A guidance document on microbiological control of cosmetic products
1 Introduction
- 1.1 Background
- 1.2 Legislation
- 1.3 Definitions
- 1.4 Human Safety
- 1.5 Dossier
- 1.6 Good Manufacturing Practice
- 1.7 Durability labeling
The purpose of this guideline is to help those concerned with the production or import of cosmetic products to maintain a good microbiological quality all through the life of the product. Cosmetics refer to products intended to be placed in contact with the various external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance and/or correcting body odours and/or protecting them or keeping them in good condition. For definition of cosmetic product in Danish see Kosmetikbekendtgørelsen, Kap.1 §3 (1) and in English see Article 1 in Council Directive 76/768/EEC (2). Contamination of cosmetics – during the production process – can cause adverse effects when used by sensitive individuals. A cosmetic product placed on the market must not cause damage to human health. This guideline will describe and recommend validated methods for measuring microbiological contamination of the cosmetic product before, during and at end of use. This guideline will also recommend how laboratories used for self-control of cosmetics products can be equipped.
This guideline is prepared by:
Ann Detmer
Claus Jørgensen
Dorte Nylén
DHI, Centre for Environment and Toxicology
The project is financed by “Virksomhedsordningen” by The Danish Agency of Environmental Protection, 2007.
Co-ordinators of The Danish EPA was:
Dorrit Skals
Anette Albjerg Ejersted
Bettina Ørsnes Andersen
1.1 Background
Due to the wide range of formulations, manufacturing procedures and conditions of consumer use, the control of microbiological growth in cosmetics is complex. Legislation in relation to microbiological growth in cosmetics is not detailed, and concerned bodies are working on developing more detailed standards. One stakeholder is PEMSAC (Platform of European Market Surveillance Authorities in Cosmetics). PEMSAC is cooperation between European authorities within cosmetics. PEMSAC has assigned a standardisation mandate to the European Standards Organisations (CEN) concerning Good Manufacturing Practices for cosmetics products, see appendix 1. GMP is supposed to ensure that products, that are not necessarily sterile contain no harmful organisms and that the benign population is of low and stable order and/or declines over the product lifetime. At the same time International Organization for Standardization, technical committee for cosmetics, ISO/TC 217 is working on a series of standards for the detection and identification of microorganisms in cosmetic products. Denmark has no representative in this technical committee. The existence of these new standards will help create safe cosmetic products.
1.2 Legislation
A cosmetic product is regulated in Council Directive 76/768/EEC of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products (Cosmetics Directive). More than 55 amendments and adaptations have changed the Cosmetic Directive through the years. The Cosmetics Directive introduces a legal responsibility for companies assuring that products reaching the market place are not only microbiologically safe but will also continue to be safe throughout the products life. In close co-operation with Member States the Commission has issued a number of ‘guidelines’ to provide a coherent interpretation of various provisions of the cosmetics-Directive in the interest of Member States authorities and stakeholders, such as industry. The Cosmetics Directive is transposed into the individual national legal frameworks (law) and in Denmark it is implemented in the Statutory Order of the ministry of the Environment no. 422 of 4. May 2006 (Bekendtgørelse nr. 422 af 4. maj 2006 om kosmetiske produkter (1). The Cosmetics and Medical Devices unit of the European Commission/ Directorate General Enterprise and Industry is in charge of administering the Cosmetics Directive and supervises a correct implementation.
The cosmetic Directive consists of a body text and eight annexes:
- Indicative list of cosmetic product types
- Officially recognized symbols (two annexes)
The "negative lists"
- Substances prohibited in cosmetics (over 1200)
- Ingredients with limitation when used (over 150)
The "positive lists"
- Colorants with limitations
- Preservatives with limitations
- UV-filters with limitations
1.3 Definitions
A cosmetic product is defined as any substance or preparation intended to be placed in contact with the various external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance and/or correcting body odours and/or protecting them or keeping them in good condition.
1.4 Human Safety
The safety of a cosmetic product in the EU is the full responsibility of the manufacturer, the first importer into the EU market or the marketer. A cosmetic product put on the market must not cause damage to human health when applied under normal or reasonably foreseeable conditions of use, according to Article 2 in the Cosmetics Directive and implemented in §10 in the Danish cosmetics regulation.
1.5 Dossier
The manufacturer or his agent or the person to whom a cosmetic product is manufactured or the person responsible for placing an imported cosmetic product on the Community market shall for control purposes keep a dossier readily accessible for inspection by the competent authorities of the Member State indicated by the address specified on the label. The dossier is not directly available in each Member State but only through the competent authority in the Member State, which the manufacturer or his agent specified on the label. The information required to produce a dossier is described in § 33 in the Danish cosmetics regulation (1) and in article 7a in the Cosmetics Directive 76/768/EEC (2). Each dossier must contain a safety assessment of the product and information on microbiological specifications of the raw materials used for production and in the product. Records should be maintained for all aspects of microbiological testing during development and manufacture of the cosmetic product. A guideline on Safety assessment of cosmetic products and how to comply a dossier is available in Guideline on safety assessment of cosmetic products: http://www.mst.dk/Udgivelser/Publications/2001/03/87-7944-336-2.htm from the Danish EPA and in a Danish Version in Environmental Guideline No 9 2000. http://www.mst.dk/Udgivelser/Publikationer/2001/03/87-7944-335-4.htm Both guidelines can be found via the homepage of the Danish EPA.
1.6 Good Manufacturing Practice
Producers of cosmetic products are legally obliged to comply with the principles and guidelines of GMP. The requirements were formulated in Directive 93/35/EEC, the 6th amendment to the Cosmetics Directive. The ISO standard DS/EN ISO 22716:2007, Cosmetics - Good Manufacturing Practices (GMP) - Guidelines on Good Manufacturing Practices, gives guidelines for the production, control, storage and shipment of cosmetic products. These guidelines cover the quality aspects of the product, but as a whole they do not cover safety aspects for the personnel engaged in the plant, nor do they cover aspects of protection of the environment. The guidelines in ISO 22716:2007 are not applicable to research and development activities and distribution of finished products. The standard can be acquired via Dansk Standards homepage, DS/EN ISO 22716:2007. COLIPA (3) and the Council of Europe (4) have also produced GMP guidelines.
GMP should ensure that products, whilst not necessarily sterile, contain no harmful organisms and that the microbiological population is of a low and stable order and/or declines over the product lifetime. GMP includes specific cleaning procedures to keep all apparatus and materials appropriately clean. Procedures also include microbiological control of raw materials, bulk and finished products, packaging material, personnel, equipment and preparation and storage rooms.
1.7 Durability labeling
From March 2005 it was legally demanded (Directive 2003/15/EC, amending Directive 76/768/EC) to label durability on cosmetic products. This Directive is implemented as § 21 in the Danish cosmetics regulation.
Indication of the exact date of durability is not mandatory for cosmetic products with a minimum durability of more than 30 months. Instead, such products must be labelled with a symbol indicating the period of time in month/year after opening (PaO), for which the product can be used without any harm to the consumer. Such a symbol is the open jar found in annex eight of the Danish cosmetics Statutory:
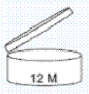
Products with durability less than 30 months must be labelled with durability period and a fixed date: best used before the end of “insert date”.
The manufacturer must have information supporting the microbiological stability of the product. The manufacturer must demonstrate that no unacceptable alterations of the product occur within the indicated durability period. Each product's PaO must be assessed with relevant methods. No official methodology is available but examples of sources of information for assessing a product’s PaO may include:
- microbiological challenge tests
- stability data
- analytical data (e.g. preservative analysis)
- type of packaging
- experience with similar formulations and products
- consumer habits and practices.
Version 1.0 January 2010, © Danish Environmental Protection Agency