The Advisory list for self-classification of dangerous substances
Technical description of the self-classifications
- 2.10 Mutagenicity
- 2.11 Carcinogenicity
- 2.12 Reproductive toxicity
- 2.13 Acute oral toxicity
- 2.14 Sensitisation by skin contact
- 2.15 Skin irritation
- 2.16 Danger to the aquatic environment
The current chapter gives the detailed description of how the advisory classifications were assigned to the chemicals in the advisory self-classification list. This includes description of the classification rules and the (Q)SARs used for predicting the dangerous properties of the chemicals.
2.10 Mutagenicity
The criteria for classification for mutagenicity are divided into 3 different categories:
Classification as mutagen, category 1 (Mut1;R46, May cause heritable genetic damage) is based on evidence of a causal association between human exposure to the substance and heritable genetic damage.
Classification as mutagen, category 2 (Mut2;R46, May cause heritable genetic damage) is based on animal studies showing mutagenity to germ cells either in assays on germ cells or by demonstrating mutagenic effects in somatic cells in vivo or in vitro as well as metabolic proof that the substances reaches the germ cells.
The criteria for classification as mutagen, category 3 (Mut3;R68, Possible risks of irreversible effects) is based either on in vivo mutagenicity tests or on cellular interactions with in vitro tests acting as supportive evidence. For this classification, it is not necessary to demonstrate germ cell mutations.
(Q)SAR based evaluation
Five models predicting genotoxicity in vivo endpoints were applied in the screening. Data for the training sets were obtained from the literature. The technical specifications for the models are given in Table 2.
Drosophila melanogaster Sex-Linked Recessive Lethal (SLRL) (in vivo)
The training set consists of data from Lee et al. /16/. In the experimental method, Drosophila melanogaster males and females are used. Males are treated with the test substance and mated individually to virgin females. The test detects the occurrence of mutations, point mutations and small deletions, in the germ line of the insect. The mutations are phenotypically expressed in males carrying the mutant gene. When the mutation is lethal in the hemizygous condition, its presence is inferred from the absence of one class of male offspring out of the two that are normally produced by a heterozygous female. The assay has a low sensitivity for genotoxins other than direct-acting agents and simple promutagens, but a very high specificity, which means that in general a positive result has considerable value for prediction of potential genotoxicity in mammals.
Mutations in mouse micronucleus (in vivo)
The training set includes data from Hayashi et al. /17/, Mavournin et al. /18/, Waters et al. /19/, and Morita et al. /20/. The test detects micronuclei produced by damage to the chromosomes or the mitotic apparatus in red blood cells. Micronuclei are small nuclei produced during cell division. They contain chromosome fragments or whole chromosomes. In the test, mice are exposed to the test substance and young red blood cells (erythrocytes) from the bone marrow are isolated and analysed for micronucleus. The test is especially relevant to assess mutagenic hazard in that it allows consideration of factors of in vivo metabolism, pharmacokinetics and DNA-repair processes.
Dominant lethal effect in rodents (in vivo)
The training set is comprised of data from Green et al. /21/ and other references. In the experimental method, mice and rats are used. Treated males are mated to virgin females according to an experimental scheme. Females are sacrificed in the second half of pregnancy and uterine contents are examined to determine the number of implants and live and dead embryos. The category of early embryonic deaths is the most significant index of dominant lethality and as such used as endpoint. The test identifies major genetic damage, mainly the induction of structural and numerical chromosomal anomalies.
Sister chromatid exchange in mouse bone marrow (in vivo)
Data from Tucker et al. /22/ are used in the training set. The sister chromatid exchange (SCE) assay detects interchange of DNA between two sister chromatids of a duplicating chromosome. Mice are exposed to the test chemical. Then a thymidine analog, bromodeoxyuridine (BrdU) is injected. If DNA exchanges occur, BrdU can be identified by use of a fluorescence technique in chromosomes in the metaphase. The test is considered to be a sensitive method for evaluating mutagenicity and may be an indicator of carcinogenicity.
Comet assay in mouse (in vivo)
The training set includes data from Sasaki et al. /23/ plus a number of physiological chemicals theoretically assumed not to have the effect (such as various amino acids, sugar molecules, fatty acids etc.). The latter was included to get a better distribution between positives and negatives in the training set for the model). Included in the training set of the model are results from eight tissue types; stomach, colon, liver, kidney, bladder, lung, brain and bone marrow. The comet assay detects DNA strand break and can be applied to virtually any organ of interest. In the experimental test, a microgel electrophoretic technique is used for detecting DNA damage at cell level. The tested chemical is positive if it produces breaks in DNA-strings, resulting in small strings of DNA that are able to migrate further in a microgel, than intact DNA strings. In the microscope, damaged DNA is seen as a “comet” while not damaged DNA appear as a dot. If appropriately performed, the test has been shown to be reliable with high sensitivity to detect DNA damage in organs that cannot be investigated in other classical mutagenicity assays.
| Model | Technical summary |
|---|---|
| Drosophila melanogaster Sex-Linked Recessive Lethal (in vivo) | MultiCASE, DK model Training set: n=377 Cross-validation 10*50% gave Sensitivity: 73.9% Specificity: 88.0% Concordance: 81.6% Domain: 48% |
| Mutations in mouse micronucleus (in vivo) | MultiCASE, DK model Training set: n=358 Cross-validation 10*50% gave Sensitivity: 30.1% Specificity: 84.5% Concordance: 66.1% Domain: 59% |
| Dominant lethal mutations in rodent (in vivo) | MultiCASE, DK model Training set: n=191 Cross-validation 10*50% gave Sensitivity: 41.3% Specificity: 95.2% Concordance: 75.9% Domain: 42 |
| Sister chromatid exchange in mouse bone marrow (in vivo) | MultiCASE, DK model Training set: n=265 Cross-validation 10*50% gave Sensitivity: 70.4% Specificity: 86.9% Concordance: 85.5% Domain: 53% |
| COMET assay in mouse (in vivo) | MultiCASE, DK model Training set: n=286 Cross-validation 10*50% gave Sensitivity: 63.3% Specificity: 93.3% Concordance: 83.9% Domain: 45% |
Table 2: Technical summary for the mutagenicity models
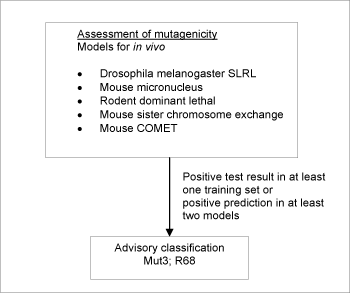
Figure 2: Schematic diagram illustrating the systematic evaluation applied to assign advisory classifications for mutagenicity.
For a substance to be selected as a probable mutagen it was necessary for the following criteria to be fulfilled: Positive prediction in two or more models, accepting only predictions where no significant deactivating fragments were detected. If one or more positive tests could be seen (as part of the training sets for the models) for any genotoxicity endpoint, this took precedence over model predictions.
When classification is proposed on basis of test data, a positive result in a single in vivo test is sufficient evidence on which to base the classification. In contrary to that, positive predictions in at least two models were required.
5,742 of the chemicals investigated in the current project met the criteria in the systematic evaluation and were assigned advisory classifications Mut3;R68.
2.11 Carcinogenicity
This endpoint can result in classification in 3 different categories:
Classification as carcinogen in category 1 (Carc1;R45, Toxic; May cause cancer, or Carc1;R49, Toxic; May cause cancer by inhalation) is based on a strong causal relationship in humans.
Classification as carcinogen in category 2 (Carc2;R45, Toxic; may cause cancer, or Carc2;R49, Toxic; may cause cancer by inhalation) is based on conclusive animal data from 2 species or 1 species with supportive evidence such as genotoxic effects in vitro or in vivo.
Classification as carcinogen in category 3 (Carc3;R40, Harmful; Possible risks of irreversible effects”) is subdivided into two:
- Well-investigated substances with restricted tumorigenic effects. It is normally based on clear data of tumour formation in one species. Mutagenicity data in vitro and in vivo can be used as supportive evidence.
- Substances that are insufficiently investigated, but raising concern for man.
(Q)SAR based evaluation
Four models predicting carcinogenicity in vivo and models predicting three genotoxicity in vitro endpoints were applied in the screening. Commercial MultiCASE training sets constitutes the basis of the carcinogenicity models. The technical specifications for the models are given in Table 3.
Carcinogenicity male and female, rats and mice (in vivo)
The models are the MultiCASE commercial models AG1-4 /24/. The training sets were constructed using the NTP (US National Toxicology Program) rodent carcinogenicity database, the Lois Gold Carcinogen Potency Database, FDA/CDER (US Food and Drug Administration / Center for Drug Evaluation and Research) archives, and the scientific literature. Training sets include both non-proprietary and proprietary data. Proprietary (confidential) data constitute around ten percent of the training sets. The open models based on the non-proprietary data were also available and consulted in the screening process.
In the experimental test, the test substance is administered by an appropriate route to the animals for a major portion of their lifespan. The highest dose level should elicit signs of toxicity, without substantially altering the normal lifespan due to effects other than tumours. During and after exposure, the animals are observed daily to detect signs of toxicity, particularly the development of tumours.
Reverse mutation test, Ames (in vitro)
The training set is from Kazius et al. /25/. The bacterial reverse mutation test detects point mutations, which involve substitution, addition or deletion of one or a few DNA base pairs. Amino-acid (histidin) requirering strains of Salmonella typhimurium are used. Mutations, which revert mutations present in the test strains and restore the functional capability of the bacteria to synthesise the amino acid (histidin), are detected. These appear by the ability of the bacteria to grow in the absence of histidin required by the parent test strain. The test is a useful tool as an initial screen for potential in vivo genotoxic activity, and has become the most extensively used in vitro short-term test in the screening for mutagenicity.
Chromosomal aberration CHO/CHL (in vitro)
This model was used by Niemela and Wedebye /28/ to evaluate the OECD principles for development and validation of (Q)SARS /27/. The Chinese Hamster Ovary (CHO) model is the commercial MultiCASE model A61 /26/ and the training set for the Chinese Hamster Lung (CHL) model was taken from Ishidata /28,29/. The in vitro mammalian chromosome aberration test identifies agents that cause structural chromosome aberrations in cultured cells. Chromosome damage is expressed as breakage of single or both chromatids, sometimes followed by reunion between chromatids or of both chromatids at an identical site. Many compounds that are positive in this test are mammalian carcinogens causing DNA damage.
Mutations in mouse lymphoma (in vitro)
The training set is comprised of data from Grant et al. /30/. The mouse lymphoma assay detects mutations affecting the heterozygous thymidine kinase (TK) locus. It identifies chemicals acting as clastogens (delete, add, or rearrange chromosome sections) as well as point mutagens. Mutations in genes coding for TK are identified. TK is involved in the phosphorylation of thymidin and subsequently in the formation of DNA. Positive chemicals may give rise to mutations in genes coding for TK. A mutation may result in loss of the ability to phosphorylate the pyrimidin analogs, which is detected by the test. The assay has a reputation for high sensitivity and low specificity of detecting genotoxic agents. However, in this exercise the model is used to give mechanistic information to chemicals already predicted to be carcinogens.
| Model | Technical summary |
|---|---|
| Carcinogenicity in male rat (in vivo) | MultiCASE, AG1 Training set: n=1381 External validation (100 chemicals): Sensitivity: 58.6% Specificity: 97.6% Concordance: 75.0% Domain: 70% |
| Carcinogenicity in female rat (in vivo) | MultiCASE, AG2 Training set: n=1376 External validation (100 chemicals): Sensitivity: 58.6% Specificity: 97.6% Concordance: 75.0% Domain: 70% |
| Carcinogenicity in male mouse (in vivo) | MultiCASE, AG3 Training set: n=1252 External validation (100 chemicals): Sensitivity: 58.6% Specificity: 97.6% Concordance: 75.0% Domain: 71% |
| Carcinogenicity in female mouse (in vivo) | MultiCASE, AG4 Training set: n=1263 External validation (100 chemicals): Sensitivity: 58.6% Specificity: 97.6% Concordance: 75.0% Domain: 71% |
| Reverse mutation test, Ames (in vitro) | MultiCASE, DK model Training set: n=4102 Cross-validation 10*50% gave Sensitivity: 84.4% Specificity: 82.5% Concordance: 83.5% Domain: 73% |
| Chromosomal aberration CHO (in vitro) | MultiCASE, A61 Training set: n=233 Cross-validation 10*50% gave Sensitivity: 32.0% Specificity: 91.2% Concordance: 69.9% Domain: 45% |
| Chromosomal aberration CHL (in vitro) | MultiCASE, DK model Training set: n=600 Cross-validation 10*50% gave Sensitivity: 57.8% Specificity: 86.5% Concordance: 74.3% Domain: 64% |
| Mutations in mouse lymphoma (in vitro) | MultiCASE, DK model Training set: n=555 Cross-validation 10*50% gave Sensitivity: 68.5% Specificity: 86.3% Concordance: 79.2% Domain: 64% |
Table 3: Technical summary for the carcinogenicity models
Identification of carcinogenic substances
For a substance to be selected as a probable carcinogen it was necessary for the following criteria to be fulfilled: Positive according to the ICSAS methodology /24/, corresponding to two or more positive carcinogenicity predictions, accepting only predictions for chemicals without significant deactivating fragments.
If one or more positive tests could was observed (as part of the training sets for the models) for any cancer endpoint, this took precedence over model predictions. As the models are heavily biased towards making a correct prediction for substances used to make them the latter criterion only resulted in little change. However, it was felt that there was no reason to artificially reduce the quality of the advisory classification by neglecting to use data, which happen to be present.
One or more negative tests in the training set of each model also took precedence over predictions of that model, except in cases where positive training set tests were present in other cancer models.
Employing this carcinogenicity identification algorithm resulted in a list of 3,726 positive predictions.
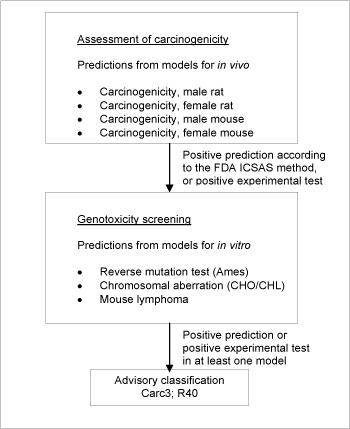
Figure 3: Schematic diagram illustrating the systematic evaluation applied to assign advisory classifications for carcinogenicity.
Identification of genotoxic carcinogens
While there are many non-genotoxic carcinogens acting by a wide variety of often-unknown mechanisms, it was chosen to focus here on chemicals likely to cause cancer through a genotoxic mechanism. Therefore, a further selection criterion for genotoxicity was set up.
As opposed to the selection criteria for mutagenicity, not all genotoxic carcinogens are necessarily clastogenic (cause loss, addition or rearrangement of parts of chromosomes). To select the genotoxic chemicals from the chemicals already predicted positive for in vivo carcinogenicity,which include genotoxic as well as non-genotoxic carcinogens, a battery of models for sensitive in vitro genotoxicity endpoints was used.
The genotoxicity criterion was a positive estimate in one or more of the models for the following in vitro genotoxicity endpoints; Reverse mutation test (Ames), chromosomal aberrations (CHO/CHL), or mutations in mouse lymphoma.
A schematic diagram of the systematic evaluation is given in Figure 3. According to these criteria, 3,726 of the chemicals assessed in the current project were identified as genotoxic carcinogens and selected for advisory classification for carcinogenicity. It is not felt that the models employed allow discrimination between classification in the three categories, so the lower classification Carc3;R40 was applied in all cases.
2.12 Reproductive toxicity
This endpoint can result in classification in 3 different categories:
Classification as toxic to reproduction in category 1 (Rep1;R60, Toxic; May impair fertility, or Rep1;R61, Toxic; May cause harm to the unborn child) is based on a strong causal relationship in humans.
Classification as toxic to reproduction in category 2 (Rep2;R60, Toxic; May impair fertility, or Rep2;R61, Toxic; May cause harm to the unborn child) is based primarily on animal data, and secondly on “other relevant information”. Data from in vitro studies, or studies on avian eggs, are regarded as “supportive evidence” and would only exceptionally lead to classification in the absence of in vivo data.
Classification as toxic to reproduction in category 3 (Rep3;R62, Harmful; Possible risks of impaired fertility, or Rep3;R63, Harmful; Possible risk of harm to the unborn child) is based primarily on animal data, and secondly on “other relevant information”. Substances in category three are insufficiently investigated, but raising concern for man.
Classification for reproductive toxicity covers a wide range of effects on either fertility or to the developing organism before and after birth (structural or functional damage). The (Q)SAR models applied in the current project only cover certain but far from all types of harm to the unborn child. Hence only certain types of mechanisms causing malformations or foetal mortality are covered.No (Q)SAR models were used for effects concerning other types of developmental toxicity and fertility.
(Q)SAR based evaluation
Three models predicting in vivo teratogenicity or fetal lethality related endpoints were applied in the assessment. A commercial MultiCASE training set constitutes the basis of one model. Data for the training sets for the two other models were obtained from the literature. The technical specifications for the models are given in Table 4.
Teratogenic risk (in vivo)
The model is the MultiCASE commercial model A49 /31/. The training set is composed of data taken from the TERIS (Teratogen Information System) and a compilation in which the FDA (US Food and Drug Administration) definitions were used to quantify risk of developmental toxicity from drugs used during pregnancy. The training set consists of clinical and epidemiologicdata. Many biological mechanisms are involved in the effects.
Drosophila melanogaster SLRL effect (in vivo)
The training set consists of data from Lee et al. (1983) /32/. In the experimental method, Drosophila melanogaster males and females are used. Males are treated with the test substance and mated individually to virgin females. The test detects the occurrence of mutations, point mutations and small deletions, in the germ line of the insect. The mutations are phenotypically expressed in males carrying the mutant gene. When the mutation is lethal in the hemizygous condition, its presence is inferred from the absence of one class of male offspring out of the two that are normally produced by a heterozygous female. The assay has a low sensitivity for genotoxins other than direct-acting agents and simple promutagens, but a very high specificity.
Dominant lethal effect in rodents (in vivo)
The training set is comprised of data from Green et al. (1985) /33/ and other references /21/. In the experimental method, mice and rats are used. Treated males are mated to virgin females according to an experimental scheme. Females are sacrificed in the second half of pregnancy and uterine contents are examined to determine the number of implants and live and dead embryos. The category of early embryonic deaths is the most significant index of dominant lethality and as such used as endpoint. The test identifies major genetic damage, mainly the induction of structural and numerical chromosomal anomalies.
| Model | Technical summary |
|---|---|
| Teratogenic risk in humans (in vivo) | MultiCASE, A49 Training set: n=323 Cross-validation 10*50% gave Sensitivity: 50.2% Specificity: 91.3% Concordance: 79.3% Domain: 48% |
| Mutations in Drosophila melanogaster SLRL (in vivo) | MultiCASE, DK model Training set: n=377 Cross-validation 10*50% gave Sensitivity: 73.9% Specificity: 88.0% Concordance: 81.6% Domain: 48% |
| Dominant lethal mutations in rodent (in vivo) | MultiCASE, DK model Training set: n=191 Cross-validation 10*50% gave Sensitivity: 41.3% Specificity: 95.2% Concordance: 75.9% Domain: 42% |
Table 4: Technical summary for the models for reproductive toxicity.
The dominant lethal test in rodents and the Drosophila SLRL test are initially meant for genotoxicity effects on germ cells, but the resulting effect is early embryonic deaths and lethal effect on offspring, respectively. Therefore, the endpoints are relevant for reproductive toxicity assessment.
In many cases, a toxicological threshold is assumed to exist for reproductive toxicity. With mutagenic chemicals this may not be the case.
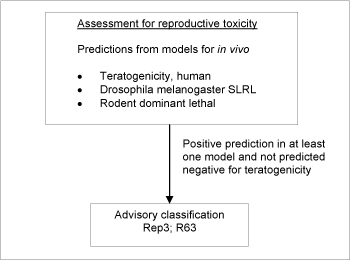
Figure 4: Schematic diagram illustrating the systematic evaluation applied to assign advisory classifications for reproductive toxicity.
For a substance to be selected as probable toxic to reproduction in the assessment, the criterion was a positive prediction in any of the three models and without a negative prediction in the teratogenic risk in humans model (see Figure ) (see also /34/).
The screening resulted in a list of 4,036 positive predictions. The models employed do not allow discrimination between classification in the three classification categories, so the lower classification Rep3;R63 was applied in all cases.
2.13 Acute oral toxicity
The formalized criteria for classification for acute oral toxicity includes a number of options of tests including fixed-dose procedure and interpretation of the various sources of information about acute oral toxicity, but is often based on acute LD50 tests in the rat for which the following classification criteria are used:
| Classification | Classification criteria |
|---|---|
| Tx;R28 (very toxic; very toxic if swallowed) | LD50 oral, rat = 25 mg/kg |
| T;R25 (toxic; toxic if swallowed) | 25 mg/kg < LD50 oral, rat = 200 mg/kg |
| Xn;R22 (harmful; harmful if swallowed) | 200 mg/kg < LD50 oral, rat = 2,000 mg/kg |
Table 5: EU criteria for classification for acute oral toxicity
2.13.1 (Q)SAR based evaluation
If test results measured in the rat were readily available (had been used to make the model) these took precedence over any predictions.
Moreover, as acute toxicity data from the mouse following a variety of different routes of administration was also available in some cases, this was used to predict rat oral LD50’s using the QAARs (Quantitative activity-activity relationships) preferentially as follows /63,64/:
| 1. | Log LD50 oral, rat = 0.190 + 0.953 * (Log LD50 oral, mouse) RTECS data 1989, n=1257, R² = 0.82 |
| 2. | Log LD50 oral, mouse = 0.682 + 0.373 * (Log LD50 iv, mouse) + 0.518 * (Log LD50 ip, mouse) RTECS data 1994, n = 286, R² = 0.766, Q² = 0.764 |
| 3. | Log LD50 oral, mouse = 0.731 + 0.841 * (Log LD50 ip, mouse) RTECS data 1994, n=286, R² = 0.724, Q² = 0.724 |
| 4. | Log LD50 oral, mouse = 0.945 + 0.802 * (Log LD50 iv, mouse) RTECS data 1994, n=286, R² = 0.689, Q² = 0.688 |
iv: intravenous
ip: intraperitonial
Table 6: QAAR equations for acute oral toxicity correlating mouse and rat data by different routes
Biological data consisting of LD50’s in mice or rats was available for about 15% of the chemicals processed.
If no test data were available, rat oral LD50 was estimated according to the Pharma Algorithms Inc. ToxBoxes (vers. 2.9) acute toxicity LD50 for Rat (oral) which is based on RTECS (Registry of Toxic Effects of Chemical substances) and ESIS (European Survey of Information Society) data for 8,631 substances /65, 67/.
In the Pharma ToxBoxes predictions of LD50 are given together with applicability domain estimates in the form of reliability indexes (RI=Reliability Index), which take into account the similarity of the query compound to the training set, the difference between predicted LD50 and experimental values for similar compounds, and the consistence of experimental values for similar compounds.
An external validation of this model using a test set with 2,167 tests from Pharma Algorithms gave a multiple R-squared of 0.524. When using a RI of 0.5, the R-squared went up to 0.639 for the 1,332 tests that met this RI cut-off.
This is a significant improvement over the TOPKAT model which was applied as basis for advisory classifications for acute oral toxicity in the 2001 version of the list. This TOPKAT model was evaluated by external validation with 1,840 chemicals resulting in an R-squared of 0.31.
| Model | Technical data |
|---|---|
| Acute toxicity LD50 for Rat (in vivo), oral | Pharma ToxBoxes version 2.9 Commercial model Training set: n=8,631 External validation with 2,167 tests gave: At RI set to 0.5; N=1,332 R2=0.639 Domain: 51% |
Table 7: Technical summary for the Pharma ToxBoxes acute toxicity model
In modern acute oral toxicity tests using small numbers of animals, statistical variation is often within a factor of 2-4, and inter-laboratory variations of up to an order of magnitude is not uncommon /66/.
The accuracy of the Pharma model is considered to be sufficient to differentiate between the three different levels of acute toxicity (“harmful”, “toxic” and “very toxic”).
A schematic diagram of the systematic evaluation is given in figure 5.
This resulted in 13,873 substances with an advisory classification of Xn;R22, 1,184 substances with an advisory classification of T;R25 and 168 substances with an advisory classification of Tx;R28. In total 15,225 substances with advisory classifications for acute oral toxicity.
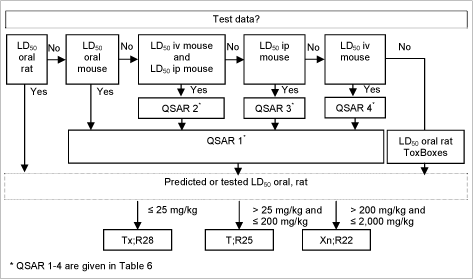
Figure 5: Diagram illustrating the systematic evaluation used to assign advisory classifications for acute oral toxicity
2.14 Sensitisation by skin contact
The current advisory classifications for sensitisation by skin contact originate from 2001 and have not been updated. The general documentation on the assessments undertaken - start list, criteria for application domain etc. - can be found in the documentation report from 2001 /5/. No attempt to search for and exclude chemicals with advisory classifications for skin sensitization, which have received harmonized EU classifications since 2001 have been made. The general advice on the use of the advisory self-classification list is to first check whether the substance in question has harmonized EU classifications and if so classify accordingly.
Classification as sensitising by skin contact, R43 (“May cause sensitisation by skin contact”), is based either on animal studies or practical experience or combinations thereof. The animal criterion is based on either an adjuvant or non-adjuvant test.
Different adjuvant tests exist, but the Magnusson-Kligmann’s method (GPMT: Guinea Pig Maximization Test) is preferred. Response in 30% of the animals results in classification. For a non-adjuvant test (for example the Büehler test) 15% responding animals is regarded as positive. The human data can be results from patch testing, case studies or epidemiological studies.
2.14.1 (Q)SAR based evaluation
Two approaches were used to estimate contact sensitisation /68,69/.
The first approach uses two TOPKAT QSTR models. The first model was used to predict “Allergy versus non-allergy”, and, in cases where this was positive, the second model was used to predict “Strong versus weak/moderate allergy”. The models used were primarily related to the GPMT. Only predictions of “Strong allergy” were considered as being likely to fulfill the EU criteria for R43.
In a second approach, predictions were also made using MultiCASE. The data set used to produce the MultiCASE models differed somewhat from the TOPKAT set, in that both data from the GPM tests and human data were represented. Only positive predictions with MultiCASE scores of > 40 (corresponding to “very active”) were selected.
| Model | Technical specifications |
|---|---|
| TOPKAT (v. 5.01 1998) No sensitisation vs Any |
N=389 GPMT Cross validation result (Q²) /68/: Sensitivity 84-94% Specificity 87-96% |
| TOPKAT (v. 5.01 1998) Strong vs Weak/Moderate |
N=266 GPMT Cross validation result (Q²) /68/: Sensitivity 88-96% Specificity 88-98% (Q²) |
| MultiCASE (v. 3.320 1999) Model A33: Allergic contact dermatitis |
N=1034 GPMT or data from human experience Cross validation result (3*10% out) /69/: Sensitivity 69 – 89% Specificity 89– 94% Chi² > 50, p<0.0001 |
Table 8: Technical summary for the models for sensitisation by skin contact
External validation of both TOPKAT and MultiCASE models was also attempted using confidential results from the EU New Chemicals program. Using the two-stage TOPKAT model (n= 64 AOK[4] predictions) 67% of positives were correctly identified, and 77% of negatives. For MultiCASE, (n= 75 AOK predictions) 45% of positives were correctly predicted, and 81% of negatives /70/.
It is difficult to know how representative “New Chemicals” are with regard to the universe of Existing Chemicals (EINECS). Generally “New Chemicals” are more complex structures with higher molecular weights. Perhaps the most surprising aspect of this exercise was to find that for over three thousand chemicals that should have been assessed for this endpoint, such a tiny percentage of useful test data could be found.
Compounds predicted as positive by either TOPKAT or MultiCASE according to the above criteria were selected, provided that they were either AOK in the first, or contained no unknown fragments or equivocal results in the latter.
While it was considered to use “positive” in both models as a criterion, in the end this seemed inefficient, not so much due to lack of concordance between model predictions, but because the acceptance domains (AOK or all fragments known) of the two methods differed considerably.
No attempt was made to further reduce the list by systematically applying expert judgment.
A schematic diagram of the systematic evaluation is given in figure 6.
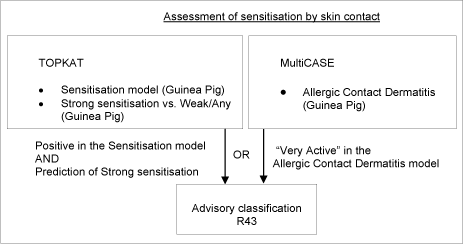
Figure 6: Schematic diagram illustrating the systematic evaluation applied to assign advisory classifications for sensitisation by skin contact
9.669 chemicals met the above criteria, for which an advisory classification of R43 was assigned. This strike many experts as being a rather large number of chemicals and while these models represent the current “state-of-the-art” it may indicate that they are over-sensitive. However, it was very difficult to obtain any reliable indication of how many Existing Chemicals would cause contact allergy if actually tested in animals or humans. Estimates of percentages of allergens on EINECS ranged from 5-25%, with some preference being expressed for 10%, which is the number of Annex I (now Annex VI of 1272/2008/EU) substances currently classified for this effect. It is not possible, however, to estimate the influence of confounders on the distribution represented in Annex I. Positive bias can have been introduced because chemicals testing positive are over-represented. Negative bias can have been caused by the fact that most of the chemicals have never been tested at all. The question of numbers remains open.
2.15 Skin irritation
Substances which cause significant inflammation of the skin determined on the rabbit according to the cutaneous irritation Annex V test method (persisting for =24h after exposure =4h) should be classified for skin irritation with Xi;R38 (Irritating to skin).
2.15.1 (Q)SAR based evaluation
If test results measured in the rabbit were readily available (had been used to make the model) these took precedence over any predictions.
Positive test data for rabbits were available for 213 of the chemicals processed.
If no test data were available, skin irritation was estimated according to the DK MultiCASE model for severe skin irritation vs. mild skin irritation. The training set for the model includes data from RTECS /71/ on 701 chemicals[5], HSDB /72/ on 31 chemicals[6], EU Annex I classifications for a total of 56 chemicals[7], and expert judgments for certain groups of chemicals for a total of 49 chemicals[8].
As the model training set contains both information on skin irritation and corrosion, positive predictions from the model may in reality be due to either of the effects.
| Model | Technical data |
|---|---|
| Skin irritation in rabbits (in vivo), severe vs mild | MultiCASE version 2009 DK model Training set: n=837 Cross-validation 10*50% gave: Sensitivity: 63.8% Specificity: 81.0% Concordance: 72.7% Domain: 49% |
Table 9: Technical summary for the model for skin irritation
The software used in the current project is unable to predict the properties of ionized compounds (salts) and therefore predictions have not been made for ionized compounds, as skin irritation is a local effect, which can be highly sensitive to pH.
A schematic diagram of the systematic evaluation is given in figure 7.
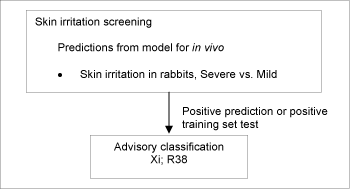
Figure 7: Schematic diagram illustrating the systematic evaluation used to assign advisory classifications for skin irritation
This resulted in 8,005 substances, which were assigned an advisory classification of Xi;R38. As the model does not discriminate between strong irritants and corrosive chemicals, the advisory classifications based on the predictions from the model should be considered as “minimum classifications”.
2.16 Danger to the aquatic environment
The classification criteria are composed of three main elements: 1) potential for rapid degradation, 2) bioconcentration potential in fish, and 3) short-term toxicity to aquatic organisms (fish, daphnia, and algae). Classifications are assigned according to the following scheme:
| Classification | Classification criteria* |
|---|---|
| N;R50 Dangerous for the environment; very toxic to aquatic organisms |
Acute toxicity = 1.0 mg/L |
| N;R50/53 Dangerous for the environment; very toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment |
Acute toxicity = 1.0 mg/L and not readily degradable or BCF**= 100 |
| N;R51/53 Dangerous for the environment; toxic to aquatic organisms; may cause long-term adverse effects in the aquatic environment |
Acute toxicity > 1 and = 10 mg/L and not readily degradable or BCF** = 100 |
| R52/53 Harmful to aquatic organisms; may cause long-term adverse effects in the aquatic environment |
Acute toxicity > 10 and = 100 mg/L and not readily degradable |
| R53 Harmful to aquatic organisms |
Solubility in water < 1 mg/L and not readily degradable and BCF** = 100 |
Table 10: EU criteria for classification for danger to the aquatic environment
* The lowest effect concentration, EC50, for fish, daphnia or algae is used
** BCF: Bioconcentration factor
(Q)SAR based evaluation
Advisory classifications were assigned on the basis of combinations of estimates for ready biodegradability, bioconcentration and acute toxicity according to the criteria in Table 5. Classification with risk phrase R53 alone was not done in this exercise, as the strong co-linearity between water solubility and bioconcentration factor made it redundant.
It is noted that compared to the classification criteria according to which abiotic degradation (and assessment of primary degradation products for their environmental hazard classification) can be used, only predictions concerning potential for rapid biodegradation was employed here. Furthermore only predictions for bioconcentration in fish were used even though the classification criteria refers to use of log Kow when reliable measured BCF data in fish are not available.
Biodegradation
Biodegradability was estimated using the Syracuse BIOWIN program /43/. Only the non-linear equation for rapid/non-rapid biodegradation (BPP2) was applied. Previous validation of this parameter compared with 304 MITI “ready/not-ready (45:259) results showed that while a relatively high percentage of “not-ready” chemicals were missed (sensitivity result was 53%), 97% of “not ready” predictions were correct (PPV, Positive Predictive Value) in this “chemical universe” of 85% not-ready chemicals /44/. MITI data was also applied by Tunkel et al /41/ who found a sensitivity of 53%, a specificity of 86% and a PPV of 83% for 884 chemicals (385 ready: 499 not-ready). These findings were largely confirmed in a comparison exercise made by the Danish EPA and based on chemicals assessed at OECD (SIAM 11-18), where 128 chemicals (59 ready:69 not-ready), which were not part of the BPP2 training set indicated a sensitivity of 54%, a specificity of 85% and a positive predictive value of 80% /38/. In other words while this model may fail to identify around half of all “non-ready” substances, the number of false predictions for not-ready biodegradability will be very low.
A total of 11,766 chemicals of the 49,292 chemicals studied were found to be “not-readily degradable” according to this criterion.
Bioconcentration
The classification and labelling guidence prefers measured data for bioconcentration, but as this rarely is available, a Log Kow of greater than three is recommended as an indication that BCF will be 100 or greater, in accordance with the linear equation of Veith /55/. While a good rule-of-thumb, this relation both over- and underestimates BCF for many classes of chemicals, and it is only applicable in the Log Kow interval 2-6.
Bioconcentration was therefore predicted using Syracuse BCFWIN /42/, a method based on a combination of Log Kow relations and structural fragment categories. This method was evaluated by its authors as having a statistical accuracy of R² = 0.74 (n = 694, S.D. 0.65, mean error = 0.47), which is a significant improvement over the standard equation of Veith (log BCF = 0.85 * Log Kow – 0.70) where predictions for the same 694 compounds had a statistical accuracy of R² = 0.32 (S.D. 1.62 and mean error = 1.12).
No attempt was made to further assess bioaccumulation potential.
For chemicals predicted to have aquatic toxicity concentrations below 10 mg/L and to be readily biodegradable, 4,662 chemicals were predicted to have BCF estimates of equal to or greater than 100.
Acute toxicity
For aquatic toxicity classifications, it is recommended to used L(E)C50-values for fish, daphnia and algae. Aquatic toxicity to fish, daphnia and algae were predicted using three models and a theoretical equation.
Fish
For acute aquatic toxicity to fish a DK MultiCASE model using 96h LD50 data on 569 chemicals from the Duluth Fathead minnow database was applied /48/. Cross-validation of this model gave a R² of 0.735. As there was insufficient test data for very hydrophobic substances the MultiCASE model was only applied for chemical substances with Log Kow of 6 or less.
Daphnia
For acute aquatic toxicity to daphnia a DK MultiCASE model using 48h EC50 data on 641 chemicals from various sources was applied /49/. Cross-validation of this model gave a R² of 0.69. As there was insufficient test data for very hydrophobic substances the MultiCASE model was only applied for chemical substances with Log Kow up to 7.
Algae
For acute aquatic toxicity to daphnia a DK MultiCASE model using EC50 data on 531 chemicals (396 tests made at the Technical University of Denmark for the Danish EPA, plus literature data from various sources) /50/ was applied. Cross-validation of this model gave a R² of 0.74.
A regression equation was used on top of MultiCASE predictions to adjust for Log Kow contribution to the toxicity:
Log EC50 (μM) = 0.593*Log EC50 (MultiCASE prediction, μM) – 0.257*Log Kow + 1.076
N = 343, R2 = 0.743, S.E = 0.853
(Log Kow below –1 were set to –1, Log Kow above 7 and less or equal to 8 were set to 5, and Log Kow above 8 were set to 1)
As there was insufficient test data for very hydrophobic substances the MultiCASE model was only applied for chemical substances with Log Kow of up to 8.
Non-polar narcosis predictions for highly hydrophobic substances
Another relationship was used for chemicals with a Log Kow of greater than six. Here, all substances were assumed to act by non-polar narcosis (minimum or baseline toxicity) , and toxicity at dynamic equilibrium (or steady state) was estimated according to a relation to the predicted bioconcentration factor in small fish:
LC50 (equilibrium) = 8.15 mmol /BCF
The choice of 8.15 mmol corresponds to the theoretical level inducing aquatic lethal effects represented by the non-polar narcosis fish (Q)SAR recommended in the REACH-guidance /51/. Non-polar narcosis Lethal Body Burden’s for fish are generally assumed to be within the range of about 2–8 mmol /53/.
While simple Log Kow relationships exist for predicting the non-polar narcotic toxicity for fish, daphnia and algae, these do not distinguish specific toxicity’s unique to any of the three taxa, and were not felt to offer any advantage over using the fish models alone, which also adequately predict non-polar narcosis. For all practical purposes, non-polar narcosis induces effects at the same concentration levels in all three taxa for chemicals with these high Log Kow values.
Aquatic toxicity screening
Using the three Multicase models and the non-polar narcosis equation, 18,809 of the chemicals assessed in the current project had acute aquatic toxicity’s of = 100 mg/L.
| Model | Technical summary |
|---|---|
| Biodegradation, Syracuse BIOWIN2 non-linear model for rapid/non-rapid aerobic biodegradation probability (BPP2) | Syracuse BIOWIN, US EPA /45/ Training set: n=295 External validation (n=304) gave Sensitivity: 53.3% Specificity: 91.1% Concordance: 58.9% PPV: 97.2% |
| Bioconcentration (BCF), Syracuse BCFWIN | Syracuse BCFWIN, US EPA /42,46/ Training set: n=694 Cross-validation gave R² = 0.74 S.D. = 0.65 Mean error = 0.47 |
| Acute toxicity to fish, Fathead minnow LC50 (96h) | MultiCASE, DK model /48/ Training set: n=569 Cross-validation 3*10% gave R² = 0.74 Domain: 52% |
| Acute toxicity to daphnia, Daphnia magna, EC50 (48h) | MultiCASE, DK model /49/ Training set: n=641 Cross-validation 3*10% gave R² = 0.69 Domain: 52% |
| Acute toxicity to algae, Pseudokirchneriella subcapitata, EC50 | MultiCASE, DK model /50/ plus Log Kow equation Training set: n=531 Cross-validation 10*50% for the two-step model gave R² = 0.74 Domain: 58% |
| Non-polar narcosis, LC50 (equilibrium) = 8.15 mmol /BCF | Theoretical equation /51-54/ |
Table 11: Technical summary for the models used for classification of danger to the aquatic environment.
Advisory classifications
A total of 18,809 of the chemicals assessed in the current project were selected according to one of the four classification categories based on the combination of model predictions as indicated in the classification criteria and shown in Figure 8. The classifications for danger to the aquatic environment were assigned to the following number of chemicals:
| N;R50 | 2,381 |
| N; 50/53 | 7,376 |
| N; R51/53 | 6,063 |
| N; R52/53 | 2,989 |
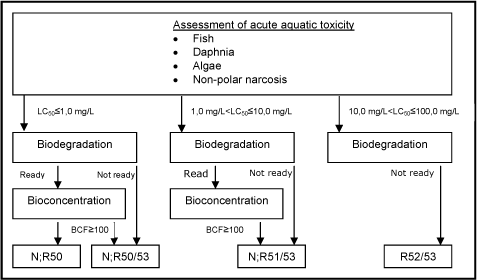
Figure 8: Schematic diagram illustrating the systematic evaluation applied to assign advisory classifications for danger to the aquatic environment.
[4]AOK means within applicability domain as defined in /5/
[5] 291 were positives and 410 were negatives. For the positives, the search criterion in RTECS was the RTECS code “SEV” for severe skin irritation and no requirements on dose or duration of exposure was made. For the negatives, the search criterion in RTECS was the RTECS code “MLD” for mild skin irritation, and moreover a requirement of 500 mg and 24H exposure was set.
[6] The 31 chemicals from HSDB were all positives; highly irritation or corrosive according to HSDB criteria.
[7] The 56 chemicals with EU classifications were either corrosive with R34 (causes burns) or corrosive with R35 (causes severe burns).
[8] Of the expert judgment groups entered, some consisted of presumably not irritating chemicals that the model was otherwise confused by and where experimental data could not be found, together with some well known groups of positives. A QMRF including the full training set with statements of source for both test data and expert judgments will be submitted for inclusion the EU QMRF inventory and is also available on request.
Version 1.0 March 2010, © Danish Environmental Protection Agency