Survey of chemical substances in cleaning products for ovens, cookers and ceramic cooktops
6 Health assessment of selected substances
- 6.1 Substances selected for assessment
- 6.2 Risk evaluation of the selected substances
- 6.3 Assessment of exposure of the consumer to the selected substances
- 6.3.1 Exposure scenarios
- 6.3.1.1 Exposure by inhalation
- 6.3.2 Assessment of N-methyl-2-pyrrolidone in oven cleaners
- 6.3.3 Evaluation of dipropylene glycol monomethyl ether (DPGME) in oven cleaners
- 6.3.4 Petroleum distillate in cleaners for ceramic cooktops
- 6.3.5 White spirit in stainless steel care products
- 6.4 Environmental assessment of selected substances
6.1 Substances selected for assessment
Based on the screening of the occurrence of the chemical substances in the products and their hazards, the following substances were selected for a more detailed assessment of the exposure of humans and the environment, respectively:
- N-Methyl-2-pyrrolidone
- Dipropylene glycol monomethyl ether
- Petroleum distillates
- White spirit
As mentioned in subsection 2.4.1, three different petroleum distillates were found in five products. Two of the petroleum distillates have been identified as types of white spirit (WHO type 1 and type 3). The third petroleum distillate is chemically closely related to white spirit type 3. In the following assessments the three petroleum distillates will be regarded as white spirit, however, considering the higher boiling point of petroleum distillate, and thus lower vapour pressure and slower evaporation.
The substances are found in cleaning products for ovens, ceramic cooktops and stainless steel. Table 6.1 gives an overview of the product types included in the exposure assessment.
| Product No. | Type of product | Hazardous substance in product |
|---|---|---|
| 1 | Oven cleaners (spray with propellant) | N-methyl-2-pyrrolidone |
| 5 | Over cleaners (gel in pump) | DPGME* |
| 3 | Ceramic cooktop cleaners | Petroleum distillates |
| 20 | Stainless steel care | White spirit |
* Dipropylene glycol monomethyl ether
6.2 Risk evaluation of the selected substances
6.2.1 N-Methyl-2-pyrrolidone
6.2.1.1 Identification and physico-chemical data for N-methyl-2-pyrrolidone
| Chemical name | N-methyl-2-pyrrolidone |
|---|---|
| Synonyms | 2-pyrrolidinone, 1-methyl-, 1-methyl-2-pyrrolidinone, methylpyrrolidone |
| CAS No. | 872-50-4 |
| EINECS No. | 212-828-1 |
| Molecular formula | C5H9NO |
| Molecular structure | 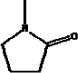 |
| Legislation: Classification according to the Danish EPA List of dangerous substances (Danish Statutory Order No. 439 of 3 June 2002)/2/ List of Undesirable Substances. The Danish EPA /15/ |
Xi; R36/38 (> 10 %) Not on the list |
| Danish National Working Environment Authority’s limit value /16/ | 5 ppm/20 mg.m-3 |
| Physical state | Transparent liquid /17/ |
|---|---|
| Molecular weight (g/mol) | 99.13 |
| Melting point (°C) | 26 /19/ |
| Boiling point (°C) | 202 /19/ |
| Vapour pressure (Pa, 25 ºC) | 70 /18/ |
| Octanol-water partition coefficient (log POW) | -0.11 /19/ |
| Water solubility (mg/L) | 2.48 ´ 10-5 /19/ |
Furthermore, N-methyl-2-pyrrolidone has low volatility and high water absorbing properties.
6.2.1.2 Health assessment of N-methyl-2-pyrrolidone
Acute toxicity
N-Methyl-2-pyrrolidone can be absorbed in the body by inhalation, through the skin and through the gastro-intestinal system and is moderately toxic by all routes of exposure /20/.
The inhalation studies made have either shown no mortality or very low mortality in the exposed animals. No LD50 values have been reported /20/.
| Study | Effect concentration | Reference |
|---|---|---|
| LD50, rat, oral | 3,914 mg.kgbw-1 | /21/ |
| LD50, rabbit, dermal | 8,000 mg.kgbw-1 | /21/ |
| NOAEL, rat, ingestion, 90-day study | 169-217 mg.kgbw-1 | /22/ |
| Reproduction toxicity | ||
| NOEL, rat, inhalation (embryonic weight) | 56 mg.kgbw-1.d-1 | /23/ |
| LOEL, rat, inhalation (embryonic weight) | 130 mg.kgbw-1.d-1 | /23/ |
| NOEL, rabbit, dermal (mother-toxicity and embryonic mortality) | 300 mg.kgbw-1.d-1 | /23/ |
| LOEL, rabbit, dermal (mother-toxicity and embryonic mortality) | 1,000 mg.kgbw-1.d-1 | /23/ |
Irritation of skin and eyes
N-methyl-2-pyrrolidone has a mild skin irritating effect. Prolonged and repeated contact may cause skin irritation /24/. N-Methyl-2-pyrrolidone has high skin permeability and may increase transport of other substances through the skin /20/.
Eye irritation, corneal lesions and conjunctivitis in humans exposed to N-methyl-2-pyrrolidone have been reported /21/. N-Methyl-2-pyrrolidone in concentrations up to 50 mg.m-3 did not show irritation of the mucous membranes of the eyes and the respiratory system in humans /39/.
Sensibilisation
No data were found indicating that N-methyl-2-pyrrolidone may cause allergy.
Toxicity and repeated exposure
Inhalation of aerosols of N-methyl-2-pyrrolidone (0, 100, 500 or 1000 mg.m-3, 6 hours/day, 5 days/week, 4 weeks) caused drowsiness and irregular breathing in all animals after 3 - 4 hours of exposure. The only hazardous effect was found in the respiratory system at the highest exposure level /20/. No effect was found in the respiratory system by inhalation of 20, 40 or 400 mg.m-3, mainly vapours /20/.
Long-term effects
Studies on reproduction toxicity show that N-methyl-2-pyrrolidone may cause harm to the unborn child /20/. After skin contact with N-methyl-2-pyrrolidone at a concentration of 750 mg.kgbw-1, pregnant rats have shown toxic effects in both dam and embryo /22/. In reproduction studies, exposure doses with no or mild toxic effects on female rats (NOAEL 620 mg.m-3, 6 hours) have shown harmful developmental effects on the rat embryos (NOAEL 360 mg.m-3, 6 hours) /20/.
In connection with the preparation of the 31st adaptation of the Substance Directive, it was discussed, as mentioned earlier, to classify N-methyl-2-pyrrolidone as reproduction toxic with T;R61 (May cause harm to the unborn child).
No carcinogenic effects of N-methyl-2-pyrrolidone have been observed /20/.
Conclusion
The critical local effect of N-methyl-2-pyrrolidone by short-term exposure is irritation of the mucous membranes of the eyes and the respiratory system in humans.
NOAEL for irritation is 50 mg.m-3.
The critical systemic effect of N-methyl-2-pyrrolidone by inhalation is drowsiness and irregular breathing. NOAEL for this effect is 100 mg.m-3 after 4 hours, corresponding to 20 mg.kgbw-1.d-1.
The critical effect at repeated exposure for N-methyl-2-pyrrolidone is effect on embryonic development: NOAEL for this effect is 360 mg.m-3, corresponding to approx. 104 mg.kgbw-1.d-1.
N-methyl-2-pyrrolidone has high skin permeability and may promote the skin permeability of other substances.
6.2.2 Dipropylene glycol monomethyl ether (DPGME)
6.2.2.1 Identification and physico-chemical data
| Chemical name | Dipropylene glycol monomethyl ether |
|---|---|
| Synonyms | DPGME Propanol, (2-methoxymethylethoxy)-, (2-methoxymethylethoxy) propanol, |
| CAS No. | 34590-94-8 |
| EINECS No. | 252-104-2 |
| Molecular formula | C7H16O3 |
| Molecular structure | 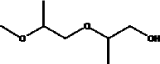 |
| Legislation: Classification according to the Danish EPA List of dangerous substances (Danish Statutory Order No. 439 of 3 June 2002)/2/ List of Undesirable Substances. The Danish EPA /15/ |
Not classified Not on the list |
| Danish National Working Environment Authority’s limit value /16/ | 50 ppm/300 mg.m-3 (H) |
| Physical state | Liquid /17/ |
|---|---|
| Molecular weight (g/mol) | 148.2 |
| Melting point (°C) | -83 /17/ |
| Boiling point (°C) | 190 /17/ |
| Vapour pressure (Pa, 20 ºC) | 37- 60 /12/ |
| Octanol-water partition coefficient (log POW) | -0.06 /12/ |
| Water solubility (mg/L) | Miscible /12/ |
6.2.2.2 Health assessment of dipropylene glycol monomethyl ether (DPGME)
Acute toxicity
DPGME is found on the Danish National Working Environment Authority’s list of organic solvents, with a limit value of 50 ppm/300 mg.m-3, as a substance that may be absorbed through the skin /16/. When exposing rats to 500 ppm (3080 mg.m-3) DPGME for 7 hours, a mild narcotic effect (narcosis) was observed. This corresponds to a systemic dose of 1035 mg.kg-1 /33/.
| Study | Effect concentration | Reference |
|---|---|---|
| LD50, rat, oral | 5,600 mg/kgbw-1 | /21/ |
| LD50, rat, dermal | 9,500 mg/kgbw-1 | /21/ |
Irritation of skin and eyes
DPGME may cause mild irritation of skin and eyes /21, 34/. DPGME has a degreasing effect on the skin /32/. Repeated skin contact (rabbits, 90 days) caused mild skin irritation /33/.
Vapours of DPGME irritate the eyes and the respiratory system /32/. The limit for irritation of the mucous membranes is reported to be 450 mg.m-3 (73 ppm) /34/.
Sensibilisation
No data on sensitising effects or effects on the immune system of DPGME were found. Glycol ethers are generally not considered to be sensitising /33/.
Toxicity at repeated exposure
Oral dosage to rats exposed to 0, 40, 200 or 1000 mg/kgbw-1.d-1 for 4 weeks caused increased saliva secretion and liver weight with histopathological changes at the highest dose. NOAEL in this study was 200 mg/kgbw-1.d-1.
No effects were found in rats or rabbits exposed by inhalation to 200 ppm (1230 mg.m-3) DPGME for 13 weeks. In other inhalation studies, where rats were exposed to concentrations of DPGME of up to 330 ppm (2030 mg.m-3) 6 hours/day for 9 days, a little increase of the liver weight was observed /33/.
Exposure of the skin of rabbits to 1 or 10 ml/kgbw-1.d-1, 5 days/week for 90 days (950, 9500 mg/kgbw-1.d-1, respectively) DPGME caused narcosis and death at the highest dose. NOAEL in this study was 950 mg/kgbw-1.d-1.
Long-term effects
No indications were found that DPGME has toxic effects on the reproduction or the development. No tests have shown mutagenic and carcinogenic properties of DPGME /33/.
Conclusion
Skin irritation is not considered to be a problem.
The critical local effect by short-term exposure to DPGME is irritation of the mucous membranes of the eyes and respiratory system. NOAEL for irritation is 450 mg.m-3.
The critical systemic effect by short-term exposure to DPGME is the effect on the nervous system. NOAEL for acute effect on the nervous system is 3080 mg.m-3, 7 hours, corresponding to 1035 mg/kgbw-1.d-1.
The critical systemic effect by repeated exposure to DPGME is increased liver weight and liver changes; NOAEL for liver effect by repeated exposure is 200 mg/kgbw-1.d-1 (4 weeks).
6.2.3 Petroleum distillate / white spirit
The name white spirit covers, as mentioned in subsection 2.4.1, several types of similar petroleum distillates. In this assessment, Stoddard solvent is used as a basis, but data from the other substances in this group have also been used.
6.2.3.1 Identification and physico-chemical data for white spirit
| Chemical name | White spirit |
|---|---|
| Types |
|
| Synonyms |
|
| CAS Nos. |
|
| EINECS Nos. |
|
| Molecular formula | - |
| Molecular structure | Complex mixtures of unbranched and branched aliphatic, naphthenic and aromatic hydrocarbons |
| Legislation: Classification according to the Danish EPA List of dangerous substances (Danish Statutory Order No. 439 of 3 June 2002)/2/ List of Undesirable Substances. The Danish EPA /15/ |
Not on the list |
| Danish National Working Environment Authority’s limit value /16/ | 25 ppm / 145 - 180 mg.m-3 |
| Odour threshold /28/ | 0.5 - 5 mg.m-3 |
* The substances have only been assessed as regards carcinogenic effect (carc cat. 2) and risk of aspiration into the lungs. The classification is valid for product concentrations ≥ 10 %.
** The classification as carcinogenic may be omitted if it can be proven that the substance contains less than 0.1 weight percentage of benzene, which is the case for almost all of the types of white spirits forming part of products on the Danish market.
| Physical state | Liquid |
|---|---|
| Molecular weight (g/mol) | - |
| Melting point (°C) | - |
| Boiling point (°C) | 150 - 200 /17/ |
| Vapour pressure (Pa, 25 ºC) | 600 /29/ |
| Octanol-water partition coefficient (log POW) | 2.1 - 6 /12/* |
| Water solubility (mg/L) | < 0.1 % /29/ |
* Estimated on the basis of data on naphtha (petroleum), hydrodesulfurized heavy (CAS No. 64742-82-1 – WHO type 1)
The physico-chemical data depend on the specific type of white spirit.
6.2.3.2 Health assessment of white spirit
Acute toxicity
Exposure to white spirit primarily occurs via inhalation, and white spirit is readily absorbed via the respiratory system. Controlled exposure of humans to 100 ppm (600 mg.m-3) for 7 hours affected the central nervous system (CNS) with symptoms as unsteady walk and prolonged reaction time. Exposure to 4000 mg.m-3 for 40 minutes also affected the CNS /30/. Prolonged inhalation of high concentrations of vapours may cause headache, dizziness, intoxication, nausea and convulsions. Exposure to very high concentrations in confined spaces may cause narcotic effects and loss of consciousness /21, 26/.
Ingestion of white spirit may cause risk of aspiration (risk of chemically induced pneumonia) because of its low viscosity and low surface tension /26, 30/. Ingestion will cause malaise in the form of stomach trouble and the same symptoms as when inhaled /21/.
White spirit does not easily penetrate intact skin. Frequent use of hand cleansers containing white spirit has caused systemic effects in the form of liver and bone marrow damage /21/.
| Study | Effect concentration | Reference |
|---|---|---|
| LCLO, rat, 8-hour exposure – no mortality | 8,200 mg.m-3 | /31/ |
| LOEL, inhalation, rat, acute narcotic effect | 1,200 mg.m-3 | /31/ |
| LD50, rat, oral | >5,000 mg/kgbw-1 | /22/ |
Irritation of skin and eyes
Prolonged and repeated skin contact may result in serious irritation eczema. In skin irritation tests, white spirit has proven slightly to moderately irritating /30/. Degreasing, drying-out properties have been observed in relation to skin contact /21, 26/.
Vapours are slightly irritating to the eyes /26/. In humans, eye irritation has been reported down to 100 ppm, corresponding to 600 mg.m-3 /30/.
Sensibilisation
White spirit may cause non-allergic contact eczema, but it has not been found allergenic /27, 31/.
Toxicity at repeated exposure
The classification with R48/20 states that the substance is harmful by prolonged exposure through inhalation. Professional exposure for 13 years to on average 240 mg.m-3 (40 ppm) white spirit caused chronic CNS-effects, so called toxic encephalopathy /31/.
Prolonged, repeated exposure (13 weeks) of rats and dogs to white spirit caused effect on liver and kidneys at air concentrations higher than approx. 500 – 900 mg.m-3 (90 – 150 ppm) /31/.
Long-term effects
No indication of genotoxicity of white spirit was found in various in vitro tests /30/. No teratogenic, embryotoxic or reproduction toxic effects of white spirit were found /21, 31, 32/.
White spirit is classified as carcinogenic (carc2;R45), but the risk is defined on the basis of its content of benzene. White spirits with less than 0.1 % benzene do not have to be classified as carcinogenic /2/.
Conclusion
The critical local effect by short-term exposure to white spirit is eye irritation. The irritating effect to the mucous membranes depends on the contents of naphthenes and aromates. In humans, eye irritation has been reported down to 100 ppm (Cirr = 600 mg.m-3).
The critical systemic effect by short-term exposure (7 hours) to white spirit is the effect on the CNS. In humans, effect on the CNS has been reported at 100 ppm (600 mg.m-3, LOAEL). This corresponds to a drawn reference value (RVacute) of 10 mg/kgbw-1.d-1 for a 60 kg person, who inhales 24 L.min-1 during light work /37/, when the absorption is set to 100 %. An adaptation factor of 10 has been used in order to use LOAEL instead of NOAEL.
The critical effect by repeated exposure to white spirit is the effect on the CNS. In humans, chronic effect on the CNS has been reported by exposure to on average 240 mg.m-3 (40 ppm) for 13 years (LOAEL). When assuming the daily work to be 7 hours, this exposure corresponds to a drawn reference value (RVchronic) of 4 mg/kgbw-1.d-1 for a 60 kg person, who inhales 24 L.min-1 during light work /37/, when the absorption is set to 100 %. An adaptation factor of 10 has been used in order to use LOAEL instead of NOAEL.
6.3 Assessment of exposure of the consumer to the selected substances
Oven cleaners are usually used indoors in kitchens. Some products can be used outdoors, e.g. for cleaning of barbecues. In the following assessments, we have only focused on indoor use.
When applying the oven cleaners, the consumer will mainly be exposed by inhalation and skin contact. Exposure by ingestion is regarded as insignificant and has not been included in the assessments. The consumer will experience acute effects on the contact points (skin and mucous membranes of the eyes and respiratory system), and following absorption and distribution in the body (systemic effects). Furthermore, systemic effects may occur in case of repeated exposure during a long period of time.
The selected substances are individually a component in different products. Two of these products are spray products, aerosol and pump, respectively, applied by spraying. The two other products are liquids, applied with a cloth (application products).
During application of spray products, aerosols of the product will be formed, which may deposit on the skin. During application of application products with a cloth, part of the product comes in contact with the skin. By skin contact, some components may be absorbed through the skin. Volatile components will be liberated into the air and may thus be inhaled.
The health risk in case of exposure to the selected critical substances has been assessed based on worst-case scenarios according to the principles in EU’s Technical Guidance Document /14/ and partly in ECHA’s Guidance on Information Requirements and Chemical Safety Assessment /38/. In the assessments, exposure by inhalation and skin contact has been taken into consideration.
6.3.1 Exposure scenarios
6.3.1.1 Exposure by inhalation
The amount of inhaled substance depends on the concentration of air. The concentration of air (Cinh) depends on the amount of used product (Qprod), the amount of substance in the product (Fcprod) and the volume of air in which the substance is distributed (Vroom). The concentration of air is calculated based on the equation (6.1) /38/.
| (6.1) |
The inhaled dose (Dinh) depends on the concentration in the inhalation air (Cinh), the respirable amount of inhaled dose (Fresp), the person’s respiration velocity (IHair), the time of exposure (Tcontact), the person’s body weight (BW) and the number of applications per day (n). The dose is calculated from the concentration of air based on the equation (6.2) /38/.
| (6.2) |
In the following calculations, it is assumed that the assessed substances are liberated 100 % to the air. Equations (6.1) and (6.2) may be used for both spray and application products.
Table 6.11 explains the symbols used in equations (6.1) and (6.2). Furthermore, a number of standard values used in the following calculations are stated. The standard values are stated in the references /14, 37, 38/. The room size (Vroom) is set to 2 m³ (the person’s immediate zone) /38/, because when using oven cleaner it is a question of a short local exposure; the person’s body weight (BW) is set to 60 kg, which is the standard weight for women /14/; the respirable amount of inhaled dose (Fresp) is set to 1; the respiration velocity (IHair) is set to 34.6 m³.d-1 (24 L.min -1 during light work) /37/; the number of applications per day (n) varies from product to product.
| Parametre | Explanation | Unit | Standard value |
|---|---|---|---|
| Cinh | Concentration of substance in air | kgsubst.m-3 | |
| Qprod | Amount of used product | kgprod | |
| Fcprod | Amount of substance in the product | kgsubst.kgprod-1 (%) | |
| Vroom | Room size | m³ | 2 /38/ |
| Dinh | Inhaled dose of the substance | kgsubst.kglgv-1.d-1 | |
| Fresp | Respirable amount of inhaled dose | - | 1 /38/ |
| IHair | Respiration velocity (light work: 24 L . min-1) |
m³.d-1 | 34.6 /37/ |
| Tcontact | Contact time per application | d | |
| BW | Body weight | kgbw | 60 /38/ |
| n | Number of applications per day | d-1 |
6.3.1.2 Exposure of mucous membranes
For a substance causing irritation of the mucous membranes of the eyes and respiratory system, the effect depends on the concentration of air of the substance. Equation (6.1) is therefore also used when assessing the irritating effect of substances causing irritation of the mucous membranes in both spray and application products.
6.3.1.3 Exposure by skin contact
Exposure of the skin to a chemical product may cause skin irritation. Furthermore, chemical substances in the product may be absorbed through the skin and cause systemic exposure of the user.
When assessing possible skin irritation, the dermal load must be known, which can be calculated with equation (6.3) /38/. The dermal load (Lder) depends on the amount of product used (Qprod), the amount of substance in the product (Fcprod), the amount of product deposited on the skin (Fcder) and the exposed skin area (Askin).
| (6.3) |
When assessing the systemic exposure, the dermal dose must be known, which can be calculated with equation (6.4) /38/. The dermal dose depends on the amount of product used (Qprod), the amount of substance in the product (Fcprod), the amount of product deposited on the skin (Fcder), the person’s body weight (BW) and the number of applications per day (n).
| (6.4) |
Table 6.12 explains the symbols used in equations (6.3) and (6.4). Furthermore, a number of standard values used in the following calculations are stated. The standard values are stated in the references /14, 37, 38/.
| Parametre | Explanation | Unit | Standard value |
|---|---|---|---|
| Lder | Amount of substance per skin area | kgsubst.m-2 | |
| Qprod | Amount of used product | kgprod | |
| Fcprod | Amount of substance in the product | kgsubst.kgprod-1 | |
| Fcder | Amount of used product on the skin | - | |
| Askin | Exposed skin area | cm² | 731 /38/ |
| Dder | Dose on the skin that may potentially be absorbed | kgsubst.kgbw-1.d-1 | |
| BW | Body weight | kgbw | 60 /38/ |
| n | Number of application per day | d-1 |
For spray products, RIVM has set standard values for the amount of product that stays in the air as drops. The fraction for aerosol sprays is 0.6 and for pump sprays the fraction is 0.2 /37/. In the following calculations it is assumed that 10 % of the amount of product that stays in the air as drops comes into contact with the surface of the hands (Askin), i.e. Fcder = 0.06, 0.02, respectively.
For the use of application products, RIVM has set a standard value of 1 % for the amount of product left on the hands (Fcder) /37/. Furthermore, RIVM assumes that application products come into contact with half of the skin area of the hands (Askin / 2) /37/.
If there is no knowledge of the absorption of a substance through the skin, it is assumed that the amount of substance, which comes into contact with the skin, is 100 % absorbed.
6.3.1.4 Systemic exposure
When estimating the systemic exposure (Dsyst), the contributions from inhalation (Dinh) and skin absorption (Dder) must be added, see equation (6.5).
| (6.5) |
6.3.1.5 Risk assessment
In the following assessments, it is assumed that the exposure is determined by further factors.
The cleaning is assumed to last 5 minutes, and the exposure is only assessed in connection with the cleaning, and not possible following exposure by inhalation. The assessments do not include possible ventilation via cooker hood during the cleaning.
The result of the risk assessment is stated as margin of exposure (MOE), which is expressed by the ratio between the NO(A)EL-value and the exposure to the relevant scenario using the above equations (6.1) – (6.5). If there is no NO(A)EL-value, the LO(A)EL is used. The definition of these concepts is stated in Table 6.13, which is used to express the health effect.
| NO(A)EL (No Observed (adverse) Effect Level) |
The highest concentration/dose of the substance with no observed effect in exposed individuals compared with a comparable control group. |
| LO(A)EL (Lowest Observed (adverse) Effect Level) |
The highest concentration/dose of the substance with the lowest observed effect in exposed individuals compared with a comparable control group. |
| MOE (Margin of Exposure) |
Is the factor that NO(A)EL is higher than the estimated exposure level for the exposed consumer. The bigger the MOE, the lesser the risk. If NO(A)EL-values are used for calculating MOE, MOE-values of 100 or above will give a reasonable certainty that the consumer will have no effects. |
6.3.2 Assessment of N-methyl-2-pyrrolidone in oven cleaners
A cleaning product for ovens (product no. 1) is selected to represent the products containing N-methyl-2-pyrrolidone. The product is an oven cleaner in an aerosol expenser. Tables 6.14 and 6.15 show the parametres, which form the basis for the calculation of exposure to N-methyl-2-pyrrolidone.
| Product type | Oven cleaner |
|---|---|
| Product form | Spray with propellant (aerosol spray) |
| Instructions | Shake the can well and spray at a distance of 25 cm in an even coat into the cold barbecue/oven on all surfaces and grills. Other objects are also sprayed from a distance of 25 cm. Wash away the foam with a damp sponge or cloth. |
| Specific gravity of the product | Estimated to 0.8 kg.L-1. |
| Concentration in the product (Fcprod) | 0.15 (15 % after evaporation of the propellant, see Chapter 4). |
| Parametre | Symbol | Value |
|---|---|---|
| Room size | Vroom | 2 m³ |
| Body weight | BW | 60 kg |
| Cleaning frequency | n | 0,14 d-1 (1 once a week) |
| Used amount per cleaning | Qprod | 8 g (10 ml) |
| Exposure time (estimate) | Tcontact | 0.0035 d (5 min) |
| Respirable fraction | Fresp | 1 |
| Respiration velocity (light work) | IHair | 34.6 m³.d-1 (24 L.min-1) /37/ |
In the following, the risk of inhalation, skin absorption and the total exposure are assessed by calculating MOE-values for these exposure routes for N-methyl-2-pyrrolidone. N-Methyl-2-pyrrolidone can be absorbed through the skin. Therefore, it is necessary to include skin absorption when calculating the systemic dose.
It is assumed that the product is used once a week for cleaning of oven, which is an expression of a worst-case consumption. As N-methyl-2-pyrrolidone is heavily volatile with a vapour pressure of 70 Pa at 25 °C, it is assumed that only 1 % evaporates during use and may be inhaled.
For products in aerosol cans with a propellant, it is assumed that 60 % of the product stays in the air /37/, and that 10 % of this amount comes into contact with the skin, i.e. 6 % of the used amount. Absorption of N-methyl-2-pyrrolidone through the skin is set to 100 % (worst-case).
The results of the exposure calculation are shown in Table 6.16. When risk assessing, the calculated exposure values are compared with the drawn reference values in Table 6.17.
| Estimate | Symbol | Calculation 1) | Value |
|---|---|---|---|
| Concentration in air | Cinh | Equation 6.1 1) | 6 mg.m-3 |
| Dose by inhalation | Dinh | Equation 6.2 1) | 0.0017 mg.kgbw-1.d-1 |
| Dermal load | Lder | Equation 6.3 2) | 0.1 mg.cm-2 |
| Dose by skin contact | Dder | Equation 6.4 2)3) | 0.17 mg.kgbw-1.d-1 |
| Systemic dose | Dsyst | Ligning 6.5 | 0.172 mg.kgbw-1.d-1 |
- It is assumed that only 1 % of N-methyl-2-pyrrolidone evaporates.
- It is assumed that 60 % of the product is found in the air as spray mist /37/, and that 10 % comes into contact with the skin, i.e. 6 % of the used amount.
- N-Methyl-2-pyrrolidone is skin permeable and absorption through the skin is set to 100 % (worst-case).
| Critical effect,see subsection 6.2.1 | Symbol | Reference value |
|---|---|---|
| Irritation of mucous membranes, eyes | - | 50 mg.m-3 |
| Systemic effect, short-term exposure | RVacute | 20 mg.kgbw-1.d-1 |
| Systemic effect, repeated exposure | RVchronic | 105 mg.kgbw-1.d-1 |
6.3.2.1 Skin irritation
The estimated dermal load with N-methyl-2-pyrrolidone is 0.1 mg.cm-2. No quantitative data were found, which would have enabled an assessment of possible skin irritation. N-Methyl-2-pyrrolidone is readily absorbed through the skin.
6.3.2.2 Exposure of the mucous membranes
The concentration of air of N-methyl-2-pyrrolidone is calculated to 6 mg.m-3for a room size of 2 m³. This is approx. 8 times lower than the concentration that caused irritation of the mucous membranes in humans (MOE = 8). It is assessed that there is a risk of irritation of the mucous membranes when using N-methyl-pyrrolidone this way.
6.3.2.3 Systemic exposure
N-Methyl-2-pyrrolidone is readily absorbed through the skin. The contribution to the systemic exposure from skin absorption is estimated to 0.17 mg.kgbw-1.d-1. The contribution to the systemic exposure from inhalation of vapours of N-methyl-2-pyrrolidone is calculated to 0.0017 mg.kgbw-1.d-1. The total systemic exposure is thus 0.172 mg.kgbw-1.d-1. When comparing the calculated exposure to the drawn reference value RVacute of 20 mg.kgbw-1.d-1 , MOE = 116 is found. If the calculated exposure is compared to the drawn reference value RVchronic of 105 mg.kgbw-1.d-1 , MOE = 610 is found. It is assessed that there is no risk of acute or chronic effects when using N-methyl-2-pyrrolidone this way.
6.3.3 Evaluation of dipropylene glycol monomethyl ether (DPGME) in oven cleaners
A cleaning product for ovens (product no. 5) has been selected to represent the products containing DPGME. The product is an oven cleaner in a can with a pump. Tables 6.18 and 6.19 show the parameters, which form the basis of the calculation of exposure to DPGME.
| Product type | Oven cleaner |
|---|---|
| Product form | Gel in a pump bottle |
| Application | Spray the gel on the surfaces of the oven. Leave the gel to work for 2-4 hours. |
| Specific gravity of the product | Not informed |
| Concentration in product (Fcprod) | 0.13 (13 %) |
| Parametre | Symbol | Value |
|---|---|---|
| Room size | Vroom | 2 m³ |
| Body weight | BW | 60 kg |
| Cleaning frequency | n | 0.14 d-1 (once a week) |
| Used amount per cleaning | Qprod | 5 g |
| Exposure time (estimate) | Tcontact | 0.0035 d (5 min) |
| Respirable fraction | Fresp | 1 |
| Respiration velocity (light work) | IHair | 34.6 m³.d-1 (24 L.min-1) /37/ |
In the following, the risk of inhalation, skin absorption and the total exposure are assessed by calculating MOE-values for these exposure routes for DPGME. DPGME can be absorbed through the skin. Therefore, it is necessary to include skin absorption when calculating the systemic dose.
It is assumed that the product is used once a week for cleaning of oven, which is an expression of a worst-case consumption. As DPGME is heavily volatile with a vapour pressure of approx. 37 - 60 Pa at 20 °C, it is assumed that only 1 % DPGME evaporates during use and may be inhaled.
For products in pump spray bottles, it is assumed that 20 % of the product stays in the air as spray mist /37/, and that 10 % of this amount comes into contact with the skin, i.e. 2 % of the used amount. Absorption of DPGME through the skin is set to 100 % (worst-case).
The results of the exposure calculation are shown in Table 6.20. When risk assessing, the calculated exposure values are compared with the drawn reference values in Table 6.21.
| Estimate | Symbol | Calculation | Value |
|---|---|---|---|
| Concentration in air | Cinh | Equation 6.1 1) | 3.3 mg.m-3 |
| Dose by inhalation | Dinh | Equation 6.2 1) | 0.00093 mg.kgbw-1.d-1 |
| Dermal load | Lder | Equation 6.3 2) | 0.018 mg.cm-2 |
| Dose by skin contact | Dder | Equation 6.4 2)3) | 0.03 mg.kgbw-1.d-1 |
| Systemic dose | Dsyst | Equation 6.5 | 0.031 mg.kgbw-1.d-1 |
- It is assumed that only 1 % of DPGME evaporates.
- It is assumed that 20 % of the product is found in the air as spray mist /37/, and that 10 % comes into contact with the skin, i.e. approx. 2 % of the used amount.
- DPGME is skin permeable and absorption through the skin is set to 100 % (worst-case).
| Critical effect, see subsection 6.2.2 | Symbol | Reference value |
|---|---|---|
| Irritation of mucous membranes, eyes | - | 450 mg.m-3 |
| Systemic effect, short-term exposure | RVacute | 1035 mg.kgbw-1.d-1 |
| Systemic effect, repeated exposure | RVchronic | 200 mg.kgbw-1.d-1 |
Skin irritation
The estimated dermal load with DPGME is 0.018 mg.cm-2. No quantitative data were found, which would have enabled an assessment of possible skin irritation. DPGME is readily absorbed through the skin.
Exposure of the mucous membranes
The concentration of air of DPGME is calculated to 3.3 mg.m-3for a room size of 2 m³. This is approx. 135 times lower than the concentration that caused irritation of the mucous membranes in humans (MOE = 135). It is assessed that there is a risk of irritation of the mucous membranes when using DPGME this way.
Systemic exposure
DPGME is readily absorbed through the skin. The contribution to the systemic exposure from skin absorption is estimated to 0.03 mg.kgbw-1.d-1. The contribution to the systemic exposure from inhalation of vapours of DPGME is calculated to 0.00093 mg.kgbw-1.d-1. The total systemic exposure is thus 0.031 mg.kgbw-1.d-1. When comparing the calculated exposure to the drawn reference value RVacute of 1035 mg.kgbw-1.d-1 , MOE = 33,400 is found. If the calculated exposure is compared to the drawn reference value RVchronic of 200 mg.kgbw-1.d-1, MOE = 6450 is found. It is assessed that there is no risk of acute or chronic effects when using DPGME this way.
6.3.4 Petroleum distillate in cleaners for ceramic cooktops
A cleaning product for ceramic cooktops (product no. 3) has been selected to represent the products containing petroleum distillate. The product is applied with a cloth. Tables 6.22 and 6.23 show the parameters, which form the basis of the calculation of exposure to petroleum distillate. For the risk assessment, the drawn RVacute and RVchronic are used for white spirit.
| Product type | Ceramic cooktop cleaner |
|---|---|
| Product form | Relatively thin |
| Application | Apply and then polish with a soft cloth |
| Specific gravity of the product | 1.07 kg.L-1 |
| Concentration in the product (Fcprod) | 0.13 (13 %) |
| Parametre | Symbol | Value |
|---|---|---|
| Room size | Vroom | 2 m³ |
| Body weight | BW | 60 kg |
| Cleaning frequency | n | 1 d-1 (every day) |
| Used amount per cleaning | Qprod | 5.35 g (5 ml) |
| Exposure time (estimate) | Tcontact | 0.0035 d (5 min) |
| Respirable fraction | Fresp | 1 |
| Respiration velocity (light work) | IHair | 34.6 m³.d-1 (24 L.min-1) /37/ |
It is assumed that the ceramic cooktops are cleaned once a day after use. As petroleum distillate is heavily volatile with a vapour pressure of approx. 13 - 60 Pa at 20 °C, it is assumed that only 1 % petroleum distillate evaporates during use and may be inhaled.
In the following, the risk of exposure of the mucous membranes and the risk of systemic exposure by inhalation are assessed. No quantitative data were found for absorption through skin of petroleum distillates, but it is known to be very limited through intact skin. It is assessed that absorption through the skin is negligible considering the short contact time. The contribution to the systemic exposure has therefore been set to naught.
The results of the exposure calculation are shown in Table 6.24. When risk assessing, the calculated exposure values are compared with the drawn reference values in Table 6.25.
| Estimate | Symbol | Calculation | Value |
|---|---|---|---|
| Concentration in air | Cinh | Equation 6.1 1) | 3.5 mg.m-3 |
| Dose by inhalation | Dinh | Equation 6.2 1) | 0.0070 mg.kgbw-1.d-1 |
| Dermal load | Lder | Equation 6.3 2) | 0.019 mg.cm-2 |
| Dose by skin contact | Dder | Equation 6.4 2)3) | 0 mg.kgbw-1.d-1 |
| Systemic dose | Dsyst | Equation 6.5 | 0.0070 mg.kgbw-1.d-1 |
- It is assumed that only 1 % of the petroleum distillate evaporates.
- It is assumed that 1 % of the product comes into contact with half of the surface of the hands (365 cm²) /37/.
- Petroleum distillate does not penetrate intact skin. Absorption through skin is set to 0 %.
| Critical effect, see subsection 6.2.3.2 | Symbol | Reference value |
|---|---|---|
| Irritation of mucous membranes, eyes | - | 600 mg.m-3 |
| Systemic effect, short-term exposure | RVacute | 10.0 mg.kgbw-1.d-1 |
| Systemic effect, repeated exposure | RVchronic | 4.0 mg.kgbw-1.d-1 |
Skin irritation
The estimated dermal load with petroleum distillate is 0.019 mg.cm-2. No quantitative data were found, which would have enabled an assessment of possible skin irritation. Petroleum distillate may degrease and dry out the skin and thus cause skin irritation. Gloves should be worn during use of the product.
Exposure of the mucous membranes
The concentration of air of petroleum distillate is calculated to 3.5 mg.m-3for a room size of 2 m³. This is approx. 170 times lower than the concentration that caused eye irritation in humans (MOE = 170). It is assessed that there is no risk of irritation of the mucous membranes when using petroleum distillate this way.
Systemic exposure
No quantitative data were found for the absorption through skin of petroleum distillate, but it is assessed that the absorption is very limited through intact skin. The contribution to the systemic exposure has therefore been set to naught.
The contribution to the systemic exposure from inhalation of vapours of petroleum distillate is calculated to 0.0070 mg.kgbw-1.d-1. When comparing the calculated exposure to the drawn reference value RVacute of 10 mg.kgbw-1.d-1 , MOE = 1430 is found. If the calculated exposure is compared to the drawn reference value RVchronic of 4 mg.kgbw-1.d-1 , MOE = 570 is found. It is assessed that there is no risk of acute or chronic effects when using petroleum distillate this way.
6.3.5 White spirit in stainless steel care products
A stainless steel care product (product no. 20) has been selected to represent the products containing white spirit. The product is applied with a cloth. Tables 6.26 and 6.27 show the parametres, which form the basis of the calculation of exposure to white spirit.
| Product type | Stainless steel care product |
|---|---|
| Product form | Fluid |
| Application | Cleans and polishes stainless steel and lacquered surfaces. Apply with a dry cloth and rub. |
| Specific gravity of the product | 0.92 kg.L-1 |
| Concentration in product (Fcprod) | 60 % /37/ |
The maximum concentration of white spirit in the product is estimated to 60 % /37/.
| Parametre | Symbol | Value |
|---|---|---|
| Room size | Vroom | 2 m³ |
| Body weight | BW | 60 kg |
| Cleaning frequency | n | 0.29 d-1 (twice a week) |
| Used amount per cleaning | Qprod | 5 g (found during practical testing) |
| Exposure time | Tcontact | 0.0035 d (5 minutes) |
| Respirable fraction | Fresp | 1 |
| Respiration volume (light work) | IHair | 34.6 m³.d-1 (24 L.min-1) /37/ |
White spirit is relatively heavily volatile with a vapour pressure of approx. 600 Pa at 25 °C. The evaporation velocity for a substance, and thus the amount of substance that evaporates per time unit, depends on the vapour pressure of the substance among others. The connection is approximately linear at low vapour pressure. The vapour pressure for white spirit is approx. 10 times higher than the vapour pressure for the three other solvents assessed in this report. White spirit is therefore estimated to evaporate approx. 10 times faster than these. As a realistic worst-case in the following exposure calculations, it is assumed that 10 % white spirit evaporates during use.
In the following, the risk of exposure of the mucous membranes and the risk of systemic exposure by inhalation are assessed. No quantitative data were found for absorption through skin of white spirit, but it is known to be very limited through intact skin. It is assessed that absorption through the skin is negligible considering the short contact time. The contribution to the systemic exposure has therefore been set to naught.
The results of the exposure calculation are shown in Table 6.28. When risk assessing, the calculated exposure values are compared with the drawn reference values in Table 6.29.
| Estimate | Symbol | Calculation | Value |
|---|---|---|---|
| Concentration in air | Cinh | Equation 6.1 1) | 150 mg.m-3 |
| Dose by inhalation | Dinh | Equation 6.2 1) | 0.088 mg.kgbw-1.d-1 |
| Dermal load | Lder | Equation 6.3 2) | 0.082 mg.cm-2 |
| Dose by skin contact | Dder | Equation 6.4 2)3) | 0 mg.kgbw-1.d-1 |
| Systemic dose | Dsyst | Equation 6.5 | 0.088 mg.kgbw-1.d-1 |
- It is assumed that only 10 % of the white spirit evaporates.
- It is assumed that 1 % of the product comes into contact with half of the surface of the hands (365 cm²) /37/.
- White spirit does not penetrate intact skin. Absorption through skin is set to 0 %.
| Critical effect, see subsection 6.2.4 | Symbol | Reference value |
|---|---|---|
| Irritation of mucous membranes, eyes | - | 600 mg.m-3 |
| Systemic effect, short-term exposure | RVacute | 10.0 mg.kgbw-1.d-1 |
| Systemic effect, repeated exposure | RVchronic | 4.0 mg.kgbw-1.d-1 |
Skin irritation
The estimated dermal load with white spirit is 0.082 mg.cm-2. No quantitative data were found, which would have enabled an assessment of possible skin irritation. White spirit may degrease and dry out the skin and thus cause skin irritation. Gloves should be worn during use of the product.
Exposure of the mucous membranes
The concentration of air of white spirit is calculated to 150 mg.m-3 for a room size of 2 m³. This is approx. 4 times lower than the concentration that caused eye irritation in humans. The irritating effect of white spirit to the mucous membranes depends on the contents of naphthenic and aromatic hydrocarbons. If the used type of white spirit only contains small amounts of especially aromatic hydrocarbons, irritation of the mucous membranes will be considerably limited when using white spirit this way.
Systemic exposure
No quantitative data were found for the absorption through skin of white spirit, but it is assessed that the absorption is very limited through intact skin. The contribution to the systemic exposure has therefore been set to naught.
The contribution to the systemic exposure from inhalation of vapours of white spirit is calculated to 0.088 mg.kgbw-1.d-1. When comparing the calculated exposure to the drawn reference value RVacute of 10 mg.kgbw-1.d-1, MOE = 113 is found. If the calculated exposure is compared to the drawn reference value RVchronic of 4 mg.kgbw-1.d-1 , MOE = 45 is found. It is assessed that there will be a risk of chronic effects when using white spirit for a long period of time.
6.4 Environmental assessment of selected substances
6.4.1 N-methyl-2-pyrrolidone
N-methyl-2-pyrrolidone is assessed to be completely biodegradable on the basis of results from various tests for biodegradability /12/. No data were found for the anaerobic biodegradability. N-methyl-2-pyrrolidone generally has low acute toxicity for aquatic organisms with EC/LC50-values > 100 mg.L-1 in standard tests for acute toxicity with algae, crustacean and fish /12/. With an estimated log POW-value of -0.38, the substance is assessed not to be bioaccumulable. N-methyl-2-pyrrolidone is assessed to constitute no risk to the environment.
6.4.2 Dipropylen glycol monomethyl ether (DPGME)
DPGME is completely degraded in a 28 days standard test for ready biodegradability /12/. No data were found for the anaerobic biodegradability. Only a few data were found for the aquatic toxicity of DPGME. These data indicate that DPGME has low acute toxicity for aquatic organisms with EC/LC50-values > 100 mg.L-1 in tests with crustacean and fish /12/. With an estimated log POW-value < 0 the substance is assessed to be not bioaccumulable. On the basis of the above data, DPGME is assessed to constitute no risk to the environment.
6.4.3 Petroleum distillates
Petroleum distillates are not completely biodegradable in standard tests for ready biodegradability /12/. Petroleum distillates may be toxic to aquatic organisms with EC/LC50-values for acute toxicity between 1 - 10 mg.L-1 in standard tests with algae, crustacean and fish /12, 13/. With estimated log POW-values between 3.3 and 8.7 petroleum distillates are potentially bioaccumulable. Petroleum distillates should thus be classified as Dangerous for the environment (N) with R51/53 (Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.). A more detailed assessment of the effect of petroleum distillates in the aquatic environment is given in Chapter 7.
6.4.4 White spirit
White spirit is assessed on the basis of data for petroleum distillates and naphtha (pertroleum), hydrogentreated, heavy /12/ to be not completely biodegradable and at the same time to be toxic to aquatic organisms with EC/LC50-values between 1 and 10 mg.L-1. With estimated log POW-values between 2.1 and 6, white spirit is potentially bioaccumulable. A more detailed assessment of the effect of white spirit in the aquatic environment is given in Chapter 7.
Version 1.0 December 2010, © Danish Environmental Protection Agency