A guidance document on microbiological control of cosmetic products
3 Laboratories
In Denmark, there is no specific regulation on quality requirements or accreditation of microbiological laboratories used in self-control of cosmetic products. This gives the producer of cosmetic products freedom but also responsibility. When conducting microbiological examinations of cosmetic products it is necessary to pay attention to personal hygiene and to use appropriate working techniques to ensure that only those microorganisms that are present in the samples are enumerated. To help producers with their own laboratories for self-control of cosmetics products establish a reasonable laboratory quality level, the following chapter on quality management is included. Matters related to the working environment are not covered. Please refer to the Danish Working Environment Authority Guideline C.0.18.
3.1 Quality Management
3.1.1 Quality management standards
Quality management is defined in ISO 9000 (6) as coordinated activities to direct and control an organisation with regard to quality. Quality management generally includes establishment of a quality policy with quality objectives, and quality planning, quality control, quality assurance and quality improvement. For laboratories, ISO 17025 (7) specifies the general requirements for the competences of testing and calibration laboratories, and its implementation will ensure compliance with ISO 9000 as well. ISO 17025 describes both management requirements and technical requirements.
If your company has implemented a quality management system in accordance with ISO 9000, all management requirements and some of the technical requirements in ISO 17025 are already implemented and will be easy to extend to the laboratory. In this case it is recommended to fully implement ISO 17025.
If your company has implemented a Good Manufacturing Practice according to DS/EN ISO 22716, a number of both management and technical requirements similar to those of ISO 17025 are implemented and will be easy to extent to the laboratory. In this case it is recommended to follow the ISO 17025 as close as reasonable for the specific laboratory.
Below, we describe the recommended minimum requirements for the control laboratory.
3.1.2 Approach to quality management in microbiology laboratories
A common approach for implementation of a practical QA/QC system is “the 5 D’s”.
- Decide where it is relevant to perform quality management
- Describe who does what, how and when
- Do what is decided and described
- Document what has actually been done
- Deem whether procedures and practices give the desired results and make improvement, if necessary
In the following paragraphs each of the D’s are described and suggestions are given on how to do the D’s.
3.1.2.1 Decide
In order to decide where it is relevant to perform quality management it is suggested first to draw a flowchart of the analytical flow from sampling to reporting the results. An example of a flowchart is seen in Figure 1.
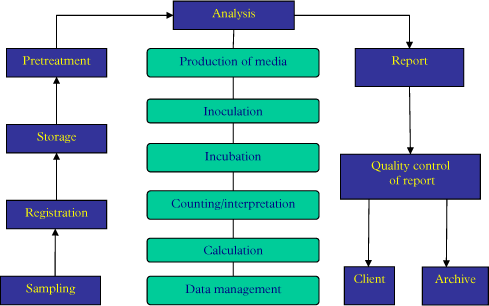
Figure 1: Example of a flowchart describing the steps of a microbial analysis.
The flow chart should identify all the critical steps of an analysis. The idea is now to carefully go through each step and decide which of steps needs a written operational procedure. In some cases more than one step may be needed. It is important to do this very carefully because missing procedures may hamper the analytical quality. On the other hand, the number of procedures should be limited to a minimum; because the more procedures to be followed the higher the chance is that one or more of them are not followed.
Deciding where to perform quality management and in what detail is a delicate balance. For instance the temperature is a critical factor in a microbial analysis, and a number of questions arise on how to manage the temperature. For instance: should the incubator temperature be monitored weekly, daily or recorded continuously? Is it OK to monitor the incubator temperature in one point, or should the spatial variation also be known? Should the thermometer be calibrated at each °C at all the temperatures, where the thermometer is used, or is it OK to intra- or extrapolate from two calibration points? The art of implementing good quality management is to find a satisfactory level of QA/QC without overdoing it. As a rule of thumb, procedures or devices that directly influence the analytical results should be given the highest priority.
3.1.2.2 Describe
When the steps requiring an operational procedure have been decided, the procedures should be written as short and clear as possible without missing any points. The procedures may be very simple consisting of a reference to a standard, for instance the analytical methods or sampling procedures, a reference to a maintenance manual, or it may be procedures entirely written by the laboratory, for instance procedures for control of incubators or thermometers. The procedures may have annexes such as templates for sample registration. A number of the procedures may be shared with other laboratories in the company, such as procedures for registration of chemicals, and calibration of pipettes and balances. It is suggested that the procedures follow a common format, which includes at least the following:
- A unique title
- The purpose of the procedure
- The process
- Responsibilities
- Name of the author and the approving person
- Date of approval, date of expiry and edition.
3.1.2.3 Do
This part is quite simple: You just have to do what was decided and described. However, this is also the hard part. The experience shows that procedures are often forgotten and left alone in the binder or in the drawer. A few things can be done to reduce the likelihood of forgotten and unfollowed procedures:
- The procedures should be reviewed by the staff doing the work to assure that the procedures are practical and in accordance with the way the work is actually carried out in the lab (assuming the work is done in a proper way).
- The procedures should be readily available to all relevant staff.
- A year plan for maintenance and calibration should be made and followed, and made readily available (e.g. posted on the wall).
- Education of and discussion among the staff members.
3.1.2.4 Document
Document what has actually been done. This requirement is included for at least four reasons:
- It provides a tool for identifying errors and thereby preventing the same errors to take place in the future work.
- It enables your company to perform internal audit to verify that the actions to be taken were actually taken.
- It enables audit to be done by an independent third party, if necessary.
- In case of complaints, or if unusual results have been obtained, the laboratory can control and prove that the quality of the analysis is sufficient and the results are reliable.
The requirement for documentation covers all operations that may influence the quality of the analysis. In a good QA/QC system, all relevant (and only the relevant) operations are described in the procedures, and must be documented. The best way is to provide templates where the work carried out can be recorded. Examples of documentation are signed templates for sampling, control and calibration of volumetric equipment, substrate control, employees’ education, quality control of reports etc.
A short rule of thumb is: it must be possible for an auditor by a signed document to verify that a certain operation has been performed when and by whom.
3.1.2.5 Deem
Even the best QA/QC system can be improved and fine-tuned. Therefore it is necessary to evaluate the system periodically. This is done through several methods.
One of the methods is audit, internal (and external), for which requirements usually are laid down in the quality management /GMP system. During the audits, inconsistencies between procedures and the actual work are identified. It must be decided in each case, if the procedure or the practise should be changed.
Another method is internal quality control, which is a program carried out by the laboratory to show that the variability is under control, using tools such as standards, replicate samples and participation in proficiency tests. If the variability is deemed to be too high, actions must be taken to improve the procedures.
A third mechanism, and maybe the most important, is the daily discussions among the staff and with colleagues from other laboratories. It is important to encourage open discussions between all lab employees, and to be willing to make changes accordingly.
3.1.3 Requirements to quality management
This section describes a recommended minimum of requirements to the microbial control laboratory. The recommendations are based on ISO 17025, “EA - 4/10 - Accreditation in Microbiological Laboratories” (8) and DS/EN ISO 22716. However, full implementation of ISO 17025 is preferable to obtain consistently reliable results.
3.1.3.1 Document control
The laboratory must establish and maintain procedures to control all documents related to the quality of the analyses. These procedures should follow the requirements of ISO 17025 or ISO 22716.
3.1.3.2 Personnel
The laboratory management must ensure the competences of all personnel involved in planning, performing, interpreting and reporting of tests and/or calibrations. Testing and calibration must be performed or supervised by an experienced person with a degree in microbiology or equivalent, or with extensive relevant experience. The staff must have relevant practical working experience and have received adequate training in basic techniques such as plate pouring, counting of colonies, aseptic techniques etc.
Where a method or a technique is not regular in use, verification of personnel performance before testing is undertaken may be necessary. Critical interval between performances of tests should be established.
The laboratory must maintain job descriptions and documentation of staff qualifications.
3.1.3.3 Environment
The laboratory must ensure that the environmental conditions do not invalidate the results or adversely affect the required quality of any measurement.
The laboratory must monitor, control and record the environmental conditions as required by the relevant methods or procedures or when they influence the quality of the result. Due attention must be paid, for example, to biological sterility and temperature.
The laboratory should be arranged so as to minimise the risks of cross contamination. This can be achieved for example by constructing the laboratory according to the “no way back” principle, where all samples and cultures only travel in one direction through the laboratory. For instance, cultures or incubated plates should never enter media and sample preparation rooms. Alternatively, activities can be separated by time and space and appropriate precautions can be taken to ensure test and sample integrity, such as use of sealed containers and hygienic practises.
It is good practice to have separate locations or clearly designated areas for:
- sample receipt and storage
- sample preparation and challenge test preparations including sterile room or sterile cupboards. Powdery products should be handled separately.
- incubation and sample examination
- media and equipment preparation including sterilisation
- decontamination
The area for washing after decontamination may be shared with other laboratories provided that transfer of substances that could adversely affect microbial growth is prevented.
Space should be sufficient to allow work areas to be kept clean and tidy.
Rooms should be appropriately ventilated and at a suitable temperature.
Reduction of contamination may be achieved by having:
- smooth surfaces on walls, ceilings, floors and benches. Tiles are not recommended as bench covering material
- concave joints between the floor, walls and ceiling;
- minimal opening of windows and doors while tests are being carried out
- sun shades placed on the outside;
- fluid conveying pipes not passing above work surfaces unless placed in hermetically sealed casings
- a dust-filtered air inlet for the ventilation system;
- separate hand-washing arrangements, preferably non-manually controlled;
- cupboards up to the ceiling;
- no rough and bare wood;
- wooden surfaces of fixtures and fittings adequately sealed;
- stored items and equipment arranged to facilitate easy cleaning;
- no furniture, documents or other items other than those strictly necessary for testing activities.
There must be a cleaning programme for laboratory fixtures, equipment and surfaces.
Laboratory coats must be worn in the laboratory and must be removed before leaving the area.
Access to the microbiology laboratory should be restricted to authorised personnel.
See also ISO 21148 General instructions for microbial examination.
3.1.3.4 Test and calibration methods and method validation
The laboratory must use appropriate methods and procedures for all tests and/or calibrations. These include sampling, handling, transport, storage and preparation of items to be tested or calibrated, and where appropriate, an estimation of the measurement uncertainty as well as statistical techniques for analysis of test and/or calibration data.
If laboratory-developed methods or non-standard methods are used, they must be validated in house. Standard methods used on matrices not specified in the standard must be validated as well.
Validation of a microbial method requires a substantial amount of work. The standards for validation (DS/EN ISO 16140 or DS/ENV ISO/TR 13843) should be followed. See also EA - 4/10. To avoid the validation procedure it is strongly recommended to use the ISO methods described in section 4. However, even when a complete validated method is used, the laboratory still needs to verify on a regular basis that performance can be met, e.g. by use of spiked samples or reference materials.
The laboratory must have and must apply procedures for estimating the uncertainty of measurements. Rigorous, metrologically and statistically valid calculation of uncertainty of microbial analyses cannot be performed. It is appropriate to base the estimate of uncertainty on repeatability and reproducibility data, combined with bias estimation from participation in proficiency testing or use of standard materials when possible. The individual components of uncertainty must be demonstrated to be under control and their contribution to the variability of the evaluated results. Some components such as pipetting, weighing and dilution effects can be readily measured and evaluated. Other components such as sample stability and sample preparation cannot be evaluated in a statistical manner but their importance should be considered. Se also section 3.1.3.6
Calculations and data transfers must be subject to appropriate checks in a systematic manner.
3.1.3.5 Equipment
The laboratory must be furnished with all items of sampling, measurements and test equipment required for the correct performance of the tests.
The laboratory must operate a documented programme for the maintenance, calibration, and performance verification of its equipment.
Maintenance of essential equipment must be carried out at specified intervals and detailed records must be kept.
The laboratory must establish a programme for the calibration and performance verification of equipment which directly influences the test result. Examples of calibration and performance checks are given in EA-4/10. Before taken into service, equipment must be checked to establish that it meets the requirements.
The temperature is an important parameter and the laboratory must have temperature measuring devices of an appropriate quality. Calibration of the devices must be traceable to national or international standards for temperature.
The stability of temperature and the uniformity of the temperature distribution in incubators, water baths and ovens must be established initially and documented with respect to typical uses. The initial validation must be checked and recorded after each significant repair or modification, and operating temperatures must be monitored and recorded.
Autoclaves must be capable of meeting the time and temperatures specified in the methods used. Pressure cookers only equipped with a pressure gauge are not acceptable. Initial validation must include operating cycles and load configurations used in practice. Temperature sensors should be positioned inside containers filled with liquid.
Monitoring of autoclaving should be carried out using a thermocouple and a recorder to produce a chart or printout, or by the use of chemical or biological indicators. Autoclave tape and indicator strips should only be used to show that the load has been processed.
Balances and weights must be calibrated traceably at regular intervals.
Laboratories must carry out initial verification of volumetric equipment and make regular checks to ensure that the equipment is performing with the required specification. Verification should not be necessary for glassware which has been certified to a specific tolerance. Equipment should be checked for the accuracy of the delivered volume against the set volume (for several different settings in the case of variable volume instruments) and the precision of the repeat deliveries should be measured. Single use disposable volumetric equipment should be obtained from ISO 9000 certified manufacturers. An initial validation of the suitability of the equipment must be carried out.
Conductivity meters, oxygen meters, pH meters and other similar instruments should be verified regularly or before each use. The buffers used for verifications purposes should be stored in appropriate conditions and should be marked with an expiry date, and their use documented.
3.1.3.6 Reagents and culture media
Laboratories must have procedures for selecting and purchasing of the services and supplies it uses that affect the quality of the tests. To ensure that the quality of the reagents and media used is appropriate for the test concerned, it is recommended to obtain reagents and media from ISO 9001 certified manufacturers. In this case an initial validation of the suitability of the equipment must be carried out. In case in-house prepared media or ready-to-use media from non-certified manufacturers are used, each batch should be validated according to ISO 11133.
Laboratories must ensure that all reagents (including stock solutions), media, diluents, and other suspending fluids are adequately labelled to indicate, as appropriate, identity, concentration, storage conditions, preparation date, validated expiry date and /or recommended storage periods. The person responsible for preparation should be identifiable from records.
The laboratory must keep a record of all purchased reagents, media etc.
3.1.3.7 Internal quality control
Internal quality control consists of all the procedures undertaken by a laboratory for the continuous evaluation of its work. The main objective is to ensure the consistency of results day-to-day and their conformity with defined criteria.
A programme of periodic checks is necessary to demonstrate that variability (i.e. between analysts and between equipment and materials etc.) is under control. All tests included in the control of the products need to be covered. The programme may involve:
- the use of spiked samples
- the use of reference materials (including proficiency testing scheme materials)
- replicate testing
It is recommended to follow option 1 described in ISO/TS 19036:2006 (9).
A laboratory may use a test at rare. It is recognised that in such cases an ongoing internal quality control programme may be inappropriate and that a scheme for demonstrating satisfactory performance which is carried out in parallel with the testing, may be more suitable.
3.1.3.8 External quality assessment (proficiency testing)
If available laboratories should regularly participate in proficiency testing which are relevant to their activities, bias should be assessed and the validity of the whole quality system should be checked.
3.1.3.9 Internal audit
The company must periodically, and in accordance with a predetermined schedule and procedure conduct internal audits of the activities of the control laboratory to verify that its operations continue to comply with the requirements of the laboratory quality management system. The internal audit programme must address all elements of the laboratory management system. Internal audit is a requirement of both ISO 9001 and ISO 22716 and can be extended to cover the laboratory as well. The audit must be carried out by specially designated personnel having both quality management competence and technical competence. If the company does not have independent technical competence, external technical advisors can be included in the audit.
Internal audit follow-up must confirm the satisfactory completion of the audit or satisfactory implementation of corrective actions.
The area of activity audited, the audit findings and corrective actions that arise from them must be recorded.
Version 1.0 January 2010, © Danish Environmental Protection Agency