Identification and assessment of alternatives to selected phthalates
Annex 4 Background data for the environmental and health assessment
Diethylene glycol dibenzoate, DEGD
| Identification of the substance | ||
| CAS No. | 120-55-8 | |
| EINECS No. | 204-407-6 | [1] |
| EINECS Name | Oxydiethylene dibenzoate | [1] |
| Synonyms | Diethylene glycol dibenzoate DEGD |
|
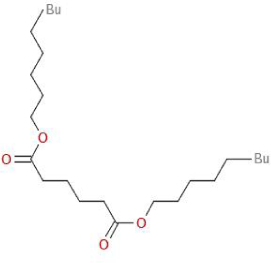
| Molecular Formula | C18H18O5 | [1] |
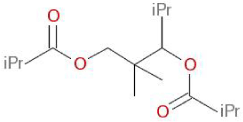
| Structural Formula | 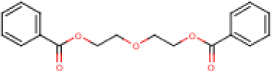 |
|
| Major Uses | Plasticizer | [3] |
| IUCLID | Is listed as a LPV chemical | [1] |
| EU classification | This substance is not classified in the Annex I of Directive 67/548/EEC as such, but it may be included in one of the group entries. | [1] |
| Physico-chemical Characteristics | ||
| Physical Form | Colourless liquid with mild ester odour | [3], [4] |
| Molecular Weight (g/mole) | 314.4 | [2] |
| Melting Point/range (°C) | 28 ºC | [2] |
| 24 ºC | [4] | |
| 33.5ºC | [6] | |
| Boiling Point/range (°C) | 236 ºC (at 0.7 kPa) | [2] |
| 225-227 ºC (at 3 mm Hg) | [3] | |
| Decomposition Temperature (°C) | > 230 ºC | [4] |
| Vapour Pressure (mm Hg at °C) | 1.3 x 10-7 mm Hg (at 25 ºC) [1.73 x 10-5Pa] 3.2 x 10-6 mm Hg (at 50 ºC) [4.26 x 10-4Pa] 5.1 x 10-4 mm Hg (at 100 ºC) [6.79 x 10-2Pa] |
[4] |
| Density (g/cm3 at °C) | 1.2 (at 20 ºC) | [2], [3] |
| Vapour Density (air=1) | 9.4 | [2] |
| Henry’s Law constant (atm/m³/mol at °C) | 7.0 x 10-10 | [4] |
| 3.0 x 10-12 at 25ºC | [3], [6] | |
| Solubility (mg/l water at °C) | Soluble in water | [3] |
| 38.3 mg/l (at 30 ºC and pH 7) | [4] | |
| Partition Coefficient (log Pow) | 3.2 | [4] |
| 3.04 | [7] | |
| pKa | - | |
| Flammability | - | |
| Explosivity | - | |
| Oxidising Properties | - | |
| Migration potential in polymer | - | |
| Flash point (ºC) | 232 ºC | [2] |
| Viscosity (mPas) | 110 (at 20 ºC) | [3] |
| Atmospheric OH rate constant cm³/(molecule sec) | 1.9 x 10-11 at 25ºC | [6] |
| Emission Data | ||
| During production | ||
| Exposure Data | ||
| Aquatic environment, incl. sediment | Diethylene glycol dibenzoate's production and use as a plasticizer may result in its release to the environment through various waste streams. | [3] |
| Terrestrial environment | Diethylene glycol dibenzoate's production and use as a plasticizer may result in its release to the environment through various waste streams. | [3] |
| Sewage treatment plant | - | |
| Working environment | NIOSH (NOES Survey 1981-1983) has statistically estimated that 25,414 workers (10,937 of these are female) are potentially exposed to diethylene glycol dibenzoate in the USA. Occupational exposure may be through inhalation and dermal contact with this compound. | [3] |
| Consumer goods | - | |
| Man exposed from environment | - | |
| ”Secondary poisoning” | - | |
| Atmosphere | - | |
| Dermal | - | |
| Toxicological data | ||
| Observations in humans | Observed symptoms: Nausea, vomiting and headaches; with continued use obdominal pain, polyuria followed by oliguria, anuria and renal failure. Also drowsiness, coma, respiratory arrest and pulmonary edema. Range of toxicity: A) The average fatal dose is difficult to estimate. Much of the data is from historical sources or from epidemics that have occurred in patients in third world countries with limited access to medical care. Extrapolation from these sources must therefore be interpreted with caution. B) The average fatal dose in people who drank a sulfanilamide elixir with diethylene glycol as the vehicle was approximately 1 ml (72% concentration of DEG) per kilogram body weight. However, the actual reported fatal doses were highly variable. C) Adult 1) Three men died after consuming approximately 2 to 3 cups (473 – 709 ml) each of 100% diethylene glycol, as an ethanol substitute. 2) A 56-year-old man died after ingesting 8 ounces (236 ml) of 100% diethylene glycol in a suicide attempt. 3) Adults who ingested sulfanilamide contaminated with diethylene glycol survived doses of 1 to 240 milliliters (of a 72% solution). D) Pediatric 1) Median diethylene glycol dose that was fatal in 85 (98%) of 87 children was estimated to be 1.34 ml/kg (range 0.22 to 4.42 ml/kg). Twelve children ingested less than 1.0 ml/kg. 2) Forty-nine children survived ingestion of a median dose of 0.67 ml/kg (range of 0.05 - 2.48 ml/kg) diethylene glycol present in contaminated acetaminophen syrup. |
[3] |
| Acute toxicity | ||
| Oral | LD50 = 4190 mg/kg body weight (rat, combined) | [4], [5] |
| LD50 = 2830 mg/kg body weight (rat) | [6] | |
| Dermal | LD50 > 2000 mg/kg body weight (rat, combined) | [4], [5] |
| LD50 = 20 ml/kg body weight (rabbit) | [6] | |
| Inhalation | LC50 (4 h, mist) > 200 mg/l (rat) | [5] |
| Other routes | - | |
| Skin irritation | No dermal reaction was reported following a single semi-occlusive application of diethylene glycol dibenzoate to intact rabbit skin for 4 hours. | [5] |
| Eye irritation | A single instillation of diethylene glycol dibenzoate into the eye of the rabbit elicited transient very slight conjunctival irritation only. No allergic skin reaction was reported in guinea pigs after repeated skin contact (intradermal and topical) using the Magnusson and Kligman method. | [5] |
| Irritation of respiratory tract | - | |
| Skin sensitisation | Not sensitising to Guinea pig | [4] |
| Subchronic and Chronic Toxicity | ||
| Oral | NOAEL = 1000 mg/kg/day (rat, 13 weeks) Diethylene glycol dibenzoate was administered to rats by dietary admixture to achieve dosages of 0, 250, 1000, 1750 or 2500 mg/kg/day over 13 weeks. Selected Control and Group 5 animals were subsequently maintained off dose for 4 weeks to assess reversibility of any treatment related changes. There were no findings of toxicological importance at a dosage of 1000 mg/kg/day or below. In animals receiving 1750 or 2500 mg/kg/day, there was an adverse effect on bodyweight gain, changes in clinical pathology parameters and an increased incidence/degree of haemosiderosis in the spleen. In addition, at 2500 mg/kg/day, a few treatment-related clinical signs were evident, minimal periportal hepatocyte hypertrophy was noted in both sexes. Plasma enzyme activities (transaminases and/or AP or OCT) were elevated at Week 13 in rats receiving 1750 or 2500 mg/kg/day with an associated minimal increase in liver weight, At necropsy, minimal periportal hepatocyte hypertrophy was detected only at 2500 mg/kg/day. The slight effects in the liver may be a physiological adaptation to treatment at the highest doses. Following 4-weeks recovery, most enzyme activities were normal, liver weights were unremarkable and there was no residual hepatic pathology. Epithelial hyperplasia was detected in the colon of males and the caecum of both sexes. In general, however, dosages of up to 2500 mg/kg/day of diethylene glycol dibenzoate were tolerated. When selected animals previously receiving 2500 mg/kg/day were maintained off-dose for 4-weeks, all treatment related changes showed evidence of, or complete, recovery. |
[4], [5] |
| No effects were reported in dogs administered up to 300 mg/kg/day of diethylene glycol dibenzoate in their diet for 90 days. | [5] | |
| Inhalation | - | |
| Dermal | - | |
| Metabolism | Metabolism in the Rat. The metabolism of diethylene glycol dibenzoate was studied after both single oral low level (50 mg/kg) and high level (750 mg/kg) doses to groups of 4 male and 4 female rats. The tissue distribution of radioactivity was studied after low-level doses. The proportions and nature of metabolites were also investigated. Virtually all of single oral doses of 50 and 750 mg/kg of diethylene glycol dibenzoate administered to Sprague-Dawley CD rats were adsorbed, metabolized and excreted in the urine within 24 hours of administration. Diethylene glycol dibenzoate was metabolized via hydrolysis of the ester bonds to benzoic acid; this free acid was then conjugated with either glycine (major pathway) or glucuronic acid (minor pathway) prior to excretion. | [4] |
| Mutagenicity, Genotoxicity and Carcinogenicity | ||
| Mutagenicity | S.typhimurium and E.coli (metabolic activator Sprague-Dawley rat liver (S9)) Cytotoxic conc: No toxicity with or without metabolic activation Genotoxic conc: No genotoxic effects observed with or without metabolic activation No evidence of mutagenic activity in this bacterial system. |
[4] |
| Mouse lymphoma (metabolic activator Sprague-Dawley rat liver (S9)) Genotoxic Effects: In the absence of S9-increases in mutant frequency were observed 350 µg/ml on Test 1 and 200 and 325 µg/ml in Test 2. The increases were not 100 above the control level and were within the historical control range. It was concluded that diethylene glycol dibenzoate did not demonstrate mutagenic potential in the absence of S9 mix. There was no substantive increases in mutant frequency observed in the presence of S9 mix. It is concluded that diethylene glycol dibenzoate did not demonstrate mutagenic potential in this in vitro gene mutation assay |
[4] | |
| Chromosome Abnormalities | Chinese Hamster Lung (metabolic activator Sprague-Dawley rat liver (S9)) Genotoxic Effects: No statistically significant increases in the proportion of aberrant cells, when compared to the solvent control, were seen in either the presence or the absence of S9 mix. A small response seen in the first test, with S9 mix, was not reproduced in the repeat test or at the later harvest. This response was not considered to be indicative of clastogenic activity. |
[4] |
| Other Genotoxic Effects | - | |
| Estrogenic activity | Rat Diethylene glycol dibenzoate for Estrogenic Activity Using Vaginal Cornification and the Uterotrophic Response in the Ovariectomized Adult Rat as the Endpoints. Diethylene glycol dibenzoate did not induce vaginal cornification at doses of 500, 1000, 1500 or 2000 mg/kg/day for 7 days by oral gavage in ovariectomized adult Spraque-Dawley (CD) rats. Diethylene glycol dibenzoate did not stimulate a uterine weight increase or an increase in the uterine weight to final body weight ratio at doses of 500, 1000, 1500 or 2000 mg/kg/day for 7 days. When compared with the vehicle control (corn oil) and positive control (diethylstilbestrol), these data demonstrate that Diethylene glycol dibenzoate did not exhibit estrogenic activity up to and including the maximally tolerated dose. |
[4] |
| Carcinogenicity | - | |
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||
| Reproductive Toxicity | Rat (38 weeks duration) The evidence from this study suggested that a dietary concentration of 10,000 ppm should be considered as the No-Observed-Adverse-Effect-Level (NOAEL) for the FO and Fl parent animals. The No-observed-Adverse-Effect-Level (NOAEL) for the developing offspring is considered to be 3300 ppm. The No-Observed-Effect-Level (NOEL) for reproductive parameters is considered to be 10000 ppm. |
[4] |
| Teratogenicity | - | |
| Developmental toxicity | Rat (20 days duration from gestation) Maternal Toxicity NOEL : 1000 mg/kg/day Clinical Signs: The general condition of females at all dosages remained satisfactory throughout the study and there were no deaths. Salivation after dosing was observed at all dosages. The incidence was dosage related but this finding was not considered to be of toxicological importance. At 1000 mg/kg/day, there were no detectable signs of maternal toxicity; there were no maternal deaths and all females had a live litter at sacrifice. Litter Responses and Fetal Changes Prenatal development NOAEL: 500 mg/kg/day. Although a small number of fetuses with cervical ribs at 1000 mg/kg/day precludes defining this dosage as a NOEL for developmental anomalies, there were no findings at this dosage that were considered indicative of any substantial disturbance of morphological development. Fetal Growth and Development NOEL: 250 mg/kg/day Post-implantation loss was higher in all treated groups compared to the concurrent Control, differences attaining significance at 500 and 1000 mg/kg/day. However values were comparable with recent background control data and it is considered that the test groups were disadvantaged by a particularly high survival rate in the Control. It was concluded that in utero survival had not been adversely affected by treatment since live litter size was unaffected and was similar in all groups. |
[4] |
| Toxicokinetics | ||
| Toxicokinetics | ||
| Ecotoxicity Data | ||
| Algae | EL50 (area under the curve 72h) = 5.2 mg/l EL50 (growth rate 0-72h) = 11 mg/l EL50 (area under the curve 96h) = 5.9 mg/l EL50 (growth rate 0-96h) = 15 mg/l |
[4] |
| Daphnia magna | EL50 (48h) = 6.7 (Daphnia magna) No-observed effect loading rate = 1.0 mg/l (Daphnia magna) |
[4] |
| Other aquatic organisms | - | |
| Fish | LL50 (96h) = 3.9 mg/l (fathead minnow) No-observed effect = 1.5 mg/l (fathead minnow) |
[4] |
| Bacteria | EC50: >10 mg/l, Bacteria (Pseudomonas putida) 10 mg/l was the highest attainable concentration that could be prepared due to the limited solubility to the test material in water and auxiliary solvent and the limitations imposed by the addition of nutrient solutions and bacterial suspension to the test material stock solution. | [5] |
| Terrestrial organisms | Acute toxicity LC50 > 1000ppm (earthworm, eisenia foetida) NOEL = 1000 ppm |
[4] |
| Sludge | Diethylene glycol dibenzoate had no inhibitory effect on the respiration rate of activated sludge at concentrations up to 100 mg/l. | [5] |
| Environmental Fate | ||
| BCF | An estimated BCF value of 120 was calculated for diethylene glycol dibenzoate, using an estimated log Kow of 3.04 and a recommended regression-derived equation. According to a classification scheme, this BCF value suggests that bioconcentration in aquatic organisms is high. | [3] |
| Aerobic biodegradation | 17% of TCO2 at 2 days 71% of TCO2 at 10 days 93% of TCO2 at 28 days Readily biodegradable |
[4] |
| Diethylene glycol dibenzoate is considered readily biodegradable in the CO2 evolution test (modified Sturm test). The mean CO2 production by mixtures of diethylene glycol dibenzoate was equivalent to 16% of the theoretical value (TCO2, 106.4 mg CO2) after 2 days of incubation and 63% after 10 days; a mean level of 83% degradation was achieved by the end of the test on Day 29. The mean BOD5 = 0.77 gO2/g Diethylene glycol dibenzoate (34% of it’s ThOD = 2.05 gO2/g) The mean COD = 2.22 gO2/g Diethylene glycol dibenzoate (109% of the ThOD) The BOD5 of Diethylene glycol dibenzoate was 32% of it’s COD. Substances are generally considered readily biodegradable in the Closed Bottle test if the ratio of BOD5:COD or ThOD is >50. Component 1 therefore cannot be considered readily biodegradable in this screening test. |
[5] | |
| Anaerobic biodegradation | Diethylene glycol dibenzoate is considered ultimately biodegradable under anaerobic conditions in the biogas production test. The level of anaerobic biodegradation, based on biogas measurements alone, was equivalent to 65% by Day 60 and the total level of biodegradation (dissolved inorganic carbon plus biogas) was calculated to be 70% of the theoretical level. | [5] |
| Abiotic degradation | The rate constant for the vapour-phase reaction of diethylene glycol dibenzoate with photochemically produced hydroxyl radicals has been estimated as 1.8 x 10-11 cm³/(molecule sec) at 25 ºC using a structure estimation method. This corresponds to an atmospheric half-life of about 20 hours at an atmospheric concentration of 5 x 10+5 hydroxyl radicals per cm³. Diethylene glycol dibenzoate has an estimated base-catalyzed hydrolysis rate of 0.16 l/mol-sec at a pH of 8, which corresponds to a half-life of 49 days at a pH of 8 and 1.3 years at a pH of 7 | [3] |
| Metabolic pathway | - | |
| Mobility | Using a structure estimation method based on molecular connectivity indices, the Koc for diethylene glycol dibenzoate can be estimated to be about 540. According to a recommended classification scheme, this estimated Koc value suggests that diethylene glycol dibenzoate has low mobility in soil. | [3] |
| Volatilization from water/soil | The Henry's Law constant for diethylene glycol dibenzoate is estimated as 3 x 10-12 atm/m³/mol using a fragment constant estimation method. This value indicates that diethylene glycol dibenzoate will be essentially nonvolatile from water surfaces. Diethylene glycol dibenzoate's estimated values for vapour pressure, 0.09 mm Hg and Henry's Law constant indicate that volatilization from dry and moist soil surfaces should not occur. | [3] |
| Terrestial fate | Based on a recommended classification scheme, an estimated Koc value of 540, determined from a structure estimation method, indicates that diethylene glycol dibenzoate will have low mobility in soil. Volatilization of diethylene glycol dibenzoate should not be important from moist soil surfaces given an estimated Henry's Law constant of 3 x 10-12 atm/m³/mol, using a recommended regression equation. Volatilization from dry soil surfaces is not expected based on an estimated vapour pressure of 0.09 mm Hg, determined from a fragment constant method. No data are available to determine the rate or importance of biodegradation of diethylene glycol dibenzoate in soil. | [3] |
| Aquatic fate | Based on a recommended classification scheme, an estimated Koc value of 540, determined from a structure estimation method, indicates that diethylene glycol dibenzoate should adsorb to suspended solids and sediment in the water. Diethylene glycol dibenzoate will be essentially non-volatile from water surfaces based on an estimated Henry's Law constant of 3 x 10-12 atm/m³/mole, developed using a fragment constant estimation method. According to a classification scheme, an estimated BCF value of 120, from an estimated log Kow, suggests that bioconcentration in aquatic organisms is high. Diethylene glycol dibenzoate has an estimated base-catalyzed hydrolysis rate of 0.16 l/mol-sec at a pH of 8, which corresponds to a half-life of 49 days at a pH of 8 and 1.3 years at a pH of 7. Insufficient data are available to determine the rate or importance of biodegradation of diethylene glycol dibenzoate in water. | [3] |
| Atmospheric fate | According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere, diethylene glycol dibenzoate, which has an estimated vapor pressure of 0.09 mm Hg at 25 ºC, will exist solely as a vapour in the ambient atmosphere. Vapour-phase diethylene glycol dibenzoate is degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-life for this reaction in air is estimated to be about 20 hours. Particulate-phase diethylene glycol dibenzoate may be physically removed from the air by wet and dry deposition. | [3] |
| Conclusion | ||
| Physical-chemical | - | |
| Emission | - | |
| Exposure | - | |
| Health | - | |
| Environment | - | |
| References | ||
| 1 | ESIS | |
| 2 | InChem, WHO IPCS | |
| 3 | HSDB, Toxnet | |
| 4 | EPA HPV | |
| 5 | MSDS, Genovique | |
| 6 | ChemId | |
| 7 | SRC physprop database | |
Dipropylene glycol dibenzoate, DGD
| Identification of the substance | ||
| CAS No. | 27138-31-4 | |
| EINECS No. | 248-258-5 | [1] |
| EINECS Name | oxydipropyl dibenzoate | [1] |
| Synonyms | Dipropylene glycol dibenzoate DGD |
|
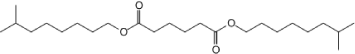
| Molecular Formula | C20H22O5 | [1] |
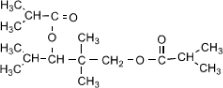
| Structural Formula | 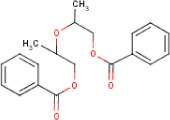 |
|
| Major Uses | - | |
| IUCLID | Is listed as a LPV chemical | [1] |
| OECD. Listed as a High Production Volume Chemical. | [4] | |
| EU classification | This substance is not classified in the Annex I of Directive 67/548/EEC as such, but it may be included in one of the group entries. | [1] |
| N, R51/53 (self classification) | [4] | |
| Physico-chemical Characteristics | ||
| Physical Form | Clear colourless liquid with a mild esterlike odour | [2] |
| Molecular Weight (g/mole) | 342.4 | [3] |
| Melting Point/range (°C) | -30ºC | [7] |
| Boiling Point/range (°C) | Decomposes above 270ºC without boiling at 762 mm Hg | [2] |
| 232ºC at 5 mm Hg | [3] | |
| 197 ºC at 1 mm Hg | [7] | |
| Decomposition Temperature (°C) | Decomposes above 270ºC without boiling at 762 mm Hg | [2] |
| Vapour Pressure (mm Hg at °C) | 1.2 x 10-6 mm Hg at 25ºC [1.59 x 10-4 Pa] 1.1 x 10-5 mm Hg at 50ºC [1.46 x 10-3 Pa] 3.8 x 10-4 mm Hg at 100ºC [0.0506 Pa] |
[2] |
| Density (g/cm3 at °C) | 1.12 | [3] |
| 1.129 at 25ºC | [7] | |
| Vapour Density (air=1) | 11.8 | [4] |
| Henry’s Law constant (atm/m³/mol at °C) | 3.8 x 10-8 | [2] |
| 1.38 x 10-8 at 25ºC | [5], [6], [7] | |
| Solubility (mg/l water at °C) | 8.69 mg/l at 30ºC, pH = 7.0 | [2] |
| 15 mg/l at 25ºC | [5], [6] | |
| Partition Coefficient (log Pow) | 3.9 | [2] |
| 3.88 | [5], [6] | |
| pKa | - | |
| Flammability | Combustible. Very slightly to slightly flammable in presence of open flames and sparks. | [4] |
| Explosivity | Not considered to present risk of explosion | [4] |
| Oxidising Properties | - | |
| Migration potential in polymer | - | |
| Flash point (ºC) | 192 | [2] |
| Viscosity | 110 cP at 25ºC | [4] |
| Atmospheric OH rate constant cm³/(molecule sec) | 3.44 x 10-11 | [5], [6] |
| Emission Data | ||
| During production | - | |
| Exposure Data | ||
| Aquatic environment, incl. sediment | - | |
| Terrestrial environment | - | |
| Sewage treatment plant | - | |
| Working environment | Inhalation and skin contact are expected to be the primary routes of occupational exposure to dipropylene glycol dibenzoate. This material is not expected to cause significant adverse human health effects when used in accordance with good industrial hygiene and safety practices are followed. | [4] |
| Consumer goods | - | |
| Man exposed from environment | - | |
| ”Secondary poisoning” | - | |
| Atmosphere | - | |
| Dermal | - | |
| Toxicological data | ||
| Observations in humans | - | |
| Acute toxicity | ||
| Oral | LD50 = 3914 mg/kg (rat, combined) | [2] |
| LD50 = 5313 mg/kg (rat) | [4] | |
| Dermal | LD50 > 2000 mg/kg (rat) | [2] |
| LD50 > 2000 mg/kg (rat) | [4] | |
| Inhalation | LC50 (mist) > 200 mg/l | [4] |
| Other routes | ||
| Skin irritation | A single semi-occlusive application of dipropylene glycol dibenzoate to intact rabbit skin for four hours elicited no dermal irritation. | [2] |
| Eye irritation | None of the treated animals showed a positive response. No corneal damage or iridial inflammation was observed. Transient hyperemia of blood vessels only was observed in all animals. These reactions had resolved in all instances by one or two days after instillation. | [2] |
| Irritation of respiratory tract | - | |
| Skin sensitisation | Dipropylene glycol dibenzoate did not produce evidence of skin sensitization (delayed contact hypersensitivity) in any of twenty test animals. Evidence of skin sensitization was produced by hexyl cinnamic aldehyde (HCA) in all ten positive controls thus confirming the sensitivity of the method. | [2] |
| Subchronic and Chronic Toxicity | ||
| Oral | NOAEL = 1000 mg/kg/day (rat, 13 weeks) Dipropylene glycol dibenzoate was administered to rats by dietary admixture to achieve dosages of 0, 250, 1000, 1750 or 2500 mg/kg/day over 13 weeks. Selected Control and Group 5 animals were subsequently maintained off dose for 4 weeks to assess reversibility of any treatment related changes. Dosages of 1000 mg/kg/day or below are considered to represent a No Observable Adverse Effect Level (NOAEL) of Dipropylene glycol dibenzoate in rats by oral administration over 13 weeks. A few minor intergroup differences were noted at 1000 mg/kg/day but were insufficient to be of toxicological importance. Higher dosages of 1750 or 2500 mg/kg/day were tolerated but the adverse effect on bodyweight was more pronounced, there were increases in circulating enzyme activities, low grade hepatocyte hypertrophy and an increased incidence and degree of hemosiderosis in the spleen in one or both sexes. At 2500 mg/kg/day, an increased incidence of minimal epithelial hyperplasia was noted in the caecum. When selected animals previously receiving 2500 mg/kg/day were maintained off dose for 4 weeks, all treatment related effects showed evidence of, or complete, recovery. |
[2] |
| Inhalation | - | |
| Dermal | - | |
| Metabolism | Studies conducted show dipropylene glycol dibenzoate is rapidly metabolized and excreted from the body. It does not accumulate in rats and this behavior is expected in other mammalian systems as well. This conclusion is supported through the test where oral doses of 14C-labeled Dipropylene glycol dibenzoate were rapidly absorbed through the gut in rats. Seventy percent of the administered dose was excreted through the urine within 48 hours as hippuric acid, and about 10% was observed in the feces. The half-life of radiocarbon in the blood was 3 hours and for other organs 2-15 hours. | [2] |
| Mutagenicity, Genotoxicity and Carcinogenicity | ||
| Mutagenicity | S.typhimurium and E.coli (metabolic activator Sprague-Dawley rat liver (S9)) Genotoxic conc: No genotoxic effects observed with or without metabolic activation No evidence of mutagenic activity in this bacterial system. |
[2] |
| Mouse lymphoma (metabolic activator Sprague-Dawley rat liver (S9)) Genotoxic effects: No effects observed with or without metabolic activation. No evidence of mutagenicity in this in vitro gene mutation assay. |
[2] | |
| Chromosome Abnormalities | Chinese hamster lung (metabolic activator Sprague-Dawley rat liver (S9)) Genotoxic effects: No statistically significant increases in the proportion of aberrant cells, when compared to the solvent control, were seen in either the presence or the absence of S9 mix. A small response seen in the first test, with S9 mix, was not reproduced in the repeat test or at the later harvest. This response was not considered to be indicative of clastogenic activity. |
[2] |
| Other Genotoxic Effects | - | |
| Estrogenic activity | Dipropylene glycol dibenzoate did not induce vaginal cornification at doses of 500, 1000, 1500 or 2000 mg/kg/day for 7 days by oral gavage in ovariectomized adult Spraque-Dawley (CD) rats. Dipropylene glycol dibenzoate did not stimulate a uterine weight increase or an increase in the uterine weight to final body weight ratio at doses of 500, 1000, 1500 or 2000 mg/kg/day for 7 days. When compared with the vehicle control (corn oil) and positive control (diethylstilbestrol), these data demonstrate that dipropylene glycol dibenzoate did not exhibit estrogenic activity up to and including the maximally tolerated dose. | [2] |
| Carcinogenicity | - | |
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||
| Reproductive Toxicity | Rat (38 weeks duration) The NOEL is 10,000 ppm for F0 and F1 parent animals and the NOAEL for survival and growth of offspring is considered to be 10,000 ppm. |
[2] |
| Teratogenicity | - | |
| Developmental toxicity | Rat (20 days duration from gestation) Maternal Toxicity NOEL : 1000 mg/kg/day Clinical Signs: The general condition of females at all dosages remained satisfactory throughout the study and there were no deaths. Salivation after dosing was observed at all dosages. The incidence was dosage related but this finding was not considered to be of toxicological importance. At 1000 mg/kg/day, there were no detectable signs of maternal toxicity; there were no maternal deaths and all females had a live litter at sacrifice. Litter Responses and Fetal Changes Prenatal development NOAEL: 500 mg/kg/day. A small number of fetuses with cervical ribs at 1000 mg/kg/day precludes defining this dosage as a NOEL for developmental anomalies, in all other respects the NOAEL for pre-natal development is concluded to be 1000 mg/kg/day. Fetal Growth and Development NOEL: 250 mg/kg/day There were no effects of treatment on pre-natal survival or growth. At 1000 mg/kg/day, treatment was associated with a small but definite increase in the number of fetuses with cervical ribs. At 1000 and 500 mg/kg/day, there were a greater number of fetuses with incomplete ossification of the 5th and 6th sternebrae compared with Controls, but this finding was not considered to be of any long term toxicological significance. |
[2] |
| Toxicokinetics | ||
| Toxicokinetics | ||
| Ecotoxicity Data | ||
| Algae | EL50 (area under the curve 72h) = 1.1 mg/l EL50 (growth rate 0-72h) = 4.9 mg/l EL50 (area under the curve 96h) = 0.96 mg/l EL50 (growth rate 0-96h) = 3.6 mg/l NOEL (area under the curve 72h) = 0.22 mg/l NOEL (growth rate 0-72h) = 1.0 mg/l NOEL (area under the curve 96h) = not observed NOEL (growth rate 0-96h) = 0.46 mg/l |
[2] |
| Daphnia magna | EL50 (24h) = 43.2 mg/l (Daphnia magna) EL50 (48h) = 19.31 mg/l (Daphnia magna) No-observed effect loading rate = 2.2 mg/l (Daphnia magna) |
[2] |
| Other aquatic organisms | - | |
| Fish | LC50 (0.25-48h) > 4.9 mg/l (fathead minnow) LC50 (72h) = 4.7 mg/l (fathead minnow) LC50 (96h) = 3.7 mg/l (fathead minnow) No-observed effect concentration = 1.2 mg/l (fathead minnow) |
[2] |
| Bacteria | EC50 > 10 mg/l (pseudomonas putida) | [4] |
| Terrestrial organisms | Under the conditions of this study, the LC50 of dipropylene glycol dibenzoate to the earthworm was found to be in excess of 1000 ppm. The NOEL was considered to be 1000 ppm. | [2] |
| Sludge | No inhibitory effect on the respiration rate of activated sludge at concentrations up to 100 mg/l. | [4] |
| Environmental Fate | ||
| BCF | - | |
| Aerobic biodegradation | 6% of TCO2 at 2 days 62% of TCO2 at 12 days 85% of TCO2 at 28 days Readily biodegradable |
[2] |
| The BOD5 of dipropylene glycol dibenzoate was 0.65 gO2/g (30% of its ThOD; 2.15 gO2/g) based on results obtained at a nominal concentration of 2 mg/l. The mean COD of dipropylene glycol dibenzoate (2.33 gO2/g) was 104% of its ThOD which confirmed that the material was completely oxidized in the COD test. The mean BOD5 of dipropylene glycol dibenzoate was 29% of its COD. For screening purposes, substances are generally considered readily biodegradable in this test if the ratio of BOD5:COD or ThOD ≥ 50%. Dipropylene glycol dibenzoate cannot therefore be considered to be readily biodegradable under the conditions of this test. Because this type of BOD test employs both a weak microbial inoculum and a relatively short incubation time, it can be considered to be a particularly stringent test of biodegradability. | [2] | |
| Anaerobic biodegradation | Dipropylene glycol dibenzoate was degraded to 46% after 60 days of incubation and 75% after 120 days of incubation, based on a nominal level of carbon in the culture at the start of the test (12 mgC). At Day 120 of the test, dipropylene glycol dibenzoate was degraded to 90% based on the theoretical carbon level (10 mgC) remaining in cultures following removal of samples for DIC analysis. The precise distribution of dipropylene glycol dibenzoate in test mixtures was not determined in this test so the level of carbon remaining in test mixtures after samples were removed for DIC analysis cannot be accurately determined. However, since the octanol:water partition coefficient for dipropylene glycol dibenzoate is relatively high (log Pow 3.9), it is likely that the material will adsorb onto sewage solids. Although the level of biodegradation calculated using the nominal level of carbon at the start of the test (12 mgC) gives the worst case estimate, it is likely to be the most accurate. Substances are considered to be ultimately degraded under anaerobic conditions in this test if the level of degradation is equal to or greater than 60%. Dipropylene glycol dibenzoate can therefore be considered ultimately biodegradable under anaerobic conditions. | [2] |
| Abiotic biodegradation | - | |
| Metabolic pathway | - | |
| Mobility | - | |
| Photodegradation | [2] | |
| Stability in water | - | |
| Transport (fugacity) | - | |
| Conclusion | ||
| Physical-chemical | - | |
| Emission | - | |
| Exposure | - | |
| Health | - | |
| Environment | - | |
| References | ||
| 1 | ESIS | |
| 2 | EPA HPV challenge programme | |
| 3 | Sigma Aldrich MSDS | |
| 4 | Genovique MSDS for Benzoflex 9-88 | |
| 5 | ChemID | |
| 6 | SRC PhysProp database | |
| 7 | MITI | |
Di-isononyl-cyclohexane-1,2dicarboxylate, DINCH
| Identification of the substance | ||
| CAS No. | 166412-78-8 (EU) 474919-59-0 (USA and Canada) |
[4] |
| EINECS No. | 431-890-2 | [1] |
| EINECS Name | 1,2-Cyclohexanedicarboxylic acid, 1,2-diisononyl ester | [2] |
| Synonyms | Di-isononyl-cyclohexane-1,2-dicarboxylate DINCH Hexamoll DINCH 1,2-Cyclohexanedicarboxylic acid, diisononyl ester (9CI) 1,2-Cyclohexanedicarboxylic acid, diisononyl ester, branched and linear |
|
| Molecular Formula | C26H48O4 | |
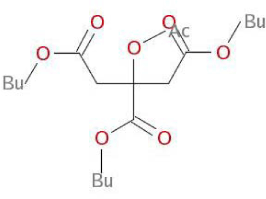
| Structural Formula |  |
|
| Major Uses | The major applications for the notified chemical will use it as a plasticiser and impact modifier in food packaging, but also in general applications such as wire and cable, automotive, plastisols and other similar applications. | [2] |
| IUCLID | - | |
| EU classification | - | |
| Physico-chemical Characteristics | ||
| Physical Form | Clear colourless liquid at 20ºC and 101.3 kPa Almost odourless |
[2], [3] |
| Molecular Weight (g/mole) | 424.6 | [2] |
| Melting Point/range (°C) | No freezing point Glass point < -90ºC Pour point = -54ºC |
[2] |
| Boiling Point/range (°C) | > 351ºC at 101.3 kPa | [2] |
| 240 – 250ºC at 7 mbar | [3] | |
| Decomposition Temperature (°C) | > 351ºC at 101.3 kPa, decomposes before boiling | [2] |
| Vapour Pressure (mm Hg at °C) | 2.2 x 10-8 kPa at 25ºC [2.2 x 10-5 Pa, 1.65 x 10-7 mmgHg] 8.9 x 10-7 kPa at 50ºC [8.9 x 10-4 Pa, 6.67 x 10-6 mmgHg] |
[2] |
| Density (g/cm3 at °C) | 0.947 at 20ºC | [2] |
| Vapour Density (air=1) | - | |
| Henry’s Law constant (atm/m³/mol at °C) | - | |
| Solubility (mg/l water at °C) | < 0.00002 g/l at 25ºC [< 0.02 mg/l] | [2] |
| Partition Coefficient (log Pow) | > 6.2 at 25ºC | [2] |
| pKa | - | |
| Flammability | Not highly flammable | [2] |
| Explosivity | Not explosive | [2] |
| Oxidising Properties | - | |
| Migration potential in polymer | - | |
| Viscosity | 44-60 mPa.s at 20ºC | [2] |
| Absorption/Desorption (log Koc) | > 5.6 at 23ºC | [2] |
| log Kow | 10 | [4] |
| Autoignition temperature | 330ºC | [2], [3] |
| Flash point | 224ºC | [2], [3] |
| Emission Data | ||
| During production | - | |
| Exposure Data | ||
| Aquatic environment, incl. sediment | - | |
| Terrestrial environment | - | |
| Sewage treatment plant | - | |
| Working environment | 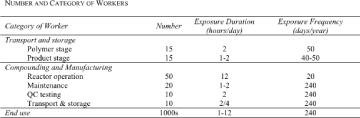 |
|
| Transport and storage The notified chemical will be transported by road to a warehouse and then to the compounding facility. Exposure of receivers and transport personnel should only occur in the event of an accidental spillage. |
[2] | |
| Compounding Incidental skin contact with the notified chemical may occur when the storemen insert the drum lance into the 200 L drum, or during connection of an IBC or isotank to the weighing vessel. Inhalation exposure to vapours may also occur during the transfer process. After mixing, intermittent skin contact may occur during the packaging process, from powdered blend or liquid plastisol. Quality control samples may also be taken at this stage, as technical personnel will make up small-scale compounds by hand in the laboratory. During the subsequent compounding of dry blend into pellets, closed systems are used, and any exposure will be incidental. However, manual operations during this process may include opening of packages, connection/insertion of lines/hoses, pumping liquid products, and eventual removal of connections and closing the containers. In addition, maintenance workers may experience skin contact with the notified chemical. For the specific formulation sites in Australia, a pproximately one third of the production time for the operators will be dedicated to running compound. During production runs (which can be up to 5 days long), the operators will work two 12-hour shifts, 5 days per week, and 48 weeks per year. Workers will prepare approximately 8 batches per day. Given the time that it takes to connect up and transfer product, the estimated period of direct contact with the notified chemical is less than 30 minutes per day for one person per shift. Local exhaust ventilation will be employed at all workplace areas where natural ventilation is considered inadequate. Workers, particularly for those operators involved in any open transfer operations, wear personal protective equipment (PPE) including overalls, safety glasses/goggles and face splash shields, protective gloves, and are assumed to operate using appropriateindustrial hygiene practices. |
[2] | |
| Product manufacture Exposure to the notified chemical may occur during the processing of PVC compound or plastisol to manufacture the end-use product. Once compounded with PVC, the notified chemical is bound within the PVC matrix and exposure is unlikely. However, during product manufacture by processes such as extrusion, calendering and injection moulding, the elevated temperatures required may result in inhalation exposure to the notified chemical, whether from vapours or aerosols. The methods for product manufacture from plastisols include spread coating, under-body coating, sealing, rotational coating, dipping and slush moulding. Although the processes are largely automated and enclosed, incidental skin contact with the notified chemical may occur during transfer of plastisol from drums to the moulding equipment. Workers are expected to wear PPE including overalls, gloves and eye protection. |
[2] | |
| End-use of products Under normal circumstances, dermal exposure to the notified chemical is not expected during handling of PVC products, as it is expected to be physically bound within the PVC matrix. Exudation may occur during any heating of plastics, leading to possible skin and inhalation exposure to low levels of the notified chemical. |
[2] | |
| Occupational exposure estimation For dermal exposure of workers involved in handling of the notified chemical during compounding and/or product manufacture, assuming non-dispersive use with some intermittent direct contact, EASE exposure modelling estimates the dermal exposure to the notified chemical to be 0-0.1 mg/cm²/day (EC, 2003). However, the use of EASE for accurately predicting dermal exposures is thought to be limited in accuracy (EC, 2003). The RISKOFDERM project, based on measurements of industrial exposures, describes exposure levels to the hands fo r the addition of liquids into "large containers (or mixers) with large amounts (many litres) of liquids" (Marquart et al, 2006). In this study, a typical case exposure was described as 0.5 mg/cm²/scenario, though a reasonable worst-case exposure was described as 14 mg/cm²/scenario. Therefore, based on a reasonable exposure frequency of once daily and a whole-hand exposure (420 cm²) to a 60 kg adult, a typical dermal exposure of 3.5 mg/kg bw/day is assumed. Worst-case, infrequent (whole-hand) exposures may be as high as 98 mg/kg bw/scenario. Assuming a closed system with LEV, a highest-probable process temperature of 220ºC (and excluding the possibility of aerosol formation), EASE estimates that the gas/vapour exposure to the notified chemical is likely to be 0-1.8 mg/m³ (0-0.1 ppm) (EC, 2003). The same value is estimated for an identical system at 25ºC. Therefore as a worst-case estimate, a 60 kg adult male worker exposed to vapours with an inhalation rate of 25.5 m³/12-hour shift during medium activity (EC, 2003), might experience inhalation exposure to the no tified chemical of 0-0.77 mg/kg bw/day. |
[2] | |
| Therefore, excluding oral exposure and assuming 10% dermal and 100% inhalation absorption (EC, 2003), the typical exposure during handling of the notified chemical is estimated to be 0.35-1.12 mg/kg bw/day. | [2] | |
| Consumer goods | The notified chemical has undergone assessment by the European Food Safety Authority in September 2006 (EFSA, 2006). For this assessment, the specific migration of the notified chemical was measured using various food simulants and representative foodstuffs, under different storage conditions. The specific migration of 10-17.8% notified chemical in plasticised PVC cling film into food simulants and foodstuffs was determined using a validated Gas Chromatography/Mass Spectrometry (GC/MS) method (Otter, 2007): | [2] |
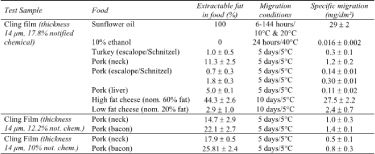 |
||
| The notified chemical was found to migrate into foods with high fat content (e.g. .29 mg/dm² into sunflower oil, and .27.5 mg/dm² into high fat cheese). The migration of the notified chemical into food like fresh meat and low fat cheese was lower than that of foods containing higher fat levels (<2.4 mg/dm²). The level of notified chemical in fresh meat at equilibrium was found to be proportional to the starting concentration in the cling film and relative to the fat content of the foods. In fatty foods, migration to equilibrium was achieved after 6 hours of contact. Likewise, extraction studies from bottle closures using isooctane (in which the notified chemical is very soluble) show that it is able to extract an equilibrium concentration of the notified chemical after 5.3 hours. For the use of the notified chemical in bottle sealing gaskets, artificial wine corks and beverage tubes, migration of the notified chemical into mineral water, grapefruit juice, soft drink or 15% ethanol was found to be very low (generally less than 0.11 mg/L, its solubility in 15% ethanol). This level of migration of the notified chemical is expected to apply for all aqueous foods (except alcoholic drinks with high ethanol content) as the low aqueous solubility of the notified chemical would limit its migration. Migration of the notified chemical from polystyrene (at the proposed use concentration) is expected to be lower than that from PVC. A test study using notified chemical-containing polystyrene sticks showed no migration of the notified chemical into olive oil or aqueous 10% ethanol (after 10 days at 40ºC) above the detection limit of the analytical method (unpublished study provided by the notifier). Very low levels of migration of the notified chemical from polystyrene into aqueous 50% ethanol were observed. For conveyor belts, migration into solid or semi-solid foods is expected to be limited by contact area and short contact times. Computer modelling of fatty food with .30 minutes contact time on a conveyor belt containing 12% notified chemical estimates specific migration rates of 12.4 mg/dm² at 20ºC and 6.6 mg/dm² at 10ºC (Otter, 2007). Therefore, assuming that migration into most foods will be considerably less than migration into oil, and that only the bottom of food is in contact with the conveyor belt (1 dm²/kg), the migration of the notified chemical is expected to be <5 mg/kg food for 0.30 minutes contact time. |
[2] | |
| Man exposed from environment | - | |
| ”Secondary poisoning” | - | |
| Atmosphere | - | |
| Dermal | Members of the public are likely to make limited dermal contact with food packaging, wires, cables and/or automotive parts containing the notified chemical. Significant exposure to the notified chemical in plastic products as a result of casual contact during handling is not expected, as it is expected to be sufficiently bound within the plastic matrix. However, as the notified chemical will not be chemically bound, it may be released from products in low levels over time (e.g. volatilisation from car upholstery). The expected dermal exposure from prolonged contact with plastics containing the notified chemical cannot be accurately estimated, but may be significant as the notified chemical may partition from the plastic into the skin over time. | |
| Toxicological data | ||
| Observations in humans | - | |
| Acute toxicity | ||
| Oral | LD50 > 5000 mg/kg (rat) | [2] |
| Dermal | LD50 > 2000 mg/kg (rat) | [2] |
| Inhalation | - | |
| Other routes | - | |
| Skin irritation | Slightly irritating (rabbit) | [2] |
| Eye irritation | Not irritating (rabbit) | [2] |
| Irritation of respiratory tract | - | |
| Skin sensitisation | No evidence of skin sensitisation | [2] |
| Subchronic and Chronic Toxicity | ||
| Oral | Rat, 28-day oral repeat dose NOAEL = 318 mg/kg (male) NOAEL = 342 mg/kg (female) |
[2] |
| Rat, 90-day oral repeat dose NOAEL = 107.1 mg/kg (male) NOAEL = 389.4 mg/kg (female) |
[2] | |
| Rat, 2-year chronic toxicity/carcinogenicity NOAEL = 40 mg/kg (male) NOAEL = 200 mg/kg (female) |
[2] | |
| Inhalation | - | |
| Dermal | - | |
| Metabolism | After oral administration DINCH showed rapid but saturable absorption and extensive elimination 24 hours after dosing approximately 80% of the radioactivity is excreted, after 48 hours more than 90 % is excreted via urine and mainly via feces.Based on the amounts of radioactivity excreted in the bile and urine, the bioavailability of 14C-1,2-yclohexanedicarboxylic acid di(isononyl)ester is estimated to be 5-6% at the high dose and 40-49 % at the low dose. There is no indication of bioaccumulation. The characterisation of metabolites after oral and intravenous administration of DINCH indicates two main pathways: the partial hydrolysis of DINCH to the mono-isonyl ester followed by conjugation to glucuronic acid, which is the most ab Undant metabolite in bile, or the hydrolysis of the remaining ester bond to yield free cyclohexane dicarboxylic acid, the predominant urinary metabolite. |
[4] |
| Mutagenicity, Genotoxicity and Carcinogenicity | ||
| Mutagenicity | S.typhimurium and E.coli (metabolic activator Aroclor 1254-induced rat liver (S9)) Not mutagenic to bacteria under the conditions of the test. |
[2] |
| Chinese hamster (CHO cells) (metabolic activator Aroclor 1254-induced rat liver (S9)) Not observed to induce mutations in CHO cells treated in vitro under the conditions of the test. |
[2] | |
| Chromosome Abnormalities | Chinese hamster (V79 cells) (Phenobabital/3-naphthoflavone-induced rat liver S9 mix) Not clastogenic to V79 cells treated in vitro under the conditions of the test. |
[2] |
| Mouse Not found to be clastogenic or aneuploidogenic under the conditions of this in vivo mouse micronucleus test. |
||
| Other Genotoxic Effects | - | |
| Carcinogenicity | Rat, 2-year chronic toxicity/carcinogenicity NOAEL = 40 mg/kg (male) NOAEL = 200 mg/kg (female) |
[2] |
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||
| Reproductive Toxicity | Rat, two-generation study (37 weeks) Under the conditions of this two-generation reproduction study, the NOAEL for fertility and reproductive performance is 1000 mg/kg bw/day for F0 and F1 generation rats of both genders. The NOAEL for general toxicity is 1000 mg/kg bw/day (F0 rats of both genders) and 100 mg/kg bw/day for the F1 male and female rats (based on tubular vacuolisation and flaky thyroid follicular colloid). The NOAEL for developmental toxicity (growth and development of offspring) was 1000 mg/kg bw/day for the F1 and F2 pups. |
[2] |
| Teratogenicity | - | |
| Developmental toxicity | Rabbit (exposure day 6 to day 29 post insemination) NOAEL = 1000 mg/kg, based on maternal and prenatal developmental toxicity. |
[2] |
| Rat (exposure day 6 to day 19 post coitum) NOAEL = 1200 mg/kg, based on maternal and prenatal developmental toxicity. |
[2] | |
| Pre-/Postnatal developmental toxicity | Rat (exposure day 6 to day 20 post partum) Based on the conditions of this study, the No Observed Adverse Effect Level (NOAEL) for reproductive performance and systemic toxicity of the parental female rats is 1000 mg/kg bw/day. The NOAEL for developmental toxicity (based on the growth and development of the offspring, including sexual organ morphology and sexual maturation) is also 1000 mg/kg bw/day for F1 progeny. |
[2] |
| Toxicokinetics | ||
| Toxicokinetics | Rat, toxicokinetics and metabolism Distribution to all organs and tissues was observed after rapid absorption. The oral bioavailability was calculated to be ~5-6% of a high dose and ~40-49% of a low dose, indicating saturation of gastrointestinal absorption. Accumulation was not observed in rats, and excretion was rapid, mainly via the faeces. Metabolism to several major metabolites: cyclohexanedicarboxylic acid (urine), monoisononyl cyclohexanedicarboxylate (faeces) & the glucuronide of monoisononyl cyclohexanedicarboxylate (bile) |
[2] |
| Ecotoxicity Data | ||
| Algae | Scenedesmus subspicatus Biomass EC50 > 100 mg/l WAF (72h) NOEC ≥ 100 mg/l WAF Growth EC50 > 100 mg/l WAF (72h) NOEC ≥ 100 mg/l WAF |
[2] |
| Daphnia magna (acute) | LC50 > 100 mg/l WAF (48h, daphnia magna) ("Water Accommodated Fraction" (WAF) NOEC = 100 mg/l WAF (48h) |
[2] |
| Daphnia magna (chronic) | Daphnia magna (21 days) NOEC ≥ 0.021 mg/l LOEC ≥ 0.021 mg/l LCD ≥ 0.021 mg/l |
[2] |
| Other aquatic organisms | - | |
| Fish | LC50 > 100 mg/l (96h, zebra fish) NOEC = 100 mg/l (96h) |
[2] |
| Bacteria | - | |
| Terrestrial organisms | LC0 > 1000 mg/kg (earth worm, 14 day) LC50 > 1000 mg/kg (earth worm, 14 day) LC100 > 1000 mg/kg (earth worm, 14 day) |
|
| Sludge | EC50 >1000 mg/L (nominal) The oxygen concentration decreased more significantly for the test substance than for the blank controls. The oxygen consumption rate did not differ between the test substance and the controls. The oxygen consumption rate of the reference substance is significantly lower than both the rate of the test substance and the blank samples and the reference met the validity criteria (EC50 = 6.5 mg/L). |
[2] |
| Higher plants | The EC50 test results, relating to dry mass of the soil, for all three species (Avena sativa, Brassica napus and Vicia sativa) are as follows: EC50 (emergence rate) >1000 mg/kg (nominal) EC50 (dry matter) >1000 mg/kg (nominal) EC50 (fresh matter) >1000 mg/kg (nominal) EC50 (shoot length) >1000 mg/kg (nominal) The NOEC/LOEC tests results relating to the dry mass of the soil for all the three species (Avena sativa, Brassica napus and Vicia sativa) are as follows: NOEC/LOEC (emergence rate) .1000 mg/kg (nominal) NOEC/LOEC (dry matter) .1000 mg/kg (nominal) NOEC/LOEC (fresh matter) .1000 mg/kg (nominal) NOEC/LOEC (shoot length) .1000 mg/kg (nominal) |
[2] |
| Environmental Fate | ||
| BCF | BCF = 189.3 DT50 = 0.5 (low conc) DT50 = 0.6 (high conc) Not likely to bioaccumulate |
[2] |
| Aerobic biodegradation | BILLEDE The substance is not readily biodegradable. |
[2] |
| Anaerobic biodegradation | - | |
| Metabolic pathway | - | |
| Mobility | - | |
| Conclusion | ||
| Physical-chemical | - | |
| Emission | - | |
| Exposure | - | |
| Health | - | |
| Environment | - | |
| References | ||
| 1 | ESIS | |
| 2 | NICNAS | |
| 3 | BASF MSDS | |
| 4 | SCENIHR | |
Di (2-ethyl-hexyl) terephthalate, DEHT, DOTP
| Identification of the substance | ||
| CAS No. | 6422-86-2 | |
| EINECS No. | 229-176-9 | [1] |
| EINECS Name | bis(2-ethylhexyl) terephthalate | [1] |
| Synonyms | Di-(2-ethyl-hexyl)-terephthalate DEHT DOTP |
|
| Molecular Formula | C24H38O4 | [1] |
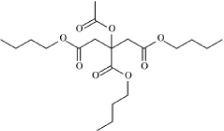
| Structural Formula | 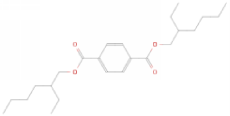 |
|
| Major Uses | Softeners | [2] |
| IUCLID | Not listed | [1] |
| EU classification | This substance is not classified in the Annex I of Directive 67/548/EEC as such, but it may be included in one of the group entries. | [1] |
| Physico-chemical Characteristics | ||
| Physical Form | Liquid | [2] |
| Colourless, mobile and highly volatile liquid with a pleasant odour. | [3] | |
| Molecular Weight (g/mole) | 390.557 | [3] |
| Melting Point/range (°C) | -48ºC | [2], [3] |
| Boiling Point/range (°C) | 383ºC at 1015 hPa | [2], [3] |
| 400ºC | [7] | |
| Decomposition Temperature (°C) | - | |
| Vapour Pressure (mm Hg at °C) | 0.0000285 hPa at 25ºC [2.85 x 10-3 Pa, 2.13 x 10-5 mmgHg] 1013 hPa at 398ºC [1.013 x 105 Pa, 757.8 mmgHg] |
[2] |
| 2.14 x 10-5 mm Hg at 25ºC [2.85 x 10-3 Pa] | ||
| 1.33 mbar at 217ºC [133 Pa , 0.99 mmHg] 5.56 x 10-10 mbar at 25ºC [5.56 x 10-8 Pa , 4.17 x 10-10 mmHg] |
[7] | |
| Density (g/cm3 at °C) | 0.984 at 20ºC | [2] |
| 0.9825 at 20ºC | [3] | |
| Vapour Density (air=1) | 13.5 | [3] |
| Henry’s Law constant (atm/m³/mol at °C) | 1.18 x 10-5 | [2] |
| 1.02 x 10-5 25ºC | [3] | |
| Solubility (mg/l water at °C) | 0.4µg/l at 22.5ºC [GLP study from 2002] The aqueous solubility of DOTP has been recently determined to be 0.0004 mg/L (0.4 ppb) at 22.5 C using the slow-stir method. Results of earlier solubility studies have been reported that are significantly higher: values of 0.35 mg/L in well water, 0.61 mg/L in sea-water, and 1.5 mg/L in de-ionized water, all at 25 C. However, these earlier studies were performed using the shake-flask technique, which is no longer considered appropriate for oily hydrophobic substances such as DOTP. |
[2] |
| Partition Coefficient (log Pow) | 8.39 | [2] |
| pKa | - | |
| Flammability | - | |
| Explosivity | - | |
| Oxidising Properties | - | |
| Migration potential in polymer | - | |
| Flash point | 238ºC open cup | [2] |
| Auto flammability | 399ºC | [2] |
| Atmospheric OH rate constant cm³/(molecule sec) | 21.9554 x 10-12 | [2] |
| 2.2 x 10-11 at 25ºC | [4] | |
| Log Kow | 8.39 | [3] |
| Emission Data | ||
| During production | Minimal potential for air pollution. Material has a very low volatility. | [2] |
| Exposure Data | ||
| Aquatic environment, incl. sediment | Bis(2-ethylhexyl) terephthalate's production and subsequent use as a plasticizer may result in its release to the environment through various waste streams | [3] |
| Terrestrial environment | - | |
| Sewage treatment plant | - | |
| Working environment | Production uses a closed system. Exposure could occur when chemical is put into drums or during quality control. | [2] |
| Occupational exposure to bis(2-ethylhexyl) terephthalate may occur through inhalation of aerosols and dermal contact with this compound at workplaces where bis(2-ethylhexyl) terephthalate is produced or used. Use data indicate that the general population may be exposed to bis(2-ethylhexyl) terephthalate via dermal contact from products containing this compound. | [3] | |
| Consumer goods | Minimal consumer exposure expected based on limited use in consumer products and low migration of the substance out of the polymer matrix in it's major use as a plasticizer. | [2] |
| Man exposed from environment | - | |
| ”Secondary poisoning” | - | |
| Atmosphere | - | |
| Dermal | - | |
| Toxicological data | ||
| Observations in humans | Primary dermal irritation There was one adverse event reported during the course of the study that was not related to test-substance exposure. Overall irritation scores ranged from 0.00 to 0.11. Since the irritation did not occur in a concentration-dependent manner, it was not considered to be related to test substance exposure. |
[2] |
| Skin sensitization Under the conditions of this study, DOTP was found to be non-irritating and did not elicit evidence of sensitization. There were nine adverse events which occurred during the study, one of them severe. One of the events (soreness at the test site location) was clearly related to test substance exposure. However, this reaction was to another material being tested, not DOTP. Another event, (papular rash) was possibly related to test substance exposure. The rest of the events were unrelated to test substance exposure. Slight erythema was observed for one to seven subjects at any given time during the induction phase of the study, and for only one subject during the challenge phase of the study. |
[2] | |
| Acute toxicity | ||
| Oral | LC50 > 5000 mg/kg (rat, combined) | [2] |
| LC50 > 3200 mg/kg (rat, male) | [2] | |
| LC50 > 3200 mg/kg (mouse, male) | [2] | |
| LDL0 = 20,000 mg/kg (mouse) | [5] | |
| Dermal | LC50 > 19670 mg/kg (guinea pig, male) | [2] |
| Inhalation | Acute Exposure/ No deaths occured following inhalation exposure of mice for 4 hr to "saturated" vapors; however, mucosal irritation, loss of coordination and decreased mobility were noted. Recovery occured in 24 hours. | [3] |
| Other routes | - | |
| Skin irritation | Slightly irritating (guinea pig, 24h) | [2] |
| Not irritating (human) | [7] | |
| Eye irritation | Slightly irritating (rabbit) | [2] |
| Irritation of respiratory tract | - | |
| Skin sensitisation | Not sensitizing (guinea pig) | [2] |
| Subchronic and Chronic Toxicity | ||
| Oral | Rat, 90-day repeat dose NOEL = 277 mg/kg (male) NOEL = 309 mg/kg (female) |
[2], [3] |
| Di(2-ethylhexyl) terphthalate was evaluated for subchronic toxicity in Charles River rats (17-20/sex/group) fed diets containing concentrations of 0, 0.1, 0.5, or 1.0% for 90 days. There were statistically significant differences between treated and control animals in the following: decreased mean corpuscular hemoglobin (1% animals, 0.5% females), mean corpuscular volume (0.5% and 1% animals), hemoglobin (1% and 0.1% males), hematocrit (1% males), and serum glucose (0.1% females), and increased relative liver weight (1% animals). Variations in red blood cell morphology were observed in all groups (and therefore not considered to be treatment-related) including microcytosis, anisocytosis, poikilocytosis, and spherocytosis. No treatment-related gross or microscopic abnormalities were observed. There were no consistent, significant, exposure-related differences between treated and control animals in the following: mortality, body weight gain, food consumption, clinical signs of toxicity, clinical chemistry (except serum glucose), and absolute and relative organs weights (except relative liver weight) | [3] | |
| Rat, 21-day repeat dose NOEL = 505 mg/kg (male) NOEL = 487 mg/kg (female) |
[2] | |
| Rat, 14-day repeat dose NOEL = 885 mg/kg |
[2] | |
| Inhalation | Rat, 10-day exposure 6h per day NOEL = 46.3 mg/m³ |
[2] |
| Dermal | - | |
| Metabolism | Rat Approximately 25% of DEHT was hydrolyzed to 2EH- after about 10 minutes. The rest of the compound remained unchanged, and there was no evidence to suggest that 2EH was metabolized further. After 30 minutes, the stoichiometry indicated 2 moles of 2EH had been formed per mole of DEHT, indicating complete hydrolysis. The half-life of DEHT was calculated to be 53.3 minutes. |
[2] |
| Rat The results obtained with DEHT are in contrast to DEHP, which is hydrolyzed to 2-ethylhexanol and the monoester MEHP. The mean total recovery of 14C was 93.0 +/- 2.2%. Most of the radioactivity was eliminated in the feces (56.5 +/- 12.1%) and urine (31.9 +/- 10.9%), with smaller amounts in expired air (3.6 +/- 0.9%). Approximately 1.4 +/- 0.6% of the dose remained in the carcass. Of the approximately one half of the material that was absorbed, 73% was excreted in the urine and 8.3% was completely metabolized to 14CO2. About half (50.5%)of the total dose (77% of the absorbed dose) was detected as unlabelled terephthalic acid (TPA) in the urine, indicating that complete hydrolysis of the diester had taken place. Almost one-third of the radioactivity in the urine (10 % of the dose) was present as glucuronide and sulphate conjugates. Several oxidation products of mono(2-ethylhexyl) phthalate (MEHT) and 2-ethylhexanol (2-EH)were identified as minor components. Between 90-95 % of the total activity in the feces was unchanged [14C]DEHT (36.6% of the total dose). MEHT (2.5% of total dose) and the glucuronide conjugate of 2-ethyl5-hydroxyhexanoic acid were also identified. Several other minor uncharacterized radiolabelled metabolites were detected that were more polar than DEHT. The mean total amount of material recovered in the urine (as unlabeled TPA) and fecal fractions (as unmetabolized DEHT) was 87.1%, indicating that a major portion of the dose passed directly through the GI tract without hydrolysis or was completely hydrolyzed to TPA and 2-EH before or after absorption. The rates of excretion of radioactivity in urine and feces revealed that more than 95 and 99% of the total radioactivity was excreted by 24 and 48 hours, respectively. The time course for expired 14CO2 was complex, with peaks at 1.3, 2.8, and 5.0 hours after dosing. Approximately 68% of the total radioactivity in expired air was eliminated after 4 hours and the remaining 32% was eliminated after 40 hours. The excretion of radioactivity in the feces, urine and expired air from animals given food 4 hours after dosing was comparable to the levels seen for animals given food immediately after dosing. |
[2], [3] | |
| Absorption | Rats readily excreted (14)C EHT given in a single dose at 100 mg/kg. 57.9%, 28.6%, and 3.6% of the (14)C was recovered in the feces, urine an expired air, respectively, within 144 hr after admin. Only 2.1% remained in the carcass | [3] |
| Absorption, distribution and excretion | The hydrolysis of di(2-ethylhexyl) terephthalate and di(2-ethylhexyl) phthalate were studied using rat gut homogenate fractions in vitro. Both isomers were hydrolysed by the intestinal fraction; however di(2-ethylhexyl) phthalate was hydrolysed to 2-ethylhexanol and mono(2-ethylhexyl) phthalate in about equal proportions whereas di(2-ethylhexyl) terephthalate was hydrolysed to 2-ethylhexanol and terephthalic acid. The half-lives for disappearance of the diesters were determined to be 12.6 min for di-(2-ethylhexyl) phthalate and 53.3 min for di(2-ethylhexyl) terephthalate. 2. The absorption and metabolism of di(2-ethylhexyl) terephthalate were studied by administering (hexyl-(14)C)di(2-ethylhexyl) terephthalate (in corn oil) by oral gavage at a dose level of 100 mg/kg to 10 adult male Sprague Dawley rats. Urine feces and expired air were collected for 144 hr and analysed for thepresence of radioactivity and feces and urine were analysed for unlabelled metabolites. 3. Radioactivity was eliminated in feces (56.5 +/- 12.1% of dose) primarily as unchanged di(2-ethylhexyl) terephthalate, small amounts of mono(2-ethylhexyl) phthalate and polar metabolites; excreted in urine (31.9 +/- 10.9% of dose) principally as mono(2-ethylhexyl) phthalate and metabolic products of 2-ethylhexanol; and expired as (14)C02 (3.6 +/-0.9% of dose). Less than 2% of the administered radioactivity was found in the carcass. Small amounts of (14)C were found in the tissues with the highest amounts found in liver and fat. 4. Metabolites identified in urine included terephthalic acid (equivalent to 51% of dose), oxidized metabolites of 2-ethylhexanol and mono(2-ethylhexyl) phthalate, and glucuronic and sulfuric acid conjugates (equivalent to about 10% of dose). 5. These findings indicate that di(2-ethylhexyl) terephthalate was hydrolysed more extensively than di(2-ethylhexyl) phthalate and consequently the urinary metabolite profiles for these two isomeric plasticizers were very different. The hydrolysis and metabolism of di(2-ethylhexyl) terephthalate were found to be similar to those of di(2-ethylhexyl) adipate in that hydrolysis of both ester bonds occurs. | [3] |
| Mutagenicity, Genotoxicity and Carcinogenicity | ||
| Mutagenicity | Salmonella typhimurium (metabolic activator induced rat liver (S9)) There was no increase in the number of revertants for any of the five strains either in the presence or absence of metabolic activation up to dose levels of 10,000 µg/plate. |
[2], [3], [6] |
| Chinese hamster (CHO cells) (metabolic activator induced rat liver (S9)) In the cultures treated with DOTP, no statistically significant increases in aberrations were observed at any dose level. |
[2] | |
| Chinese hamster (CHO cells) (metabolic activator induced rat liver (S9)) DOTP is negative in the CHO/HGPRT mammalian cell mutation assay at dose levels up to 20 nl/ml (the limit of the test). |
[2] | |
| Salmonella typhimurium (metabolic activator induced rat liver (S9) No mutagenic activity was detected with unmetabolized DEHT, and there was no evidence that mutagenic substances were excreted in the urine from rats dosed with DEHT. |
[2], [3] | |
| Chromosome Abnormalities | - | |
| Other Genotoxic Effects | - | |
| Estrogenic activity | NOAEL = 2000 mg/kg (rat, female) | [2] |
| Carcinogenicity | Bis(2-ethylhexyl) terephthalate was evaluated for combined chronic toxicity and carcinogenicity. The test substance was administered in the diets of male and female Fischer-344 inbred rats at concentrations of 20, 142, and 1000 mg/kg/day. Clinical evaluations revealed no treatment-related signs, however, eye opacities (cataracts) occurred frequently in all groups. At 1000 mg/kg/day, body weights and female liver weights were reduced. There were no consistent reductions in food consumption. There were no treatment-related effects evident from the gross and histopathologic examinations conducted at 6 and 12 months. At 18 months, two basic lesions of the females in the 1000 mg/kg/day level appear to be associated with treatment. These were hyperplasia and/or transitional cell adenomas of the urinary bladder and adenomas or adenocarcinomas of the uterus. | [3] |
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||
| Reproductive Toxicity | Rat, two-generation study NOAEL = 3000 ppm (Parental) NOAEL = 3000 ppm (F1 offspring) NOAEL = 3000 ppm (F2 offspring) |
[2] |
| Teratogenicity | Rat (exposure day 0 to 20 gestation) NOEL = 6000 ppm (maternal) NOEL = 10000 ppm (teratogenicity) |
[2] |
| Rat (exposure day 14 to postnatal day 3) NOEL = 750 mg/kg (maternal) NOEL = 750 mg/kg (teratogenicity) |
[2] | |
| Other Toxicity Studies | Skin absorption (in vitro) Dematomed human cadaver skin specimen The absorption rate of DEHT through dermatomed human skin was found to be 0.103 +/- 0.052 µg/cm²/hr. According to the criteria set forth by Marzulli (1969), DEHT would be considered an "extremely slow" penetrant relative to other chemical species. The mean damage ratio for skin treated with DEHT was determined to be 1.14 +/- 0.23. This value is within the range of damage ratios for skin exposed to physiological saline (Dugard et al 1884). Therefore, under the conditions of this study, DEHT did not cause significant damage to the skin. |
[2] |
| Male development NOEL > 750 mg/kg No effect on male organ development |
[7] | |
| Uterotrophic assay NOEL > 2,000 mg/kg No estrogenic activity Results of several robust studies following established guidelines to assess both reproductive and developmental toxicity potential have been conducted on Eastman 168 Plasticizer. The results of these studies indicate there is no evidence of such toxicities when tested in the diet at very high levels 1.0% (rats) and 0.7% (mice). Experimental studies have been conducted to evaluate the potential of DEHT to alter normal postnatal development in males and act as an estrogen agonist in females (uterotrophic assay). DEHT had no “endocrine”-like effects in either study. |
[7] | |
| Toxicokinetics | ||
| Toxicokinetics | - | |
| Ecotoxicity Data | ||
| Algae | EC50 > 0.86 mg/l (selenastrum capricornutum, 3h) NOEC ≥ 0.86 mg/l (selenastrum capricornutum, 3h) |
[2] |
| Daphnia magna (acute) | EC50 > 1.4 µg/l (daphnia magna, 48h) NOEC ≥ 1.4 µg/l (daphnia magna, 48h) |
[2] |
| EC50 > 984 mg/l (planorid snail, 96h) NOEC ≥ 984 mg/l (planorid snail, 96h) |
[2] | |
| EC50 > 624 µg/l (eastern oyster, 96h) NOEC ≥ 624 µg/l (eastern oyster, 96h) |
[2] | |
| Daphnia magna(chronic) | EC50 > 0.76 µg/l (daphnia magna, 21 day) NOEC ≥ 0.76 µg/l (daphnia magna, 21 day) LOEC > 0.76 µg/l (daphnia magna, 21 day) MATC > 0.76 µg/l (daphnia magna, 21 day) |
[2] |
| Other aquatic organisms | - | |
| Fish (acute) | LC50 > 984 mg/l (fresh water fish, 96h) NOEC ≥ 984 mg/l (fresh water fish, 96h) |
[2] |
| EC50 > 1000µg/l (Fathead minnow, 96h) NOEC ≥ 1000µg/l (Fathead minnow, 96h) |
[2] | |
| LC50 > 0.25 mg/l (Salmo gairdneri, 7 day) NOEC ≥ 0.25 mg/l (Salmo gairdneri, 7 day) |
[2] | |
| Fish (chronic) | LLC ≥ 0.28 mg/l (salmo gairdneri, 71 days) NOEC ≥ 0.28 mg/l (salmo gairdneri, 71 days) |
[2] |
| Bacteria | - | |
| Terrestrial organisms | - | |
| Sludge | EC50 > 10 mg/l (3h) NOEC ≥ 10 mg/l (3h) |
[2] |
| Terrestrial plants | EC50 > 1400 µg/l (lolium perenne, 14 days) NOEC ≥ 1400 µg/l (lolium perenne, 14 days) |
[2] |
| EC50 > 1500 µg/l (Williams 82 soybean, 14 days) NOEC ≥ 1500 µg/l (Williams 82 soybean, 14 days) |
[2] | |
| EC50 > 1400 µg/l (Raphanus sativus, 14 days) NOEC ≥ 1400 µg/l (Raphanus sativus, 14 days) |
[2] | |
| Environmental Fate | ||
| BCF | BCF = 393 | [2] |
| An estimated BCF of 25 was calculated in fish for bis(2-ethylhexyl) terephthalate, using an estimated log Kow of 8.39 and a regression-derived equation. According to a classification scheme, this BCF suggests the potential for bioconcentration in aquatic organisms is low | [3] | |
| Aerobic biodegradation | 40.2 % (28 days) Biodegradable |
[2] |
| BOD20 = 0.15 g/g COD = 2.7 g/g ThOD = 2.58 g/g |
[2] | |
| 56% in 28 days | [7] | |
| Anaerobic biodegradation | - | |
| Metabolic pathway | - | |
| Mobility | The Koc of bis(2-ethylhexyl) terephthalate is estimated as 2,000, using a water solubility of 4 mg/l and a regression-derived equation. According to a classification scheme, this estimated Koc value suggests that bis(2-ethylhexyl)terephthalate is expected to have slight mobility in soil. | [3] |
| Abiotic degradation | The rate constant for the vapor-phase reaction of bis(2-ethylhexyl) terephthalate with photochemically-produced hydroxyl radicals has been estimated as 2.2 x10-11 cm³/molecule-sec at 25ºC using a structure estimation method. This corresponds to an atmospheric half-life of about 18 hours at an atmospheric concentration of 5 x 10+5 hydroxyl radicals per cm³. A base-catalyzed second-order hydrolysis rate constant of 0.16 L/mole-sec was estimated using a structure estimation method; this corresponds to half-lives of 1.4 years and 51 days at pH values of 7 and 8, respectively. Bis(2-ethylhexyl) terephthalate does contain chromophores that absorb at wavelengths > 290 nm and therefore may be susceptible to direct photolysis by sunlight. | [3] |
| Volatilization | The Henry's Law constant for bis(2-ethylhexyl) terephthalate is estimated as 1.0 x10-5 atm m³/mole using a fragment constant estimation method. This Henry's Law constant indicates that bis(2-ethylhexyl) terephthalate is expected to volatilize from water surfaces. Based on this Henry's Law constant, the volatilization half-life from a model river (1 m deep, flowing 1 m/sec, wind velocity of 3 m/sec) is estimated as 7.3 days. The volatilization half-life from a model lake (1 m deep, flowing 0.05 m/sec, wind velocity of 0.5 m/sec) is estimated as 59 days. Bis(2-ethylhexyl) terephthalate's Henry's Law constant indicates that volatilization from moist soil surfaces may occur. Bis(2-ethylhexyl) terephthalate is not expected to volatilize from dry soil surfaces based upon an estimated vapor pressure of 2.1 x10-5 mm Hg, determined from a fragment constant method. | |
| Conclusion | ||
| Physical-chemical | - | |
| Emission | - | |
| Exposure | - | |
| Health | - | |
| Environment | - | |
| References | ||
| 1 | ESIS | |
| 2 | SIDS Dossier. OECD HPV Chemical programme | |
| 3 | HSDB | |
| 4 | SRC physprod database | |
| 5 | ChemID | |
| 6 | CCRIS | |
| 7 | EASTMANN | |
Sulfonic acids, C10 – C18-alkane, phenylesters, ASE
| Identification of the substance | ||
| CAS No. | 91082-17-6 | |
| EINECS No. | 293-728-5 | [1] |
| EINECS Name | Sulfonic acids, C10-21-alkane, Ph esters | [1] |
| Synonyms | Sulfonic acids, C10-C18-alkane, phenylesters ASE |
|
| Molecular Formula | - | |
| Structural Formula | - | |
| Major Uses | - | |
| IUCLID | HPV | [1] |
| EU classification | This substance is not classified in the Annex I of Directive 67/548/EEC as such, but it may be included in one of the group entries. | [1] |
| Physico-chemical Characteristics | ||
| Physical Form | Liquid | [2] |
| Light yellow clear liquid | [3] | |
| Molecular Weight (g/mole) | - | |
| Melting Point/range (°C) | < -15ºC | [2] |
| Boiling Point/range (°C) | 200ºC at 13hPa | [2] |
| Decomposition Temperature (°C) | Decomposes on heating > 200ºC | [3] |
| Vapour Pressure (mm Hg at °C) | < 0.0001 hPa at 20ºC [0.01 Pa, 7.5 x 10-5 mmHg] | [2] |
| Density (g/cm3 at °C) | 1.055 g/cm³ at 20ºC | [2] |
| Vapour Density (air=1) | - | |
| Henry’s Law constant (atm/m³/mol at °C) | - | |
| Solubility (mg/l water at °C) | 0.002 g/l at 22ºC [2 mg/l] | [2] |
| Partition Coefficient (log Pow) | > 6 | [3] |
| pKa | - | |
| Flammability | - | |
| Explosivity | - | |
| Oxidising Properties | - | |
| Migration potential in polymer | - | |
| Flash point | 210-240ºC | [2] |
| Auto flammability | - | |
| Atmospheric OH rate constant cm³/(molecule sec) | - | |
| Log Kow | - | |
| Emission Data | ||
| During production | - | |
| Exposure Data | ||
| Aquatic environment, incl. sediment | - | |
| Terrestrial environment | - | |
| Sewage treatment plant | - | |
| Working environment | - | |
| Consumer goods | - | |
| Man exposed from environment | - | |
| ”Secondary poisoning” | - | |
| Atmosphere | - | |
| Dermal | - | |
| Toxicological data | ||
| Observations in humans | - | |
| Acute toxicity | ||
| Oral | LD50 = 26,380-31,650 mg/kg (rat) | [2] |
| LD50 > 15,825 mg/kg (rat) | [2] | |
| Dermal | LD50 > 1055 mg/kg (rat) | [2] |
| Inhalation | - | |
| Other routes | - | |
| Skin irritation | Not irritating (rabbit, 24h exposure) | [2] |
| Not irritating (human, 8h exposure) | [2] | |
| Eye irritation | Not irritating (rabbit) | [2] |
| Irritation of respiratory tract | - | |
| Skin sensitisation | - | |
| Subchronic and Chronic Toxicity | ||
| Oral | Rat, 90-day repeat dose (male and female) NOAEL = 3000 ppm No death, no effect on behaviour, reduced body weight gain, increased feed (female) and water consumption (males) at the high dose level, absolute and relative liver weight is significantly deose-related at alle dose levels, kidney weight only increased at the high dose level, no substance-related histopathological effects (47 organs and tissue), opthalomological, hematological and clinical-chemical parameters within the normal range, slightly increased tromboplastin/time at the high dose. |
[2] |
| Rat, 25 day repeat dose (female) Test substance intake 360 and 1230 mg/kg bw per day No death, no effect on behaviour, no substance related histopathological effects (30 organs), haematological and clinical-chemical parameters within normal range. Absolute and relative liver weight significantly increased at 1230 mg/kg bw. |
[2] | |
| Rat, 43 day repeat dose Dose = 100 ppm (ca. 7.5 mg/kg bw per day) Treatment time related increasing amounts of test substance in the fat tissue (up to 25µg sulfonic acid C10-21-alkane) No accumulation was observed in the liver. |
[2] | |
| Rat, 28 day repeat dose Dose = 1000 ppm (ca. 75 mg/kg bw per day) Day 21: 235µg sulfonic acid C10-21-alkane per g fat tissue Day 43: 100µg sulfonic acid C10-21-alkane per g fat tissue An elimination half life of 15 days was calculated for the fat tissue. No accumulation was observed in the liver. |
[2] | |
| Rat, 49 day repeat dose Dose = 1000 ppm (ca. 75 mg/kg bw per day) Day 49: 290µg sulfonic acid C10-21-alkane per g fat tissue No accumulation was observed in the liver. |
||
| Rat, 6 weeks repeat dose Dose = 530 mg/kg bw Males: 2 days a week Females: daily No death, normal behaviour, no substance-related histopatholical effects (m/f 8/9 organs), male rats: no substance-related alteration in oxygen consumption, female rats: Allen-Doisy test negative. |
[2] | |
| Rat, 1 year repeat dose Dose: 265 and 530 mg/kg bw (2 days a week) Normal weight gain, no substance-related histopathological effects (m/f 9/10 organs), haematological parameters within the normal range, roentgenological findings at teeth and ankle-joint within normal range. |
[2] | |
| Inhalation | - | |
| Dermal | - | |
| Mutagenicity, Genotoxicity and Carcinogenicity | ||
| Mutagenicity | Salmonella typhimurium (metabolic activator induced rat liver (S9)) Negative |
[2] |
| Chinese hamster (V79 cells) (metabolic activator induced rat liver (S9)) Negative in the CHO/HGPRT mammalian cell mutation assay at dose levels up to 75µg/ml (the limit of the test). |
[2] | |
| Chromosome Abnormalities | Chinese hamster (V79 cells) (rat liver S9 mix) Not clastogenic to V79 cells treated in vitro under the conditions of the test. |
[2] |
| Other Genotoxic Effects | - | |
| Carcinogenicity | - | |
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||
| Reproductive Toxicity | Rat, two-generation study Dose: 530 mg/kg bw F0-generation: no effect on fertility F1-generation: normal weight gain, normal weight of endocrine organs, normal first oestrus F2-generation: no effect on fertility and body weight gain F3- generation: no effect on fertility and body weight gain |
[2] |
| Estrogenic activity | Negative Allen-Doisy test (see above) | [2] |
| Teratogenicity | - | |
| Toxicokinetics | ||
| Toxicokinetics | - | |
| Ecotoxicity Data | ||
| Algae | Non-toxic in a saturated aqueous solution (test concentration at 10 g/l). Scenedesmus subspicatus, 72h) | [2] |
| Invertebrates (acute) | Immobilization of test organisms (daphnia magna, 48h) at 10,000 mg/l | [2] |
| No immobilization of test organisms (daphnia magna, 48h) at 100 mg/l to 1000 mg/l | [2] | |
| Other aquatic organisms | - | |
| Fish (acute) | NOEC ≥ 10,000 mg/l (fresh water fish, 48h) | [2] |
| LC0 ≥ 100 mg/l (fresh water fish, 96h) | [2] | |
| LC50 > 10,000 mg/l (zebra fish, 48h) | [3] | |
| Bacteria | < 20 % inhibition at 1.2 mg/l (photobacterium phosphoreum, 30 minutes) | [2] |
| No inhibition at 500 mg/l (photobacterium phosphoreum, 30 minutes) | [2] | |
| Terrestrial organisms | - | |
| Sludge | EC50 > 10,000 mg/l (3h) | [2] |
| Environmental Fate | ||
| BCF | - | |
| Aerobic biodegradation | 31% after 28 days | [2] |
| Anaerobic biodegradation | - | |
| Metabolic pathway | - | |
| Mobility | - | |
| Conclusion | ||
| Physical-chemical | - | |
| Emission | - | |
| Exposure | - | |
| Health | - | |
| Environment | - | |
| References | ||
| 1 | ESIS | |
| 2 | IUCLID dataset | |
| 3 | MSDS Sigma-Aldrich | |
Glycerol Triacetate, GTA
| Identification of the substance | ||
| CAS No. | 102-76-1 | |
| EINECS No. | 203-051-9 | [1] |
| EINECS Name | triacetin | [1] |
| Synonyms | Glycerol triacetate GTA |
|
| Molecular Formula | C9H14O6 | [1] |
| Structural Formula | 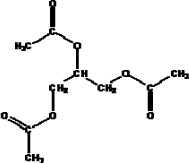 |
|
| Major Uses | Since triacetin has a variety of applications including as a plasticizer for cigarette filters and cellulose nitrate, solvent for the manufacture of celluloid, photographic films, fungicide in cosmetics, fixative in perfumery, component in binders for solid rocket fuels and a general purpose food additive, release of triacetin to the environment may occur at the production sites, specific industrial sites and consumers depending on the conditions of use in Japan | [2] |
| IUCLID | HPV | [1] |
| EU classification | This substance is not classified in the Annex I of Directive 67/548/EEC as such, but it may be included in one of the group entries. | [1] |
| Physico-chemical Characteristics | ||
| Physical Form | Liquid | [2] |
| Colourless liquid with a fruity or fatty odour and a mild sweet tast that becomes bitter in cons. above 0.05% | [3] | |
| Molecular Weight (g/mole) | 218,21 | [2] |
| pH | 7 | [2] |
| Melting Point/range (°C) | 3ºC | [2] |
| Boiling Point/range (°C) | 258ºC at 1,013 hPa | [2], [3] |
| Decomposition Temperature (°C) | - | |
| Vapour Pressure (mm Hg at °C) | 0.00248 mmHg at 25ºC [0.33 Pa] 0.003306hPa at 25ºC |
[2] |
| < 0.01 hPa at 20ºC [1 Pa, 0.0075 mmHg] | [4] | |
| Density (g/cm3 at °C) | 1.1562 at 25ºC | [2], [4] |
| Vapour Density (air=1) | 7.52 | [3] |
| Henry’s Law constant (atm/m³/mol at °C) | 1.23 x 10-8 | [2] |
| 1.2 x 10-8 at 25ºC | [3] | |
| Solubility (mg/l water at °C) | 70 g/l at 25ºC [70,000 mg/l] | [2], [4] |
| 58 g/l at 25ºC [58,000 mg/l] | [2], [4] | |
| Partition Coefficient (log Pow) | 0.21 at 25ºC | [2] |
| pKa | None | [2] |
| Flammability | Not flammable | [2], [4] |
| Auto flammability | 432ºC | [2], [4] |
| Explosivity | Not explosive | [2], [4] |
| Oxidising Properties | Stable at normal temperatures and pressures under fire exposure conditions | [2] |
| Migration potential in polymer | - | |
| Flash point | > 145ºC | [2], [4] |
| 138ºC | [3], [4] | |
| Koc | 10.5 | [2] |
| logKow | 0.36 | [3] |
| Atmospheric OH rate constant cm³/(molecule sec) | 7.810 x 10-12 | [2] |
| 8.5 x 10-12 at 25ºC | [3] | |
| Explosion limits | Lower: 1.05% Upper: 7.73% |
[6] |
| Emission Data | ||
| During production | The production process consists of the batchwise controlled reaction of glycerol, acetic acid and acetic anhydride in a closed reaction system with adequate cooling facilities. This is followed by purification using vacuum distillation. The pungent nature of the raw materials demands a totally enclosed plant. | [4] |
| Exposure Data | ||
| Aquatic environment, incl. sediment | Environmental exposure: emission to aquatic compartment from waste water and evaporative emissions associated with its use in the perfume and cosmetic industries and its use as a solvent and CO2 remover from natural gas, and disposal of consumer products containing triacetin. | [2] |
| SURFACE WATER: Triacetin was detected in a sample of Tennessee River water collected in Apr 1973 (concn not reported). | [3], [4] | |
| RAIN/SNOW: Triacetin was found in 1 out of 10 snow samples collected early March, 1998 from Finland, Russia and Siberia. The sample that contained 0.8 µg/kg of triacetin was from Moscow State University, Russia. | [3] | |
| Terrestrial environment | - | |
| Sewage treatment plant | Triacetin's production and use as a topical antifungal, fixative in perfumery, plasticizer, specialty solvent, as well as its use in the manufacture of cosmetics and removal of carbon dioxide from natural gas may result in its release to the environment through various waste streams. | [3] |
| EFFLUENT CONCENTRATIONS: Triacetin was detected in secondary effluent samples from a rapid infiltration site in Fort Polk, LA sampled November 4-5, 1980 at concentrations of 0.024 and 0.51 µg/l. | [3] | |
| Working environment | Occupational exposure: inhalation and dermal route in the industries. | [2] |
| NIOSH has statistically estimated that 18,436 workers (4,103 are female) are potentially exposed to triacetin in the USA. Occupational exposure to triacetin may occur through inhalation and dermal contact with this compound at workplaces where triacetin is produced or used. The general population may be exposed to triacetin via inhalation and dermal contact from use of consumer products containing this compound. | [3] | |
| Consumer goods | Consumer exposure: intake and dermal/inhalation route through the use as a food additive and topical antifungal and perfume fixative or cigarette filter, respectively. | [2] |
| Due to its uses triacetin soon becomes diffused into small quanties and there is little possibility of large scale human contact or environmental effect after it leaves the manufacturers first line customers' premises. Its use is adhesive may give skin contact. As a carrier and solvent for food soft drinks flavouring materals ingestion will occur. Very small amounts can be found in cigarette smoke through a filter tip using triacetin as the plasticizer. | [4] | |
| Man exposed from environment | - | |
| ”Secondary poisoning” | - | |
| Atmosphere | - | |
| Dermal | - | |
| Toxicological data | ||
| Observations in humans | Very mild skin reaction One case report of contact eczema |
[2] |
| Ingestion: 7.8 mg/day/adult | [2] | |
| Commercial triacetin may contain diacetin, as well as monoacetin, and when applied to human eyes causes severe burning, pain and much redness of the conjunctiva, but no injury. Diacetin causes considerably more discomfort than pure triacetin. | [2], [4] | |
| Glycerol triacetate appears to be innocuous when swallowed, inhaled or in contact with the skin, but may cause slight irritation to sensitive individuals. | [2], [4] | |
| A case of allergic contact eczema in a 29 year-old patient in a cigarette factory is reported, which was based on sensitisation towards the triacetin used for the production of cigarette filters. The allergy was demonstrated in a patch test. In addition to triacetin, the di- and mono acetate of glycerol also produced positive tests. It seems reasonable to regard the reaction as an expression of a group sensitisation towards glycerol acetate. | [2] | |
| A Duhring-chamber test was conducted on 20 healthy volunteers The test substance was applied as 50% dilution for 24 hours. Result: Only very mild skin reactions were observed. The substance has good skin compatibility. | [2], [4] | |
| No skin reactions occurred in 33 volunteers treated with 20% triacetin in petrolatum in an attempt to induce skin sensitisation using the maximization test. | [2] | |
| Triacetin (20 % in petrolatum) did not irritate the skin of 33 volunteers when tested in a 48-hr covered patch test. | [2] | |
| Acute toxicity | ||
| Oral | LD50 > 2,000 mg/kg (rat, combined) | [2], [4] |
| LD50 = 3,000 mg/kg (rat) | [2], [4] | |
| LD50 = 6,400-12,800 mg/kg (rat) | [2], [4] | |
| LD50 = 12,700 mg/kg (rat) | [2] | |
| LD50 = 9,300 mg/kg (mouse, male) | [2], [4] | |
| LD50 = 1,800 mg/kg (mouse, male) LD50 = 1,100 mg/kg (mouse, female) |
[2] | |
| LD50 = 3,200-6,400 mg/kg (mouse) | [2], [4] | |
| LD50 = 1,100 mg/kg (mouse) | [4] | |
| LD50 = 3,000 mg/kg (mouse) | [4] | |
| LD50 > 2,000 mg/kg (rabbit) | [4] | |
| LDL0 = 150 mg/kg (frog) | [4] | |
| Dermal | LD50 > 2,000 mg/kg (rabbit) | [2], [4] |
| LD50 > 5,000 mg/kg (rabbit) | [2], [4] | |
| LD50 > 20 ml/kg (guinea pig) | [2], [4] | |
| Inhalation | LD50 > 1,721 mg/m³ (rat, combined, 4h) No lethal effects observed |
[2], [4] |
| NOAEL = 73,700 mg/m³ (5 days) | [2] | |
| Other routes | - | |
| Skin irritation | Not irritating (rabbit) | [2], [4] |
| Eye irritation | Not irritating (rabbit) | [2], [4] |
| Irritation of respiratory tract | - | |
| Skin sensitisation | Not sensitising (guinea pig) | [2] |
| Subchronic and Chronic Toxicity | ||
| Oral | NOAEL = 1,000 mg/kg (rat, male) NOAEL = 1,000 mg/kg (rat, female) |
[2] |
| NOAEL = 10 g/kg per day (rat, 20% of diet) | [2] | |
| Inhalation | NOAEL = 2,220 mg/m³ (rat, 90 day) | [2] |
| NOAEL = 250 ppm (rat, 64 day) | [4] | |
| NOAEL = 8271 ppm (rat, 64 day) | [4] | |
| Dermal | - | |
| Metabolism | Triacetin has been administered iv to mongrel dogs. The majority of infused triacetin underwent intravascular hydrolysis, and the majority of the resulting acetate is oxidized. Triacetin was found to be hydrolyzed by human intestinal lipase. | [3] |
| Triacetin is rapidly hydrolysed in vitro by all tissues of the organism including the gastrointestinal tract. | [3] | |
| Groups of female mongrel dogs to study the metabolic effects of isocaloric and hypercaloric infusions of 5% v/v aqueous triacetin. A primed, continuous infusion of 5 µmol/kg (0.3 µCi/kg/min) [13C]-acetoacetate and 1.0 µCi/kg (0.01 µCi/kg/min) [3H]-glucose was continued for 6 hr. Three hours after the start of the isotope infusion, dosing with triacetin was started. Six animals were infused at a rate of 47 µmol/kg/min and seven were infused at a rate of 70 µmol/kg/min triacetin for 3 hr. Blood and breath samples were taken at 15 to 30-min intervals. A group of four animals was infused with 70 µmol/kg/min glycerol and used as the control for the hypercaloric infusion. During isocaloric infusion of triacetin, plasma acetate and free fatty acid concentrations were significantly increased at 30 and 60 min, respectively, and remained elevated. During hypercaloric infusion, plasma acetate concentration increased progressively throughout the study, whereas the plasma free fatty acid concentration did not change. Plasma pyruvate and lactate concentrations were significantly decreased after 30 and 90 min, respectively, and throughout the study with both isocaloric and hypercaloric infusion. The plasma insulin concentrations were modestly increased during both infusions. Plasma glucose concentration was significantly decreased during isocaloric triacetin infusion; a slight but significant increase was observed with hypercaloric infusion. Glucose clearance decreased significantly in both groups during the last hour of triacetin infusion. Plasma ketone body concentrations increased significantly by 60 min, and they remained elevated with isocaloric infusion and increased progressively with hypercaloric infusion of triacetin; the increased concentrations were due to increased ketone body production. During the last hour of infusion, resting energy expenditure was significantly increased with isocaloric triacetin. | [3] | |
| Absorption | Triacetin is more rapidly absorbed from the gastrointestinal tract in 3 hours than the other fats tested. Triacetin has been shown to be a source of liver glycogen and when fed in amounts equal in caloric value to 15% glucose it was utilized as efficiently as was glucose. | [3] |
| Mongrel dogs were used to determine the systemic, hindlimb, gut, hepatic, and renal uptake of acetate during infusion of a 5% v/v aqueous solution of triacetin. A primed, continuous infusion of [1-14C]-acetate was continued for 7 hr with 10 animals. Three hours after the start of the tracer infusion, the animals were infused with triacetin at a rate of 47 µmol/kg/min for 4 hr. Blood and breath samples were taken at 15-min intervals for the last 30 min. Steady-state conditions were achieved in plasma acetate concentrations and specific activity and in expired [14-C02]. Plasma acetate concentrations were 1180, 935, 817, 752, and 473 µmol/L (all values approximate) in the aorta, renal vein, portal vein, femoral vein, and hepatic vein, respectively. The acetate turnover rate during triacetin infusion was 2214 µmol/min; systemic acetate turnover accounted for 68% of triacetin-derived acetate. | [3] | |
| Mutagenicity, Genotoxicity and Carcinogenicity | ||
| Mutagenicity | S.typhimurium and E.coli (metabolic activator Sprague-Dawley rat liver (S9)) Cytotoxic conc: No toxicity with or without metabolic activation up to 5,000µg/plate (five strains) Genotoxic conc: No genotoxic effects observed with or without metabolic activation |
[2] |
| S.typhimurium and E.coli (metabolic activator Sprague-Dawley rat liver (S9)) Cytotoxic conc: No toxicity with or without metabolic activation up to 5,000µg/plate (four strains) Genotoxic conc: No genotoxic effects observed with or without metabolic activation |
[2], [4] | |
| Chromosome Abnormalities | Chinese Hamster Lung (metabolic activator Sprague-Dawley rat liver (S9)) Triacetin induced structural chromosome aberrations on shortterm treatment with an exogenous metabolic activation system at the maximum concentration of 2.2 mg/ml (10 mM). However, triacetin decreased pH of the medium at 2.2 mg/ml on short-term treatment with an exogenous metabolic activation system. Therefore, chromosome aberrations induced with triacetin were likely to be caused by lowering pH of the medium rather than by damaging DNA per se. It is, however, recognized that changes in pH of the medium caused by triacetin can induce such artifacts in this assay. Polyploidy was not induced under any of the conditions on continuous and short-term treatment with and without an exogenous metabolic activation system. With metabolic activation: Not observed up to 1.2 mg/ml for 6h exposure. The 50 % inhibition of cell proliferation was calculated to be 1.8 mg/ml. Without metabolic activation: Not observed up to 2.2 mg/ml for 24- and 48-h exposure. Genotoxic effects: Equivocal. |
[2] |
| Other Genotoxic Effects | - | |
| Carcinogenicity | - | |
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||
| Reproductive Toxicity | NOAEL = 1,000 mg/kg per day (rat m/f, reproduction) F1 NOAEL = 1,000 mg/kg per day (rat, offspring) Triacetin did not exert any toxic effects on reproductive parameters including the mating index, fertility index, gestation length, number of corpora lutea or implantations, implantation index, gestation index, delivery index and parturition or maternal behaviour at delivery and lactation. General parental toxicity: Triacetin had no effects on clinical signs, body weight, food consumption, and organ weight or necropsy findings. No histopathological changes ascribable to the compound were observed in either sex. There were no haematological or blood chemical parameters in males. The NOAEL for reproductive toxicity is thus considered to be 1,000 mg/kg bw/day for both sexes. |
[2] |
| Teratogenicity | NOAEL = 1,000 mg/kg per day (maternal toxicity) NOAEL = 1,000 mg/kg per day (teratogenicity) No teratological or other developmental effects were observed at any dose. General parental toxicity: Triacetin had no effects on clinical signs, body weight, food consumption, and organ weight or necropsy findings. No histopathological changes ascribable to the compound were observed in either sex. There were no haematological or blood chemical parameters in males. Pregnancy/litter data: Triacetin did not exert any toxic effects on reproductive parameters including the mating index, fertility index, gestation length, number of corpora lutea or implantations, implantation index, gestation index, delivery index and parturition or maternal behaviour at delivery and lactation Toxicity to offspring: On examination of neonates, there were no significant differences in numbers of offspring or live offspring, the sex ratio, the live birth index, the viability index or body weight. No abnormal findings ascribable to the compound were found for external features, clinical signs or necropsy of the offspring. The NOAEL for reproductive and developmental toxicity is considered to be 1,000 mg/kg bw/day for parental animals and offspring. |
[2] |
| Other Toxicity Studies | Sheep, 175 days (male and female) Each of two basal diets (pelleted, ground hay (H), and a pelleted mixture of the same hay and corn meal (HC) was supplemented singly with triacetin (Ac3) and glycerol (G). A given animal was fed continuously one of the four diets at one of two levels of intake (approximately, 1 or 2.2 times the maintenance level) during the 175-day feeding period. The mean rates with which the metabolizable energy (ME) ingested above the maintenance level of intake was utilized for body-energy gain, were: (in %) H + Ac3, 59.9; HC + G, 59.5; HC + Ac3, 63.7; and HC + G, 61.8(p > 0.3). Ignoring the kind of basal diet, utilization rates were 62.0 and 61.2 % for the ME provided by the diets containing triacetin and glycerol, respectively. The mean pooled net utilization of ME for body-energy gain by females (65.5%) was markedly greater (P<0.01) than that by males (57.6 %). In a series of respiration-calorimetric experiments, the net utilization of ME provided by the acetic acid moiety of triacetin was 76.4%, between days 50 and 70 of continuous feeding. Conclusion: In a 175 day feeding study with sheep, 62 % of metabolic energy was utilized. Concentration of triacetin in food was 10 %. No data on toxicity are reported. |
[2], [4] |
| Toxicokinetics | ||
| Toxicokinetics | Dog Significant acetate uptake was demonstrated in all tissues (liver, 559 ± 68; intestine, 342 ± 23; hindlimb, 89 ± 7; and kidney, 330 ± 37 µmol/min). Conclusion: During intravenous administration in dogs, the majority of infused triacetin undergoes intravascular hydrolysis, and the majority of the resulting acetate is oxidized. Thus, energy in the form of short-chain fatty acids can be delivered to a resting gut via intravenous infusion of a short-chain triglyceride. |
[2] |
| Dog There were no changes in serum P or Ca. The serum Mg concentration decreased from 0.7 ± 0.03 to 0.57 ± 0.03 mmol/L (p < 0.001) by 90 min and remained at this level for the remainder of the study. The triacetin infusion did not influence fractional urinary Mg excretion; thus, the decrease in serum Mg was likely because of an increase in cellular transport of this cation. Conclusion: An isocaloric infusion of the short-chain triglyceride triacetin in dogs resulted in modest increases in plasma acetate but did not significantly affect serum Ca or P concentrations. Serum Mg decreased by approximately 20 %, probably because of cellular uptake rather than accelerated excretion. Triacetin administered to dogs at a rate approximating resting energy expenditure has no demonstrable adverse effects on mineral metabolism. |
[2] | |
| Rat Triacetin caused no overt toxic effects at any point during the study. As the proportion of triacetin in the diet increased from 0 to 50 or 90 % of the lipid energy, cumulative nitrogen balance increased 50 or 120 %, respectively (p < 0.05). Whole-body and tissue leucine kinetics (determined during the last 2.5 hr of the 7-day study) were unaffected by the lipid composition of the diet. Plasma acetate concentration was not significantly different among groups. Conclusion: These results indicate that incorporation of triacetin in nutritionally balanced total parenteral nutrition formulas improves nitrogen balance with no overt toxic effects. |
[2] | |
| Ecotoxicity Data | ||
| Algae | EC50 > 1,000 mg/l (Selenastrum capricornutum, 72h) NOEC = 556 mg/l (Selenastrum capricornutum, 72h) Growth inhibition: growth rate and biomass |
[2] |
| Growth rate EC50 > 940 mg/l (Selenastrum capricornutum, 72h) NOEC = 460 mg/l (Selenastrum capricornutum, 72h) Biomass (AUG) EC50 > 1000 mg/l (Selenastrum capricornutum, 72h) NOEC = 560 mg/l (Selenastrum capricornutum, 72h) |
[5] | |
| Daphnia magna (acute) | EC50 = 888 mg/l (daphnia magna, 24h) EC50 = 768 mg/l (daphnia magna, 48h) EC0 = 309 mg/l (daphnia magna, 48h) |
[2] |
| EC50 > 974.4 mg/l (daphnia magna, 24h) EC50 = 810.9 mg/l (daphnia magna, 48h) EC0 = 541.1 mg/l (daphnia magna, 48h) |
[2] | |
| EC50 = 380 mg/l (daphnia magna, 48h) EC0 = 65 mg/l (daphnia magna, 48h) |
[2] | |
| EC0 = 65 mg/l (daphnia magna, 24h) EC50 = 380 mg/l (daphnia magna, 48h) EC100 = 1000 mg/l (daphnia magna, 48h) |
[4] | |
| EC50 = 770 mg/l (daphnia magna, 48h) Acute immobilization |
[5] | |
| Daphnia magna (chronic) | EC50 > 100 mg/l (daphnia magna, 21d, reproduction) NOEC = 100 mg/l (daphnia magna, 21d, reproduction) LC50 > 100 mg/l (daphnia magna, 14d, parental) LC50 > 100 mg/l (daphnia magna, 21d, parental) |
[2] |
| EC50 > 94 mg/l (daphnia magna, 21d) NOEC > 94 mg/l (daphnia magna, 21d) |
[5] | |
| Other aquatic organisms | - | |
| Fish (acute) | LC50 > 100 mg/l (Oryzia latipes, 96h) | [2], [5] |
| LC50 = 165.3 mg/l (Pimephales promelas, 96h) | [2] | |
| LC50 = 174 mg/l (Cyprinus carpio, 48h) | [2] | |
| LC50 = 170 mg/l (Leuciscus idus, 48h) | [2] | |
| LC50 = 300 mg/l (Branchydanio rerio, 96h) | [2] | |
| LC0 = 100 mg/l (Cyprinus carpio, 48h) LC50 = 174 mg/l (Cyprinus carpio, 48h) LC100 = 320 mg/l (Cyprinus carpio, 48h) |
[4] | |
| LC0 = 100 mg/l (Leuciscus idus, 48h) LC50 = 170 mg/l (Leuciscus idus, 48h) LC100 = 300 mg/l (Leuciscus idus, 48h) |
[4] | |
| Fish (chronic) | LC50 > 100 mg/l (Oryzia latipes, 14d) LC0 = 100 mg/l (Oryzia latipes, 14d) |
[2], [5] |
| Bacteria | EC0 > 541.6 mg/l (Pseudomonas putida, 18h) | [2], [4] |
| EC0 = 10,000 mg/l (Pseudomonas putida, 30 minutes) | [2], [4] | |
| NOEC = 3,000 mg/l (Pseudomonas putida, 16h) FOEC = 10,000 mg/l (Pseudomonas putida, 16h) |
[2], [4] | |
| Terrestrial organisms | - | |
| Environmental Fate | ||
| BCF | 1.3 | [2], [4] |
| An estimated BCF of 1 was calculated for triacetin, using a log Kow of 0.25 and a regression-derived equation. According to a classification scheme, this BCF suggests the potential for bioconcentration in aquatic organisms is low. | [3] | |
| Aerobic biodegradation | 77% after 14 days based on BOD 94% after 14 days based on TOC Readily biodegradable |
[2] |
| 64% after 28 days based on ThCO2 (10 mg/l) 93% after 28 days based on ThCO2 (20 mg/l) Readily biodegradable |
[2], [4] | |
| Triacetin, present at 100 mg/l, reached 91-94% of its theoretical BOD in 4 weeks using an activated sludge inoculum at 30 mg/l and the Japanese MITI test. Using a rapid infiltration system for treating primary and secondary effluents, triacetin, present in a feed solution at a concentration of 0.094 µg/l, was not detected in the column effluent. The specific loss process was not identified. | [3] | |
| 79% after 30 days (5 mg/l) Readily biodegradable |
[4] | |
| 93% after 4 weeks based on BOD 97% after 4 weeks based on TOC Readily biodegradable |
[5] | |
| Anaerobic biodegradation | - | |
| Abiotic degradation | The rate constant for the vapour-phase reaction of triacetin with photochemically-produced hydroxyl radicals has been estimated as 8.5 x10-12 cm³/(molecule-sec) at 25º C using a structure estimation method. This corresponds to an atmospheric half-life of about 1.9 days at an atmospheric concentration of 5 x 10+5 hydroxyl radicals per cm³. A base-catalyzed second-order hydrolysis rate constant of 6.2 x 10-1 l/(mole-sec) was estimated using a structure estimation method; this corresponds to half-lives of 130 and 12 days at pH values of 7 and 8, respectively. Triacetin does not contain chromophores that absorb at wavelengths à 290 nm and therefore is not expected to be susceptible to direct photolysis by sunlight. | [3] |
| Photodegradation | T½ = 48h (indirect photolysis) | [2], [4] |
| Metabolic pathway | - | |
| Mobility | - | |
| Stability in water (abiotic) | Stable at pH 4 at 50ºC T½ = 60.4 days at pH 7 at 25ºC T½ = 16.5 h at pH 9 at 25ºC |
[2] |
| T½ = 130 days at pH 7 T½ = 1.3 h at pH 9 T½ = 13 days at pH 8 |
[4] | |
| Soil adsorption/mobility | The Koc of triacetin is estimated as 33, using a log Kow of 0.25 and a regression-derived equation. According to a classification scheme, this estimated Koc value suggests that triacetin is expected to have very high mobility in soil. | [3] |
| Based upon a measured water solubility of 58 g/l at 25ºC, the Koc-value can be estimated to be 10.5 from a regression derived equation. This Koc-value indicates very high soil mobility. | [4] | |
| Volatilization from water/soil | The Henry's Law constant for triacetin is estimated as 1.2 x10-8 atm/m³/mol derived from its vapour pressure, 0.00248 mm Hg, and water solubility, 58,000 mg/l. This Henry's Law constant indicates that triacetin is expected to be essentially non-volatile from water surfaces. Triacetin's estimated Henry's Law constant indicates that volatilization from moist soil surfaces is not expected to occur. Triacetin is not expected to volatilize from dry soil surfaces based upon a vapour pressure of 0.00248 mm Hg | [3] |
| Based on a water solubility of 58 g/l and a vapour pressure of 0.00248 mmgHg at 25ºC, a Henry's law constant of 1.23 x 10-8 atm/m³/mol is estimated. This value indicates that the compound is essentially non-volatile from water. | [4] | |
| Terrestrial fate | Based on a classification scheme, an estimated Koc value of 33, determined from a log Kow of 0.25 and a regression-derived equation, indicates that triacetin is expected to have very high mobility in soil. Volatilization of triacetin from moist soil surfaces is not expected to be an important fate process given an estimated Henry's Law constant of 1.2 x10-8 atm/m³/mol derived from its vapour pressure, 0.00248 mm Hg, and water solubility, 58,000 mg/l. Triacetin is not expected to volatilize from dry soil surfaces based upon a vapour pressure of 0.00248 mm Hg. A theoretical BOD of 91-94% in 4 weeks using an activated sludge inoculum and the Japanese MITI test suggests that biodegradation may be an important environmental fate process in soil | [3] |
| Aquatic fate | Based on a classification scheme, an estimated Koc value of 33, determined from a log Kow of 0.25 and a regression-derived equation, indicates that triacetin is not expected to adsorb to suspended solids and sediment. Volatilization from water surfaces is not expected based upon an estimated Henry's Law constant of 1.2 x 10-8 atm/m³/mol, derived from its vapour pressure, 0.00248 mm Hg, and water solubility, 58,000 mg/l. A base-catalyzed second-order hydrolysis rate constant of 6.2 x 10-1 l/mol/sec was estimated using a structure estimation method; this corresponds to half-lives of 130 and 12 days at pH values of 7 and 8, respectively. According to a classification scheme, an estimated BCF of 1, from its log Kow and a regression-derived equation, suggests the potential for bioconcentration in aquatic organisms is low. A theoretical BOD of 91-94% in 4 weeks using an activated sludge inoculum and the Japanese MITI test suggests that biodegradation may be an important environmental fate process in water. | [3] |
| Atmospheric fate | According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere, triacetin, which has a vapour pressure of 0.00248 mm Hg at 25ºC, is expected to exist solely as a vapour in the ambient atmosphere. Vapour-phase triacetin is degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals, the half-life for this reaction in air is estimated to be 1.9, calculated from its rate constant of 8.5 x 10-12 cm³/(molecule-sec) at 25º C that was derived using a structure estimation method. Triacetin does not contain chromophores that absorb at wavelengths à 290 nm and therefore is not expected to be susceptible to direct photolysis by sunlight. | [3] |
| Conclusion | ||
| Physical-chemical | - | |
| Emission | - | |
| Exposure | - | |
| Health | - | |
| Environment | - | |
| References | ||
| 1 | ESIS | |
| 2 | OECD SIDS final | |
| 3 | HSDB | |
| 4 | IUCLID dataset | |
| 5 | MITI | |
| 6 | MSDS Sigma Aldrich | |
Trimethyl pentanyl diisobutyrate, TXIB
| Identification of the substance | ||
| CAS No. | 6846-50-0 | |
| EINECS No. | 229-934-9 | [1] |
| EINECS Name | 1-isopropyl-2,2-dimethyltrimethylene diisobutyrate | [1] |
| Synonyms | Trimethyl pentanyl diisobutyrate TXIB |
|
| Molecular Formula | C16H30O4 | [1] |
| Structural Formula |
|
|
| Major Uses | Plasticizer | |
| IUCLID | HPV | [1] |
| EU classification | This substance is not classified in the Annex I of Directive 67/548/EEC as such, but it may be included in one of the group entries. | [1] |
| Physico-chemical Characteristics | ||
| Physical Form | Liquid | [2] |
| Colourless liquid with a slight odour | [4] | |
| Molecular Weight (g/mole) | 286.41 | [2] |
| Melting Point/range (°C) | < - 10ºC | [2] |
| -70ºC | [3], [4], [5] | |
| Boiling Point/range (°C) | 280ºC at 1,013 hPa | [2], [3], [4], [5] |
| Decomposition Temperature (°C) | - | |
| Vapour Pressure (mm Hg at °C) | 8.8 x 10-2 Pa at 25ºC [6.6 x 10-4 mgHg] | [2] |
| Density (g/cm3 at °C) | 0.941 | [3], [5] |
| 0.945 at 20ºC | [4] | |
| Vapour Density (air=1) | 9.9 | [4] |
| Henry’s Law constant (atm/m³/mol at °C) | - | |
| Solubility (mg/l water at °C) | 15 mg/l at 25ºC | [2] |
| 1-2 mg/l at 20.5ºC | [4] | |
| Partition Coefficient (log Pow) | > 4.11 at 25ºC | [2] |
| 4.1 | [4] | |
| pKa | - | |
| Flammability | - | |
| Explosivity | - | |
| Oxidising Properties | - | |
| Migration potential in polymer | - | |
| Flash point | 140ºC (open cup) | [2], [5] |
| 113ºC (closed cup) | [3] | |
| 128ºC (closed cup) | [4] | |
| Explosion limits | Lower: 0.48% Upper: 3.1% |
[3], [4], [5] |
| Auto ignition point | 424ºC | [5] |
| Emission Data | ||
| During production | - | |
| Exposure Data | ||
| Aquatic environment, incl. sediment | - | |
| Terrestrial environment | - | |
| Sewage treatment plant | - | |
| Working environment | - | |
| Consumer goods | - | |
| Man exposed from environment | - | |
| ”Secondary poisoning” | - | |
| Atmosphere | - | |
| Dermal | - | |
| Toxicological data | ||
| Observations in humans | - | |
| Acute toxicity | ||
| Oral | LD50 > 3,200 mg/kg (rat) | [2], [4] |
| LD50 > 6,400 mg/kg (mouse) | [2], [4] | |
| LD50 > 2,000 mg/kg (rat, higest dose) | [6] | |
| Dermal | LD50 > 20 ml/kg (guinea pig) | [2], [4] |
| LD50 > 2,000 mg/kg (rabbit, higest dose) | [6] | |
| Inhalation | 453 ppm/6h (rat) | [2] |
| LC50 > 5.3 mg/l (rat, 6h) | [4] | |
| Other routes | - | |
| Skin irritation | Moderate irritating (Guinea pig) | [2] |
| Slightly irritating (Guinea pig) | [4] | |
| Not irritating (human) | [6] | |
| Eye irritation | Not irritating (rabbit) | [4] |
| Irritation of respiratory tract | - | |
| Skin sensitisation | Not sensitizing (Guinea pig) | [4] |
| Not sensitizing (Human) | [6] | |
| Subchronic and Chronic Toxicity | ||
| Oral | NOAEL = 30 mg/kg per day (rat) LOEL = 150 mg/kg per day (rat) The results in clinical observations did not reveal any effects attributable to the administration of test substance, and there were no mortality in all groups. Depressions of body weight gain were observed in male rats receiving 750 mg/kg/day, and food consumption of female rats receiving 750 mg/kg/day was greater than those of control. As the results of hematology, there were no essential effects of test substance. In blood clinical examination, increases in creatinine and total bilirubin were observed in rats receiving 150 and 750 mg/kg/day, and increases in total protein were observed in male rats receiving 750 mg/kg/day, suggesting that those changes were due to the effect on kidneys and liver. In organ weight analysis, increases in liver weight were observed in male rats receiving 150 and 750 mg/kg/day, moreover increases in kidneys weights were observed in male rats receiving 750 mg/kg/day. As the results of gross findings, increases in incidence of brown colored livers were observed in male rats receiving 750 mg/kg/day. As the results of histopathological findings, increases in grade of basophilic change of the renal tubular epithelium and degeneration of hyaline droplet were observed in male rats receiving 150 mg/kg/day or more. Moreover, necrosis and fibrosis of the proximal tubule, dilatation of the distal tubule, decreased fatty change and swelling of the liver cells were observed in male rats receiving 750 mg/kg/day. |
[2], [5] |
| Rat, 103-day repeat dose NOAEL = 0.1% LOAEL = 1% |
[4] | |
| Rat, 52 or 99-day repeat dose NOAEL = 0.1% LOAEL = 1% |
[4] | |
| Dog, 13 weeks repeat dose (6 days a week) NOAEL = 1% |
[4] | |
| Inhalation | - | |
| Dermal | - | |
| Adsorption, distribution, metabolism and elimination | Rat TXIB was rapidly adsorped, metabolized and excreted. The major route of elimination was urine (47 – 72% total dose) within 5 - 10 days and the majority of this occurring in the first 72 hours. Radioactivity in feces accounted for 14 – 31% of the dose with elimination being essentially complete by 7 days with the majority isolated after 48 hours. Radiolabeled CO2 was not detected. In total, excretions accounted for 95-99% of the dose. Residual radioactivity of treated animals approached control by two weeks. Identification of metabolites showed the feces to contain both 2,2,4-trimethyl pentanediol (TMPD) and TXIB-3-14C indicating esterase cleavage of the two isobutyrates. A small potion of the absorbed material in the urine was unchanged TXIB-3-14C while the majority consisted of metabolites consistent with complete cleavage to the glycol (TMPD) parent molecule. Although much of the urinary metabolite was unidentified it does, nonetheless, represent rapidly cleared material. |
[6] |
| Reversibility of liver effects | Weight change There were no mortalities or statistically significant changes noted in body weights, growth rates, or in food consumption and efficiency in any of the three experiments. There were no differences in absolute organ weights in any of the animals in any of the three experiments. All organs microscopically examined in all experiments appeared normal. However, all animals (M&F) fed diets containing 1.0% TXIB for 51 days, 99 days, or the last 47 days of experiment 3 showed significant increases in relative liver weight. Other relative organ weight effects were noted in the kidneys of males and females fed 1.0% TXIB for 51 days (but not 99 days or the last 47 days in experiment 3). Females also showed increases in relative thyroid and brain weights after 99 days of exposure. There were no statistically significant effects noted in the hematology or clinical chemistry parameters analyzed (Note: The manuscript in which these data were published indicated that the SGOT values were elevated in males fed 0.1 and 1.0% TXIB for either 52 or 99 straight days and for females exposed for 99 days. Enzyme levels were still elevated at both doses in males and females in experiment 3 under both the exposure scenarios i.e., test diet for 52 days than control diet for 47 days or control for 52 days than test diet. The manuscript noted their elevation although significant was not manifested in a dose or time related manner and were within historical control values for all groups.) Dose levels of material consumed in experiment one for 51 days at 1.0% were 708 mg/kg (M) and 747 mg/kg (F); while 0.1% animals received either 70 mg/kg (M) or 68 mg/kg (F). In experiment two animals on the 1.0% diet received 824 mg/kg (M) and 853 mg/kg (F); the 0.1% test diet animals received 79 mg/kg (M) and 87 mg/kg. In experiment three animals on the 1.0% diet for the first 52 days received 959 mg/kg (M) and 947 mg/kg (F) while those on the 0.1% test diet received 94 mg/kg (M) and 79 mg/kg. Animals who received 1.0% test diets for the second half of the experiment (Days 52-99) received 558 mg/kg (M) and 614 mg/kg (F); those on the 0.1% test diet averaged 55 mg/kg (M) and 59 mg/kg. |
[6] |
| Liver enzyme changes Males and females fed 1.0% TXIB for either 52 or 99 days showed significant increases in p-nitroanisole demethylase. Males and females fed TXIB for 52 days also had elevated UDP-bilirubin-glucuronyl transferase and UDP-p-aminophenol glucuronyl transferase levels increased. Interestingly only the UDP-bilirubin-glucuronyl transferase level was increased after 99 days of feeding and only in females. Importantly, none of the four enzymes were elevated in experiment three in which animals were fed control diets for 47 days after being fed 1.0% TXIB for the first 52 days. Seven daily IP injections of 100 mg/kg TXIB resulted in elevated levels of UDP-p-aminophenol glucuronyl transferase only. |
[6] | |
| Mutagenicity, Genotoxicity and Carcinogenicity | ||
| Mutagenicity | S.typhimurium and E.coli (metabolic activator Sprague-Dawley rat liver (S9)) Negative with og without metabolic activation. Cytotoxic conc: No toxicity with or without metabolic activation up to 5,000µg/plate (five strains) Genotoxic conc: No genotoxic effects observed with or without metabolic activation |
[2], [5] |
| Chromosome Abnormalities | CHL cells Negative with og without metabolic activation. Cytotoxic conc: With = 0.018 mg/ml (continuous treatment) Without = 0.04 mg/ml (short-term treatment) With > 1.30 mg/ml (short-term treatment) Genotoxic conc: No genotoxic effects observed with or without metabolic activation |
[2], [5] |
| Other Genotoxic Effects | - | |
| Carcinogenicity | - | |
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||
| Reproductive Toxicity | Rat NOAEL = 750 mg/ kg per day (parental) NOAEL = 750 mg/kg per day (F1, offspring) The results observed in mating, fertility and estrous cycle did not reveal any effects attributable to the administration of test substance. Observation at delivery, all gestation animals delivered of pups, normally and there were not a treatment-related effect throughout the lactation period. The external examination of pups revealed no effects attributable to the administration of test substance. The body weights of pups showed the favourably growths until day 4 of lactation. The necropsy of stillborn, dead pups until day 4 of lactation and newborns at day 4 of lactation did not reveal any effects attributable to the administration of test substance. |
[2] |
| Rat NOAEL = 4.5 ppm (276 mg/kg in males and 359 mg/kg in females) NOEL = 15.0 ppm (approx. 1,000 mg/kg) (teratogenicity) |
[6] | |
| Teratogenicity | - | |
| Toxicokinetics | ||
| Toxicokinetics | - | |
| Ecotoxicity Data | ||
| Algae | EC50 = 8.0 mg/l (Selenastrum capricornutum, 72h) NOEC = 5.3 mg/l (Selenastrum capricornutum) |
[2] |
| Daphnia magna (acute) | EC50 = 300 mg/l (daphnia magna, 24h) | [2] |
| EC50 > 1.46 mg/l (daphnia magna, 48h) NOEC = 1.46 mg/l (daphnia magna, 48h) |
[4] | |
| EC50 > 1.55 mg/l (Dugesia tigrina, 96h) NOEC = 1.55 mg/l (Dugesia tigrina, 96h) |
[4] | |
| EC50 > 1.55 mg/l (Lumbriculus variegatus, 96h) NOEC = 1.55 mg/l (Lumbriculus variegatus, 96h) |
[4] | |
| EC50 > 1.55 mg/l (Helisoma trivolvis, 96h) NOEC = 1.55 mg/l (Helisoma trivolvis, 96h) |
[4] | |
| Daphnia magna (chronic) | LC50 > 32 mg/l (daphnia magna, 24h, mortality) LC50 = 45 mg/l (daphnia magna, 48h, mortality) LC50 = 20 mg/l (daphnia magna, 96h, mortality) LC50 = 13 mg/l (daphnia magna, 7 d, mortality) LC50 = 12 mg/l (daphnia magna, 14 d, mortality) LC50 = 12 mg/l (daphnia magna, 21 d, mortality) EC50 = 5.6 mg/l (daphnia magna, 21 d, reproduction) NOEC = 3.2 mg/l (daphnia magna, reproduction) LOEC = 1.0 mg/l (daphnia magna, reproduction) |
[2] |
| Other aquatic organisms | EC50 > 1.55 mg/l (Asellus intermedium, 96h) NOEC = 1.55 mg/l (Asellus intermedium, 96h) |
[4] |
| EC50 > 1.55 mg/l (Gammarus fasciatus, 96h) NOEC = 1.55 mg/l (Gammarus fasciatus, 96h) |
[4] | |
| Fish (acute) | LC50 = 18 mg/l (Oryzias latipes, 24h) LC50 = 18 mg/l (Oryzias latipes, 48h) LC50 = 18 mg/l (Oryzias latipes, 72h) LC50 = 18 mg/l (Oryzias latipes, 96h) |
[2] |
| LC50 > 1.55 mg/l (pimpephales promelas, 96h) NOEC = 1.55 mg/l (pimpephales promelas, 96h) |
[4] | |
| Bacteria | - | |
| Terrestrial organisms | - | |
| Environmental Fate | ||
| BCF | 5.2-31 (0.3 µg/l, 6 weeks at 25ºC) 6.0-17 (0.03 µg/l, 6 weeks at 25ºC) |
[2] |
| 0.6-0.8 (1 mg/l, 6 weeks) < 1.0 (0.1 mg/l, 6 weeks) |
[5] | |
| Aerobic biodegradation | 4-82% in 28 days (BOD) 2-84% in 28 days (TOC) 3-100% in 28 days (GC) Inherently biodegradable |
[2] |
| > 99.9% in 12 days Inherently biodegradable |
[4] | |
| ThOD = 2.40 g O2 per g TXIB (calculated) | [4] | |
| Anaerobic biodegradation | - | |
| Photodegradation | T½ = 90.7 years Rate: 1.21 x 10-14 mol/l/sec |
[2] |
| Stability in water | Stable at pH 4 and 7 T½ = 179 days at pH 9 |
[2] |
| Metabolic pathway | - | |
| Mobility | - | |
| Conclusion | ||
| Physical-chemical | - | |
| Emission | - | |
| Exposure | - | |
| Health | - | |
| Environment | - | |
| References | ||
| 1 | ESIS | |
| 2 | OECD SIDS final | |
| 3 | MSDS Sigma Aldrich | |
| 4 | IUCLID dataset | |
| 5 | MITI | |
| 6 | EASTMAN TXIB study | |
Acetyl tributyl citrate, ATBC
| Identification of the substance | ||||||||||||
| CAS No. | 77-90-7 | |||||||||||
| EINECS No. | 201-067-0 | [1] | ||||||||||
| EINECS Name | tributyl O-acetylcitrate | [1] | ||||||||||
| Synonyms | Acetyl tributyl citrate ATBC 1,2,3-Propanetricarboxylic acid, 2-(acetyloxy)-, tributyl ester Citric acid, tributyl ester, acetate Citroflex ® A-4 |
|||||||||||
| Molecular Formula | C20H34O8 | [1] | ||||||||||
| Structural Formula |
|
|||||||||||
| Major Uses | Acetyl tributyl citrate (ATBC) is used as a plasticizer with aqueous- and solvent-based polymers, including acrylic, methacrylic, ethyl cellulose, hydroxypropyl methyl cellulose, nitrocellulose, vinyl acetate, vinyl chloride, vinyl pyrrolidone, vinylidene chloride, and urethane polymer systems. ATBC is used in the following applications: • Medical plastics: Aqueous pharmaceutical coatings; extra-corporeal tubing. • Food contact products: Food wraps and films; beverage tubing; crown liners; food containers; tinplate lubricant; aluminum foil coatings. • Cellulosics: Nitrocellulose-based explosives/propellants. • Other industrial uses: Children’s toys; animal ear tags; ink formulations; adhesives; pesticide inerts. |
[2] | ||||||||||
| IUCLID | LPV in EU | [1] | ||||||||||
| HPV in US | [2] | |||||||||||
| EU classification | This substance is not classified in the Annex I of Directive 67/548/EEC as such, but it may be included in one of the group entries. | [1] | ||||||||||
| Physico-chemical Characteristics | ||||||||||||
| Physical Form | Liquid | [3] | ||||||||||
| Colourless liquid with a very faint sweet herbaceous odour and a mild fruity flavour. | [4] | |||||||||||
| Molecular Weight (g/mole) | 402.5 | [2] | ||||||||||
| Melting Point/range (°C) | - 59ºC | [2] | ||||||||||
| - 80ºC | [4], [7] | |||||||||||
| - 75ºC | [6] | |||||||||||
| Boiling Point/range (°C) | 326ºC at 760 mmg Hg | [2] | ||||||||||
| 172-174ºC at 1 mm Hg | [4], [7] | |||||||||||
| 173ºC | [6] | |||||||||||
| Decomposition Temperature (°C) | - | |||||||||||
| Vapour Pressure (mm Hg at °C) | 5.2 x 10-2 mm Hg at 20ºC [6.93 Pa] | [2] | ||||||||||
| 4.6 x 10-6 mm Hg at 25ºC [6.13 x 10-4 Pa] 1 mm Hg at 173ºC [133Pa] |
[4], [6], [7] | |||||||||||
| Density (g/cm3 at °C) | 1.050 | [3] | ||||||||||
| 1.046 at 25ºC | [4], [6] | |||||||||||
| Vapour Density (air=1) | - | |||||||||||
| Henry’s Law constant (atm/m³/mol at °C) | 3.8 x 10-10 at 25ºC | [4], [6], [7] | ||||||||||
| Solubility (mg/l water at °C) | < 100 mg/l | [2] | ||||||||||
| 5 mg/l | [4], [7] | |||||||||||
| Partition Coefficient (log Pow) | 4.29 | [5], [7] | ||||||||||
| pKa | - | |||||||||||
| Flammability | - | |||||||||||
| Explosivity | - | |||||||||||
| Oxidising Properties | - | |||||||||||
| Migration potential in polymer | Migration /from food packaging/ in the cheese wrapped in vinylidene chloride copolymer films (exposure 5 days, temperature 5ºC) was found at the level of 6.1 ppm or 2.0-8.0 mg/kg, and into wrapped cake, at the level of 3.2 ppm. Migration from plasticized vinylidene chloride-vinyl chloride copolymer film in fatty or aqua-type foods was determined at the levels from 0.4 mg/kg after minimal contact during microwave cooking of a soup to 79.8 mg/kg for use of the film during the microwave cooking of peanut-containing cookies. Migration /citric acid, acetyl tributyl ester/ plasticizer from plasticized polyvinylidene chloride-polyvinyl chloride films into both olive oil and distilled water during microwave heating was studied. The amount of /citric acid, acetyl tributyl ester/ migrating into olive oil after heating for 10 min was 73.9 mg/L, into distilled water it was 4.1 mg/L after heating for 8 min. | [4] | ||||||||||
| log Kow | 4.92 at 22ºC | [2] | ||||||||||
| 4.3 | [4] | |||||||||||
| Atmospheric OH rate constant cm³/(molecule sec) | 14.45 x 10-12 | [2] | ||||||||||
| 1.4 x 10-11 at 25ºC | [4], [7] | |||||||||||
| Flash point | 113ºC (closed cup) | [3] | ||||||||||
| 204ºC | [4], [6] | |||||||||||
| Emission Data | ||||||||||||
| During production | - | |||||||||||
| Exposure Data | ||||||||||||
| Aquatic environment, incl. sediment | Surface water: Acetyl tributyl citrate was identified in 2 water samples taken from the River Lee, Great Britain at trace levels. | [4] | ||||||||||
| Terrestrial environment | - | |||||||||||
| Sewage treatment plant | - | |||||||||||
| Working environment | NIOSH has statistically estimated that 106,668 workers (98,183 of these are female) are potentially exposed to acetyl tributyl citrate in the US. Occupational exposure to acetyl tributyl citrate may occur through inhalation and dermal contact with this compound at workplaces where acetyl tributyl citrate is produced or used. The general population may be exposed to acetyl tributyl citrate via dermal contact with consumer products containing actyl tributyl citrate and by the ingestion of food containing this compound. | [4] | ||||||||||
| Consumer goods | - | |||||||||||
| Man exposed from environment | - | |||||||||||
| ”Secondary poisoning” | Acetyl tributyl citrate's production and use as a plasticizer for vinyl, rubber and cellulosic resins and as a flavour ingredient may result in its release to the environment through various waste streams. | [4] | ||||||||||
| Atmosphere | - | |||||||||||
| Dermal | - | |||||||||||
| Toxicological data | ||||||||||||
| Observations in humans | - | |||||||||||
| Acute toxicity | ||||||||||||
| Oral | LD50 > 30 ml/kg (rat) | [2] | ||||||||||
| LD50 > 50 ml/kg (rat) | [2] | |||||||||||
| LD50 = 31.4 g/kg (rat) | [4] | |||||||||||
| LD50 = 4 g/kg (mouse) | [4] | |||||||||||
| LD50 > 50 ml/kg (cat) | [4] | |||||||||||
| Dermal | - | |||||||||||
| Inhalation | - | |||||||||||
| Other routes | - | |||||||||||
| Skin irritation | Slight irritation (Guinea pig) | [4] | ||||||||||
| Eye irritation | Slight ittitation (rabbit) | [4] | ||||||||||
| Irritation of respiratory tract | - | |||||||||||
| Skin sensitisation | - | |||||||||||
| Subchronic and Chronic Toxicity | ||||||||||||
| Oral | Rat, 6 weeks repeat dose NOAEL = 5% LOAEL = 10% |
[2] | ||||||||||
| Rat, 8 weeks repeat dose NOEL = 10% LOEL > 10% |
[2] | |||||||||||
| Cat, 2 months repeat dose NOEL < 5ml/kg LOEL = 5 ml/kg |
[2] | |||||||||||
| Rat, 90-day repeat dose NOEL = NOAEL = 300 mg/kg LOEL = 1000 mg/kg |
[2] | |||||||||||
| Rat, 2 years repeat dose NOAEL = 2000 ppm LOAEL = 20,000 ppm In the main study, a transient reduction in body weight gain was observed in animals in all three treated groups, 200, 2000 and 20000 ppm. This decrease in body weight gain was not seen in the additional study of animals treated for one year at dietary concentrations of 200 and 2000 ppm. Since this finding was not reproducible it is considered to be and artifact. Statistical analysis indicated that there were no significant differences between the body weights of the treated animals compared to the concurrent controls. There were no treatment-related clinical observations. Twelve of the 60 rats fed test diets and eight of the 40 control rats died prior to scheduled sacrifice. There was no significant difference in time of death or percentage mortality among the three treated groups and controls. Inflammatory disease of the lungs was the most frequent finding necropsy of these animals, it is likely that this was caused by infection rather than treatment with ATBC. Lymphoid tumors of the pleural and abdominal cavities, with some infiltration of the associated organs, were seen in both treated and control animals at comparable rates and, therefore, were not considered to be treatment-related. Careful examination of the endocrine system did not reveal evidence of abnormality in any of the animals. There were no significant differences between treated and control animals in comparisons of the pathological findings. |
[2] | |||||||||||
| Rat, 13 weeks repeat dose NOAEL = 100 mg/kg per day (males) NOAEL = 300 mg/kg per day (females) LOEL = 300 mg/kg per day (males) LOEL = 1000 mg/kg per day (females) At the completion of the in utero phase, rats that had been exposed to ATBC from before conception, through gestation and continuously from the time of birth were selected (20 unrelated males and 20 unrelated females per dose group for the main study; and 10 unrelated males and 10 unrelated females for the control and high dose recovery groups) and transferred to the 13-week study. There were no significant intergroup differences in the body weights of the animals at the start of the 13-week study. In the 13-week toxicity phase of the study, administration of ATBC via the diet to Han Wistar rats at doses as high as 1000 mg/kg/day that had already received direct and indirect exposure to the test material from before conception did not produce any marked toxicity. Treatment at 1000 mg/kg/day resulted in a slight reduction in body weight gain in both sexes, which was considered to be a nonspecific indicator of toxicity. Liver weights were increased and hepatic hypertrophy occurred at 1000 mg/kg/day in both sexes. Hepatic hypertrophy resulting from an induction of metabolizing enzymes as an adaptive response to treatment is a common finding following administration of high doses of xenobiotics, and is not considered to be toxicologically significant. Weak peroxisome proliferation was measured in males at 300 mg/kg/day and both sexes at 1000 mg/kg/day. Peroxisome proliferation is universally recognized as a rodent specific effect and not relevant to hazard characterization for humans. Slight variations in urinary composition and in plasma electrolyte concentrations suggested an effect on renal function at the higher dose levels. In view of the slight nature of these changes, which were all shown to be reversible and within normal historical control ranges, and the lack of histopathological changes in the kidneys, the possible effect on renal function is considered to be due to adaptation to the excretion of high levels of the test material and/or metabolites and is not considered to be of any toxicological significance. |
[2] | |||||||||||
| Inhalation | - | |||||||||||
| Dermal | - | |||||||||||
| Absorption, metabolism and excretion | Orally administered ATBC is extensively absorbed and rapidly metabolized and excreted by the rat. Measured levels of ATBC and radioactivity in the dosing solutions ranged from 90 to 115% of the target concentrations. No signs of toxicity were observed following dosing. One rat in the Rate of Absorption Study died following blood collection. The probable cause of death was complication from the blood collection procedure. Absorption and Elimination Study: Between 99 and 102% of the administered radioactivity was recovered in the urine, feces, cage wash, expired CO2, tissues, and carcass by study end (48 hours). The following table provides the results of the recovered radiolabeled material.
Rate of Absorption Study: Absorption of the radioactive dose was rapid (absorption T½ = 1.0 hour) and extensive (at least 67% of 14C dose absorbed). Peak concentrations of radioactivity in blood were observed 2 to 4 hours post-dosing. Most of the absorbed radiolabel was rapidly eliminated with a halflife of 3.4 hours for blood. Metabolism of absorbed 14C-ATBC was rapid and essentially complete. At least 9 radiolabeled metabolites were found in urine and at least 3 in feces. The labeled metabolites in urine were more polar than ATBC and less polar than citric acid. Urinary metabolites of ATBC which were positively identified were acetyl citrate, monobutyl citrate, acetyl monobutyl citrate, dibutyl citrate, and acetyl dibutyl citrate (two isomers). The major labeled urinary metabolite was tentatively identified as monobutyl citrate. Unchanged ATBC representing about 7% of the dose was found in feces. |
[2], [4] | ||||||||||
| Metabolism | Both ATBC and the intermediate metabolite TBC undergo rapid metabolism in both human serum and rat liver homogenates, which would be expected to yield the principal metabolites acetic acid, citric acid and butanol. The butanol would then be expected to further oxidize to butanoic acid and assimilated by ß-oxidation. Although a direct stoichiometry of butanol formed from ATBC and TBC was not observed, these results are partially explained based on the fact that butanol also is metabolized in the rat liver homogenate at a rate of 37 nmoles/ml/hr. It also may be suggested that an initial single or double debutylation may yield products which are less readily hydrolyzed in the system; products which would be, as fully ionizable carboxylic acids, readily excreted in vivo. Human serum results with ATBC and TBC: The metabolism of ATBC in human serum was a linear decline in the concentration of ATBC of the 48 hour period, after which only 25% of the starting material remained. An estimated half-life of 32 hours was obtained. In addition, only traces of TBC were detected from the deacetylation of ATBC to TBC. The metabolism of TBC in human serum showed an exponential decline in the levels of TBC with complete conversion observed in the 24 hour sample. An estimated half-life of 4 hours was obtained. Rat liver homogenate results with ATBC and TBC: The metabolism of ATBC in liver homogenate was linear and rapid decline in the concentration of ATBC the first hour of the 9 hour period examined. From the slope of the linear decline, an estimated half-life of only 10 minutes can be obtained. Not even traces of TBC were detected from the deacetylation of ATBC to TBC was seen. The metabolism of TBC in rat liver homogenate showed a nearly instantaneous and complete metabolism of TBC in 15 minutes. The metabolism was so rapid that the T0 data indicated only 35 µg/ml even though 100 µg/ml was added. A repeat incubation was conducted to try to capture an earlier time point in the conversion, but a comparable value (42 µg/ml) was again obtained. Thus, a half-life of seconds could only be estimated. |
[2] | ||||||||||
| Results with butanol capillary GC analysis in human serum and rat liver homogenate: Butanol levels generated from ATBC were maximal at 1 to 2 hours, representing a level of 279 nmoles/ml at 2 hours. This represents a 37% (279 nmoles/750 nmoles) of the theoretical amount produced. Butanol levels generated from TBC, also maximal at 1 to 2 hours yielded 436 nmoles/ml, or 58% (436 nmoles/750 nmoles) of the theoretical amount. Therefore, with 3 moles of butanol theoretically produced from one mole of ATBC or TBC, the amounts observed were 1.11 mole equivalents from ATBC and 1.74 mole equivalents from TBC. | ||||||||||||
| Metabolism | The results of this study confirm that the end products of ATBC hydrolysis in hu mans are unquestionably citric, acetic and butyric acid. In human serum, ATBC was hydrolyzed relatively slowly (half-life approximately 7 hours) into the equivalent of 2 moles of n-butanol. One butyl ester group of ATBC did not appear to undergo hydrolysis, most probably due to the lower affinity for the butyl group at the 2 position. Hydrolysis in rat liver ho mogenate took place much faster (half-life < 30 minutes). Approximately 2.3 moles of n-butanol were recovered. As shown by a separate experiment, this amount is an underestimation of the true recovery value, the loss of the analyte being due to its consumption by liver enzymes, such as alcohol dehydrogenase. |
[2] | ||||||||||
| The metabolism of acetyl tributyl citrate was evaluated using groups of male rats (number of animals, weights, and strain not stated). Both the absorption and metabolism of 14C-Acetyl tributyl Citrate proceeded rapidly, and the following metabolites were identified: acetyl citrate, monobutyl citrate, acetyl monobutyl citrate, dibutyl citrate, and acetyl dibutyl citrate. | [4] | |||||||||||
| Mutagenicity, Genotoxicity and Carcinogenicity | ||||||||||||
| Mutagenicity | S.typhimurium (metabolic activator Sprague-Dawley rat liver (S9)) The test substance showed no evidence of mutagenic activity when tested in this bacterial system with and without activation. Cytotoxic conc.: Not cytotoxic up to 5000 µg/plate Genotoxic effects: Negative with and without S-9 activation |
[2] | ||||||||||
| S.typhimurium (metabolic activator Sprague-Dawley rat liver (S9) and Syrian Golden Hamster) The test substance showed no evidence of mutagenic activity when tested in this bacterial system in the presence of both rat and hamster liver S-9 and in the absence of microsomal activation. Cytotoxic conc.: Not cytotoxic up to 10,000 µg/plate Genotoxic effects: Negative with and without S-9 activation |
[2] | |||||||||||
| S.typhimurium The test substance showed no evidence of mutagenic activity when tested in this bacterial system in the absence of microsomal activation Cytotoxic conc.: Not cytotoxic up to 495 µg/plate Genotoxic effects: Negative without metabolic activation |
[2] | |||||||||||
| Rat lymphocytes (metabolic activator Sprague-Dawley rat liver (S9)) The test substance showed no evidence of mutagenic activity in the presence and absence of an S-9 metabolic activation system. Cytotoxic conc.: None Genotoxic effects: Negative with and without metabolic activation |
[2] | |||||||||||
| Mouse lymphoma cells (metabolic activator S rat liver (S9)) The test substance showed no evidence of mutagenic activity when tested in this mammalian cell gene mutation assay both with and without metabolic activation. Cytotoxic conc.: Complete toxicity was observed during the initial toxicity test at concentrations of 514 µg/ml and above for nonactivated cultures and from 1028 µg/ml and above for the S-9 activated cultures. A dose-dependent increase in toxicity was observed in the mutagenicity assay, with an average Total Growth of 16%, 6% and 3% in the nonactivated cultures at concentrations of 70, 150, and 230 µg/ml, respectively with complete toxicity at 310 µg/ml and above. For the S-9 activated cultures, the average Total Growth was 16% and 8% at 410 and 480 µg/ml, respectively, with complete toxicity at 550 µg/ml. Genotoxic effects: Negative with and without metabolic activation. |
[2] | |||||||||||
| Chinese hamster ovary cells (metabolic activator Sprague-Dawley rat liver (S9)) The test substance showed no evidence of mutagenic activity when tested in this mammalian cell gene mutation assay. Genotoxic effects: Negative with and without metabolic activation. |
[2] | |||||||||||
| Chromosome Abnormalities | - | |||||||||||
| Genotoxicity | An acute dose-range finding toxicity study with 3 male Han Wistar rats indicated that a maximum of 2000 mg/kg could be used for the unscheduled DNA synthesis (UDS) assay. A lower dose of 800 mg/kg was also selected. Groups of 5 male rats were treated once with the solvent corn oil, the test substance (at 800 or 2000 mg/kg) or the required positive control, by oral gavage at a dose volume of 10 mL/kg. The positive controls used were 75 mg/kg 2-acetamindofluorene (2-AAF) suspended in corn oil (12-14 hr experiment) and 10 mg/kg dimethylnitrosamine (DMN) dissolved in purified water (2-4 hr experiment). This test substance did not induce unscheduled DNA synthesis in freshly prepared primary cultures of hepatocytes from rats dosed at up to 2000 mg/kg under the conditions employed in this assay. | [4] | ||||||||||
| The mutagenicity of acetyl tributyl citrate was evaluated using the Ames test and the following Salmonella typhimurium strains: TA98, TA100, TA1535, TA1537, and TA1538. Acetyl tributyl citrate (29.71 mg/mL DMSO) solutions containing 9, 50, 99, and 495 µm were tested on all strains without metabolic activation. Nitrofluorene served as the positive control.Acetyl tributyl citrate was not mutagenic in any of the strains tested with or without metabolic activation. | [4] | |||||||||||
| The mutagenicity of acetyl tributyl citrate was evaluated using the L5178Y (TK+/TK-) mouse lymphoma suspension/plate assay. Acetyl tributyl citrate, in DMSO, was tested at concentrations of 10 to 230 µg/mL (without metabolic activation) and 200 to 480 µg/mL (with metabolic activation). The test substance was not mutagenic with or without activation. | [4] | |||||||||||
| Other Genotoxic Effects | - | |||||||||||
| Carcinogenicity | Three groups of 1-month-old rats (Sherman strain /20 rats per group) were fed diets containing 200, 2,000 and 20,000 ppm acetyl tributyl citrate, respectively for 2 years. Compared to the control group, transient reduction of the growth rate was noted in all three test groups during week 5 to 15 of the study; however, the difference was not statistically significant. The difference in mortality between the test and control groups was also not statistically significant. Twelve test animals and 8 control animals died spontaneously. Differences in behaviour between test and control animals were not observed and the incidence of diarrhea in test animals was no greater than that noted for controls. At necropsy, inflammatory disease of the lungs was the most frequent finding. Pulmonary lesions ranged from bronchitis to severe suppurative and infectious necrotizing pneumonitis. Practically all rats (test and controls) had appreciable amount of passive congestion of the viscera; however, it was assumed that these were agonal. The pathological findings between test and control groups were not statistically significant; the endocrine organs were free of abnormalities. | [4] | ||||||||||
| Cytotoxicity | The in vitro cytotoxicity of acetyl tributyl citrate in HeLa cell cultures (human cell line) was evaluated using the metabolic inhibition test, supplemented by microscopy of cells after 24 hours of incubation (the MIT-24 test system). After 24 hours, cell viability was determined by microscopy. Two endpoints of cytoinhibition (total and partial inhibition) were estimated after 24 hours, based on the absence or scarcity of spindle-shaped cells, and, after 7 days The following values for minimal inhibitory concentration were reported for acetyl tributyl citrate: 13 mg/mL (for total inhibition at 24 hours), 3.8 mg/mL (for partial inhibition at 24 hours), and 5.7 mg/mL (for total and partial inhibition at 7 days). Acetyl tributyl citrate caused little toxicity in HeLa cell cultures. | [4] | ||||||||||
| The cytotoxicity of acetyl-tributyl-citrate and dibutyl-sebacate was studied in cultured mammalian cells. The impetus for the study was a report that acetyl-tributyl-citrate and dibutyl-sebacate, which were plasticizers found in polyvinylidene-chloride film used for packaging food, could leach out and diffuse into the foods. Human KB cells, monkey Vero cells, and canine MDCK cells were incubated with acetyl-tributyl-citrate or dibutyl sebacate over a range of concentrations for 72 hours. Cytotoxicity was evaluated by determining the extent of growth inhibition. Doses of acetyl-tributyl-citrate and dibutyl-sebacate that inhibited growth by 50% were calculated from the data. Both compounds inhibited the growth of all cells in a dose dependent manner. The inhibited growth by 50% of acetyl-tributyl-citrate in the various types were: 44.7 µg/mL in KB cells; 39.9 ug/m: in Vero cells; and 42.1 µg/mL in MDCK cells. The inhibited growth by 50% of dibutyl-sebacate in these cells were: KB cells, 1,549 µg/ml; Vero cells, 1,510 ug/ml; and MDCK cells, 1,549 µg/mL. /It was/ concluded that when comparing the results of this study with those obtained previously using tricresyl-phosphate,triphenyl-phosphate (TPP), butylated-hydroxyanisole, and butylated-hydroxytoluene in human KB cells, acetyl-tributyl-citrate is more toxic than TCP and more toxic than TPP. Acetyl-tributyl-citrate is less toxic than BHA, but shows toxicity similar to that of BHT. DBS is much less toxic than either BHT or BHA. KB, Vero, and MDCK cells show similar sensitivity to acetyl-tributyl-citrate and DBS. | [4] | |||||||||||
| Neurotoxicity | Blood pressure expt in rabbits revealed that tributyl citrates produced complete loss of blood pressure when admin in toxic doses. Tributyl citrates also found to have local anesthetic action in rabbit experiments & to block neural transmission in rats when placed in contact with a nerve trunk. | [4] | ||||||||||
| Acetyl tributyl citrate (in 3% acacia, applied to sciatic nerve) induced complete, reversible nerve block during electrical stimulation of the sciatic nerve-anterior tibialis muscle in white rats. Complete blockage of contralateral reflex was also demonstrated | [4] | |||||||||||
| Three drops of a 5% suspension of acetyl tributyl citrate in a 3% gum acacia medium was instilled into the conjunctival sac of the eye of a rabbit. Corneal reflex action was temporarily abolished (local anesthetic effect). | [4] | |||||||||||
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||||||||||||
| Reproductive Toxicity | Ray, 2-generation reproduction, oral, repeated dose NOAEL = 100 mg/kg per day (parental) NOAEL = 100 mg/kg per day (offspring) F0 and F1 adult data: No treatment-related clinical observations were noted throughout the study in either F0 or F1 parental animals. Body weights of F0 parents and F1 females were largely unaffected by treatment with ATBC; however, body weights of the F1 parental males in the 300 and 1000 mg/kg/day groups were consistently lower that controls and appeared to be related to treatment. Body weights of the F0 females in the 1000 mg/kg/day group at the end of pregnancy (gestation days 21 or 22) was significantly lower than control values. Water consumption of the F0 and F1 parental animals fed ATBC at a level of 1000 mg/kg/day were consistently lower than concurrent controls throughout the study. Mating, gestation and fertility of the F0 and F1 generations were unaffected by treatment. There were no abnormalities seen at necropsy that were considered to be treatmentrelated. Offspring toxicity: The body weights of the pups from the 300 and 1000 mg/kg/day dose groups were slightly lower than those of the controls, and slightly higher mortality also was observed in these groups. It was considered that these effects are a consequence of the reduced water intakes in the dams at these dose levels rather than a direct effect of ATBC. No other treatment-related effects were observed in the parameters evaluated. |
[2] | ||||||||||
| Teratogenicity | Rat, 12 months, oral NOEL = 50 mg/kg (parental toxicity) NOEL = 250 mg/kg (developmental toxicity) ATBC is rapidly and extensively absorbed, and then rapidly metabolised and virtually completely excreted by the rat. Developmental toxicity was not observed at dose levels as high as 1000 mg/kg/day in a two-generation reproductive toxicity study nor in a 13-week toxicity study with an in utero exposure phase. The metabolites that have been positively identified in the urine of rats (acetyl citrate, monobutyl citrate, acetyl monobutyl citrate, dibutyl citrate and two isomers of acetyl dibutyl citrate) have been demonstrated to undergo rapid clearance from the body and are not suspected to be developmental toxicants. Also, other ATBC metabolites, acetic acid, citric acid, butyric acid, tributyl citrate and butanol, do not pose a concern for developmental toxicity |
[2] | ||||||||||
| Toxicokinetics | ||||||||||||
| Toxicokinetics | ||||||||||||
| Ecotoxicity Data | ||||||||||||
| Algae | EC50 = 0.148 mg/l (Selenastrum capricornutum, 96h) | [2] | ||||||||||
| Daphnia magna (acute) | EC50 = 7.82 mg/l (Ceriodaphnia dubia, 48h) NOEC = 60.2 mg/l (Ceriodaphnia dubia, 24h) NOEC = 4.82 mg/l (Ceriodaphnia dubia, 48h) LOEC > 60.2 mg/l (Ceriodaphnia dubia, 24h) LOEC = 8.7 mg/l (Ceriodaphnia dubia, 48h) |
[2], [4] | ||||||||||
| Other aquatic organisms | - | |||||||||||
| Fish (acute) | LC50 = 59 mg/l (Fundalus heteroclitus, 96h) NOEC = 10 mg/l (Fundalus heteroclitus, 96h) |
[2], [4] | ||||||||||
| LC50 = 38-60 mg/l (Lepomis macrochirus, 96h) NOEC = 10 mg/l (Lepomis macrochirus, 96h) |
[2], [4] | |||||||||||
| EC50 = 3.5 mg/l (Pimephales promelas, 24h) NOEC = 2.62 mg/l (Pimephales promelas, 24h) LOEC = 5.01 mg/l (Pimephales promelas, 24h) EC50 = 2.8 mg/l (Pimephales promelas, 48h) NOEC = 1.28 mg/l (Pimephales promelas, 48h) LOEC = 2.62 mg/l (Pimephales promelas, 48h) EC50 = 1.9 mg/l (Pimephales promelas, 7 days) NOEC = 1.28 mg/l (Pimephales promelas, 7 days) LOEC = 2.62 mg/l (Pimephales promelas, 7 days) |
[2], [4] | |||||||||||
| Bacteria | - | |||||||||||
| Terrestrial organisms | - | |||||||||||
| Environmental Fate | ||||||||||||
| BCF | An estimated BCF of 250 was calculated for acetyl tributyl citrate, using a water solubility of 5 mg/l and a regression-derived equation. According to a classification scheme, this BCF suggests the potential for bioconcentration in aquatic organisms is high, provided the compound is not altered physically or chemically once released into the environment. | [4] | ||||||||||
| Aerobic biodegradation | 14% at day 5 26% at day 21 Standard BOD test |
[2] | ||||||||||
| > 90% in 5 h Sewage solumn degradation |
[2] | |||||||||||
| Acetyl tributyl citrate, present at an initial concentration of 30 mg/l, reached 80% of the theoretical BOD in 4 weeks with an activated sludge inoculum in the modified MITI test. 82% BOD 93% TOC |
[4], [6] | |||||||||||
| Anaerobic biodegradation | - | [4] | ||||||||||
| Abiotic degradation | The rate constant for the vapour-phase reaction of acetyl tributyl citrate with photochemically-produced hydroxyl radicals has been estimated as 1.4 x 10-11 cm³/(molecule-sec) at 25ºC using a structure estimation method. This corresponds to an atmospheric half-life of about 27 hours at an atmospheric concentration of 5 x 10+5 hydroxyl radicals per cm³. A base-catalyzed second-order hydrolysis rate constant of 5.8 x 10-2 L/(mol-sec) was estimated using a structure estimation method; this corresponds to half-lives of 3.8 years and 140 days at pH values of 7 and 8, respectively. | |||||||||||
| Photodegradation | T½ = 0.740 days | [2] | ||||||||||
| Stability in water | T½ = 139.3 days at pH 8 T½ = 3.8 days at pH7 |
[2] | ||||||||||
| Metabolic pathway | - | |||||||||||
| Mobility | - | |||||||||||
| Volatilization from water | The Henry's Law constant for acetyl tributyl citrate is estimated as 3.8 x 10-10 atm/m³/mol using a fragment constant estimation method. This Henry's Law constant indicates that acetyl tributyl citrate is expected to be essentially nonvolatile from water surfaces. Acetyl tributyl citrate is not expected to volatilize from dry soil surfaces based upon an estimated vapour pressure of 4.6 x 10-6 mm Hg, determined from a fragment constant method. | [4] | ||||||||||
| Soil adsorption/mobility | The Koc of acetyl tributyl citrate is estimated as 1,800, using a water solubility of 5 mg/l and a regression-derived equation. According to a classification scheme, this estimated Koc value suggests that acetyl tributyl citrate is expected to have low mobility in soil. | [4] | ||||||||||
| Terrestrial fate | Based on a classification scheme, an estimated Koc value of 1,800, determined from a water solubility of 5 mg/l and a regression-derived equation, indicates that acetyl tributyl citrate is expected to have low mobility in soil. Volatilization of acetyl tributyl citrate from moist soil surfaces is not expected to be an important fate process given an estimated Henry's Law constant of 3.8 x 10-10 atm/m³/mol, using a fragment constant estimation method. Acetyl tributyl citrate is not expected to volatilize from dry soil surfaces based upon an estimated vapour pressure of 4.6 x 10-6 mm Hg, determined from a fragment constant method. Based on limited data, acetyl tributyl citrate is expected to biodegrade readily in the soil environment; 80% of the theoretical BOD was reached in 4 weeks using an activated sludge inoculum and the Japanese MITI test. | [4] | ||||||||||
| Aquatic fate | Based on a classification scheme, an estimated Koc value of 1,800, determined from a water solubility of 5 mg/l and a regression-derived equation, indicates that acetyl tributyl citrate is expected to adsorb to suspended solids and sediment. Volatilization from water surfaces is not expected based upon an estimated Henry's Law constant of 3.8 x 10-10 atm/m³/mol, developed using a fragment constant estimation method. According to a classification scheme, an estimated BCF of 250, from its water solubility and a regression-derived equation, suggests the potential for bioconcentration in aquatic organisms is high. Estimated hydrolysis half-lives of 3.8 years and 140 days at pH values of 7 and 8, respectively, were deteremined using an estimated base-catalyzed second-order hydrolysis rate constant of 5.8 x 10-2 l/(mol-sec). Based on limited data, acetyl tributyl citrate is expected to biodegrade readily in the aquatic environment; 80% of the theoretical BOD was reached in 4 weeks using an activated sludge inoculum and the Japanese MITI test. | [4] | ||||||||||
| Atmospheric fate | According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere, acetyl tributyl citrate, which has an estimated vapour pressure of 4.6 x 10-6 mm Hg at 25ºC, determined from a fragment constant method, is expected to exist in both the vapour and particulate phases in the ambient atmosphere. Vapour-phase acetyl tributyl citrate is degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 27 hours, calculated from its rate constant of 1.4 x 10-11 cm³/(molecule-sec) at 25ºC that was derived using a structure estimation method. Particulate-phase acetyl tributyl citrate may be physically removed from the air by wet and dry deposition. | [4] | ||||||||||
| Conclusion | ||||||||||||
| Physical-chemical | - | |||||||||||
| Emission | - | |||||||||||
| Exposure | - | |||||||||||
| Health | - | |||||||||||
| Environment | - | |||||||||||
| References | ||||||||||||
| 1 | ESIS | |||||||||||
| 2 | EPA HPV programme | |||||||||||
| 3 | MSDS Sigma Aldrich | |||||||||||
| 4 | HSDB | |||||||||||
| 5 | ChemID | |||||||||||
| 6 | MITI | |||||||||||
| 7 | SRC physprop database | |||||||||||
Diisononyl adipate, DINA
| Identification of the substance | ||
| CAS No. | 33703-08-1 | |
| EINECS No. | 251-646-7 | [1] |
| EINECS Name | diisononyl adipate | [1] |
| Synonyms | Diisononyl adipate DINA |
|
| Molecular Formula | C24H46O4 | [1] |
| Structural Formula |
|
|
| Major Uses | Rubber and plastic products Electrical and electronic products |
[3] |
| IUCLID | HPV | [1] |
| EU classification | This substance is not classified in the Annex I of Directive 67/548/EEC as such, but it may be included in one of the group entries. | [1] |
| Physico-chemical Characteristics | ||
| Physical Form | Liquid | [2] |
| Colourless liquid with a faint odour | [5] | |
| Molecular Weight (g/mole) | 399 | [3] |
| Melting Point/range (°C) | - 68ºC | [2], [4], [5] |
| - 60ºC | [3] | |
| Boiling Point/range (°C) | 224-228ºC at 7 hPa > 250ºC at 1013 hPa |
[2], [4] |
| 233ºC at 5 mm Hg | [3] | |
| 239-244ºC at 7 mBar | [5] | |
| Decomposition Temperature (°C) | - | |
| Vapour Pressure (mm Hg at °C) | < 0.1 hPa at 20ºC [10 Pa, 7.5 x 10-2 mmHg] 1013 hPa at 430ºC (under argon) [1.013 x 105 Pa, 759.8 mmHg] |
[2] |
| 0.9 mm Hg at 200ºC [119.9 Pa] | [3] | |
| < 0.075 mm Hg at 20ºC [9.99 Pa] | [4] | |
| Density (g/cm3 at °C) | 0.923 at 20ºC | [2], [4] |
| 0.918-0.922 at 20ºC | [5] | |
| Vapour Density (air=1) | - | |
| Henry’s Law constant (atm/m³/mol at °C) | 2.9 x 10-5 | [3] |
| Solubility (mg/l water at °C) | < 1 mg/l at 20ºC | [2], [4] |
| 0.00022 mg/l at 20ºC | [3] | |
| < 0.01 g/l at 25ºC [< 10mg/l] | [5] | |
| Partition Coefficient (log Pow) | 9.56 – 10.4 at 25ºC | [2], [4] |
| 9.24 | [5] | |
| pKa | - | |
| Flammability | Not flammable | [2] |
| Explosivity | Not explosive | [2] |
| Oxidising Properties | No oxidising properties | [2], [5] |
| Migration potential in polymer | - | |
| Flash point | 223ºC open cup | [2] |
| 232ºC | [4] | |
| 215ºC | [5] | |
| Auto flammability | 380ºC | [2] |
| 330ºC | [5] | |
| LogKow | 9.24 | [3] |
| Explosion limit | Lower = 1.8% (179.5ºC and 18.8 hPa) Upper = 2.4% (209.7ºC and 24.6 hPa) |
[5] |
| Emission Data | ||
| During production | - | |
| Exposure Data | ||
| Aquatic environment, incl. sediment | - | |
| Terrestrial environment | - | |
| Sewage treatment plant | - | |
| Working environment | Maximum number of potentially exposed workers: between 100 and 999 (including those of manufacturing, industrial processing and use) | [3] |
| Consumer goods | - | |
| Man exposed from environment | - | |
| ”Secondary poisoning” | - | |
| Atmosphere | - | |
| Dermal | - | |
| Toxicological data | ||
| Observations in humans | - | |
| Acute toxicity | ||
| Oral | LD50 > 5000 mg/kg (rat) | [2] |
| LD50 > 10,000 mg/kg (rat) | [3] | |
| Dermal | LC50 > 3160 mg/kg (rabbit) | [3] |
| Inhalation | - | |
| Other routes | - | |
| Skin irritation | Not irritating (rabbit) | [2] |
| Eye irritation | Not irritating (rabbit) | [2] |
| Irritation of respiratory tract | - | |
| Skin sensitisation | Not sensitisation (human) | [5] |
| Subchronic and Chronic Toxicity | ||
| Oral | Rat, 13 weeks repeat dose NOAEL = 500 mg/kg NOEL = 500 mg/kg per day (male) NOEL = 150 mg/kg per day (female) |
[2], [3] |
| Dog, 13 weeks repeat dose Hepatocytic hypertrophy and aspermatogenesis in top dose animals. |
[2] | |
| Dog, 13 weeks repeat dose LOAEL = 822 – 1644 mg/kg per day NOAEL = 274 mg/kg per day |
[3] | |
| Inhalation | - | |
| Dermal | - | |
| Mutagenicity, Genotoxicity and Carcinogenicity | ||
| Mutagenicity | S.typhimurium (metabolic activator (S9)) The test substance showed no evidence of mutagenic activity when tested in this bacterial system with and without activation. Cytotoxic conc.: Not cytotoxic up to 5000 µg/plate Genotoxic effects: Negative with and without S-9 activation |
[2] |
| Mouse lymphoma (metabolic activator (S9)) Negative up to 100µl/ml |
[2], [3] | |
| S.typhimurium (metabolic activator (S9)) The test substance showed no evidence of mutagenic activity when tested in this bacterial system with and without activation. Cytotoxic conc.: Not cytotoxic up to 1000 µg/plate Genotoxic effects: Negative with and without S-9 activation |
[2] | |
| S.typhimurium (metabolic activator (S9)) Cytotoxic conc.: Not cytotoxic up to 1000 µg/plate Genotoxic effects: Negative with and without S-9 activation |
[3] | |
| Chromosome Abnormalities | - | |
| Other Genotoxic Effects | - | |
| Carcinogenicity | - | |
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||
| Reproductive Toxicity | NOAEL = 400 mg/kg per day LOAEL = 800 mg/kg per day |
[3] |
| Teratogenicity | - | |
| Developmental toxicity | NOAEL = 400 mg/kg per day (maternal) LOAEL = 800 mg/kg per day (maternal) NOAEL = 200-400 mg/kg per day (offspring) |
[3] |
| Toxicokinetics | ||
| Toxicokinetics | - | |
| Ecotoxicity Data | ||
| Algae | EC50 > 100 mg/l (Green algae, 72h) | [5] |
| Daphnia magna | NOEC > 100 mg/l (daphnia magna, 21 days) | [2] |
| EC50 > 100 mg/l (daphnia magna, 48h) | [5] | |
| Other aquatic organisms | - | |
| Fish (acute) | LC50 > 500 mg/l (Leuciscus idus, 96h) | [2] |
| NOEC = 2.2 x 10-4 mg/l (Oncorhynchus mykiss, 96h) | [3] | |
| Bacteria | EC10 > 10,000 (Pseudomons putida, 30 min) EC50 > 10,000 (Pseudomons putida, 30 min) EC90 > 10,000 (Pseudomons putida, 30 min) TKG > 10,000 (Pseudomons putida, 30 min) |
[2] |
| Terrestrial organisms | - | |
| Activated sludge | EC20 > 1000 mg/l (Sludge, 30 min) | [2] |
| Environmental Fate | ||
| BCF | 1102 – 2031 (21 days) | [2] |
| 11,000 (35 days at 15ºC) | [2] | |
| 3.2 | [3] | |
| Aerobic biodegradation | 82% after 28 days Readily biodegradable |
[2] |
| > 90% after 28 days Readily biodegradable |
[2] | |
| 73% after 28 days Redily biodegradable |
[3] | |
| Anaerobic biodegradation | - | |
| Photodegradation | T½ = 0.4 days | [3] |
| Metabolic pathway | - | |
| Mobility | - | |
| Stability in water | 4.6 years at pH 7 169 days at pH 8 |
[3] |
| Conclusion | ||
| Physical-chemical | - | |
| Emission | - | |
| Exposure | - | |
| Health | - | |
| Environment | - | |
| References | ||
| 1 | ESIS | |
| 2 | IUCLID dataset | |
| 3 | EPA HPV programme | |
| 4 | MITI | |
| 5 | MSDS BASF | |
12-(Acetoxy)-stearic acid, 2,3-bis(acetoxy)propyl ester)
| Identification of the substance | ||
| CAS No. | 36150-63-3 (COMGHA) 330198-91-9 (Component A, ca. 84%) 33599-07-4 (Component B, ca. 10%) |
[1] |
| EINECS No. | 451-530-8 | [2] |
| EINECS Name | - | |
| Synonyms | COMGHA Grinsted soft'n'safe Acetylated monoglycerides of fully hydrogenated castor oil. Acetic acid esters of monoglycerides of fully hydrogenated castor oil. 12-(Acetoxy)-stearic acid, 2,3-bis(acetoxy)-propylester |
|
| Molecular Formula | C27H48O8 (Component A) C25H46O6 (Component B) |
[1] |
| Structural Formula | 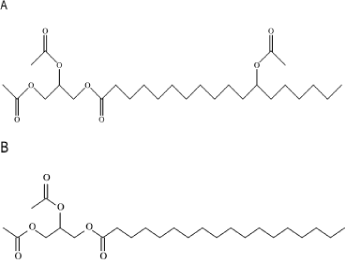 |
|
| Major Uses | Plasticizer | [1] |
| IUCLID | - | |
| EU classification | ||
| Physico-chemical Characteristics | ||
| Physical Form | Greasy substance with a slightly acid odour | [4] |
| Molecular Weight (g/mole) | 500.7 (Component A) 442.6 (Component B) |
[1] |
| Melting Point/range (°C) | - 21.5ºC | [1] |
| Boiling Point/range (°C) | 300ºC at 1 atm (decomposition) | [1] |
| Decomposition Temperature (°C) | - | |
| Vapour Pressure (mm Hg at °C) | < 2.8 x 10-4 Pa at 100ºC [2.1 x 10-6 mmHg] | [1] |
| 1.1 x 10-7 Pa at 25ºC [8.25 x 10-10 mmHg] 4.8 x 10-8 Pa at 20ºC [3.6 x 10-10 mmHg] |
[3] | |
| Density (g/cm3 at °C) | 1.0030 at 20ºC | [3] |
| Vapour Density (air=1) | - | |
| Henry’s Law constant (atm/m³/mol at °C) | - | |
| Solubility (mg/l water at °C) | 0.007 g/l [7 mg/l] | [1] |
| < 0.33 mg/l at 20ºC pH ca. 6.8 | [3] | |
| Partition Coefficient (log Pow) | 6.42 | [4] |
| pKa | - | |
| Flammability | - | |
| Explosivity | - | |
| Oxidising Properties | - | |
| Migration potential in polymer | - | |
| Log Kow | 6.4 | [1] |
| Koc | Immobile and remains preferably in soil | [3] |
| Flash point | 244ºC at 101.3 kPa | [3] |
| Auto ignition tempetature | ca 370ºC | [3] |
| Emission Data | ||
| During production | - | |
| Exposure Data | ||
| Aquatic environment, incl. sediment | - | |
| Terrestrial environment | - | |
| Sewage treatment plant | - | |
| Working environment | - | |
| Consumer goods | - | |
| Man exposed from environment | - | |
| ”Secondary poisoning” | - | |
| Atmosphere | - | |
| Dermal | - | |
| Toxicological data | ||
| Observations in humans | - | |
| Acute toxicity | ||
| Oral | LC50 > 2000 mg/kg (rat) | [3] |
| Dermal | - | |
| Inhalation | - | |
| Other routes | - | |
| Skin irritation | Not irritating (rabbit) | [3] |
| Eye irritation | Not irritating (rabbit) | [3] |
| Irritation of respiratory tract | - | |
| Skin sensitisation | Not a skin sensitizer | [3] |
| Subchronic and Chronic Toxicity | ||
| Oral | Rat, 2-weeks No signs of toxicity (3%, 7.5% of the diet) |
[3] |
| Rat, 90 days (extream dose) NOAEL < 3 ml/kg per day |
[3] | |
| Rat, 90 days (adequate dose) NOAEL = 5000 mg/kg per day |
[3] | |
| Inhalation | - | |
| Dermal | - | |
| Metabolism | Hydrolysis of the compound is incomplete and that a proportion of the administered dose passes through the gastrointestinal tract and is excreted unchanged. | [1] |
| No significant absorption across gastrointestinal epithelium | [3] | |
| Mutagenicity, Genotoxicity and Carcinogenicity | ||
| Mutagenicity | Not mutagenic in the Ames test | [3] |
| Not clastrogenic in the in vitro mammalian cytogenetic test | [3] | |
| Chromosome Abnormalities | Not mutagenic in the in vitro mammalian cell gene mutation test | [3] |
| Other Genotoxic Effects | - | |
| Carcinogenicity | - | |
| Reproductive Toxicity, Embryotoxicity and Teratogenicity | ||
| Reproductive Toxicity | - | |
| Teratogenicity | - | |
| Other Toxicity Studies | - | |
| Toxicokinetics | ||
| Toxicokinetics | - | |
| Ecotoxicity Data | ||
| Algae | EC50 = 106 mg/l (72h) 70-95% loss in concentration over test period |
[3] |
| Invetebrates | EC50 = 0.92 mg/l (daphnia magna, 48h) | [3] |
| Other aquatic organisms | - | |
| Fish | LC100 = 0.28 mg/l (zebra fish, 96h) | [3] |
| Bacteria | - | |
| Terrestrial organisms | - | |
| Activated sludge | EC20 > 143 mg/l EC50 > 143 mg/l No inhibitory effect of respiration rate |
[3] |
| Environmental Fate | ||
| BCF | - | |
| Aerobic biodegradation | 98% after 28 days Ready biodegradable |
[3] |
| Anaerobic biodegradation | - | |
| Metabolic pathway | - | |
| Mobility | - | |
| Conclusion | ||
| Physical-chemical | - | |
| Emission | - | |
| Exposure | - | |
| Health | - | |
| Environment | - | |
| References | ||
| 1 | SCENIHR | |
| 2 | ESIS | |
| 3 | Danisco | |
| 4 | MSDS Danisco | |
Version 1.0 November 2010, © Danish Environmental Protection Agency