Fate of Pesticides in Surface Waters, Laboraty and Field Experiments
Appendix D. Experiment with filters
10.18 D.1 Introduction In order to evaluate the usefulness of filters for separation of dissolved and sorbed pesticide, an experiment to determine the take up of pesticide by different filters was performed.
10.19 D.2 Materials Solutions of radiolabelled pendimethalin, ioxynil and bentazone in three concentrations were used.
Three filter types were tested:
10.20 D.3 Experimental procedure A set-up of glass filtering equipment was used. Filtering was made in this order:
For each solution, all three filter types were tested using the following procedure. Three mL of the solution was poured through the first filter and 2 mL of the filtrate was mixed with 15 mL scintillation liquid and the activity was determined using LSC. The remaining filtrate was discarded. Using the same filter, another 3 mL was filtered and again 2 mL was collected for determination of activity. Finally, the filter was transferred to a scintillation vial and the activity was determined. A new type of filter was mounted in the equipment and the procedure was repeated. After filtering with all three filter types had been performed, the same procedure was performed with the next, stronger solution. The glass equipment was cleaned between pesticides. For control, the same procedure was performed without filter.
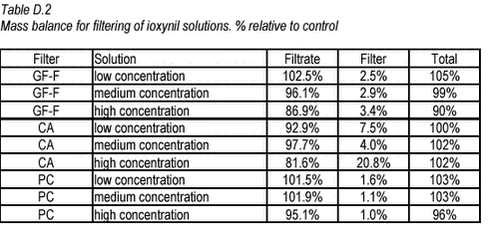
10.21 D.4 Results Table D.1 Mass balance for filtering of bentazone solutions. % relative to control
The results show that only the glass fibre filter has any potential for use for separation of sorbed and freely dissolved pendimethalin while all three types of filters can apparently be used for ioxynil and bentazone. In general, there was no difference between first and second filtering. But for pendimethalin, less pendimethalin was retained in the second filtering indicating that the filter could be saturated. |