Survey of Chemical Substances in Consumer Products No. 104, 2010
Survey and health assessment of mercury in compact fluorescent lamps and straight fluorescent lamps
Contents
- Compact fluorescent lamps contain small quantities of health-hazardous mercury
- What is mercury
- Forms of mercury in compact and straight fluorescent lamps
- Why is mercury a health problem?
- Calculation of level of mercury vapours without harmful effects
- Which levels can be found after breakage of lamps in a home in the short-term perspective?
- Which levels can be found after breakage of lamps in a home in the long-term perspective?
- 1.1 Purpose and contents
- 1.2 How do compact fluorescent lamps and straight fluorescent lamps work?
- 1.3 Relevant legislation - RoHS
- 1.4 Summary
- 2.1 Description of types of fluorescent lamps
- 2.2 Survey of quantities of mercury
- 2.3 Survey of types of mercury compounds in fluorescent lamps
- 2.4 Future developments
- 2.5 Summary
3 Release of mercury from broken lamps
- 3.1 Mercury compounds and quantities
- 3.2 Release from broken lamps
- 3.3 Impact from flooring and evaporation of mercury after accidents
- 3.4 Description of accident
- 3.5 Release of mercury to the outer environment
- 3.6 Risk of breakage
- 3.7 Discussion and summary
4 Health assessment of mercury vapours
- 4.1 Description of mercury
- 4.2 Absorption and metabolism in human body
- 4.3 Human intake of mercury
- 4.4 Measurement of mercury exposure
- 4.5 Mercury toxicity
- 4.6 Limit values
- 4.7 Summary
5 Exposure and risk assessment
Preface
This ”Survey and health assessment of mercury in compact fluorescent lamps and straight fluorescent lamps” has been commissioned by the Danish Environmental Protection Agency and carried out by FORCE Technology in the period from December 2009 to May 2010.
The project has been monitored by a steering committee from the Danish Environmental Protection Agency consisting of:
- Jette Rud Larsen Heltved, and
- Elisabeth Paludan
The project has been carried out by the following FORCE Technology staff members:
- Pia Brunn Poulsen, project manager, risk assessment
- Hanna Kristina Merrild, survey, and
- Allan Astrup Jensen, health assessment of mercury and quality assurance
Summary and conclusions
- Compact fluorescent lamps contain small quantities of health-hazardous mercury
- What is mercury
- Forms of mercury in compact and straight fluorescent lamps
- Why is mercury a health problem?
- Calculation of level of mercury vapours without harmful effects
- Which levels can be found after breakage of lamps in a home in the short-term perspective?
- Which levels can be found after breakage of lamps in a home in the long-term perspective?
Compact fluorescent lamps contain small quantities of health-hazardous mercury
Compact fluorescent lamps represent one of the most efficient solutions available today to improve energy efficiency of house lighting – but compact and straight fluorescent lamps contain small amounts of the element mercury, which is hazardous to health. With this project, the Danish Environmental Protection Agency will examine whether there is a health risk associated with breakage of a compact or a straight fluorescent lamp in a private home.
Therefore, the following is examined
- types of compact and straight fluorescent lamps on the Danish market for private use, and
- quantities of mercury and mercury compounds in these fluorescent lamps.
Based on this information, a theoretical risk assessment is made of a potential accident with breakage of a fluorescent lamp emitting mercury vapour in a private home.
The assessment is made partly as a theoretical calculation of quantities of mercury that expectedly will evaporate, when a compact fluorescent lamp or a straight fluorescent lamp breaks in a home; and partly through an assessment of measured concentrations in a home in the weeks after an accident with breakage of a compact fluorescent lamp. Concentrations are compared with known values for concentrations where health hazardous effects have been seen.
The project has been commissioned by the Danish Environmental Protection Agency and carried out but FORCE Technology in the period from December 2009 to May 2010.
What is mercury
Mercury is a metallic element appearing as a free metal as well as in inorganic and metal organic compounds. Furthermore mercury can be mixed with other metals to form amalgams. Mercury (Hg0) is the only metal which is liquid under normal pressure and temperature, and it appears as a heavy, odour-free silver liquid with a relatively high steam pressure at room temperature. Handling of liquid mercury will therefore mean exposure to invisible and imperceptible mercury vapours. Mercury vapours are seven times heavier than air and will disperse along the floor in a room with insufficient ventilation.
Forms of mercury in compact and straight fluorescent lamps
In compact/straight fluorescent lamps mercury is used either in the form of a HgFe compound, in the form of amalgams or in the form of metallic mercury. This solid or liquid mercury species will be in equilibrium with mercury as vapour. There will be a small quantity of mercury as vapour inside the fluorescent lamp, and that, among other factors, causes the lamp to light.
If one or more compact or straight fluorescent lamps break in a home, mercury vapours will be released to the indoor air as long as residues of the lamp have not been completely removed.
Consumers will be better protected against exposure to mercury in the lamps, if the lamps use encapsulated mercury (in the form of a tablet or as amalgam) compared with the use of liquid mercury – for the very reason that the mercury will be bound.
Why is mercury a health problem?
Mercury vapours are toxic and a health problem, because the vapours are extensively absorbed through the lungs during inhalation. In contrast, absorption is low of the poorly soluble metallic mercury through the skin and from the gastrointestinal tract. Furthermore, mercury vapour readily passes the blood-brain and the placental barriers, and it can in that way have an impact on the central nervous system and the unborn child.
Most data on health effects of mercury vapour originate from occupational exposures. The lungs are the target organs at very high exposures to mercury vapours as in the working environment. The effects are irritation and corrosion of the respiratory tract, and at a few hours of exposure to 1-3 mg Hg/m³ (1000-3000 µg Hg/m³) a fatal acute chemical pneumonia can develop. There is immediate danger to life and health at exposure levels of about 10 mg Hg/m³ (10,000 µg Hg/m³).
At prolonged high exposure to mercury vapours at levels of > 0.100 mg/m³ (>100 µg/m³) the critical organ is the central nervous system. At these levels serious damage with classical poisoning symptoms such as tremor, insomnia, depression, mental unbalance, irritability, memory loss, abnormal shyness and gingivitis can be expected.
Minor acute toxic effects (such as hand tremor or memory loss) in humans can be anticipated at long-term exposure to 0.025-0.050 mg Hg/m³ (25-50 µg Hg/m³). This value of 0.025 mg Hg/m³ (25 µg Hg/m³) is identical to the Danish occupational threshold limit value and the Threshold Limit Value (TLV) by the American Conference of Governmental Industrial Hygienist (ACGIH).
Calculation of level of mercury vapours without harmful effects
Based on a health assessment of mercury vapour DNEL values (Derived No Effect Level) of both short-term (30 minutes’ clean-up) and long-term (if a proper clean-up has not been carried out after an accident with a broken lamp) exposure have been calculated in this report. The calculated DNEL values for short-term (DNELshort value = 33 µg Hg/m³) and long-term (DNELlong value = 0.4 µg Hg/m³) exposure are close to the Danish occupational threshold limit value (25 µg Hg/m³) and the USEPA Reference Concentration (RfC) – a long-term concentration with no harmful effects (0.3 µg Hg/m³), respectively.
LOAEL (Lowest Observed Adverse Effect Level) and NOAEL values (No Observed Adverse Effect Level) used for the calculation of the DNEL values in this project, as well as the different threshold limit values that exist for mercury vapour, are all based on observations from the working environment of adult humans and their exposure to mercury vapours. As the documentation is based on occupational exposure, it is not known whether children are more sensitive to exposure to mercury vapours. However, children are generally considered more sensitive than adults towards toxic effects. Children have a lower respiration volume than adults and will therefore inhale smaller amounts of the toxic mercury vapours, but children also have a lower body weight than adults. Furthermore, their nervous system is under development, and as described in Chapter 4 the critical organ at prolonged high exposure to mercury vapours is exactly the central nervous system. No assessment has been made in this project whether these concentrations will present a special risk to children that are present in a room where a fluorescent lamp breaks. The DNEL values have, however, been calculated by use of a safety factor of 10 thus accounting for individual differences between humans. This safety factor should therefore account for the fact that children are more sensitive than adults towards toxic effects. However, information has been found showing that exposure to mercury vapours is a special risk for pregnant women, since mercury vapours can pass the placenta barrier and harm the unborn child.
The calculated DNEL values are then compared to the calculated worst-case concentrations during clean-up and to concentrations measured in tests after clean-up of broken fluorescent lamps. These concentrations derive from different tests found in literature.
Which levels can be found after breakage of lamps in a home in the short-term perspective?
For the calculation of short-term exposure (30 minutes’ clean-up), a calculation model has been used that accounts for ventilation in the room. The calculation model uses a specific measure for the volume of air surrounding the exposed person – in this report called the breathing zone. This specific measure has been taken from the ECHA Guidance Chapter R.15 (2008), which describes that for short-term local exposure the volume of air immediately surrounding the person can be set at 2 m³. The calculations show the following:
- No ventilation: By using the assumption that 10 % of the mercury has evaporated during the first 30 minutes, the concentration in the room without ventilation will exceed the DNELshort value and thereby present a health risk – regardless of the amount of mercury in the lamps. Calculated concentrations of mercury vapours (for =5 mg Hg in a compact fluorescent lamp) exceeding the DNELshort value with up to 8 times have been calculated. This is, however, an overestimation as no ventilation (zero) is a fictive value since this corresponds to a theoretical air tight room with no ventilation through cracks and fissures.
- Ordinary ventilation: Is slow reduction of concentration of mercury vapours in the room. It will take up to two hours before the concentration of mercury vapours in the breathing zone falls below the DNELshort value at an ordinary ventilation rate – and if only one lamp is broken (for =5 mg Hg in a compact fluorescent lamp).
- Strong ventilation: Is of significant importance with respect to reducing concentration of mercury to non-harmful levels in the home after accidents. After 10 minutes of ventilation with all windows and doors open, the mercury concentration in the breathing zone of 2 m³ will be below the DNELshort value for compact fluorescent lamps with a low content of mercury (=2.5 mg Hg), and will therefore not present an acute risk. After 15 minutes of strong ventilation, the mercury concentration in the breathing zone is below the DNELshort value for compact fluorescent lamps with the presently permitted content (=5 mg Hg), and after 30 minutes the mercury concentration is below the DNELshort value for all calculated contents of mercury in fluorescent lamps.
The calculations are based on an accident with one single compact or straight fluorescent lamp. The calculations thereby indicate that if more than one lamp breaks, the evaporation of mercury is larger, and thus the need for ventilation is greater.
However, the calculations are subject to many uncertainties. Amongst others:
- The calculation model assumes that the entire amount of mercury (here 10 % of the total content of Hg in the lamp) evaporates instantly, when the accident happens, as the calculations do not account for the evaporation rate. Mercury does, however, evaporate quickly (7 % evaporates within a few minutes), and therefore the overestimation of using 10 % is not high.
- It is assumed in the calculations that mercury vapours only disperse within the breathing zone of 2 m³ and not beyond this area. It is also assumed that the vapours are distributed evenly inside this volume. That assumption can cause an overestimation.
- The model assumes that consumers are exposed to the entire amount of mercury during the entire time of exposure – i.e. the 30 minutes it takes to clean up (except the amount of mercury that is removed through ventilation). Therefore, the model does not account for the fact that the real concentration of mercury in the breathing zone will be lower, if the exposure source (the broken lamp) is removed from the room before the end of the exposure time. If the mercury and lamp residues are removed quickly, the calculations are hence overestimated.
- The model assumes that mercury vapours are evenly distributed by use of a fan, and therefore ventilation of the room will result in an even ventilation of the mercury vapours in the room. It has not been investigated further, whether strong ventilation is as effective regarding air exchange near the floor, where the mercury vapours are concentrated, compared with air exchange higher in the room. However, investigations show that air exchange from a window also has an effect on the concentration of mercury near the floor.
- The DNELshort value is calculated by use of a LOAEL value for an exposure for a few hours (not specified further). It is assumed in this report that clean-up in the worst case will take 30 minutes. If clean-up is quick, e.g. 10 minutes, the exposure time will be significantly shorter, meaning that the exposure will be significantly lower than calculated.
When the uncertainties and the assumptions of the calculations are taken into account, the conclusion is that there is no health risk associated with a short-term exposure to mercury vapour released, after breaking a fluorescent lamp in a home, provided that lamp residues are removed quickly, and the room is ventilated immediately.
Which levels can be found after breakage of lamps in a home in the long-term perspective?
This is a situation where not all residues of the broken lamp are removed and therefore people can be exposed to mercury vapours in a prolonged time period. For this scenario, it has not been possible to carry out a calculation of the concentration of mercury vapours in the room, as it depends on many factors, such as ventilation, how well clean-up has been performed etc. Evaporation of mercury can in principle continue as long as residues of mercury are left in the room.
Consequently, the calculated DNELlong value has been compared with concentrations of mercury vapours measured in various studies with broken lamps described in literature.
These studies show that mercury from a broken lamp can evaporate in a cleaned-up home for several weeks/months after the accident. In some cases it took several weeks before the measured values were below the US long-term concentration without harmful effects of 0.0003 mg Hg/m³ (0.3 µg Hg/m³) and thereby also under the calculated DNELlong value of 0.0004 mg Hg/m³ (0.4 µg Hg/m³).
Extra ventilation after an accident is therefore important – especially in connection with ordinary cleaning/vacuuming in the home, which can cause mercury-containing dust to be stirred up. Ventilation has a substantial effect in terms of lowering mercury concentrations to non-harmful levels in homes after accidents with a broken fluorescent lamp.
For long-term exposure to mercury vapours the conclusion is that if all mercury residues are not removed properly (i.e. thorough clean-up), they may constitute a health risk. Thorough clean-up soon after the accident will remove most of the mercury. Furthermore, it is important to continue ventilation after an accident, as mercury vapours can be released from non-visible residues of the broken lamp for several weeks/months after the accident.
1 Introduction
- 1.1 Purpose and contents
- 1.2 How do compact fluorescent lamps and straight fluorescent lamps work?
- 1.3 Relevant legislation - RoHS
- 1.4 Summary
1.1 Purpose and contents
Fluorescent lamps are among the most efficient solutions available today to improve energy efficiency for home lighting (Wesnæs et al, 2009). Compact fluorescent lamps and straight fluorescent lamps contain, however, small quantities of mercury – and mercury is hazardous to human and the environment.
The purpose of this project is to study whether there is a health risk associated with compact fluorescent lamps or straight fluorescent lamps that accidentally break in private homes.
The study covers a survey of:
- types of compact and straight fluorescent lamps on the Danish market for private use, and
- quantities of mercury and mercury compounds in these fluorescent lamps.
Furthermore a risk assessment (exposure and health assessment) has been made of mercury releases from broken fluorescent lamps.
The project has been carried out with limited means and in a short period of time. Therefore it does not give a detailed study of all relevant aspects relating to compact fluorescent lamps/straight fluorescent lamps.
1.2 How do compact fluorescent lamps and straight fluorescent lamps work?
Compact fluorescent lamps and straight fluorescent lamps consist of one or more bent or straight glass tubes with an electrode at each end. A compact fluorescent lamp is merely a more compact kind of a straight fluorescent lamp, where the tube has been integrated with the electrodes needed to start and stabilise the electric current through the tube.
According to information received from manufacturers of compact and straight fluorescent lamps, lamps contain mercury either in the form of a HgFe tablet, amalgam[1] or as metallic mercury. Mercury is added with different dosage technologies in which the mercury is encapsulated in a tablet, a pill or as amalgam, or it is dosed as liquid mercury.
Mercury in solid or liquid form is in equilibrium with vapour-phase mercury. Therefore, a small amount of vapour-phase mercury will be present in the lamp. In order for the lamp to work there must be a balance between the solid-phase and the vapour-phase mercury so that the atmosphere in the glass tube is saturated with mercury vapour. The glass tube/the container in a fluorescent lamp furthermore contain an inert gas (e.g. argon) under low pressure and the glass tube is coated on its inner side with a thin layer of fluorescent phosphor (www.datalyse.dk).
When the glass tube is subjected to voltage the electrode is heated and electrons are released. The temperature in the tube increases and the mercury evaporates further. When the free electrons hit the mercury atoms the latter exite, i.e. are lifted to higher energy levels. Exited mercury atoms will decay after a certain time and thereby emit ultraviolet light together with a small amount of visible light. The ultraviolet light is converted into visible light when it hits the phosphor coating on the inner side of the tube (Aucott et al., 2003; www.datalyse.dk).
According to information from one of the interviewed manufacturers some of the mercury will in its decay be bound to the glass and the phosphor coating. The amount of bound mercury will increase with the lifetime of the lamp. Normally this can be seen by the inner side of the glass and the inner phosphor coating turning grey. The areas near the electrodes will turn black with time.
According to Truesdale et al. (1992) mercury will probably also be found in metal form in used compact fluorescent lamps/straight fluorescent lamps, since the inactive atmosphere in the tube should prevent significant oxidation of mercury.
1.3 Relevant legislation - RoHS
According to the RoHS Directive 2002/95 (Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment) mercury in compact fluorescent lamps is exempted from the rule on a maximum content of 0.1 % in homogeneous materials. Maximum permitted mercury content in compact fluorescent lamps is 5 mg/lamp. For straight fluorescent lamps maximum permitted mercury content depends on the lifetime of the fluorescent tube and the type of phosphor coating on the inner side of the tube. For ”halophosphate”[2] permitted amount is 10 mg/lamp, and permitted amount of triphosphate[3] is 5 mg/lamp for normal lifetime and 8 mg/ lamp for long lifetime (RoHS EU, 2002). For compact fluorescent lamps and straight fluorescent lamps complying with European ecolabel criteria the mercury limit value is 4 mg/compact fluorescent lamp or 5 or 8 mg/straight fluorescent lamp depending on the lifetime (EU, 2002). See Table 1-1.
Table 1-1 Maximum permitted content of mercury in compact fluorescent lamps and straight fluorescent lamps (RoHS EU, 2002), (EU, 2002).
| RoHS | EU ecolabel criteria | |
|---|---|---|
| Compact fluorescent lamps | 5 mg Hg | 4 mg Hg |
| Straight fluorescent lamps general purpose (halophosphate) | 10 mg Hg | |
| Straight fluorescent lamps general purpose (triphosphate normal lifetime) | 5 mg Hg | 5 mg Hg |
| Straight fluorescent lamps general purpose (triphosphate long lifetime) | 8 mg Hg | 8 mg Hg |
All exceptions in the RoHS directive are now being revised and according to the Danish Environmental Protection Agency it is planned to lower the limit values for straight fluorescent lamps and compact fluorescent lamps. It is being discussed to lower the maximum permitted content of mercury to 3.5 mg Hg or maybe as low as 2 mg Hg for certain types of compact fluorescent lamps.
1.4 Summary
Compact fluorescent lamps and straight fluorescent lamps contain small quantities of mercury. Mercury that is hazardous to health is needed to make the fluorescent lamps give off light. In addition, fluorescent lamps contain an electrode, an inert gas and have a thin phosphor coating on the inner side of the glass. When electric current is induced to the lamp electrons are released exiting mercury atoms, which are lifted to higher energy levels. In the subsequent decay ultraviolet light is emitted and converted to visible light when it hits the phosphor coating on the inner side of the glass. During the lifetime of the lamp more and more mercury will be bound to the phosphor coating.
Quantities of mercury in a fluorescent lamp are regulated in the RoHS Directive allowing no more than 5 mg of mercury per compact fluorescent lamp (and slightly higher values for straight fluorescent lamps). These values are being revised now, and work is ongoing to reduce the limit values for mercury contents in fluorescent lamps further.
[1] Amalgam is a mercury alloy. An alloy is composed of one or more elements of which at least one of the elements is a metal. (Den Store Danske, 2009).
[2] According to dataanalyse.dk the most common halophosphate compound is calcium fluorophosphate Ca5F(PO4)3.
[3] According to dataanalyse.dk rare soil types and, often, four to five different phosphates are added to obtain better colour value.
2 Survey
- 2.1 Description of types of fluorescent lamps
- 2.2 Survey of quantities of mercury
- 2.3 Survey of types of mercury compounds in fluorescent lamps
- 2.4 Future developments
- 2.5 Summary
The survey of compact fluorescent lamps has been conducted through internet searches and contact to various dealers, importers, manufacturers and suppliers of compact fluorescent lamps and straight fluorescent lamps.
Internet searches
Information gathered in internet searches mostly concerned types of compact fluorescent lamps and straight fluorescent lamps as defined by different manufacturers. Only to a limited extent information about actual quantity of mercury in different products was found. Normally, manufacturers just state that they are in compliance with current legislation.
Contact to manufacturers/importers
The survey has only to a limited extent been able to clarify which form of mercury is contained in products. A limited number of importers, dealers, manufacturers and suppliers were, however, able to deliver more detailed information concerning mercury amounts and mercury compounds used in compact fluorescent lamps/straight fluorescent lamps. Table 2-1 gives an outline of information received from different manufacturers and importers through websites and personal contact.
Table 2-1. Information received from manufacturers/importers
| Manufacturer/Dealer | Info about quantity of mercury | Info about type of mercury |
|---|---|---|
| A | + | + |
| B | + | + |
| C | + | - |
| D | + | + |
| E | + | - |
| F | - | - |
| G | + | + |
2.1 Description of types of fluorescent lamps
Several different categories of types of compact fluorescent lamps and straight fluorescent lamps are in use. In the following the categories used in the RoHS Directive, the EU ecolabel scheme and Danish tax legislation are described along with the categories of products used by manufacturers/dealers and the Danish energy saving trust.
RoHS
The RoHS Directive (RoHS EU, 2002) distinguishes between:
- Compact Fluorescent Lamps = CFL
- Straight Fluorescent Lamps for general purposes
- Straight Fluorescent Lamps for special purposes
Ecolabel criteria
European ecolabel criteria (EU, 2002) for light bulbs divide products into the same types as the RoHS Directive. Furthermore, the ecolabel criteria divide single-ended light bulbs into the following two groups:
- Light bulbs with integral ballast (CFL compact fluorescent lamps)
- Light bulbs without integral ballast (straight fluorescent lamps - pin based lamp).
Figure 2-1 shows examples of light bulbs with integral ballast and without integral ballast. ‘Ballast’ means a device which serves mainly to limit the current of the lamp(s) to the required value in case it is connected between the supply and one or more discharge lamps. A ballast may also include means for transforming the supply voltage, dimming the lamp, correcting the effect factor and, either alone or in combination with a starting device, providing the necessary conditions for starting the lamp(s) (EU, 2009).
Light bulbs with integral ballast have a normal base, i.e. bayonet or screw base. This means that the consumer can replace incandescent bulbs with this type of lamp in their normal socket directly. Light bulbs without integral ballast (single and double-ended straight fluorescent lamps) are tubes that are pin based. Due to this pin base they can only be used in sockets designed for this type of lamp, i.e. they cannot directly replace incandescent lamps. Non-integral lamps are single-ended while straight fluorescent lamps are double-ended.
Figure 2-1. Light bulb with integral ballast (left ) and without integral ballast (right) (General Electrics, 2009).
SKAT (Danish Tax and Customs Administration)
According to SKAT’s guidelines on excise duties 2009-2 all single-ended low energy fluorescent lamps (compact fluorescent lamps) are exempted from ”excise duty on incandescent lamps etc. and electronic fuses” (Skat, 2009). SKAT thus distinguishes between compact fluorescent lamps and straight fluorescent lamps through the number of sockets:
- Compact fluorescent lamps are single-ended
- Straight fluorescent lamps are double-ended.
Straight fluorescent lamps are furthermore divided into two categories, of which one is subject to excise duty:
- ”Straight fluorescent lamps with a power of 20 or 40 watt and emitting ultraviolet light. The tube is coated on the inner side with a specific fluorescent material. Lamps are designed for use in standard sockets.”
Straight fluorescent lamps exempt from excise duty are described as:
- ”Straight fluorescent lamps with a power of 400 watt emitting ultraviolet light. The length of the tube is 12 cm and it is equipped at each end with a main and a secondary electrode. They contain mercury vapours and cannot be used directly due to the fact that they have no socket. The lamp is used in surface treatment and hardening of lacquers in industry”.
This categorisation follows that of the RoHS Directive and thus distinguishes between straight fluorescent lamps for general purposes and straight fluorescent lamps for special industrial purposes. Only the first type of straight fluorescent lamps is included in this survey.
Manufacturers/importers
Manufacturers and importers (such as Philips, 2009; Osram, 2009; Megaman, 2009) often divide compact fluorescent lamps in general types according to:
- Function, such as dimmed, night lamp, outdoor;
- Shape, such as pear, globe, candle, spiral (See Figure 2-2 for examples);
- With/without integral ballast.
Danish energy saving trust
The Danish energy saving trust also divides compact fluorescent lamps according to shape (pear, rod, other) (Elsparefonden, 2009). See Figure 2-2.
Figure 2-2: Various types of lamps. Pear (top left), globe (top right), candle (lower left) and spiral (lower right) (General Electrics, 2009).
One of the interviewed manufacturers state that pear, globe and candle shaped lamps as shown in Figure 2-2 contain a tube within the outer casing as illustrated in Figure 2-3 below.

Figure 2-3. Example of lamp with casing (from the list of A-lamps of the Danish energy saving trust)
This manufacturer states that this additional casing may be an extra protection of the consumer since in some cases only the outer glass breaks in case of accidents. On the other hand this extra casing means that the light yield (lumen value) is reduced by at least 10 % due to reflection and absorption in the outer casing. It is possible to change the absorption features of the outer casing in order to give the lamp more pleasant colour features (e.g. less bluish and more reddish spectre). According to the manufacturer no extra mercury is added to compensate for the loss of light yield (lumen).
Furthermore, the same manufacturer has stated that the compact fluorescent lamp without the extra casing is presently most popular on the market, since it is normally cheaper. The extra casing increases production costs and thus the lamp sales price.
2.2 Survey of quantities of mercury
The survey showed that quantities of mercury in fluorescent lamps vary much. Differences are seen not only from one manufacturer to the other, but also between types of lamps from the same manufacturer. No pattern has been found regarding types of lamps whether divided according to lamps with integral ballast, without integral ballast, straight fluorescent lamps or shape of lamps.
According to Manufacturer D quantities of mercury depend on the following factors some of which have an influence on quantities of mercury over time (also described by Snijkers-Hendrickx et al. (2007)):
- Size of lamp. The larger lamp the larger surface of glass and phosphor coating to be impacted by the mercury, and thereby requirement for larger quantity of mercury.
- Lifetime of the lamp. The longer lifetime the more mercury can be bound to the phosphor coating during the lifetime of the lamp.
- Use pattern. Number of switch on/off cycles. Electrodes ”consume” mercury during the life of the lamp. Long life fluorescent lamps thereby require ”surplus” mercury (Sigai & Nesting).
- Dosage technology. If mercury is added in the form of a tablet or as amalgam, more accurate quantities of mercury can be added compared with dosage of liquid mercury.
- Material composition. Different materials used for fluorescent lamps (glass, phosphor coating, etc.) have different capacity to bind mercury through the lamp life. In addition, impurities in the glass material may have a decisive role for consumption of mercury in the lamp life.
- Use of additives. Addition of antioxidants or, for example, a protective layer of aluminium oxide between the phosphor coating and the glass may help reduce quantities of mercury bound to the fluorescent lamp in the lamp life.
- Type of phosphor coating. Quantities of mercury bound to the phosphor coating depend highly on the type of phosphor coating used.
Several manufacturers also told that they are working continuously to minimise mercury contents in their products and that the content is therefore expected to decrease in the future. Thanks to improved technologies quantities of mercury can be lowered without affecting the lifetime or performance of the lamp (SAES Getters, 2009; Snijkers-Hendrickx et al., 2007). According to Snijkers-Hendrickx et al. (2007) a gradual shift in dosage technology is furthermore seen towards the use of solid mercury, i.e. in the form of tablets or as amalgam.
In Commission Regulation (EC) No. 244/2009 with regard to ecodesign requirements for non-directional household lamps it is required that the mercury content of a lamp (in mg) must be indicated as from 1 September 2010 (EU, 2009). Since mercury contents must be known in the future it may become a future competitive parameter to minimise mercury contents, even when they are below the limit values of the RoHS directive and the ecolabel criteria. Examples have been seen that manufacturers already today indicate the mercury content on their packaging. For example a 10 Watt bulb from PRO light is on the market indicating a mercury content of ”<3 mg Hg”. Some manufacturers also indicate the mercury content on their website (e.g. Philips).
Contacts to different manufacturers have given information about mercury contents between 1.2 and 4.9 mg for compact fluorescent lamps with integral ballast. For non-integral compact fluorescent lamps contents between 1.4 and 4.4 mg Hg have been given and for straight fluorescent lamps contents have been stated between 1.4 and 9.5 mg Hg. See Table 2-2. Quantities of mercury in compact fluorescent lamps differ among manufacturers. One manufacturer has stated that his compact fluorescent lamps have a maximum of 2 mg Hg and another manufacturer states that by far most of his compact fluorescent lamps (80 % of those with integrated ballast) have an average Hg content of 4 mg.
Table 2-2 Content of mercury in compact fluorescent lamps/straight fluorescent lamps – information given by manufacturers
| Compact fluorescent lamps with integral ballast |
Compact fluorescent lamps with non-integral ballast |
Straight fluorescent lamps |
|---|---|---|
| 1.2 – 4.9 mg Hg | 1.4 – 4.4 mg Hg | 1.4 – 9.5 mg Hg |
| (data from seven manufacturers/importers) | (data from one manufacturer/importer) | (data from two manufacturers/importers) |
These figures cover lamps manufactured today and thus complying as a minimum with limit values in the RoHS Directive. However, older lamps are still in use, probably with higher mercury contents. For example, old straight fluorescent lamps can contain 15-20 mg of mercury (Hansen et al., 2008).
As a matter of comparison German magazine Öko-test has tested compact fluorescent lamps in October 2008. Here, values for compact fluorescent lamps between 2 and 6 mg Hg per lamp are reported (Öko-test, 2008).
2.3 Survey of types of mercury compounds in fluorescent lamps
Three manufacturers/importers have told which mercury compounds are added to different compact fluorescent lamps and straight fluorescent lamps. The mercury is used in the fluorescent lamps either in the form of a HgFe compound, in the form of amalgam or in the form of metallic mercury.
According to the manufacturers/importers bismuth (Bi), tin (Sn), indium (In), silver (Ag), lead (Pb) and zinc (Zn) are found in different combinations in the amalgams used. Following combinations have been reported:
- ZnSnHg
- BiInHg
- BiInPbHg
- BiSnPbHg
- BiInSnAgHg
- BiInSnPbHg
Based on information from manufacturers/importers it is not possible to find a correlation between type of fluorescent lamp and type of mercury compound used.
According to Groth (2008) consumers are better protected against exposure to mercury in fluorescent lamps using encapsulation of mercury (in the form of tablets or as amalgam) compared with liquid mercury in the fluorescent lamps – due to the fact that the mercury is bound.
Compact fluorescent lamps with an extra casing also give better protection of the consumer since in some cases only the outer casing breaks during accidents in homes.
2.4 Future developments
As described in Snijkers-Hendrickx et al. (2007) manufacturers are working to reduce quantities of mercury in compact fluorescent lamps and straight fluorescent lamps, among other things through work with/research in technologies for dosage of mercury to the lamp, the materials the lamp are made of and the use of different additives. All these issues have an impact on the quantity of mercury needed in a fluorescent lamp.
Research is also made in how to reduce releases of mercury when a fluorescent lamp breaks. Lee et al. (2009) has shown that the use of a nano-selenium barrier can reduce evaporation of mercury.
The purpose of this project has merely been to focus on the risk arising from mercury-containing compact fluorescent lamps/straight fluorescent lamps breaking in homes. Therefore no further details on technological developments in this field are given here.
2.5 Summary
A brief survey of the market has been made by contacting manufacturers/importers of compact fluorescent lamps and straight fluorescent lamps, and by conducting searches on the internet.
Different types of compact fluorescent lamps and straight fluorescent lamps are available. The general categorisation is based on form (e.g. pear, globe, candle or spiral) or the function (e.g. dimmed, outdoor etc.) Another categorisation is lamps with or without integral ballast. A lamp with integral ballast has a normal fitting, i.e. bayonet or screw base that can be used in a normal socket directly.
Compact fluorescent lamps are single-ended and straight fluorescent lamps are double-ended.
Seven different manufacturers/importers of compact fluorescent lamps and straight fluorescent lamps were interviewed for information about quantities of mercury in fluorescent lamps and about types of mercury used in these lamps.
The result of the survey was the following:
- Compact fluorescent lamps can contain between 1.2 and 4.9 mg Hg per lamp
- Straight fluorescent lamps can contain between 1.4 and 9.5 mg Hg per lamp
The content of mercury in compact fluorescent lamps is typically lower than the statutory limit value of 5 mg Hg. So for the exposure calculations it will be relevant to use different values. The content of mercury in a lamp depends on many factors such as use pattern, mercury dosage technology as well as size, age, lifetime and material composition of the lamp.
Three manufacturers/importers have stated that mercury in the lamps is found in the form of a HgFe tablet, in the form of amalgam or in the form of metallic mercury. No correlation has been found between the type of lamp and the type of mercury compound used.
Some sources state that consumers are better protected against exposure to mercury in a fluorescent lamp using a HgFe tablet or amalgam compared with liquid mercury, since this mercury is bound. One manufacturer also states that a compact fluorescent lamp with extra casing can also give better protection of the consumer since in some cases only the outer casing breaks during accidents in homes.
3 Release of mercury from broken lamps
- 3.1 Mercury compounds and quantities
- 3.2 Release from broken lamps
- 3.3 Impact from flooring and evaporation of mercury after accidents
- 3.4 Description of accident
- 3.5 Release of mercury to the outer environment
- 3.6 Risk of breakage
- 3.7 Discussion and summary
This chapter describes information found in various literature regarding release of mercury when compact/straight fluorescent lamps break.
Quantities of mercury released when a lamp breaks depend on the quantity of mercury in the lamp, the form of the mercury (i.e. the mercury compound, unbound/bound to the material in the lamp and casing in some cases) and other factors such as room temperature (Aucott et al., 2003). The form of mercury also depends on age and use pattern of the lamp (Aucott et al., 2003). These factors are described in more detail below.
3.1 Mercury compounds and quantities
Aucott et al. (2003) states based on information from industry that a compact fluorescent lamp generally contains less than 0.02 mg of vapour-phase mercury at room temperature (temperature of lamp when not in use). In addition, around 0.1 mg of mercury is bound as solid mercury compounds such as HgO.
At an operating temperature of around 40° C the share of vapour-phase mercury increases, but according to industry quantities do not exceed 0.05 mg. According to another report industry states that only 0.5 % of mercury is in vapour-phase when the lamp is on and 0.3 % is in vapour-phase when the lamp is off. This corresponds to 0.025 mg and 0.015 mg of mercury respectively for a lamp with a total content of 5 mg of mercury (NEMA, 2000).
Jang et al. (2005) states that for new straight fluorescent lamps around 0.17 % of metallic mercury is in vapour-phase while for used straight fluorescent lamps this amounts to 0.04 %. This corresponds to 0.009 mg and 0.002 mg of mercury respectively for a straight fluorescent lamp with a total content of 5 mg of mercury.
One manufacturer states that mercury is only in vapour-phase when the lamp is on. When off, it is bound to, for instance, the phosphor coating on the inner side of the glass (Manufacturer C). This, however, is not in line with information from industry stated above from Aucott et al. (2003). Another manufacturer states that mercury vapour is not released from the added amalgam at room temperature (Manufacturer G). Manufacturer D states that when the lamp is on the major part of mercury is in metallic form regardless of the compound added.
According to Truesdale et al. (1992) mercury is probably also found in metal form in used compact/straight fluorescent lamps, since the inactive atmosphere in the lamp should prevent significant oxidation of the mercury. Due to repeated evaporation and condensation during use it seems probable that the mercury is more evenly distributed at the end of the lifetime of the lamp than at the start (Truesdale et al., 1992; Aucott et al., 2003). This more evenly distributed mercury is expected to have a larger evaporation due to larger surface (Aucott et al., 2003). Along with the ageing of the lamp during use an increasing amount of the metallic mercury is converted into mercury compound (mainly HgO) and is mainly bound to the phosphor coating on the inner side. Thereby, it is no longer accessible for release as vapour (Manufacturer D; Truesdale, 1992; Aucott et al., 2003; NEMA, 2000; Raposo et al., 2003; Snijkers-Hendrickx, 2007). According to NEMA (2000) an average of 1.5 mg of mercury was bound to the glass per used lamp in 1994 – at that time the average content of mercury per lamp was at 22.8 mg. In other words, 6.5 % of the total quantity of mercury was bound to the glass.
Bound mercury can be released again if the glass/phosphor coating is heated to a minimum of 400° C in a given period of time (Manufacturer D; Raposo et al., 2003). This factor is exploited, for instance, in recycling operations. Experience from recycling has shown that most mercury in used straight fluorescent lamps can be removed from the lamps together with the phosphor coating (Truesdale et al., 1992). It is also possible that some of the mercury forms amalgam with the electrode material (Truesdale, 1992; Jang et al., 2005).
3.2 Release from broken lamps
In an USEPA report an estimate of mercury emissions to the air from breakage of used lamps is given at around 6.8 % of total mercury contents. This estimate is based, among others, on 12 measurements of mercury concentrations in the phosphor coating on the inner glass surface. Concentrations were between 0.0868 % and 1.02 %. USEPA assumes in its calculations that 100 % of metallic mercury is in vapour-phase and that quantities of mercury in vapour-phase correspond to 0.2 % of total quantities of mercury at the end of the lifetime of the lamp. There is no statement of the period of time over which these 6.8 % of total mercury content is expected to evaporate, but the project deals with emissions of mercury in waste treatment of compact/straight fluorescent lamps (USEPA, 1998).
As described above there are large variations in quantities of mercury in vapour-phase in a lamp (between 0.04 and 0.5 % or 0.002 – 0.05 mg Hg). Basically, this is expected to depend on the temperature (whether the lamp is on or off) and on the inner volume of the lamp. Saturated mercury vapour has a concentration of 20 mg Hg/m³ at 25 °C. This may result in an amount of around 0.001 mg Hg in a ball with a radius of 5 cm. Naturally, the concentration of mercury vapour will increase along with the temperature.
Chandrasekhar (2007) has made a calculation model for concentrations of mercury in a room when mercury is released from a compact fluorescent lamp that breaks. The calculation model takes account of the background concentration of mercury in the surrounding air and of air exchange/ventilation in the room. Model calculations show that with open windows and extra ventilation from a fan it is possible to reach levels below recommended limit values in less than 20 minutes after a lamp breaks.
A fan is used in the model, partly to allow for the assumption that the mercury concentration is evenly distributed in the entire room (otherwise the heavy mercury vapours will concentrate along the floor), and partly to ensure faster ventilation. The calculation assumed that all mercury in a lamp evaporates momentarily at the time t=0, i.e. the initial concentration in a room of 32.6 m³ will be 0.150 mg Hg/m³, if it is assumed that the broken lamp contains 5 mg mercury.
These concentrations are far above the recommended limit values and higher than concentrations measured by Aucott et al. (2003) in practical tests – as described below. But it is well in line with the fact that up to 0.140 mg/m³ were measured in the air in connection with mercury spills in a home (Baughman, 2006).
Aucott et al. (2003) has made measurements of rates of release of mercury vapour from used straight fluorescent lamps. Two straight fluorescent lamps with an assumed average content of 4.55 mg of mercury[4] were broken in a plastic drum with a volume of 0.121 m³. Following concentrations were measured at different temperatures:
- 0.651 mg/m³ at 5° C after two minutes
- 1.152 mg/m³ at 15° C after one minute
- 1.440 mg/m³ at 30° C after one minute[5].
If it is assumed that the concentration is the same in the entire drum this means that around 0.9 %, 1.5 % and 1.9 % of the mercury content evaporates during the first one to two minutes. It is also stated that between 4 and 7 % (depending on temperature) of the total mercury content is released during the first minutes. Aucott et al. (2003) further states that between 17 and 40 % (depending on temperature) of the mercury content will be released during a period of two weeks and that one third of this is released during the first 8 hours after breakage of the fluorescent lamp.
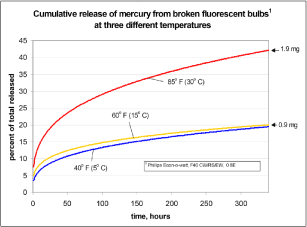
Figure 3-1 Cumulative amount of released mercury from one broken fluorescent tube Aucott et al, 2003).
Figure 3-1 shows a graph of cumulative release of mercury at tests with one broken fluorescent lamp. In these tests only metallic mercury was measured and therefore it is not known whether mercury is also released in other forms. Aucott et al. (2003) describes a formula for the rate of release of mercury per time unit, but this formula (as illustrated with the graph above) only applies to the average content of mercury of 4.55 mg of mercury per straight fluorescent lamp.
Stahler et al. (2008) has also made measurements of release of mercury from compact fluorescent lamps in a room of around 12.7 m² and with a floor-to-ceiling height of around 3 m², corresponding to a volume of around 39 m³. The tests were made with different lamps, flooring, ventilation scenarios and cleaning scenarios. Many different tests were made, but one lamp was broken at a time after which a new test (new lamp with new flooring material etc.) was analysed. The concentration of mercury was measured at a height of around 30 cm (corresponding to the inhalation height of a child) and at a height of around 1.5 m (corresponding to the inhalation height of an adult). To simulate worst case new compact fluorescent lamps were broken with a hammer, and measurements showed a tendency to higher concentrations at 30 cm height than at 1.5 m height in tests without vacuuming. This reflects that mercury vapours are very heavy and concentrate near the floor. The tests showed that when one compact fluorescent lamp broke mercury concentration in the air of the room often exceeded 0.0003 mg Hg/m³ for a period of time (corresponding to the USEPA reference long-term concentration without hazardous effects (RfC)). Short fluctuations with concentrations above 0.05 mg/m³ (upper measurable limit) were also registered. In comparison, Danish occupational threshold limit values for mercury vapours are at 0.025 mg Hg/m³ during a workday (AT, 2007).
It was seen, however, that a short period of ventilation of the room (open window) in most cases reduced the mercury concentration significantly, both in 30 cm’s height and in 1.5 m’s height. For all tests (a total of six) the concentration in 30 cm’s height decreased to below 0.0003 mg Hg/m³ within 9½ minutes after breakage of the lamp. Concentrations increased again, however, when the room was no longer ventilated, especially for some types of lamps as well as during and after vacuuming. Measurements showed that there are large differences between different types of compact fluorescent lamps, and between the period of time before mercury concentrations decrease to below 0.0003 mg/m³.
One test was also conducted with a cracked lamp instead of a broken lamp as well as one test where lamps were warm further to use. The results from these tests were similar to results from previous studies (Stahler et al., 2008).
The main conclusion of the Stahler et al. (2008) study was that the release of mercury vapour is much more variable for scenarios with compact fluorescent lamps from different manufacturers than between different accident and cleaning scenarios with compact fluorescent lamps from the same manufacturer. In other words, release of mercury depends more on type of lamp, i.e. especially quantities of mercury in the lamp and it may also depend on the mercury compound found in the lamp. In the study six different brands of compact fluorescent lamps with different effect were used so results from the study are assumed to represent a general picture if a compact fluorescent lamp should break in a home.
Stahler et al. (2008) is concerned about postponing cleaning after an accident with a broken compact fluorescent lamp. Three tests were made with exactly the same type of lamp, but with cleaning after 1 minute and 46 minutes after the accident. It was seen that even if the initial mercury concentration was the same in both 30 cm’s and 1.5 meter’s height, the delay meant that the mercury spread in the room and resulted in higher average concentrations (at both 30 cm’s and 1.5 meter’s height) – even if a window was opened immediately after the accident in all three cases. Due to the high concentrations measured just after the accident (up to 0.05 mg Hg/m³) Stahler et al. (2008) recommends to wait – not too long, but some 5-15 minutes – before cleaning.
Literature describes that compact fluorescent lamps are available that are covered by a silicone film, which will reduce the risk of release of mercury during breaks. [6] As mentioned above consumers may be better protected against mercury exposure through use of dosage techniques with encapsulated mercury compared with liquid mercury or if an additional outer casing is used for compact fluorescent lamps and only this outer casing breaks.
3.3 Impact from flooring and evaporation of mercury after accidents
Stahler et al. (2008) has also studied the impact of flooring and vacuuming on mercury concentrations in the air after accidents, i.e. when compact/straight fluorescent lamps break in a home.
It was common to all types of flooring (long and short-pile carpets and laminate wooden floor) that even if they looked clean all types of flooring still released mercury even after cleaning and vacuuming. In the tests significantly higher concentrations of mercury were measured after cleaning if the floors were impacted physically (e.g. walking, vacuuming or washing) than if the floors were left untouched. Measurements of mercury concentrations in the room (unventilated) were continued until the concentration was below 0.0003 mg Hg/m³. In most cases it took up to four days before the concentration decreased to below 0.0003 mg Hg/m³ for wooden floors, but for two of ten measurements for wooden floors it took more than 20 days before the concentration dropped to below 0.0003 mg Hg/m³. For carpet flooring it took generally longer before the concentration decreased to below 0.0003 mg Hg/m³ – 6, 15, > 27, 34, 52 and > 59 days respectively[7] for a total of six measurements. In all cases the carpets were impacted physically (simulated vacuuming) several times after cleaning. Results showed that the concentration close to the floor may reach as high as 0.029 mg Hg/m³ several weeks after removal of the lamp (measured after vacuuming/impacting of the flooring material).
The tests showed that particularly carpets seemed to contain more ”mercury residues” after cleaning compared with wooden floors. The wooden floor used was a laminate wooden floor. It may well be that an old wooden floor with, for instance, broad planks with large spacing would be just as hard to clean as a carpet, since droplets of mercury may compile in the spaces and be difficult to collect.
The surveys in Stahler et al. (2008) also showed that it is not expedient in the first cleaning stage to use a vacuum-cleaner for cleaning broken compact fluorescent lamps/straight fluorescent lamps. The vacuum-cleaner will disperse the mercury and become contaminated with mercury to an extent that it is difficult to clean. However, by removing the dust bag and cleaning the mouthpiece and hoses thoroughly, for instance with wet tissues, the concentration of mercury in the vacuum-cleaner can be reduced.
3.4 Description of accident
One of the interviewed manufacturers describes that the older the lamp, the more mercury will be bound to the glass and the phosphor coating on the inner side of the glass. Normally, this is seen by a greying of the inner side of the glass and the inner phosphor coat. The area near the electrodes will gradually turn black. In addition, in some cases it is possible to see fine dispersed mercury drops when a lamp breaks. Due to the breakage of the lamp some of the phosphor coating may be loosened from the glass surface. For the consumer it is therefore relevant to remove all visible glass, powder and mercury droplets, if any, after an accident with a broken lamp.
3.5 Release of mercury to the outer environment
Using the study by Aucott et al. (2003) to indicate how much mercury will evaporate to the outer environment when a broken fluorescent lamp is discarded in the waste bin – before incineration of the waste – the following will be the result: As mentioned between 17 and 40 % of the mercury will be released in the course of a two-weeks period. Amounts depend on the temperature but also a number of other factors such as air volume surrounding the mercury. Evaporation will presumably be lower if the mercury is packed airtight, for instance.
USEPA estimates that around 11 % of the mercury in a fluorescent lamp will be released to the air or water when the lamp is landfilled as waste. Evaporation of mercury to the outer environment is not dealt with further in this report since it is not the purpose of the study. However, it is evident that ventilation in connection with breakage of a fluorescent lamp will also contribute to mercury in the surrounding air.
3.6 Risk of breakage
It is difficult to indicate the frequency of breakage of fluorescent lamps. It has only been possible to find trade figures from the UK showing that less than 1 % of lamps break (Defra, 2009). This has not been studied in detail in this project since focus is on health impacts from broken fluorescent lamps in private homes.
3.7 Discussion and summary
The below tables sum up the most significant figures from the different studies. The first table shows values for content of mercury in vapour form and amounts bound to the glass before a lamp breaks. The second table shows concentrations of mercury measured at different times after breakage of a fluorescent lamp as well as relevant references. The values originate mostly from tests and, primarily, maximum values are stated.
Table 3-1 Values for contents of Hg in vapour form and bound to the glass – before breakage
| Compact fluorescent lamps | Straight fluorescent lamps | |
|---|---|---|
| Mercury in vapour form within the lamp | Max. 0.05 mg Hg (Aucott et al., 2003) Max. 0.025 mg Hg (0.5 %) in warm lamp and max. 0.015 mg Hg (0.3 %) in cold lamp (for lamp with total of 5 mg Hg) (NEMA, 2000) |
New lamps contain around 0.17 % Hg in vapour form. Used lamps contain around 0.04 % Hg in vapour form. (Jang et al., 2005) |
| Mercury bound to the glass | 6.5 % of total quantity of mercury is bound to the glass in used lamps (NEMA, 2000) | |
Table 3-2 Values for concentrations of mercury in accidents with broken compact fluorescent lamps/straight fluorescent lamps
| Compact fluorescent lamps | Straight fluorescent lamps | |
|---|---|---|
| Maximum concentration/ ”peak” values | Theoretical calculation without ventilation: 0.150 mg Hg/m³ (Chandrasekhar, 2007) |
|
| Measurements during accident in a private home: 0.140 mg Hg/m³ (Baughmann, 2006) Measurements during test (room 39 m³): 0.05 - > 0.1 mg Hg/m³ (Stahler et al., 2008) |
||
| Concentration one minute after accident | Test where two lamps are broken in a drum: 1.152 mg Hg/m³ at 15 °C 1.440 mg Hg/m³ at 30 °C (Aucott et al., 2003) |
Measurements during test (2 lamps with 4.55 mg Hg): 1.440 mg Hg/m³ (at 30 °C) corresponding to 1.9 % has evaporated (Aucott et al., 2003) |
| Concentration a few minutes after accident | Test where two lamps are broken in a drum: 0.651 mg Hg/m³ at 5 °C after two minutes (Aucott et al., 2003) |
Measurements during test (2 lamps with 4.55 mg Hg): 4 – 7 % Hg has evaporated depending on temperature (Aucott et al., 2003) |
| Concentration 8 hours after accident | Measurements during test (2 lamps with 4.55 mg Hg): 6 – 13 % Hg has evaporated depending on temperature (Aucott et al., 2003) |
|
| Concentration two weeks after accident | Measurements during test (2 lamps with 4.55 mg Hg): 17 – 40 % Hg has evaporated depending on temperature (Aucott et al., 2003) |
|
| Concentration > 59 days after accident after cleaning | Measurements during test (room 39 m³): > 0.0003 mg Hg/m³. Measurements were made until the value 0.0003 mg Hg/m³ was no longer exceeded. It took between < 4 days to > 59 days depending on flooring type. (Stahler et al., 2008) |
|
Information shows that around 0.5 % of total quantities of mercury (maybe up to 1 % or a maximum of 0.05 mg Hg) will evaporate immediately from a broken compact fluorescent lamp. When it is warm (30 °C) up to around 2 % may have evaporated after one minute and up to 7 % after a few minutes. After 8 hours up to 13 % of total quantities of mercury contained in the fluorescent lamp may have evaporated. It is not stipulated precisely, but it can be read from the graph (Figure 3-1) that around 10 % will have evaporated within around 30 minutes – the maximum time presumed necessary for cleaning after an accident. Therefore it is assumed that 10 % of the total amount of mercury evaporates in 30 minutes in the exposure calculations.
Thus, in practice a maximum of 0.5 % of the total amount of mercury will presumably evaporate momentarily after which in the following minutes up to 7 % of the total amount of mercury contained in the lamp will evaporate and up to 10 % after 30 minutes. In the worst-case calculations it is therefore assumed that 10 % of the total amount of mercury will evaporate immediately when the lamp breaks.
A number of measured values show that maximum concentrations can easily exceed the occupational threshold limit value – even with a very high factor. These maximum values, however, are only present for a brief period of time. Both calculation models and tests show that the concentration of mercury decreases very quickly to low levels far below relevant limit values through continuous ventilation of the room.
Tests also show that concentrations of mercury are higher 30 cm above floor height than 1.5 m above floor height. In addition to the height above floor level the concentration of mercury in the room after breakage of a lamp depends on the quantities of mercury in the lamp and, possibly, the form in which mercury is present in the lamp.
In one study a certain concern is expressed for postponing cleaning after breakage of a compact fluorescent lamp, since three different tests with the same broken lamp showed that a long waiting time (46 minutes) compared with a short period of time (1 minute) before cleaning up resulted in higher average concentrations (both in 30 cm’s and in 1.5 metre’s height) – despite the fact that a window was opened immediately after the accident in all three cases. Due to the high concentrations measured immediately after the breakage (up to 0.05 mg Hg/m³) it is recommended in the study to put off cleaning for a few minutes – some 5-15 minutes, but no longer than that.
Furthermore, tests have shown that in a room cleaned after an accident and where visible residues of the fluorescent lamp and mercury have been removed, concentrations of mercury can exceed the USEPA RfC value (reference concentration without harmful effects) for short periods of time during and after vacuuming: tests show that when floors containing mercury residues are impacted physically, concentrations at floor height may increase substantially.
Particularly carpets seem to contain more ”mercury residues” after cleaning compared with a smooth wooden laminate floor. Old wooden floors with large spaces may, however, be just as difficult to clean as a carpet.
A vacuum cleaner can easily be contaminated with mercury if it is used to vacuum glass residues from the fluorescent lamp and thereby release mercury vapours to the indoor climate for a long period of time.
One of the interviewed manufacturers stated that during an accident it is relevant to remove all visible glass, powder and even droplets of pure mercury. Both glass and powder contain mercury.
Several references state that the broken lamp should be removed using materials that are subsequently discarded, i.e. use cardboard pieces to pick up pieces of broken glas and adhesive tape to collect other physical residues. In this way it is avoided that cleaning utensils such as brooms and vacuum cleaners are contaminated with mercury so that they release mercury vapours in the weeks and months after the accident. Pieces from the broken lamp and various collection tools should be placed in a closed container, e.g. a canning jar, to avoid spreading of mercury vapours. After removal of residues the contaminated area should be cleaned.
[4] The manufacturer stated a content in straight fluorescent lamps between 4.4 and 4.7 mg Hg. Therefore, an average content of mercury of 4.55 mg has been assumed.
[5] These concentrations are far below the concentration for saturated mercury vapours (20 mg Hg/m³ at 25 °C). This means that the drum used for measuring mercury concentrations has been large enough to measure maximum quantity of mercury that can evaporate.
[6] http://www.defra.gov.uk/environment/business/products/roadmaps/lightlamps.htm; http://www.megaman.cc/global/greenroom/silicone_protection.php; http://www.clear-lite.net/docs/_sub_products_1.html).
[7] For two of the tests the concentration of 300 ng Hg/m³ was not reached before the test ceased after 27 and 59 days, respectively.
4 Health assessment of mercury vapours
- 4.1 Description of mercury
- 4.2 Absorption and metabolism in human body
- 4.3 Human intake of mercury
- 4.4 Measurement of mercury exposure
- 4.5 Mercury toxicity
- 4.6 Limit values
- 4.7 Summary
Manufacturers/importers contacted in connection with the survey have stated that mercury used in compact and straight fluorescent lamps is either metallic mercury or mercury amalgam.
If one or more compact or straight fluorescent lamps break in a home, mercury vapour may be released to the indoor air, as long as the residues have not been removed completely. Therefore, this health assessment has main focus on exposure to mercury vapour through inhalation.
4.1 Description of mercury
Mercury (Hg) is a metallic element that may occur as the free metal or in inorganic and metal organic compounds. Furthermore, mercury can be mixed with other metals forming amalgams, for example with silver and copper for dental fillings. Inorganic compounds are found in the oxidation levels +1 and +2 as mercury(I) (mercurous, Hg2²+) and mercury(II) (mercuric, Hg²+) salts. Some salts readily dissolve in water, such as mercury(II) nitrate, and others such as mercury(II) sulfide are completely insoluble. Metal organic mercury compounds are insoluble in water, but dissolve in certain organic solvents.
Mercury (Hg0) is the only metal that is liquid under normal pressure and temperature. It appears as a heavy, odour-free silvery liquid, which is practically insoluble in water and has a relatively high vapour pressure at room temperature. Occurrence of liquid mercury will therefore result in exposure to the invisible and odour-free mercury vapours. At room temperature air saturated with mercury will have a concentration of around 14 mg Hg/m³ or 500 times the current occupational threshold limit value. Mercury vapours are seven times heavier than air and will disperse along the floor in a room with insufficient ventilation (Clarkson et al., 2003).
Identification
| Chemical name | Mercury (metallic mercury) |
| CAS No. | 7439-97-6 |
| EINECS Nr. | 231-106-7 |
| Gross formula | Hg |
| Molecular weight | 200.59 g/mol |
| Atomic number | 80 |
Physico-chemical data
| Physical state | Silvery liquid |
| Melting point | -39 oC |
| Boiling point (1 atm) | 356 oC |
| Density (20oC) | 13.58 g/mL |
| Vapour pressure (20oC) | 0.0012 mm Hg/ 0,17 Pa |
| Relative vapour density (air=1) | 6.9 |
| Evaporation rate (BuAc=1) | 4 |
| Water solubility (20oC) | 0.025 mg/L |
Classification
| List of harmonised classification (ECBs Annex 1, 2009) |
Yes | Repr. Cat. 2; R61, T+; R26, T; R48/23, N; R50-53 I.e. May cause harm to the unborn child (R61). Very toxic by inhalation (R26). Toxic: danger of serious damage to health by prolonged exposure through inhalation (R48/23). Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment (R50/53). |
| List of undesired substances (Danish EPA, 2004) |
Yes | Mercury and mercury compounds |
4.2 Absorption and metabolism in human body
As described above liquid mercury is sparingly soluble and relatively inert. In case of ingestion of a few drops of mercury less than 0.01 % will be absorbed in the body through the gastrointestinal tract and this exposure will therefore not present an acute risk, but only add a minimal contribution to the mercury load (WHO 1980).
Absorption of metallic mercury through undamaged skin is also assessed to be very limited. Even though it has not been studied quantitatively, absorption through intact skin for metallic mercury is assessed to be minimal due to the very low absorption rate of the gastrointestinal tract and the physical properties of metallic mercury (ATSDR, 1999).
By contrast, mercury is readily absorbed through the respiratory tract (around 80 %) after inhalation of mercury vapours (WHO 1980).
It has been estimated that upon exposure to mercury vapours 2.6 % of the mercury is absorbed through the skin and the remaining 97.4 % is absorbed through the respiratory tract (WHO, 2003).
Mercury absorbed can be measured in the blood, where it is equally distributed in the plasma and the red blood cells. In the red blood cells extensive oxidation takes place of metallic mercury (Hg0) into mercury(II) ions (Hg²+), which may bind strongly to sulfurous proteins. Oxidation is catalysed by the enzyme catalase (Holmes et al., 2009).
Mercury vapour is different from inorganic mercury compounds and reminds of methyl mercury in being transported easily and readily across the blood-brain barrier and the placental barrier, causing affects on the central nervous system and the unborn child. In the brain metallic mercury is transformed to inorganic mercury compounds as methyl mercury is (Holmes et al., 2009).
A recent study with rats showed an interplay between mercury vapour and methyl mercury and the resulting level of mercury in the brain of offspring. Low dietary exposure to methyl mercury and parallel exposure to mercury vapour increased the level of mercury in the brain of offspring. Thus, the study concluded that human foetus exposed to both methyl mercury and mercury vapour has increased risk of impaired neurodevelopment compared with exposure to the two forms of mercury separately (Ishitobi et al., 2010).
Body half-life of mercury after exposure to mercury vapour is 35-90 days (WHO 1980). Retention in the brain is however somewhat longer. Mercury is particularly long-term accumulated in the kidneys.
Metallic mercury is excreted in exhaled air, sweat and saliva. After conversion to mercury(II) compounds these species may be excreted in urine through the kidneys (Berlin 1977).
4.3 Human intake of mercury
The predominant route of exposure to mercury for the general public is through food, especially from fish and other seafood. The daily average intake through food is estimated at 2-3 μg mercury – almost exclusively in the form of methyl mercury (see Table 4-1). This is an organic form of mercury, which can be formed by micro-organisms in the aquatic environment, and which has a particular tendency to accumulate in aquatic food chains. Methyl mercury can just as mercury vapours be transported easily and readily across the blood-brain and the placental barriers – and is thereby similarly problematic. For methyl mercury a ”secure” limit value has been set at a daily intake of 0.1 μg Hg/kg body weight. (Clarkson et al., 2003).
An additional contribution of mercury may origin from dental amalgam fillings, which contains around 50 % of mercury. It is estimated that 0.2 μg mercury is released from each amalgam filling per day (Richardson et al., 2009). WHO estimates that the ”intake” from dental fillings may amount to between 1.2 and 27 μg mercury per day (Holmes et al., 2009), but that the absorption in the gastrointestinal tract as mentioned above will be limited (see Table 4-1).
WHO estimates that the daily average mercury intake by inhalation of ambient air is at 0.04-0.2 µg Hg per day, based on an air concentration of 0.002-0.01 μg Hg/m³ (Holmes et al., 2009).
Mercury in the form of thiomersal – an ethyl mercury compound – is in some cases used as a preservative in vaccines. This use may also cause mercury loads. It has been calculated that children subject to an ordinary children’s vaccine programme with mercury preserved vaccine from birth and until the age of 6 months will be exposed to more than 0.1 μg Hg per day per kg of body weight (IPCS, 1980; Clarkson et al, 2003).
Table 4-1 Estimated daily average intake of mercury in various forms for average population.
| Source of exposure | Daily intake and absorption of different types of mercury (µg/day) | ||
|---|---|---|---|
| Elementary mercury | Inorganic mercury | Methyl mercury | |
| Air (level: 2—10 ng/m³) Dental amalgam |
0.04–0.2 (0.03–0.16) 1.2–27 (1–21.6) |
Minimal 0 |
0.008 (0.0069) 0 |
| Food – Fish (100 g/week containing 0.2 mg Hg/kg) – Other Drinking water Total |
0 0 0 1.2–27 (1–22) |
0.60 (0.06) 3.6 (0.36) 0.05 (0.005) 4.3 (0.43) |
2.4 (2.3) 0 0 2.41 (2.31) |
Source: WHO (2003, 2005).
4.4 Measurement of mercury exposure
Human mercury load can be measured by the content of mercury in blood, hair or urine. The blood-mercury level is a good indicator for recent exposure, since the half-life of mercury in blood is 3 days. Mercury in hair is a good indicator for long-term or historical (previous) exposure to mercury.
In 1979 blood samples from 264 Danes – selected randomly among the Danish population – were analysed for mercury. Average concentration amounted to 1.5 μg Hg/L, and the highest concentration was 13 μg Hg/L. A slightly lower number of people had taken hair samples and here the average concentration amounted to 0.6 ppm Hg with a maximum of 3.1 ppm Hg. There was a clear correlation between contents in blood and hair (Bach, 1980).
Intake of mercury contaminated fish may result in 5-10 times higher mercury concentrations in blood and hair than in the general population. Among residents from the Faroe Islands and Greenland, with a particularly large intake of fish and marine mammal, mercury in blood may even be more than 50 times higher than the background level (Grandjean et al., 1997).
In the Bach (1980) study, mercury was not measured in urine, but other studies have shown that non-exposed persons excrete less than 0.5 μg Hg/L corresponding to around 1.5 μg Hg/g creatinine. Persons with amalgam fillings excrete slightly more - 2-4 μg Hg/L (ATSDR, 1999).
Occupational mercury exposure can also be significant. Workers with a long-term exposure to mercury vapours had an average concentration of 10 μg Hg/L blood, while non-exposed persons only had 6.5 μg Hg/L in their blood. In urine concentrations were 11 and 2.3 μg Hg/g creatinine respectively. A significant correlation was seen between values in blood and urine and values in air and urine for these exposures (Berlin, 1977).
The correlation between mercury in air and blood and between mercury in air and urine does not appear for ordinary people for whom the major part of mercury intake is in the form of methyl mercury in food.
4.5 Mercury toxicity
In most ordinary population studies for clarification of the health hazards of mercury, exposure to methyl mercury through food plays a predominant role. Therefore, it is difficult to determine the impact of an additional exposure to mercury vapours, unless that is very substantial, such as the levels occurring in the working environment in the past.
An experimental study (Berlin, 1977) showed no observable effects in studies with dogs exposed to 0.1 mg Hg/m³ for 7 hours per day for 5 days per week for 83 weeks. This experimental no-observed-adverse-effect-level (NOAEL) of 0.1 mg Hg/m³ (100 µg/m³), however, did not take into account neurophysiological and psychological effects.
Most data on health effects are derived from occupational exposures. At very high exposure levels for mercury vapours in the working environment the lungs are the target organs. These very high exposures cause irritation and corrosion of the respiratory tract, and after a few hours’ exposure to 1-3 mg Hg/m³ (1000-3000 µg/m³) acute fatal chemical pneumonia may occur (Milne et al., 1970, quoted from Berlin, 1977). Corresponding exposure of test animals for 8 hours daily for some months was also fatal (WHO, 1980).
At prolonged high exposure to mercury vapours (>100 µg/m³ or > 0.1 mg/m³) the critical organ is the central nervous system where poisoning symptoms can occur such as tremor, insomnia, depression, mental unbalance, irritability, amnesia, abnormal shyness and gingivitis (Berlin, 1977, WHO, 1980).
Slightly toxic effects in humans are to be expected from exposures corresponding to levels of 50 μg Hg/L of blood or 150 μg Hg/L of urine (Holmes et al., 2009). This probably corresponds to 0.025-0.050 mg/m³ (25-50 µg Hg/m³). Therefore a value of 0.025 mg/m³ (25 µg Hg/m³) is often used as the lowest-observed-adverse-effect level (LOAEL value).
For long-term exposure to mercury vapour LOAEL has been determined at 0.014 mg/m³ (14 µg/m³). The effect was subtle neurological changes in the central nervous system and poor control of movements (Richardson et al., 2009).
Exposure to mercury vapour is particularly risky for pregnant women since mercury vapours can penetrate the placental barrier and harm the unborn child. A study is available of a pregnant woman exposed for a long period of time to mercury vapours (0.020-0.060 mg Hg/m³ (20-60 µg Hg/m³)) from mercury spilled on a carpet in her home. She had no poisoning symptoms but the mercury level in urine was elevated (230 µg/L). The child was also born with elevated mercury levels, but an examination at the age of 2 showed normalised levels (Caravati et al., 2008).
The no-effect-level (NOAEL) at long-term exposure has been estimated at 0.01 mg Hg/m³ (10 µg/m³) (Berlin, 1977).
No information is available about the no-effect-level of mercury for short-term exposure of humans (Groth, 2008; TNO, 2008), but NIOSH states that there is immediate danger to life and health at exposure to 10 mg/m³ (10,000 µg/m³) – a value, which is relatively close to the saturated concentration at 20 oC of 14 mg/m³.
A poisoning case has been described in the USA from exposure to mercury released from broken straight fluorescent lamps at a waste site near a nursery (Tunnessen et al., 1987). A child of 2 yrs acquired the mercury disease ”acrodynia”, which manifests itself, among other things, by strong pain, weight loss and skin changes such as blush and peeling. The entire family of five had elevated mercury levels in urine, with the mother showing the highest level, but only the 2 year old child - having the second highest concentration - acquired the disease. This may be because a child of that age plays near the ground and is more sensitive, but there are also indications that some people are genetically more sensitive to the harmful effects of mercury.
4.6 Limit values
In February 2010 FAO/WHO set a provisional tolerable weekly intake (PTWI) of inorganic mercury of 4 μg Hg/kg body weight (WHO 2010). This value replaces an older (1978) PTWI for total mercury of 5 μg Hg/kg body weight. The new PTWI for inorganic mercury is assessed to be useful for intake of mercury through other food than fish and shellfish. For fish and shellfish the PTWI from 2003 for methyl-mercury of 1.6 μg Hg/kg body weight still applies, corresponding to 0.23 μg Hg/kg body weight/day. FAO/WHO also assessed that the upper estimated limit for the average weekly intake of total mercury from food other than fish and shellfish of 1 µg/kg body weight for adults and 4 µg/kg body weight for children was below the new PTWI for inorganic mercury.
The occupational threshold limit value (time-weighed average) for long-term exposure to metallic mercury and inorganic compounds is 0.025 mg Hg/m³ (25 µg Hg/m³) (AT, 2007). Present biological occupational limit values (BEI) are 35 μg Hg/g creatinine for urine before work and 15 μg Hg/L blood after work. For short-term exposure a limit value of 0.5 mg/m³ (500 µg/m³) has been recommended by WHO (WHO, 1980).
Regarding harmful effects of long-term exposure to methyl mercury from marine food chains Danish-Faroese studies of Faroese children exposed, among others, for mercury in the embryonic stage, have been very important (Grandjean et al., 1997). Based on these studies USEPA recommended in 2001 a Reference Dose (RfD) for methyl mercury of 0.1 μg/kg/day (USEPA MeHg, 2009), or five times below a previous WHO threshold value for methyl mercury. Biomarkers in the Faroese study were mercury concentration in umbilical cord blood and the mother’s hair. The lowest concentration of methyl mercury in a mother’s hair, where statistically significant negative effects on the development of the central nervous system were observable in Faroese children, was 15 ppm.
USEPA has set a Reference concentration (RfC) for mercury vapours of 0.0003 mg/m³ (0,3 µg/m³) (USEPA Hg, 2009). This value is based on (un)certainty factors (a total of 30) and a LOAEL of 0.025 mg Hg/m³ (25 µg Hg/m³) in the working environment during a normal workday/week. This LOAEL corresponds to the Danish occupational threshold limit value mentioned above. Corrected for long-term exposure[8] (from working environment to normal population) the LOAEL becomes 0.009 mg Hg/m³ (9 µg Hg/m³). These values are based on adverse effects such as tremor and memory loss.
Agency for Toxic Substances Disease Registry (ATSDR) set in 1999 a minimum risk level (MRL) of 0.0002 mg Hg/m³ (0,2 µg Hg/m³). In addition, TSDR has recommended concentration limits after cleaning and spillage indoors (ATSDR, 1999).
In 2005 EPA in California established a long-term Reference Exposure Level (REL) of 0.00003 mg Hg/m³ (0.03 µg Hg/m³) on the basis of a LOAEL of 0.025 mg Hg/m³ (25 µg Hg/m³) in the working environment (OEHHA, 2008). An adjustment to normal population exposure gave a LOAEL of 0.009 mg Hg/m³ (9 µg Hg/m³) (corresponds to USEPA above). This was converted to REL by using larger (un)certainty factors than those used by USEPA:
- An (un)certainty factor of 10, due to the fact that it is not a NOAEL,
- An (un)certainty factor of 30 for particular sensitivity of children, variation between individuals and for sensitivity of nervous system under development.
CalEPA has furthermore set a limit value of 0.0018 mg/m³ (1.8 µg/m³) for an exposure of one hour (Groth, 2008).
A new Canadian assessment assumes a LOAEL value of 0.006 mg Hg/m³ (6 µg Hg/m³). With an (un)certainty factor of 100 for uncertainty and modifying factors the result is a REL of 0.00006 mg Hg/m³ (0,06 µg Hg/m³) (Richardson et al., 2009).
A summary of information about limit values long-term mercury exposure is given in Table 4-2.
Table 4-2 Limit values etc. for mercury exposure (Caravati et al., 2008; Richardson et al., 2009 a.o.).
| Air concentration (mg/m³) | Explanation | Authority |
|---|---|---|
| 10 | Immediately dangerous to life and health (IDLH) | NIOSH |
| 0.1 | Permissible exposure limit (PEL-TWA) | OSHA |
| 0.5 | Limit value for short-term exposure | WHO |
| 0.05 | Recommended occupational threshold limit value (TWA) | NIOSH |
| 0.025 | Recommended occupational threshold limit value (TLV/GV) | ACGIH/AT |
| 0.03 | Recommended concentration after commercial cleaning | ATSDR |
| 0.001 | Recommended breathing zone limit in private home after spillage | ATSDR |
| 0.0018 | Reference Exposure Level (REL), short-term concentration (1 hour) | CalEPA |
| 0.0003 | Long-term concentration without harmful effects (RfC) | USEPA |
| 0.0002 | Daily exposure without risk (MRL) | ATSDR |
| 0.00006 | Reference Exposure Level (REL), long-term concentration | Health Canada |
| 0.00003 | Reference Exposure Level (REL), long-term concentration | CalEPA |
| 0.000002 – 0.00001 | Background level of air concentration | WHO |
IDLH = Immediately Dangerous to Life and Health. Represents maximum concentration for a substance for which you can avoid irreversible effects after 30 minutes’ exposure
PEL = Permissible Exposure Limit determined by OSHA (US Occupational Safety and Health Administration).
TWA = Time-weighed average concentration, limit value proposal from US National Institute for Occupational Safety and Health NIOSH.
TLV = Threshold Limit Value is an occupational threshold limit value proposed by American Conference of Governmental Industrial Hygienists (ACGIH)
GV = Limit value, Danish limit value for occupational health from the Danish Working Environment Authority
RfC = Reference Concentration, developed by USEPA
MRL = Minimum Risk Level, determined by ATSDR, Agency for Toxic Substances Disease Registry
4.7 Summary
Under normal pressure and temperature mercury is a liquid metal that appear as a heavy, odour-free silvery liquid with a relatively high vapour pressure. Mercury vapours, which are seven times heavier than air, will disperse along the floor of a room with insufficient ventilation.
At inhalation of mercury vapours about 80 % will be absorbed through the lungs, while the absorption of the sparingly soluble and inert metallic mercury through the skin is < 2 %, and the absorption in the gastrointestinal tract is < 0.01 %.
In the general population the largest source of mercury is through intake by food especially fish. Daily average intake from food has been estimated at 2-3 μg of mercury, and almost exclusively in the form of methyl mercury. Among the population of the Faroe Islands and Greenland, who have a particularly high intake of fish and marine mammal, mercury in the blood can be more than 50 times higher than background loads. Similarly, people in particularly exposed jobs may have 10 times more mercury in their blood.
Background exposure to mercury can also occur from dental amalgam, and WHO has assessed this to be between 1.2 and 27 μg mercury/day, but with limited absorption in the gastrointestinal tract (see Table 4-2).
Mercury vapours are different from inorganic mercury compounds by readily penetrating the blood-brain and placental barriers, which may cause effects on the central nervous system and the unborn child. In the brain metallic mercury, like methyl mercury, is transformed to inorganic mercury (Hg²+). The biological half-life of mercury in the body after exposure to mercury vapours is 35-90 days. Retention time in the brain is, however, somewhat longer. Metallic mercury can be excreted with sweat and saliva and through exhalation. After oxidation into inorganic mercury it may be secreted with the urine through the kidneys. Excess mercury in the body is accumulated particularly in the kidneys.
Mercury exposure of humans can be measured by analysing the content of mercury in blood, hair or urine. Average concentration of mercury in the blood of Danes was determined 30 years ago at 1.5 μg Hg/L, and the corresponding concentration in hair was 0.6 ppm Hg. There was a signifikant correlation between contents in blood and hair.
Measurement of mercury in urine for non-exposed persons has shown that they excrete less than 0.5 μg Hg/L urine corresponding to around 1.5 μg Hg/g creatinine. People with amalgam fillings excrete slightly more (2-4 μg Hg/L) and people in particularly exposed jobs may have a ten times’ higher excretion.
Most data on health effects from mercury vapours derive from occupational exposure. At very high exposures to mercury vapours in the working environment the lungs are the target organ. The respiratory tract is irritated and corroded, and after a few hours’ exposure fatal acute chemical pneumonia may occur. At an exposure to 10 mg Hg/m³ (10,000 µg Hg/m³) there is acute mortal danger. No information is available about no-effect-level of mercury in short-term exposure of humans.
At prolonged high exposure to mercury vapours (>100 µg/m³ or > 0.1 mg/m³) the critical organ is the central nervous system, where poisoning symptoms such as tremor, insomnia, depression etc. may occur. Exposure to mercury vapour is particularly risky for pregnant women since mercury vapours can penetrate the placental barrier and harm the unborn child.
At lower concentrations slightly toxic effects (e.g. hand tremors and memory loss) in humans are to be expected after long-term exposure to 0.025-0.050 mg Hg/m³ (25-50 µg Hg/m³). This 0.025 mg Hg/m³ (25 µg Hg/m³) level, which is identical to the present occupational threshold limit value, is often used as the LOAEL value. However, a recent study states a LOAEL of 0.014 mg Hg/m³ (14 µg Hg/m³) for prolonged exposure to mercury vapours; subtle neurologic changes of the central nervous system and poor control of movements have been described. Furthermore, 0.010 mg Hg/m³ (10 µg Hg/m³) has been proposed as NOAEL at long-term exposure.
FAO/WHO has recommended a provisional tolerable weekly intake (PTWI) for inorganic mercury of 4 μg Hg/kg body weight/week and 1.6 μg Hg/kg body weight/week for methyl mercury. In comparison, daily average intake of methyl mercury is 2-3 μg Hg/day (corresponding to 0.2 – 0.3 μg Hg/kg body weight/week).
A LOAEL of 0.025 mg Hg/m³ (25 µg/m³) has been established in the working environment for a normal workday/week. Corrected for permanent exposure[9] (from working environment to normal population) the LOAEL became 0.009 mg Hg/m³ (9 µg Hg/m³). This value has been used by USEPA (together with a certainty factor of 30 for particular sensitivity for children, variation between individuals and for sensitivity of nervous system under development) to set a reference concentration (RfC) for mercury vapours of 0.0003 mg/m³ (0.3 µg/m³). CalEPA used a further certainty factor of 10 since the basis was a LOAEL, not a NOAEL, and arrived at a long-term limit value of 0.00003 mg Hg/m³ or 0.03 µg Hg/m³. CalEPA has furthermore set a limit value of 0.0018 mg/m³ or 1.8 µg Hg/m³ for a one hour exposure.
Agency for Toxic Substances Disease Registry (ATSDR) has set a minimum risk level (MRL) of 0.0002 mg Hg/m³ (0.2 µg Hg/m³) and recommends concentration limits after cleaning outdoors and spillage indoors of 0.03 and 0.001 mg Hg/m³ respectively (i.e. 30 and 1 µg Hg/m³ respectively).
A Canadian assessment uses a LOAEL value of 0.006 mg Hg/m³ (6 µg Hg/m³) and a certainty factor of 100, setting a long-term exposure limit of 0.00006 mg Hg/m³ (0.006 µg Hg/m³).
[8] I.e. multiplied by a factor 5/7 to take into account all seven days of the week and not only five workdays, and a factor 10/20 to take into account a respiratory volume of 10 m³ for a workday and 20 m³ for 24 hours.
[9] I.e. multiplied by a factor 5/7 to take into account all seven days of the week and not only five workdays, and a factor 10/20 to take into account a respiratory volume of 10 m³ for a workday and 20 m³ for 24 hours.
5 Exposure and risk assessment
In this chapter theoretical concentrations to which consumers may be exposed if compact fluorescent lamps or straight fluorescent lamps break in the consumer’s private home are calculated. Then associated health risks are assessed.
5.1 Exposure levels
As described in the health assessment of mercury released from a broken fluorescent lamp, inhalation is the primary exposure route for mercury. Absorption through the skin of mercury vapours can happen to a limited extent, but since skin absorption only accounts for around 2-3 % compared with inhalation a calculation is only made of health risks from inhalation of mercury vapours from broken compact fluorescent lamps and straight fluorescent lamps.
Both the short-term exposure, e.g. during clean-up from an accident with breakage of a fluorescent lamp in the private home, and a long-term exposure in case, for instance, of insufficient cleaning exposing continuously consumers to mercury vapours, are interesting in a risk assessment context.
Therefore, relevant exposure levels for both short-term and long-term exposure are dealt with.
5.1.1 Exposure scenarios
The two exposure scenarios under assessment are two situations where exposure to mercury is highest. In the first situation a person picks up the broken compact fluorescent lamp and is exposed shortly to a high concentration of mercury.
In the second situation the broken compact fluorescent lamp is not completely cleaned up and thereby people are exposed to mercury for a longer period of time. For this scenario it is not possible to calculate the concentration of mercury in the home, since it depends on many factors such as ventilation, level of cleaning etc. In principle evaporation of mercury may take place as long as there are mercury residues in the room. Tests have been conducted where concentrations of mercury have been measured above the US RfC value (long-term concentration without harmful effects) of 0.0003 mg/m³ several weeks after breakage of a compact fluorescent lamp.
Following exposure scenarios are assessed:
- Scenario 1: A fluorescent lamp breaks and cleaning is done within 30 minutes. Exposure is calculated for an adult person. Concentrations are calculated with and without ventilation of the room.
- Scenario 2: Situation where cleaning after breakage of a fluorescent lamp is insufficient. Values measured in tests of breakages of fluorescent lamps are compared with various limit values.
5.1.2 Calculation method
For the short-term exposure scenario a model is used as presented in Chandrasekhar (2007) for how the mercury concentration in a room will fall over time after breakage of a fluorescent lamp when ventilation of the room is taken into account. Chandrasekhar has set up the following formula:
![]()
Where
| Ct | = | Concentration of Hg in the room at the time t | µg/m³ |
| C0 | = | Background concentration of Hg, which is typically up to 10 ng/m³ (0.01 µg/m³). However, the background concentration is set at zero since it is negligible and is expected to be incorporated in the NOAEL/LOAEL values based on human observation. | µg/m³ |
| QHg | = | Quantity of mercury in broken lamp | µg |
| Vroom | = | Volume of room | m³ |
| A | = | Ventilation of room | m³/min |
| t | = | Time for which concentration is calculated | min |
Chandrasekhar assumes that the entire quantity of mercury from the lamp will evaporate immediately (time 0 min). This is found exaggerated since tests conducted by, among others, Aucott et al. (2003) showed that only a minor part (up to 7 %) of mercury in the lamp will evaporate within the first few minutes. Chandrasekhar furthermore assumes in the model that a fan is used. This fan contributes to ensuring that the concentration in the entire room is assumed to be the same – since heavy mercury vapours are otherwise naturally concentrated near the floor.
5.1.3 Calculation values
The following values are used for the calculation of the exposure scenarios. The values are explained in more detail below.
Table 5-1 Values used for calculation of exposure scenarios
| Parameter | Scenario 1 | Scenario 2 |
|---|---|---|
| Quantity of mercury (QHg) | No distinction is made between compact fluorescent lamp and straight fluorescent lamp. Following values are used: 1,2 mg, 1,4 mg, 2 mg, 2,5 mg, 3,5 mg, 4,9 mg, 5 mg, 7 mg, 8 mg, 9,5 mg, 13 mg, 15 mg and 40 mg Hg per fluorescent lamp. |
No distinction is made between compact fluorescent lamp and straight fluorescent lamp. Following values are used: 1,2 mg, 1,4 mg, 2 mg, 2,5 mg, 3,5 mg, 4,9 mg, 5 mg, 7 mg, 8 mg, 9,5 mg, 13 mg, 15 mg and 40 mg Hg per fluorescent lamp. |
| Duration of exposure (t) | 30 minutes | 24 hours/day |
| Volume of room (Vroom) | 2 m³ | |
| Ventilation (A) of room | No ventilation, standard ventilation (0,02 m³/min), and draught, i.e. all doors and windows open (0, 14 m³/min) |
Quantity of mercury QHg
According to the Danish Environmental Protection Agency it is being considered at the moment to change the maximum permitted concentration of Hg in compact fluorescent lamps. It is being considered to reduce the present limit of 5 mg to 3.5 mg Hg or as low as 2.5 or 2 mg Hg per lamp. Similarly, new limits are being discussed for straight fluorescent lamps and for special forms of compact fluorescent lamps with levels of 7 mg Hg, 13 mg Hg, 15 mg Hg and as much as 40 mg Hg for compact fluorescent lamps/straight fluorescent lamps above 400 W. To reflect possible health risks associated with broken fluorescent lamps containing these new and revised amounts, calculations will be made with these values as well.
The existing limit for straight fluorescent lamps is at 5, 8 and 10 mg Hg respectively per straight fluorescent lamp depending on lifetime and phosphor added. Therefore, risk assessments will be made with these amounts as well.
The survey in connection with the present study has shown, however, that compact fluorescent lamps today have a content of mercury between 1.2 and 4.9 mg Hg per lamp, and straight fluorescent lamps have a content between 1.4 and 9.5 mg Hg per lamp. It is relevant to draw up risk assessments for these minimum and maximum values.
For the calculation the following values have therefore been used (since in practice no distinction is made between a compact fluorescent lamp and a straight fluorescent lamp):
- 1.2 mg, 1.4 mg, 2 mg, 2.5 mg, 3.5 mg, 4.9 mg, 5 mg, 7 mg, 8 mg, 9.5 mg, 13 mg, 15 mg and 40 mg Hg per lamp.
According to REACH Guidance Documents it should generally be assumed that 100 % of the mercury evaporates immediately. However, tests have shown that this would give an overestimated exposure.
Information from literature/industry shows that between 0.04 and 0.5 % of total mercury in the lamp will be in vapour form. As described it depends on temperature and inner volume of the lamp how large a quantity of mercury vapour is found in the lamp. Saturated mercury vapours have a concentration of 20 mg Hg/m³ at 25 °C. Sources from literature state amounts between 0.002 and 0.05 mg mercury in vapour form in a lamp. With the stated rates and the above quantities of mercury in a lamp this corresponds to between 0.0005 and 0.2 mg mercury. This quantity of mercury vapours within a lamp will disperse immediately in case of breakage. The mercury in the broken lamp can now evaporate further.
Experience from tests (Aucott et al., 2003) as described in Chapter 3 shows that around 10 % of total quantities of mercury in a fluorescent lamp will have evaporated within the first 30 minutes. However, this is subject to reservations for the following reasons:
- It is uncertain whether release of mercury depends on the type of amalgam used in the different lamps,
- Age of lamp may have an impact, and
- Not least, the concentration of mercury in the lamp has an impact on the release of mercury.
Despite these reservations a value of 10% is used in the calculations since it is found to be more realistic than a 100 % momentary release.
In practice, thus, a maximum of 0.5 % of total quantities of mercury will evaporate momentarily and in the subsequent 30 minutes up to around 10 % of total quantities of mercury in the lamp will evaporate (7 % already after a few minutes). For the worst-case calculations it is assumed, however, that the 10 % of the total mercury amounts will evaporate immediately when the lamp breaks.
Duration of exposure t
It is assumed that the cleaning scenario in the worst case lasts 30 minutes and that the person is exposed to the same concentration of mercury for all 30 minutes - corresponding to the initial concentration at the time 0. It is assumed for the long-term exposure that in the worst case it will be 24 hours per day in order to take into account homebound persons.
Volume of room Vroom
It is stated in the ECHA Guidance Chapter R.15 (2008) that for short-term local exposure the volume of a room can be set at 2 m³ in order to represent the air immediately surrounding the exposed person. This value is used as the only value for the cleaning scenarios since mercury vapours are heavy, and the concentration of mercury in a room will be unevenly distributed where most mercury is found nearest to the place of breakage. According to, among others, Stahler et al. (2008) the concentration of mercury will be larger in, for example, 30 cm’s height than in 1.5 metre’s height. Thus, it will give an incorrect picture to “dilute” the mercury concentration on the entire volume of the room.
The 2 m³ immediately next to the exposed person can thus also be used as an estimate for the concentration in the lower 30-50 cm from the floor surrounding the place of the accident.
Ventilation A of room
The concentration of mercury in the room is calculated for three different rates of ventilation: No ventilation, normal ventilation and ventilation with all windows and doors open. No ventilation means an air exchange rate of zero and corresponds to a fictitive situation where the concentration is constant in the period of time applied. Normal ventilation is defined by the Danish Environmental Protection Agency as 0.6 times per hour. According to the ECHA Guidance Chapter R.15 (2008) air exchange in a room with all windows and doors open is at either 4.2 or 6.2 times per hour. Here, the most conservative value of 4.2 times per hour is used.
Thus, the following values for ventilation of the room are used:
- No ventilation – corresponding to 0 m³/min (note that this is a fictive value, since there will always be minor leaks in a house).
- Normal ventilation – corresponding to 0.02 m³/min for a volume of 2 m³ (room size x air exchange per hour / 60 minutes).
- Strong ventilation (all windows and doors open) – corresponding to 0.14 m³/min for a volume of 2 m³ (room size x air exchange per hour / 60 minutes).
5.1.4 Exposure calculations
5.1.4.1 Scenario 1: Short-term exposure for 30 minutes
As described above the short-term exposure is calculated with and without ventilation.
A calculation using the assumption of no ventilation is the worst case and a fictive calculation, since there will always be some leaks in a house.
The calculation of the concentration in the breathing zone during an accident with a broken compact fluorescent lamp or straight fluorescent lamp is done by dividing the quantity of mercury released from the lamp by 2 m³, which is the volume chosen for the breathing zone. The quantity of mercury released is calculated as 10 % of the total amount of mercury in the lamp (i.e. 1.2 to 40 mg). The results are shown in Table 5-2 below.
Table 5-2 Calculated concentrations in the breathing zone of mercury with a broken compact fluorescent lamp/straight fluorescent lamp in a room when it is assumed that only 10 % of total quantity of mercury will evaporate during the first few hours. The meaning of the green cells is discussed later.
To allow for the impact from ventilation the mercury concentration in the breathing zone has been calculated with the formula described in Chandrasekhar (2007). The concentration has been calculated for 13 different concentrations of mercury in compact/straight fluorescent lamps and for three different scenarios regarding ventilation (none, standard and all doors/windows open). Chandrasekhar uses a fan in his model to ensure that the concentration of the heavy mercury vapours is the same in the entire room. This will not be the case in practice, so in this study a small volume is used, corresponding to a breathing zone of 2 m³. Thus, it is assumed in the calculations that the mercury is not dispersed over the breathing zone of the 2 m³, which will be, for instance, around 30-50 cm just above the site of the accident in an area of 2-3 metres x 2 metres around the site of the accident. In the calculations it is assumed as above that only 10 % of the mercury of the fluorescent lamp will evaporate momentarily at the time 0.
5.1.4.2 Scenario 2: Exposure over longer time
Stahler et al. (2008) has shown in tests that even if cullet and mercury have been removed mercury concentrations above the USEPA long-term concentration without harmful effects (RfC = 0.0003 mg/m³) can be measured in the hours after breakage of a fluorescent lamp when windows and doors are closed again – even if there are no visible residues of the broken lamp. The same test also showed that it may take from a few days to more than 60 days before the concentration just above the floor dropped to below RfC.
The same study has also shown that mercury concentrations of up to 0.029 mg Hg/m³ can be measured several weeks after removal of lamp residues. These high concentrations were measured just above floor height and after vacuuming and impacting of the flooring material (simulation of walking/crawling on the floor). Carpets in particular seem to contain more ”mercury residues” after cleaning compared with wooden floors, but it is not difficult to imagine a wooden floor with large spaces that can easily collect as much mercury as a carpet. Thus, it is relevant to compare this value with the limit values for long-term exposure to mercury, even if it in this case is a high, short-term value that will drop when ventilation or airing brings down the concentration. The measured value of 0.029 mg Hg/m³ is thus not an expression of average concentration in the room.
Real-life tests conducted by Stahler et al. (2008) thus show that mercury residues can remain in the flooring for several weeks after the accident. It is not possible to calculate the concentration of mercury in the home based on the calculation formula from the ECHA Guidance Document Chapter R.15, since it depends on many factors such as ventilation and level of cleaning and the calculation formula does not take this into consideration. In addition it is uncertain for how long time mercury will evaporate. This would require more sophisticated calculations or use of, for instance, a computer model for calculation of consumer exposure, such as ConsExpo, as described in the REACH Guidance Document Chapter R.15. However, this has not been possible within the limits of this project.
In the following section the measured values are compared with relevant limit values for mercury at short-term and long-term exposure.
5.2 Risk assessment
5.2.1 Calculation method
For calculation of the risk of health-hazardous effects when a fluorescent lamp breaks the ECHA ”Guidance on information requirements and chemical safety assessment” (ECHA Guidance Chapter R.8 and R.15, 2008) has been used. These documents describe how to derive a DNEL[10] value from a NOAEL or a LOAEL[11] value.
The calculated DNEL value (endpoint specific) is calculated as:
![]()
Where
| DNEL | = | Derived No Effect Level |
| NOAELcorr or LOAELcorr | = | No or Lowest Observed Adverse Effect Level (corrected) |
| AFn | = | Assessment Factors ((un)certainty factors) |
The risk is found by dividing the calculated exposure with the calculated DNEL value – and the so-called RCR value (Risk Characterisation Ratio) is thus calculated. If the exposure is larger than the DNEL value, there is a health risk for the calculated exposure scenario (RCR >1) (ECHA Guidance Chapter R.8, 2008).
For inhalation the DNEL value is stated in the unit mg/m³. This value is thus compared with the calculated exposure, corresponding to the concentration of mercury in the room (measured in mg Hg/m³), to which the consumer is exposed.
5.2.2 DNEL values
As described in the ECHA REACH Guidance Chapter R.8 (2008) the following types of (un)certainty factors should be used after correction for differences between experimental and expected human exposure conditions:
- Interspecies differences
- Intraspecies differences
- Differences in duration of exposure
- Issues related to dose-response
- Quality of whole database
Short-term exposure (DNELshort)
As described in the health assessment in Chapter 4 no information about NOAEL values for short-term exposure to mercury is available. Most data on health effects from mercury vapours are derived from occupational exposures. At very high exposures to mercury vapours in the working environment the lungs are the target organ. At a few hours’ exposure to 1-3 mg Hg/m³ (1000-3000 µg/m³) acute fatal chemical pneumonia can occur. Even if the value of 1-3 mg Hg/m³ is a very high concentration of mercury vapour with very serious effects and even if the value is applicable for a few hours’ exposure and thus covers a longer period of exposure than for cleaning after breakage of a fluorescent lamp, the value of 1 mg Hg/m³ is used as the LOAEL value, since it is the lowest reliable value identified for short-term exposure.
The value is derived from occupational exposures in the working environment and the time of exposure is close to identical to the cleaning scenario, so the value of 1 mg Hg/m³ is used directly as the LOAEL value.
The LOAEL value is based on observations of humans, i.e. there is no (un)certainty factor (= 1) for interspecies differences. As standard is used a factor 10 as (un)certainty factor for intraspecies differences. For differences in duration of exposure no (un)certainty factor (= 1) is used since the LOAEL value is based on acute effects. For issues relating to dose-response the REACH Guidance Documents state an (un)certainty factor of 3-10 to convert from LOAEL to NOAEL, but it is stated that an (un)certainty factor of 3 should be used in most cases. For the quality of the whole database a further (un)certainty factor may be used. Altogether, an (un)certainty factor of 1 x 10 x 1 x 3 x 1 = 30 is used. This (un)certainty factor of 30 results in:
DNELshort value = 0.033 mg Hg/m³ (33 µg Hg/m³).
This value, after calculation with the (un)certainty factors, is close to the Danish occupational threshold limit value, which is set at 0.025 mg Hg/m³ (25 µg Hg/m³).
Long-term exposure (DNELlong)
Most data on health effects of long-term exposure to mercury vapours is derived from occupational exposure. Many existing limit values for mercury vapours are based on a LOAEL value of 0.025 mg Hg/m³ (25 µg Hg/m³ – identical to the Danish occupational threshold limit value). A recent study (Richardson et al., 2009) recommends LOAEL at 0.014 mg Hg/m³ (14 µg Hg/m³) for long-term exposure to mercury vapours.
A NOAEL value for long-term exposure of 0.010 mg Hg/m³ (10 µg Hg/m³) is used to determine the DNEL value since a LOAEL requires an additional certainty factor of at least 3.
The NOAEL value is converted to long-term exposure (as described in section 4.7) by multiplying with 5/7, as well as a factor 10/20 to take into account all seven days of the week and the total respiration volume of 24 hours. This gives 0.004 mg/m³ as the corrected NOAEL value for an exposure of 24 hours a day over a long period of time.
The NOAEL value is based on observations of humans, i.e. there is no (un)certainty factor (= 1) for interspecies differences. As standard is used a factor 10 as (un)certainty factor for intraspecies differences. For differences in duration of exposure no (un)certainty factor (= 1) is used since the NOAEL value is already based on chronic effects. For issues relating to dose-response it is stated that an (un)certainty factor of 3-10 can be used to convert from LOAEL to NOAEL. Here, it is not necessary to convert values, since it is a NOAEL value, so no (un)certainty factor is needed. For the quality of the whole database a further (un)certainty factor may be used. Altogether, an (un)certainty factor of 1 x 10 x 1 x 1 x 1 = 10 is used. This (un)certainty factor of 10 results in:
DNELlong value = 0.0004 mg Hg/m³ (0.4 µg Hg/m³).
This value is close to the USEPA long-term reference concentration without harmful effects (RfC) of 0.0003 mg Hg/m³ as stated in Table 4-2.
5.2.3 Risk assessment scenario 1: Short-term exposure for 30 minutes
The short-term exposure is calculated above in section 5.1.4 ‘Exposure calculations with and without ventilation’ (see Table 5-2).
It is seen that the calculated concentrations in the breathing zone, regardless of quantities of mercury in the fluorescent lamp, exceed the DNELshort value of 0.033 mg Hg/m³ (33 µg Hg/m³), when it is assumed that there is no ventilation in the room and assuming a momentary evaporation of 10 % of the total amount of mercury at the time t=0. This is an exceedance of around 2 to 60 times. With a content of 5 mg Hg in the lamp the exceedance is 8 times the DNELshort value and 10 times the Danish occupational threshold limit value.
However, this calculation is as mentioned a worst-case calculation and a fictive calculation, since there will always be some natural ventilation.
In Table 5-2 are also stated calculated concentrations in the breathing zone, when ventilation is taken into account. The values marked by green background are below the DNELshort value of 0.033 mg Hg/m³ (33 µg Hg/m³).
Calculations show that after 10 minutes’ ventilation with all doors and windows open the mercury concentration in the breathing zone of 2 m³ will be below the DNELshort value for compact fluorescent lamps with a low content of mercury (= 2.5 mg Hg) and thereby not constitute an acute risk. After 15 minutes’ strong ventilation the concentrations in the breathing zone will be below the DNELshort value for compact fluorescent lamps with the presently permitted content of mercury (= 5 mg Hg), and after 30 minutes the mercury concentration is below the DNELshort value for all calculated levels of mercury in compact fluorescent lamps and straight fluorescent lamps.
Thus, calculations show that with the assumption that 10 % of mercury has evaporated during the first 30 minutes the concentration of mercury in private homes will be above the DNELshort value and thus constitute a risk – unless strong ventilation is ensured. Ventilation has a very significant impact in view of lowering concentrations of mercury down to non-hazardous levels in the home during an accident.
However, it should be noted that the DNELshort value has been calculated based on a LOAEL value for an exposure of a few hours (not specified in more detail). In a cleaning scenario the cleaning time will probably only be about 10 minutes – in the worst case 30 minutes as assumed in the calculations. In case of fast clean-up the exposure time may be shorter than the one for which the DNELshort value has been calculated and this means that the exposure to mercury in this shorter period of time is in reality much lower and thereby does not constitute a health hazard.
Generally, however, many factors have an impact on the assessment of the real risk:
- These calculations assume momentary evaporation at the time 0 and show how the concentration in the breathing zone decreases over time through strong ventilation.
- It is assumed that the concentration of mercury does not decrease during the first 30 minutes, which it will do when the source of contamination – the broken lamp – is removed.
- The distribution of mercury vapours in the room has not been studied in detail.
- It has not been studied in detail whether strong ventilation works just as efficiently on air exchange at floor level where mercury vapours are concentrated, as it does on air exchange higher up in the room. The Stahler et al. (2008) study shows, however, that ventilation from a window also has an effect on concentrations at floor height.
However, the tests show that a few minutes will pass before 7 % of mercury has evaporated and calculations show that 10-15 minutes’ strong ventilation can reduce concentrations significantly. It is thus important to pick up mercury quickly before too much of it evaporates. Coldness will reduce the rate of evaporation and heating will increase the rate of evaporation. It may be relevant to close doors to other rooms so that mercury vapours are not dispersed in the home.
Calculations are based on breakage of one fluorescent lamp. Thus, calculations indicate that if several fluorescent lamps break at the same time it will be necessary to ensure strong ventilation immediately and continue this ventilation for a long time after cleaning.
5.2.4 Risk assessment scenario 2: Exposure over longer time
Concentrations measured for a long period of time after breakage of a fluorescent lamp are stated above in section 5.1.4 ‘Exposure calculations’.
The measured peak value of 0.029 mg Hg/m³ is far above the DNEL value for long-term effects (0.0004 mg Hg/m³), but below the DNEL value for short-term exposure (0.033 mg Hg/m³). However, the values cannot be compared since the high experimental value was measured briefly in connection with, for instance, vacuuming and only just above floor height. The measured values are thereby not an expression of the average concentration in the room.
Stahler et al. (2008) showed however through tests that it may take from a few days to more than 60 days before the concentration just above floor height drops to below 0.0003 mg Hg/m³ – which is the US reference concentration (long-term concentration without harmful effects), which was used as a benchmark. Thereby, it cannot be excluded that there may be a risk of harmful effects (particularly for children crawling on the floor) after cleaning of a broken fluorescent lamp if there is not much focus to thorough and regular airing – also in the months after the accident.
Thus, experience from Stahler et al. (2008) shows that it is very important to continue airing after the accident – in particular in connection with cleaning and specifically if the accident happened on a carpet – also when it looks clean to the naked eye.
5.3 Summary and discussion
Short-term exposure
For the exposure scenario with 30 minutes’ exposure to mercury vapours during cleaning after breakage of a fluorescent lamp a LOAEL value of 1 mg Hg/m³ has been used to calculate the DNELshort value. This LOAEL value covers a few hours’ occupational exposure and thereby a period of exposure longer than the assumed 30 minutes, though still relatively close to this.
Practical tests as described in Chapter 3 show that up to 7 % of the mercury in a fluorescent lamp evaporates during the first few minutes and that up to 13 % evaporates during the first 8 hours after breakage of a fluorescent lamp. Therefore it has been assumed that 10 % of the total amount of mercury in a fluorescent lamp evaporates within 30 minutes.
In the calculations it has been assumed that 10 % evaporates momentarily at the time 0 and that the resulting concentration is constant for 30 minutes, if there is no ventilation. Calculations show that with this assumption the concentration of mercury in the indoor air will exceed the DNELshort value and thus constitute a risk. After 10 minutes of ventilation with all windows and doors open the mercury concentration in the breathing zone of 2 m³ will be below the DNELshort value for compact fluorescent lamps with low content of mercury (= 2,5 mg Hg), and thus not constitute an acute risk. After 15 minutes of strong ventilation the concentration in the breathing zone will be below the DNELshort value for compact fluorescent lamps with the presently permitted content of mercury (= 5 mg Hg) and after 30 minutes the mercury concentration will be below the DNELshort value for all calculated levels of mercury in compact and straight fluorescent lamps.
However, there are a number of uncertainties in the calculations; for example, momentary evaporation at the time 0 has been assumed, and the DNELshort value is based on occupational exposure to mercury during a few hours. These uncertainties and assumptions in the calculations together with fast clean-up, for instance during 10 minutes, means that the exposure time will be significantly shorter than the 30 minutes’ period which again means that the resulting exposure is lower than calculated and does not constitute a health risk.
However, many factors play a role in the assessment of the risk in question. Thus, there are a number of uncertainties in the calculations, for example:
- The model assumes that the entire quantity of mercury (here 10 % of total quantities of mercury in the lamp) evaporates in the very instant of the accident since the calculations do not take into account the rate of evaporation. However, mercury evaporates quickly (7 % evaporated within a few minutes), so the overestimation is not large.
- It is assumed in the calculations that mercury vapours only disperse to the breathing zone of 2 m³ and not beyond this zone and that vapours are evenly distributed in this volume. This assumption may lead to an overestimation.
- The model assumes that the consumer is exposed to the entire quantity of mercury in the entire period of exposure – the 30 minutes it takes to clean up – though without the quantity reduced by ventilation. Thereby, the model does not take into account the fact that the concentration of mercury in the breathing zone will decrease as the source of exposure (the broken lamp) is picked up during the period of exposure.
- The model assumes that mercury vapours are evenly distributed through the use of a fan so airing will also lead to even ventilation of mercury vapours in the room. It has not been studied in detail whether strong ventilation is just as efficient for air exchange at floor height where mercury vapours are concentrated as for air exchange higher up in the room. However, the study by Stahler et al. (2008) shows that ventilation from a window also has an effect on concentrations at floor height.
- The DNELshort value has been calculated on the basis of a LOAEL value for exposure for a few hours (not specified in more detail). In a cleaning scenario the cleaning time will probably be shorter – in the worst case 30 minutes as assumed in the calculations. In case of clean-up of, for instance, 10 minutes the exposure time will be significantly shorter and the resulting exposure lower than calculated.
Calculations are based on breakage of one fluorescent lamp. Calculations thus indicate that if several fluorescent lamps break at the same time it is necessary to ensure strong ventilation immediately. Generally, it is important to pick up mercury quickly and continue ventilation for a relatively long period after cleaning.
Long-term exposure
For the scenario with long-term exposure to mercury vapours in case of insufficient cleaning after a broken fluorescent lamp a NOAEL value of 0.010 mg Hg/m³ (10 µg Hg/m³) has been used to calculate the DNELlong value.
This DNELlong value is compared with measured levels from practical tests of broken compact/straight fluorescent lamps. In the tests cleaning had been done after the accident.
Calculations and a literature review also show that it is important to pick up mercury in case of an accident, since mercury remaining in a home may constitute a health risk.
The practical tests conducted by Stahler et al. (2008) showed that a cleaned-up home after breakage of a fluorescent lamp can still give off mercury to the indoor air for several weeks/months after the accident. In some cases it took several weeks before measured values had decreased to below the US long-term concentration without harmful effects (0.0003 mg Hg/m³) and under the calculated DNELlong value of 0.0004 mg Hg/m³. Thus, it is also important to ensure extra ventilation after breakage of a lamp – particularly in connection with general cleaning/vacuuming of the home which may cause mercury-containing dust to be stirred up. Ventilation has a substantial impact in relation to lowering concentrations of mercury to non-harmful levels in the home after an accident.
[10] DNEL = Derived No Effect Level
[11] NOAEL = No Observed Adverse Effect Level, LOAEL = Lowest Observed Adverse Effect Level
6 Discussion and conclusion
Mercury is a metal that is liquid under normal pressure and temperature and it appears as a heavy, odour-free silvery liquid with a relatively high vapour pressure at room temperature. Particularly mercury vapours constitute a health problem since large parts of mercury vapours are absorbed through the lungs during inhalation whereas absorption of the poorly soluble and inert metallic mercury through the skin and the gastrointestinal tract is minimal. Furthermore, mercury vapour can also easily pass the blood-brain barrier and the placenta barrier and can in that way have an impact on the central nervous system and the unborn child. Long-term exposure of the central nervous system can lead to symptoms such as tremor and memory loss.
In fluorescent lamps mercury is used either in the form of a HgFe compound, as amalgam or in the form of metallic mercury. This mercury in solid or liquid form will be in equilibrium with mercury in vapour form. Therefore, a small quantity of mercury in vapour form will be present within the lamp and this mercury vapour causes, among other substances, the lamp to light.
If one or more fluorescent lamps break in a home invisible and odour-free mercury vapour is released to the indoor air as long as the residues from the lamp/-s have not been completely removed. Mercury vapour is seven times heavier than air and will disperse along the floor in a room with insufficient ventilation.
In this study it was investigated whether the quantities of mercury present in fluorescent lamps constitute a health risk if a lamp breaks in a home. Assessments have been carried out of risks in the short term (30 minutes’ cleaning) and in the long term in the event of insufficient cleaning after breakage of a lamp.
A worst-case calculation has been made of concentrations of mercury vapour in the breathing zone during accidents with a broken lamp with different contents of mercury. These calculations, which also take into account ventilation, show that concentrations of mercury in the breathing zone during cleaning exceed the DNELshort value (calculated concentration without effects), which means that there may be health effects when exposed to mercury vapour. The calculation model, however, is subject to several uncertainties. The calculations are worst-case calculations and the DNELshort value has been calculated based on data for an exposure time of a few hours. In addition, the calculation model shows that strong ventilation is an important factor in terms of bringing down the level of mercury vapour to a non-health hazardous level in the breathing zone. Removing the source of contamination – the broken lamp – as fast as possible also has a substantial impact.
Therefore the conclusion is that when uncertainties and assumptions are taken into consideration there is no risk of health effects through short-term exposure to mercury when a mercury-containing lamp breaks if residues are picked up immediately and ventilation is ensured.
Evaporation of mercury will take place as long as there are still mercury residues present in the room.
For prolonged exposure to mercury in a home it has not been possible within the frames of the present project to make a calculation of the concentrations of mercury in a home since it depends on many factors such as ventilation, level of cleaning etc. Therefore, a DNELlong value has been compared with concentrations of mercury vapour measured in tests conducted after cleaning of broken fluorescent lamps. These concentrations derive from various tests found in a literature study.
Literature describes that it has been demonstrated in practical tests that a cleaned-up home after breakage of a fluorescent lamp can still release mercury vapours for several weeks/months after the accident. In some cases it took several weeks before measured values just above floor height were below DNELlong value. Thereby crawling children may be exposed to concentrations with health-hazardous effects in the long-term perspective. In connection with general cleaning/vacuuming in a home mercury-containing dust may be stirred up.
Thus, the conclusion regarding exposure to mercury vapours in the long term is that there may be a risk of health effects in case ventilation and removal of all mercury-containing residues (i.e. thorough cleaning) is not ensured. Thorough cleaning after breakage of the lamp is thus important and so is ventilation for a long period, since mercury vapours may be released from invisible residues of the broken lamp.
7 References
AT, 2007. At-vejledning. Stoffer og materialer – C.0.1. Grænseværdier for stoffer og materialer. August 2007. In Danish.
http://www.at.dk/~/media/3FA26655715740ED84EA28EC1191FB62.ashx
AT, 2009. Hvad skal man gøre når en elsparepærer eller et lysstofrør går i stykker? In Danish. http://www.at.dk/TEMAER/Kort%20information/~/media/
0C54B74C77484BADAC7C90D85C751ABB.ashx
ATSDR, 1999. Toxicological profile for mercury. Agency for Toxic Substances & Disease Registry. http://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=115&tid=24
Aucott M., McLinden M., Winka, M., 2003. Release of Mercury from Broken Fluorescent Bulbs. J Air Waste Manag Assoc 2003; 53: 143-151.
Bach, 1980. Elsa Bach. Voksnes belastning med kviksølv. Hellerup: DIKE, 1980. In Danish.
Baughman, 2006. Baughman TA. Elementary mercury spills. Environ Health Perspec 2006; 114: 147-152.
Berlin, 1977. Berlin M. Mercury. I: Friberg L (ed).Toxicology of metals, Vol. II. EPA-600/1-77-022. Washington: USEPA, 1977. p. 301-344.
Caravati et al., 2008. Caravati EM, Erdman AR, Christianson G, Nelson LS, Woolf AD, Booze LL, et al. Elemental mercury exposure: An evidence-based consensus guideline for out-of-hospital management. Clin Toxicol 2008; 46: 1-21.
Chandrasekhar, 2007. Remediation of Indoor Airborne Mercury Released from Broken Fluorescent Lamps. Florida Department of Environmental Protection, Tallahassee, Florida. Chandrasekhar TM, 15.6.2007. http://www.dep.state.fl.us/waste/quick_topics/publications/shw/
mercury/Mercury_CFL_Dynamics-final.pdf
http://www.dep.state.fl.us/waste/quick_topics/publications/shw/mercury/
Mercury_CFL_Model.xls
Clarkson et al, 2003. Clarkson TW, Magos L, Myers GJ. The toxicology of mercury – current exposures and clinical manifestations. N Engl J Med 2003; 349: 1731-1737.
Defra, 2009. Department for Environment, Food and Rural Affairs. http://www.defra.gov.uk/environment/business/products/roadmaps/
lightbulbs.htm
Den Store Danske, 2009. In Danish.
http://www.denstoredanske.dk/It,_teknik_og_naturvidenskab/Kemi/
Metallurgi_og_korrosion/legering
ECBs Annex 1, 2009. Listen over harmoniseret klassificering. ECBs Annex 1 til stofdirektivet med 30. og 31. tilpasning til det gamle stofdirektiv. In Danish. http://www.mst.dk/NR/rdonlyres/5F38F880-FCEB-4BC3-9870-
6CF8E2BB497A/0/LOFS_Annex1.xls
ECHA Guidance Chapter R.8, 2008. Guidance on information requirements and chemical safety assessment. Chapter R.8: Characterisation of dose [concentration]-response for human health. May, 2008.
ECHA Guidance Chapter R.15, 2008. Guidance on information requirements and chemical safety assessment. Chapter R.15: Consumer exposure estimation. May, 2008.
Elsparefonden, 2009. In Danish.
http://application.sparel.dk/asp/a-paere/query/paerewiz/liste.asp
Energitjenesten. Beskrivelser om LED belysning. In Danish. http://www.energitjenesten.dk/files/resource_4/friske/
Lyskilder_med_dioder.pdf
http://www.dba.dk/asp/sektion/artikler/detail.asp?ArtikelId=44785
Energy Star, 2007. CFL and mercury: Overview of EPA efforts, Peter Banwell, Energy Star Lighting Partner Meeting, March 13, 2007. http://www.energystar.gov/ia/partners/downloads/meetings/
MercuryRecycling_Banwell.pdf (page 12).
Energy Star, 2008. Frequently Asked Questions. Information on Compact Fluorescent Light Bulbs (CFLs) and Mercury. July 2008. http://www.energystar.gov/ia/partners/promotions/change_light/
downloads/Fact_Sheet_Mercury.pdf
EU, 2002. Commission Decision of 9 September 2002 establishing revised ecological criteria for the award of the Community eco-label to light bulbs and amending Decision 1999/568/EC, Official Journal of the European Communities, 10.9.2002, L 242/44-242/49. http://eur-lex.europa.eu/LexUriServ/
LexUriServ.do?uri=OJ:L:2002:242:0044:0049:EN:PDF
EU, 2009. Commission Regulation (EC) No. 244/2009 of 18 March 2009 implementing Directive 2005/32/EC of the European Parliament and of the Council with regard to ecodesign requirements for non-directional household lamps. Official Journal of the European Union. 24.3.2009. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?
uri=OJ:L:2009:076:0003:0016:EN:PDF
General Electrics, 2009. http://genet.gelighting.com/LightProducts/Dispatcher?
REQUEST=APPLICATIONSSUBPAGE&CHANNEL=
Consumer&APPLICATION=General+Purpose&CATEGORY=Lamps
Grandjean et al., 1997. Grandjean P, Weihe P, White R, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methyl-mercury. Neurotoxicol Teratol 1997; 20: 1-12
Groth, 2008. Shedding light on mercury risks from CFL breakage. Prepared by Edward Groth, PhD for The Mercury Policy Project, February 2008. http://mpp.cclearn.org/wp-content/uploads/2008/08/
final_shedding_light_all.pdf
Hansen E, von Freiesleben NE, Høibye L, Slot IL, Nielsen JB, Hansen CL, Hansen JH, 2008. Miljømæssige og økonomiske konsekvenser af øgede offentlige grønne indkøb. Miljøprojekt Nr. 1218, 2008, Miljøstyrelsen. In Danish.
Holmes et al., 2009. Holmes P, James KAF, Levy LS. Is low-level environmental mercury exposure of concern to human health? Sci Total Environ 2009; 408: 171-182
IPCS, 1976. IPCS. Environmental Health Criteria 1. Mercury. Geneva: WHO, 1976.
IPCS, 1980. IPCS. Environmental Health Criteria 101. Methyl-mercury. Geneva: WHO, 1980.
Ishitobi et al., 2010. Ishitobi H, Stern S, Thurston SW, Zareba G, Langdon M, Gelein R, Weiss B. Organic and Inorganic Mercury in Neonatal Rat Brain after Prenatial Exposure to Methyl-mercury and Mercury Vapor. Environ Health Perspec, 2010; 118; 242-248.
Jang M, Hong SM, Jae K, 2005. Characterization and recovery of mercury from spent fluorescent lamps. Waste Manag 2005; 25: 5-14.
KEMI, 2009. Kvicksilver i lågenergilampor och lysrör. In Swedish. http://www.kemi.se/templates/Page____5266.aspx
Lee et al., 2009. Lee B, Sarin L, Johnson NC, Hurt RH. A Nano-Selenium Reactive Barrier Approach for Managing Mercury over the Life-Cycle of Compact Fluorescent Lamps. Environ Sci Technol 2009; 43: 5915-5920.
Megaman, 2009. http://www.megaman.cc/global/index.php
Miljøstyrelsen, 2004. ”Listen over uønskede stoffer 2004”. Orientering fra Miljøstyrelsen nr. 8, 2004. Miljøstyrelsen. In Danish.
Miljøstyrelsen, 2006. ”Metoder til fastsættelse af kvalitetskriterier for kemiske stoffer i jord, luft og drikkevand med henblik på at beskytte sundhed”. Vejledning fra Miljøstyrelsen nr. 5, 2006. In Danish.
NEMA, 2000. Environmental Impact Analysis: Spent Mercury-Containing Lamps: A Summary of Current Studies, January, 2000 (Fourth Edition), Prepared by the National Electrical Manufacturers Association.
OEHHA, 2008. Office of Environmental Health Hazard Assessment. California Government. TSD for noncancer RELs. Appendix D. Individual acute, 8-hour, and chronic reference exposure level summaries. Mercury Reference Exposure Levels.
http://www.oehha.ca.gov/air/hot_spots/2008/
AppendixD1_final.pdf#page=214
Raposo C, Windmöller CC, Junior WAD, 2003. Mercury speciation in fluorescent lamps by thermal release analysis. Waste Manag 2003; 23: 879-886.
Richardson et al, 2009. Richardson GM, Brecher RW, Scobie H, Hamblen J, Samuelian J, Smith C. Mercury vapour (Hg0): Continuing toxicological uncertainties, and establishing a Canadian reference exposure level. Regul Toxicol Pharmacol 2009; 53: 32-38.
RoHS EU, 2002. DIRECTIVE 2002/95/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment.
http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?
uri=OJ:L:2003:037:0019:0023:en:PDF
SAES Getters, 2009. Mercury Dispensers for Fluorescent Lamps. Tilgængelig på: http://www.saesgetters.com/default.aspx?idPage=292
Sigai & Nesting. Pantent application: Low-Mercury-Consuming Fluorescent Lamps. Inventors: A. Gary Sigai David C. Nesting, Agents: PHILIPS INTELLECTUAL PROPERTY & STANDARDS. http://www.faqs.org/patents/app/20090146545
Skat, 2009. Punktafgiftsvejledning 2009-2, Del I Andre afgifter, I.1 Glødelamper mv. og elektriske sikringer. Version: Punktafgiftsvejledning 2009-2 (Gældende fra den 15. juli 2009). In Danish. http://www.skat.dk/SKAT.aspx?oId=111210
&vId=202322&i=899&action=open#i111210
Snijkers-Hendrickx et al., 2007. Snijkers-Hendrickx I, Lauwerijssen P, Milewski P, Bruyndoncx V. Low-mercury containing discharge lamps. Sustainable and environmental friendly lighting solutions. 2007. 11th International Symposium on the Science and Technology of Lamps, LS11, May 20-24, 2007, Shanghia, China.
Stahler D, Ladner S, Jackson H, 2008. Maine Compact Fluorescent Lamp Study. Maine Department of Environmental Protection. www.maine.gov/dep/rwm/homeowner/cflreport/cflreport.pdf
TNO, 2008. TNO Memo to the Dutch lamp recycling organisation LightRec, 28 Ocotber 2008. TNO 034 UT 2008-00093 M&L. Modtaget fra Miljøstyrelsen.
Truesdale RS, Beaulieu SM, Pierson TK, 1992. Management of Used Fluorescent Lamps: Preliminary Risk Assessment – Final Report. Submitted to U.S Environmental Protection Agency. RTI Project No. 94U-5400-010.
Tunnessen et al., 1987. Tunnessen WW, McMahon KJ, Baser M. Acrodynia: Exposure to mercury from fluorescent light bulbs. Pediatrics 1987;79:786-789.
USEPA, 1998. Mercury emissions from the disposal of fluorescent lamps, Revised Model – Final Report Post-OMB Review, March 31, 1998, Office of Solid Waste, U.S. Environmental Protection Agency.
USEPA, 2009. What to do if a fluorescent or other mercury-containing light bulb breaks. http://www.epa.gov/hg/spills/#fluorescent
USEPA Hg, 2009. Integrated Risk Information System. Mecury, elemental.
http://www.epa.gov/ncea/iris/subst/0370.htm.
USEPA MeHg, 2009. Integrated Risk Information System. Methyl-mercury. http://www.epa.gov/iris/subst/0073.htm
Wesnæs M, Thestrup J, Remmen A, 2009. Environmental Screening and Evaluation of Energy-using Products (EuP). Final Report, Miljøprojekt Nr. 1308 2009, Miljøstyrelsen.
WHO, 1980. Recommended health-based limits in occupational exposure to heavy metals. Geneva: WHO, 1980.
WHO, 2003. Consice International Chemical Assessment Document 50. Elemental mercury and inorganic mercury compounds: Human health aspects. First draft prepared by J.F. Risher, Agency for Toxic Substances and Disease Registry (ATSDR), Atlanta, Georgia, USA. World Health Organization, 2003. http://www.who.int/ipcs/publications/cicad/en/cicad50.pdf
WHO, 2005. WHO (World Health Organisation). Mercury in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. Document WHO/SDE/WSH/05.08/10, 2005. Geneva, World Health Organisation. http://www.who.int/water_sanitation_health/dwq/chemicals/mercuryfinal.pdf.
WHO, 2010. Joint FAO/WHO Expert Committee on Food Additives. Seventy-second meeting. Rome, 16-25 February 2010. Summary and conclusions, issued 16th March 2010. Food and Agriculture Organization of the United Nations, WHO, 2010. http://www.who.int/foodsafety/chem/summary72_rev.pdf
Wikipedia. Searches at Wikipedia. E.g. http://da.wikipedia.org/wiki/Lysstofr%C3%B8r, http://en.wikipedia.org/wiki/Fluorescent_lamp#Principles_of_operation.
http://da.wikipedia.org/wiki/Amalgam
Öko-test, 2008. Öko-test nr. 10, oktober 2008. Test Energiesparlampen. Keine Luchten. In German.
www.datalyse.dk. Sparepærer er ikke bare sparepærer. In Danish.http://www.datalyse.dk/carl/sparpare.htm
Version 1.0 August 2010, © Danish Environmental Protection Agency