG (1997), the reference dose of n-hexane is 0.06 mg/bw kg/day with neurotoxicity as the critical effect. TPHCWG (1997) states, that n-hexane has unique toxic properties compared to petroleum products and petroleum mixtures, and therefore it is concluded that the reference dose for n-heptane should be used instead. It is calculated to be 2 mg/kg bw/day as n-heptane on the basis of tests seems to be 38 times less neurotoxic than n-hexane. However, tests with commercial hexane (mixture as mentioned above with 53% hexane) result in a reference dose of 5 mg/kg bw/day. TPHCWG states the value to be the recommended reference dose for petroleum mixtures for C5-C8, of course, if the total hexane amount is below 53%.
Correspondingly, TPHCWG (1997) states a reference dose of 0.1 mg/kg bw/day for C>8-C16 aliphatic petroleum products in the light of calculations from several studies. In this case, the critical effect is hepatic and hematologic changes (i.e. cell changes in liver and blood). Three studies state the same reference dose of 0.1 mg/kg bw/day.
Baars et al. (2001) discuss TDI values (Tolerable Daily Intake) for a long range of substances. Among them are petroleum distillates (TPH). Baars et al. (2001) state a TDI value of 2 mg/kg bw/day for the C5-C8 fraction and a TDI value for the C>8-C16 fraction of 0.1 mg/kg bw/day.
IARC (IARC 47, 1998 and IARC 45, 1998) state the following assessments of a number of petroleum distillates:
- Petroleum distillates – non-classifiable with regard to carcinogenicity in humans (IARC group 3)
- Petroleum – non-classifiable with regard to carcinogenicity in humans (IARC group 3)
- Benzine – may be carcinogenic to humans (IARC group 2B)
- Jet fuel – non-classifiable with regard to carcinogenicity in humans (IARC group 3)
- Marine diesel fuel – may be carcinogenic to humans (IARC group 2B)
- Light diesel fuel – non-classifiable with regard to carcinogenicity in humans (IARC group 3)
- Heavy fuel oils – may be carcinogenic to humans (IARC group 2B).
As stated in Table 8.4Table 8.4, most of the petroleum products stated on the safety data sheets of the products for interior car care have to be labelled with R45 according to the List of Dangerous Substances, i.e. may cause cancer. However, there are notes to the substances describing that the classification carcinogenic can be left out for the petroleum distillates if it can be demonstrated that the substance contains less than 0.1% (w/w) benzene. However, none of the petroleum products are market with R45 according to the safety data sheets and therefore it must be anticipated that they contain less than 0.1% benzene (only a few safety data sheets directly state that the content of benzene is less than 0.1%).
Critical effect
On the basis of Baars et al. (2001), who reassessed TDI for petroleum products, the found TDI values of 2 and 0.1 mg/kg bw/day are used for the C5-C8 fraction and C>8-C16 fraction, respectively. The critical effect of the C5-C8 fraction is neurotoxic effects and hepatic and haematological changes (cell changes in liver and blood) for the C>8-C16 fraction.
8.4 Health assessment of butane
Occurrence and application
Butane is used widely and for many applications. Butane is used as lighter gas, as propellant in aerosol containers/spray cans, in small blowtorches. Butane is also used for organic synthesis (e.g. for the production of ethylene) and as raw material for the production of synthetic rubber. Pure butane is used to calibrate instruments and as additive in foodstuffs (IPCS, 1997), (TOXNET HSDB).
Limit value in working environment
The occupational threshold limit value of butane is 1200 mg/m³ (500 ppm), (DWEA, 2007).
| Chemical name | Butane |
|---|---|
| Synonyms | n-butane butylhydrid methylethylmethane |
| CAS No. | 106-97-8 |
| EINECS No. | 203-448-7 |
| Gross formula | C4H10 |
| Molecule structure |  |
| Legislation: List of Dangerous Substances (Executive Order 923, 2005) List of Undesirable Substances (Information from DEPA no. 8, 2004) DEPA Self classification (DEPA project no. 635, 2001) |
Fx; R12 (Extremely flammable) When pure butane is in question i.e.content of 1.3-butadien < 0.1%, Otherwise also Carc1 R45 (May cause cancer) and Mut2 R46 (May cause heritable genetic damage). No No |
| State of matter | Colourless gas | Chemfinder |
|---|---|---|
| Molar weight (g/mol) | 58.123 | Chemfinder |
| Melting point | -138 °C | TOXNET ChemIDplus |
| Boiling point | - 0.45 °C | Chemfinder |
| Vapour pressure | 1820 mmHg | TOXNET ChemIDplus |
| Octanol/water distribution coefficient (log POW) |
2.89 | TOXNET ChemIDplus |
| Solubility in water | 61.2 mg/L (at 25 °C) | TOXNET ChemIDplus |
Absorption and distribution
Inhalation studies with mice and rats that were given lethal butane doses show that butane is absorbed and distributed to i.a. fatty tissue, brain, spleen, liver and kidney (TOXNET HSDB), (IUCLID, 2000f).
Dermal absorption of butane vapours has not been reported. Dermal absorption of butane is not expected to take place to a large degree as skin contact only is brief due to the volatility of butane (TOXNET HSDB).
Butane is very volatile and therefore it must be expected that butane also can be exhaled with the expiratory air (TOXNET HSDB).
Studies have not been found that show the absorption of butane in percent for skin absorption or inhalation. In connection with the exposure calculations 100% absorption via inhalation is therefore used and 10% is used for skin contact (based on butane being volatile) as worst-case.
Acute toxicity
Butane mainly shows health damaging effects by displacing oxygen, i.e. large concentrations of butane can result in suffocation. Concentrations of 15% butane in the air can result in sensitisation of the heart muscles and dysrhythmias (seen in humans) (IPCS, 1997). Exposure to smaller amounts of butane can result in symptoms such as i.a. euphoria, psychic excitement, dimmed sight and speech, coughing and vomiting (IPCS, 1997). Butane is used as lighter gas and the effects of butane have made it popular to sniff lighter gas. Exposure to larger amounts of butane can give rise to hallucinations, delusions, tinnitus, CNS depression, lethargy, headache, coma and, finally, sudden death due to lack of oxygen (IPCS, 1997). Tests with rats exposed to different concentrations of butane for 4 hours demonstrated a LC50 value of 658 mg/l (corresponding to 658.000 mg/m³). After exposure it was ascertained that butane accumulates in several organs. A similar test with mice exposed to different concentrations of butane for 2 hours gave a LC50 value of 680 mg/l (corresponding to 680.000 mg/m³) (IUCLID, 2000f). In comparison, the limit value of butane is 1200 mg/m³ (DWEA, 2007).
Local irritation
According to IUCLID (2000f), butane does not irritate the eyes. IPCS (1997) states that butane vapours can seem irritating to the throat if condensed butane gas is sprayed directly into the throat. Butane sprayed directly on the skin from a spray can result in frostbite (TOXNET HSDB).
Analysis project no. 49 of the Danish Environmental Protection Agency ”Emission of chemical substances from products made of exotic wood” (Witterseh, 2004) states a LCI value (Lowest Concentration of Interest) of 200 mg/m³ for butane. That LCI value is developed especially for indoor climate considerations. The critical effect from that value is irritation.
Allergy
No information was found about possible sensitising properties of butane.
Long-term, repeated exposure and mutagenic effects
In a 90-day inhalation test with rats, rats were exposed to a concentration of 1017 and 4489 ppm, respectively, (corresponding to 2.417 and 10.670 mg/m³ according to the conversion formula shown in Box 8.1). No deaths or other significant toxicological effects were observed. NOAEL was determined to 4489 ppm (or 10.670 mg/m³). The subsequent check of the animals showed mild hydrocarbon kidney effects, but there were no signs of kidney effects. The test was not carried out with pure butane but with two gas mixtures of 50% pentane and 50% butane, respectively, and 50% isopentane and 50% isobutane (IUCLID, 2000f).
In a 21-day inhalation test with rats, no significant toxic effects were observed at concentrations of 0.12 mg/l, 1, 15 mg/l and 11.8 mg/l, respectively, of a mixture consisting of 25% butane and the rest isobutane, pentane and isopentane. The duration of exposure was 6 hours per day, 5 days a week. In the light of the study, NOAEL is determined to 11.8 mg/l (or 11.800 mg/m³) (IUCLID, 2000f).
Ames test with butane is negative i.e. that butane does not show genetic effects (IUCLID, 2000f).
No information was found about tests showing hereditary genetic damage.
IARC has not assessed butane in relation to carcinogenicity. If the content of 1.3-butadien is more than 0.1%, then butane will have to be classified as carcinogenic.
Critical effect
The critical effect of butane seems to be CNS depression. However, no information has been found about levels concerning when damages arise (besides death). Only a few long-termed tests are described in literature. The tests were not carried out with butane only, but with a mixture of butane, pentane, isobutane and isopentane.
The tests stated a NOAEL of 11.8 mg/l (highest dose applied during tests – none of the tests gave toxic effects). The value is not stated per kg body weight. If it is anticipated, that a rat weighs max. 520 g[14] and that the respiratory volume of a rat is max. 130 ml/min[15], then that will correspond to a NOAEL of 4.248 mg/kg bw/day (Ace Animals Inc., 2007), (Rat Forum, 2007). Corrections have not been made for the fact that the rats, as described in the test, only inhaled the mixture 6 hours per day and 5 days a week.
If a safety factor of 1000 is used (10 for interspecies variation, 10 for intraspecies variation and 10 for sub-chronic to chronic), then that gives a tolerable dose of 4.2 mg/kg bw/day. That TDI value is used in the exposure calculations.
8.5 Health assessment of ethyl acetate
Occurrence and application
Ethyl acetate appears as a natural flavouring agent in i.a. sugar cane, rum and whisky (Jensen, 2003). In addition, it also appears naturally in wine (Department of the Environment and Water Resources Australia, 2006). The most important application of ethyl acetate regarding amount is as technical solvent in varnish and lacquer products for surface treatment. Besides, it is also used as solvent for plastics, fatty substances, nitrocellulose, synthetic resin and colours, e.g. for serigraphy. A smaller amount is used in laboratories or for chemical synthesis of perfume, medicine, photo chemicals and artificial silk and leather (Jensen, 2003).
Other consumer products that contain ethyl acetate comprise car paint, ink, lubricating oils, moisturizing lotion, nail varnish, nail varnish remover, paint diluents and artificial flavour additives (Department of the Environment and Water Resources Australia, 2006) and products for interior car care.
In 1985, global production of ethyl acetate amounted to app. 300.000 tons. The annual consumption of ethyl acetate in Denmark has declined from 3.370 tons in 1984 to 1.140 tons in 1999, but due to a reduced limit value for other solvents over recent years it is possible that the consumption of ethyl acetate might increase again (Jensen, 2003).
Limit value in working environment
According to the Danish Working Environment Authority limit values for air pollution the limit of ethyl acetate is 540 mg/m³ (150 ppm) (Danish Working Environment Authority, 2007).
| Chemical name | Ethyl acetate |
|---|---|
| Synonyms | Ethyl acetate ester Acetoxy ethane Acetidin |
| CAS No. | 141-78-6 |
| EINECS No. | 205-500-4 |
| Gross formula | C4H8O2 |
| Molecule structure |  |
| Legislation: List of Dangerous Substances (Executive Order 923, 2005) List of Undesirable Substances (Information from DEPA no. 8, 2004) DEPA Self classification (DEPA project no. 635, 2001) |
F; R11: Highly flammable XI: R36: Irritating to eyes; R66: Repeated exposure may cause skin dryness or cracking; R67: Vapours may cause drowsiness and dizziness. No No |
| State of matter | Colourless liquid with a pleasant fruity smell. | Chemfinder |
|---|---|---|
| Molar weight (g/mol) | 88.106 | Chemfinder |
| Melting point | -83.6 °C | Chemfinder |
| Boiling point | 77.1 °C | Chemfinder |
| Vapour pressure | 93 hPa at 20 °C 124.79 hPa at 25 °C |
IUCLID |
| Octanol/water distribution coefficient (log POW) |
0.71 | IUCLID |
| Solubility in water | Moderately water-soluble. 8 g/100 ml |
Chemfinder |
Absorption and distribution
Ethyl acetate is easily absorbed through the skin, lungs and the gastrointestinal tract. However, a considerable amount of ethyl acetate as liquid on the skin will evaporate before it passes through the skin as the substance in volatile (Jensen, 2003).
Ten men and women (between 18 and 25 years) were in a test exposed to 344-501 mg ethyl acetate/m³ air for 4 hours. The results demonstrated intake via the respiratory passages of 63.2% (men) and 56.7% (women) and elimination through the respiratory passages of 3% (men) and 2.5% (women). In addition, there was a respiratory retention of 60.2% (men) and 54.1% (women). According to the authors of the study, the results indicated that ethyl acetate quickly is transformed in the body (IUCLID, 2000g). That is confirmed by Jensen (2003) who states that ethyl acetate is an ester that decomposes (hydrolyzed) quickly in the body by means of enzymes to ethanol and acetic acid which again can be degraded to CO2 and water.
A 63.2 % absorption of ethyl acetate via inhalation is used as worst-case in the calculations. No immediate data exists on absorption via skin contact. The only existing information is, that it is ”easily” absorbed through the skin (Jensen, 2003), and that a considerable amount of ethyl acetate evaporates from the skin before absorption. However, in the calculations a value of 100% is used as worst-case.
Acute toxicity
Short-term exposure of high concentrations of ethyl acetate first of all results in irritation of eyes, nose and throat. Then come headache, nausea, vomiting, sleepiness and unconsciousness (Department of the Environment and Water Resources Australia, 2006).
According to Jensen (2003) the acute toxicity of ethyl acetate in humans and animals is very low and therefore ethyl acetate does not have to be classified as harmful. Nevertheless, the toxicity should not be underestimated as intake through the mouth can cause inflammation of the throat, stomach ache and diarrhea. Exposure to very high concentrations can result in liver damages (Jensen, 2003) and anaesthesia (Department of the Environment and Water Resources Australia, 2006).
The U.S. National Toxicology Program has made the below summary of LC50 values related to acute toxicity of ethyl acetate. The values are based on information in NTP (2006).
| Study type | Rute | Type | Result | Unit |
|---|---|---|---|---|
| LC50 | Inhalation | Mouse | 45 | mg/m³/2H |
| LC50 | Inhalation | Rat | 1.600 | ppm/8H |
| LCLo | Inhalation | Cat | 61 | gm/m³ |
| LCLo | Inhalation | Guinea pig | 77 | mg/m³/1H |
| LD50 | Intraperitoneal | Mouse | 709 | mg/kg |
| LD50 | Oral | Guinea pig | 5.500 | mg/kg |
| LD50 | Oral | Mouse | 4.100 | mg/kg |
| LD50 | Oral | Rabbit | 4.935 | mg/kg |
| LD50 | Oral | Rat | 5.620 | mg/kg |
| LD50 | Subcutaneous (hypodermic needle) | Cat | 3.000 | mg/kg |
| LD50 | Subcutaneous (hypodermic needle) | Guinea pig | 3.000 | mg/kg |
| LDLo | Subcutaneous (hypodermic needle) | Rat | 5.000 | mg/kg |
Local irritation and allergy
Ethyl acetate has degreasing properties and therefore it is moderately irritating to skin, mucous membrane and respiratory passages. Toxic as well as allergic skin eczema can appear. At air concentrations of 200 ppm (720 mg/m³) the smell of the vapour is unpleasant, while at 400 ppm (1440 mg/m³) mild irritation of the eyes, nose and throat appeared (Jensen, 2003). According to IUCLID (2000g) a study showed irritation of the eyes in humans exposed to 400 ppm for 72 hours. However, humans will typically experience considerable irritation at that concentration and therefore they will not remain exposed to such a concentration for a very long time (TOXNET, HSDB).
According to HSDB (TOXNET), a study did not demonstrate irritation or sensitisation during a skin test on 25 persons (exposure of a 10% ethyl acetate solution in petroleum).
Long-term, repeated exposure and mutagenic effects
Long-term exposure to ethyl acetate can result in ”misty vision” and damages on lungs, heart, liver and kidneys (Department of the Environment and Water Resources Australia, 2006). In addition, long-term dermal exposure to ethyl acetate can make the skin dry out and crack (NTP, 2006).
Very limited knowledge exists on the possible long-term effects of ethyl acetate at low exposure; however, the substance does not seem to have reproduction damaging effects but many good long-term tests exist that could clarify possible hereditary genetic damage or cancer risks. As ethyl acetate is quickly transformed in the body to rather harmless compounds (ethanol and acetic acid) it is not likely that the substance under normal working environment conditions would have substantial chronic effects (Jensen, 2003). According to HSDB (TOXNET) and Dutia (2004) ethyl acetate also has the reputation of being one of the least toxic of the volatile organic solvents.
A study on rats was reported in IRIS and it demonstrated an oral reference dose of 0.9 mg/kg bw/day based on a NOEL value of 900 mg/kg bw/day and a safety factor of 1000 as it is a sub-chronic study (extra safety factor of 10 from sub-chronic study to chronic). The investigated factors were i.a. body weight and food intake, clinical signs of toxicity, mortality and influence on blood and urine. The study took 90 days and involved 120 rats that (in groups of 30) were exposed to 0, 300, 900 and 3600 mg, respectively, of ethyl acetate/kg/day. At doses of 3600 mg/kg/day there were significant toxic effects that resulted in weight loss, at doses of 900 mg/kg/day there were no effects.
In another study with mice, reference is made to a NOAEL value of 0.02 mg/L air. For a period of 90 days the mice were exposed to ethyl acetate via inhalation (doses: 0; 0.002; 0.01; 0.043 mg/L air). At 0.01 and 0.043 mg/L there were after 15 and 30 days effects related to muscular activity and the internal organs. Therefore, a NOAEL value of 0.02 mg/L air was determined.
Critical effect
The critical effect of ethyl acetate is effects on blood, urine, body weight and food intake. The described NOEL value of 900 mg/kg bw/day above (reference dose of 0.9 mg/kg bw/day) is used in the subsequent calculations.
8.6 Health assessment of 1-methoxy-2-propanol (PGME)
Occurrence and application
In 2003, a total of 188.000 tons of PGME was produced in Europe. From 2001- 2003 production increased - mainly due to increased export. The superior demand in the EU is constant (EU Risk Assessment, 2006).
In the EU, PGME is mainly used as solvent in paint and coatings (38.5%), printer colours (8.5%), washing powder and detergents (5.3%), leather agents (1.3%), the electronic industry (1%), agriculture (0.8%), cosmetics/personal care (0.7%), adhesives (0.2%), metal cleaning (0.2%) and oil dispergents (0.1%). In addition, a large amount is used (42%) in synthesis of other chemicals. The figures are based on information from the year 2001 to 2003 (EU Risk Assessment Report, 2006).
Other consumer products containing PGME, i.a. comprise paint, varnish, car care products, window cleaning agents, oven cleaner, pesticides, dyes and ink, and swimming pool cleaners (OECD SIDS, 2001). A study of 150.000 products in Switzerland demonstrated that 1.5% of the products contained PGME, and the largest part of the products contained between 1 and 10% PGME, while a few contained up to 10-50% (EU Risk Assessment Report, 2006).
It should be mentioned that PGME typically is found in two isomers: 1-methoxy-2-propanol and 2-methoxy-1-propanol, of which the latter is anticipated to be more toxic as it can be transformed to 2-methoxy propionic acid. However, commercial PGME typically consists of 95% of the non-toxic ismers (Tobiassen et al., 2003). The main part of toxicological studies concerning PGME, concern the non-toxic isomer to which the below studies refer unless otherwise stated.
Limit value in working environment
According to the limit value list of the Danish Working Environment Authority concerning air pollution the limit for 1-methoxy-2-propanol is 50 ppm (185 mg/m³) (DWEA, 2007). According to HSDB the odour limit of PGME is 10 ppm (37 mg/m³).
| Chemical name | Propylene Glycol Monomethyl ether |
|---|---|
| Synonym | 1-methoxy-2-propanol 1-methoxy-2-hydroxy propane 1-methoxypropan-2-ol Polypropylene glycol methyl ether PGME |
| CAS No. | 107-98-2 |
| EINECS No. | 203-539-1 |
| Gross formula | C4H10O2 |
| Molecule structure |  |
| Legislation: List of Dangerous Substances (Executive Order 923, 2005) List of Undesirable Substances (Information from DEPA no. 8, 2004) DEPA Self classification (DEPA project no. 635, 2001) |
R10: S(2-)24 No No |
| State of matter | Clear, colourless liquid with ether-like smell | EU Risk Assessment Report (2006) |
|---|---|---|
| Molar weight (g/mol) | 90.1218 | Chemfinder |
| Melting point | -97 °C | Chemfinder |
| Boiling point | 119.6 °C | Chemfinder |
| Vapour pressure | 11.5 hPa at 20 °C 16.4 hPa at 25 °C |
IUCLID (2000h) EU Risk Assessment Report (2006) |
| Octanol/water distribution coefficient (log POW) | -0.437 -0.49 |
IUCLID (2000h) EU Risk Assessment Report (2006) |
| Solubility in water | 10 g/100 ml at 19 °C | Chemfinder |
Absorption and distribution
A report from the Danish Environmental Protection Agency (Tobiassen et al., 2003) on health hazardous effects of selected pesticide compounds, among them PGME, states that PGME seems to be absorbed via all ways of exposure. Toxicological studies have not indicated that an accumulation of the substance takes place. Elimination mainly takes place by demethylation and oxidation to CO2, which subsequently is exhaled. Conjugation and liberation via the urine also take place but are less important (Tobiassen et al., 2003).
The above is confirmed by a test on rats that received one single dose radioactively marked PGME. Within 48 hours, the rats liberated 50-60% PGME as CO2 in the expiratory air, while 20% was liberated via the urine as glucoronid conjugators, sulphate conjugators and propylene glycol (Miller et al., 1983 i OECD SIDS, 2001). Another study has demonstrated that after 10 tests with 6 hours of exposure to PGME (inhalation, 3.000 ppm), PGME was completely eliminated in rats 24 hours after the last exposure (Margot and Nolan, 1987 i OECD SIDS, 2001).
A study (Johansson, 1990) mentions a test where drugged rats absorbed 87% of PGME by inhalation. The rats were exposed to 1000 ppm.
Other studies concerning absorption via inhalation do not immediately exist and therefore it is as worst-case considered to be 100%. It should be noted that the studies mentioned above indicate that PGME is liberated from the body completely within 24-48 hours (however, based on tests with rats).
In relation to skin absorption, a study with human skin demonstrated an absorption rate of 1.17 mg/cm²/hour for undiluted PGME (Dugard et al., 1984). According to Johansson (1990) a test has been reported with percutaneous intake (through the skin) of PGME in vitro (isolated epidermis from humans), where intake through the skin was 1.2 mg/cm² per hour. That value is not immediately applicable in the exposure calculations and therefore a dermal absorption of 100% is used as worst-case.
PGME vapours that are exposed to sunlight are decomposed rather quickly as a result of reactions with photo chemically created hydroxyl radicals. The half-life period of PGME is in the air estimated to 3.1 hour (OECD SIDS, 2001).
Acute toxicity
The acute toxicity of PGME is anticipated to be low. Oral LD50 values for PGME in experiments with rats were found from >5.000 mg to 6.100 mg/kg (BASF AG, 1964, 1979; Rowe et al., 1954; Smyth et al., 1941, 1962 in OECD SIDS, 2001). Oral LD50 values for other animal studies have turned out to be 10.800 mg/kg (mice), 1.840-5.300 mg/kg (rabbits) and 4.600-9.000 mg/kg (dogs). LD50 values for PGME conveyed via the skin on rabbits was 13-14 g/kg (OECD SIDS, 2001). The study by Tobiassen et al. (2003) confirms that PGME has a low acute toxicity.
Investigations where two people inhaled 50-1000 ppm (2050 ppm in one single test) PGME were carried out. Exposure duration for concentrations of up to 250 ppm took up to 7 hours, while exposure took max. 2 hours for concentrations of up to 2050 ppm. The investigations showed that at 10 ppm the smell was noticeable. At concentrations exceeding 100 ppm the test persons experienced a temporary odour irritation, but after 2 hours they experienced minor irritation in the eyes. At concentrations exceeding 300 ppm the persons experienced mild eye and nose irritation within the first 5 minutes, but after 1 hour the irritations were almost unbearable. Severe irritation was measured at 750 ppm, whereas 1000 ppm indicated CNS depression. Neurological, clinical, chemical and general medicine studies have not shown significant abnormalities. However, all test persons experienced a quick ”odour habituation” which can result in a risk for people who are exposed to high doses without being conscious about it. In the meantime, PGME vapours are believed to contain sufficient warnings (heavy smell) and therefore that should not happen (IUCLID, 2000h).
Human exposure to PGME in a concentration exceeding 150 ppm is expected to be self-regulating due to irritation effects (OECD SIDS, 2001). According to a study from the United States and Canada, it is recommended that the use of PGME should not exceed 100 ppm in an 8 hour period, while the limit according to the Danish WEA is 50 ppm.
Local irritation and allergy
In animal studies (rabbits) PGME was found to be non-irritating for the skin and less irritating for the eyes (OECD SIDS, 2001). According to IUCLID (2000h) less irritation of the eyes has been reported after exposure (of rabbits) with PGME (no values stated).
IUCLID (2000h) reports one single test with guinea pigs where PGME is non- sensitising.
Long-term, repeated exposure and mutagenic effects
Laboratory animals exposed to PGME via inhalation developed effects such as CNS depression (anaesthesia), adapted changes in the liver, and reduced weight increase. The NOAEL values range from 300 to 5.000 ppm in tests with rats that took from 11 days to 6 months (OECD SIDS, 2001).
Tests with monkeys that inhaled PGME during a period of 6 months resulted in NOEL values of 800 ppm (Rowe et al., 1954 in OECD SIDS, 2001).
With regard to reproductive effects a NOAEL value of 300 ppm (adult rats) and 1000 ppm (rat offspring) was reported in a two-generation study with exposure to PGME via inhalation (Liberacki et al., 1997[16]; Carney et al, 1999 in OECD SIDS, 2001). The 300 ppm corresponded to 396 mg PGME/kg bw/day (the figures are stated in the source). At that value, there were no effects in the parent rats. A NOEL value of 1.325 mg/kg/day is stated for effects on rat offspring. However, it should be mentioned that a solution of PGME was used where 2% consisted of the previously mentioned ß-isomer of PGME that can be transformed to 2-methoxy propionic acid (2-MPA, which is a known animal teratogenic agent, i.e. causes malformation on foetuses).
Another study demonstrated a NOEL of 200 to 600 ppm for inhalation of PGME, 6 hours a day for 10 days. The study has not been further described (Doe et al., 1983 in OECD SIDS, 2001).
A study with rats exposed to PGME via inhalation showed a NOAEL value of 1.500 ppm (for dam), 1.500 ppm (teratogenically) and 3.000 ppm (embryotoxic, i.e. harmful to the foetus) (Hanley et al., 1984 in OECD SIDS, 2001). The effects observed at 3.000 ppm were minor CNS depressions and reduced appetite and weight.
Laboratory animals exposed to dermal exposure of PGME developed skin effects such as peeling, minor inflammation and thickening of the skin. In addition, large dermal doses can result in narcosis (narcosis of the body up to anaesthesia) and death. Two studies in which PGME was applied to the skin showed a NOEL value of < 1000 mg/kg (3 week study) and a NOEL value of 2 ml/kg (corresponding to app. 2000 mg/kg) (90-day study) (Calhoun and Johnson, 1984; Rowe et al., 1954 in OECD SIDS, 2001). Another study demonstrated a NOEL of 1000 mg/kg for systematic effects, while a LOEL of 4 ml/kg turned out to give a weak narcotic effect.
In general, studies with laboratory animals demonstrated that PGME is neither teratogenic nor embryotoxic when it is inhaled or after intake. In addition, PGME is not assumed to be carcinogenic (OECD SIDS, 2001). Furthermore, the study by Tobiassen et al. (2003) concludes that PGME is anticipated to have low systematic toxicity and that the critical values are irritation of eyes, CNS depression and effects on mucous membrane and respiratory passages.
Critical effect
An inhalation effect related to irritation effects on the eyes after two hours of exposure is 100-150 ppm (374-560 mg/m³ value converted according to the formula in Box 8.1).
As further information concerning the study that states a NOAEL value of 200 ppm cannot be found, focus is on the study that demonstrates a NOAEL value of 396 mg/kg/day (determined from 300 ppm) for reproductive effects in a rat test as other studies also have stated effects at values around 300 ppm.
If a safety factor of 100 is used (10 for interspecies variation and 10 for intraspecies variation) that gives a tolerable dose of 3.96 mg/kg bw/day. That TDI value is used in exposure calculations.
8.7 Health assessment of benzyl chloride
Occurrence and application
Benzyl chloride is used as intermediate substance in organic synthesis for the production of benzyl alcohol, dyes, perfumes, resin, softening agents (phthalates), pesticides and as a preliminary stage to penicillin (OECD SIDS, 1998), (TOXNET HSDB).
In 1993, the production of benzyl chloride in Japan was app. 7.800 tons (OECD SIDS, 1998), in 1982 the production in the USA was estimated to be 49.900 tons and in 1989 the industry capacity in the Western world was estimated to be 144.200 tons/year (TOXNET HSDB).
Limit value in working environment
The occupational threshold limit value of benzyl chloride is 5 mg/m³ (1 ppm), (Danish Working Environment Authority, 2007).The limit value is a threshold value that never must be exceeded. Benzyl chloride is marked as carcinogenic on the limit value list of the Danish Working Environment Authority.
| Chemical name | Benzyl chloride |
|---|---|
| Synonyms | Chloromethyl benzene chlorophenylmethane α-chlor toluene α -tolyl chloride |
| CAS No. | 100-44-7 |
| EINECS No. | 202-853-6 |
| Gross formula | C7H7Cl |
| Molecule structure | 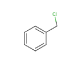 |
| Legislation: List of Dangerous Substances (Executive Order 923, 2005) List of Undesirable Substances (Information from DEPA no. 8, 2004) DEPA Self classification (DEPA project no. 635, 2001) |
Carc2;R45 (May cause cancer) Xn;R22-48/22 (Harmful if swallowed. Harmful: danger of serious damage to health by prolonged exposure if swallowed) T;R23 (Toxic by inhalation) Xi;R37/38-41 (Irritating to respiratory system and skin. Risk of serious damage to eyes) Yes No |
| State of matter | Colourless to slightly yellow liquid with unpleasant smell |
TOXNET HSDB |
|---|---|---|
| Molar weight (g/mol) | 126.9 | TOXNET HSDB |
| Melting point | -45 °C -43 °C |
TOXNET ChemIDplus OECD SIDS, 1998 |
| Boiling point | 179 °C | TOXNET ChemIDplus |
| Vapour pressure | 1.23 mmHg at 25 °C | TOXNET ChemIDplus |
| Octanol/water distribution coefficient (log POW) |
2.3 2.66 |
TOXNET ChemIDplus OECD SIDS, 1998 |
| Solubility in water | 525 mg/l at 25 °C App. 1.2 g/l |
TOXNET ChemIDplus OECD SIDS, 1998 |
Absorption and distribution
Tests with rats, where radioactive marked benzyl chloride was given orally, demonstrated that benzyl chloride is absorbed through the gastrointestinal tract. The concentrations were highest in the stomach, stomach content, ileum and the duodenum. 76% of the intake amount was liberated through the kidney in the course of 72 hours. Around 7% was liberated through the expiratory air as CO2, while less than 1.3% existed as benzyl chloride or benzyl chloride metabolic in the expiratory air in the course of the 72 hours. Benzyl alcohol, benzaldehyde and acetylsysteine were found as metabolic of benzyl chloride in the urine (Saxena and Abdel-Rahman (1989) in OECD SIDS, 1998).
No information was found about the absorption of benzyl chloride through the skin or during inhalation. Therefore, 100% dermal absorption and 100% absorption during inhalation is anticipated in the exposure calculations which also is consistent with the molar weight and log Pow of benzyl chloride that are below 500 g/mol and between -1 and 4, respectively.
Acute toxicity
Benzyl chloride is labelled toxic by inhalation (R23) and Harmful: danger of serious damage to health by prolonged exposure if swallowed (R48/22).
Oral LD50 values are 1231 mg/kg bw for rats and 1500 mg/kg bw for mice. In connection with inhalation, the LC50 values are 740 mg/m³ and 390 mg/m³ for rats and mice, respectively (OECD SIDS, 1998).
Local irritation and allergy
Benzyl chloride is regarded as irritating for skin, eyes and respiratory organs. 0.5 ml benzyl chloride on rabbit skin for 24 hours resulted in a considerable blush, swelling and subsequent cell damage. Rabbits and cats exposed to 462 mg/m³ (95 ppm) 8 hours/day for 6 days gave symptoms of eye and respiratory irritation. Irritation of mucous membrane and infection in the conjunctivitis of the eye appeared when exposed to benzyl chloride for 2 hours in concentrations from 100-1000 mg/m³ (21-205 ppm) (however, it has not been stated for which animals) (OECD SIDS, 1998).
IUCLID (2000i) as well as OECD SIDS (1998) refer to certain animal tests that all indicate that benzyl chloride is sensitising. IUCLID (2000i) reports of minimum sensitising doses of 0.0006 mg/kg bw for rats (given as 30 daily oral doses).
Long-term, repeated exposure and mutagenic effects
In a test that took 26 weeks, rats were orally given concentrations of between 6.4 and 107.1 mg benzyl chloride/kg bw/day. Doses were given 3 times a week. All rats that were given doses of 53.6 and 107.1 mg/kg bw/day, respectively, died within two-three weeks. The cause of death was mainly acute and chronic infection in the gastritis but edema of the heart and acute cell death in the heart muscles was also observed - often in the dead rats. At lower doses, hyperplasia also appeared in the stomach after new cell formation and cell death in the hearth muscles (resulted in death). NOEL was set to 12.9 mg/kg bw/day for male rats and 6.4 mg/kg bw/day for female rats (OECD SIDS, 1998).
Reproduction studies with rats, where doses of 50 and 100 mg/kg bw/day, respectively, were given to the dam from day 6 to 15 of the pregnancy showed no toxic effects in the dam. The number of live born foetuses and the average birth weight were not influenced. The only substantial change was a reduced birth length at doses of 100 mg/kg bw/day. NOEL was therefore set to 50 mg/kg bw/day for foetus toxicity (OECD SIDS, 1998).
Benzyl chloride has demonstrated genotoxic effects in Ames test (IUCLID, 2000i).
IARC (1999) assesses that there is sufficient waterproof in experimental animals of the carcinogenic properties of benzyl chlorides. However, the IARC assessment only covers a simultaneous exposure of chlormethyl benzene (benzyl chloride), dichlormethyl benzene, trichlormethyl benzene and benzoyl chloride. Therefore, IARC assesses that a combined exposure to the above-mentioned substance probably is carcinogenic to humans (group 2A) although there is no clear obviousness for humans (OECD SIDS, 1998).
Critical effect
The critical effect of benzyl chloride is acute and chronic infection in the gastritis. NOEL was set to 6.4 mg/kg bw/day.
If a safety factor of 1000 is used (10 for interspecies variation, 10 for intraspecies variation and 10 for sub-chronic to chronic) that gives a tolerable dose of 0.006 mg/kg bw/day. That TDI value is used in the exposure calculations.
[9] According to the List of Dangerous Substances, some of the petroleum destillates identified in the investigated products have to be classified as carcinogenic (Carc2) with R45 unless the benzene content is < 0.1%. In connection with two of the products it has not been stated if the benzene content is < 0.1% so the classification Carc2 can be omitted. According to the safety data sheet the products are not classified as Carc2, R45, i.e. the benzene content is presumably below 0.1%, but as mentioned earlier that has not been stated.
[10] On the safety data sheets of some of the investigated products it is not stated if the content of butadiene is < 0.1%. According to the safety data sheets the products are not classified as Carc1, R45, i.e. the content of butadiene is presumably below 0.1% but as mentioned earlier that has not been stated.
[11] According to the List of Dangerous Substances, some of the petroleum distillates identified in the investigated products have to be classified as carcinogenic (Carc2) with R45 unless the benzene content is < 0.1%. In connection with two of the products it has not been stated if the benzene content is < 0.1% so the classification Carc2 can be omitted. According to the safety data sheet the products are not classified as Carc2, R45, i.e. the benzene content is presumably below 0.1%, but as mentioned earlier that has not been stated.
[12] On the safety data sheets of some of the investigated products it is not stated if the content of butadiene is < 0.1%. According to the safety data sheets the products are not classified as Carc1, R45, i.e. the content of butadiene is presumably below 0.1% but as mentioned earlier that has not been stated.
[13] Jet fuels are medium distillates of petroleum crude oil with a boiling point between 150-300 ºC (ATSDR, 1999a).
[14] Weight of Sprague Dawley rats which the test is based on is 250-300 g for female rats and 450-520 g for male rates according to http://aceanimals.com/SpragueDawley.htm.
[15] Found on http://gray.hmgc.mcw.edu/pipermail/rat-forum/2000-April/000531.html. 130 ml/min corresponds to app. 2% of the respiratory volume of a human at rest.
[16] Original source could not be procured but a description of the test method was found on the following site: http://www.americanchemistry.com/s_acc/sec_directory.asp?CID=1478&DID=5629
Version 1.0 December 2010, © Danish Environmental Protection Agency