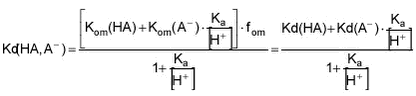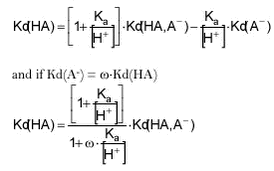Fate of Pesticides in Surface Waters, Laboraty and Field ExperimentsAppendix F. The influence of pH on sorption coefficientsBentazone and ioxynil are reasonably strong acids and they will be dissociated at neutral pH. The level of dissociation can be calculated from:
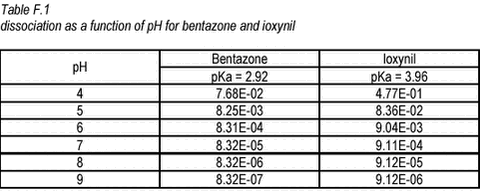
where áa is the fraction of the total amount present as non-dissociated (neutral) acid. áa is given for different pHs in the table below.
From Table F.1 it can be seen that, at pH around 7, only 0.008% of the bentazone will occur in the neutral form. The remainder will be charged. Thus, the Kd values determined in this study at pH around 7 are for a mixture of the neutral and charged form but mainly for the charged form. The hydrophobicity of organic compounds falls drastically when the compound becomes charged and if the dissolution into organic matter is the main responsible mode of sorption. Kd decreases drastically. The relationship between Kd for the charged and the neutral acid is called ù. If only the sorption/dissolution to organic matter is considered, the following expression can be written
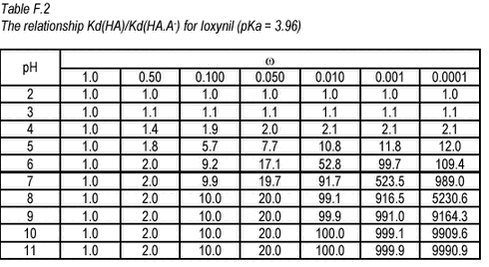
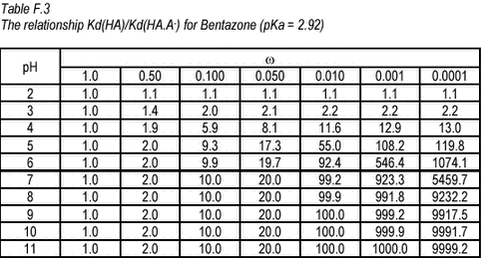
Equation 6 This relationship has been tabulated as function of ù and pH in Tables F.2-F.3 below.
For bentazone, Kow has been measured at different pH (Chapter 2). At low pH, at which the neutral HA form dominates, a Kow of 219 was reported. At high pH, atwhich the charged A- form dominates, a Kow of 0.28 has been reported. This gives a Kow-ù around 0.001, which means that for the neutral form Kow(HA) is 900 times higher than the Kow(HA.A-) at pH around 7. The relationship between Kd(HA) and Kd(HA.A-) will be similar because Kd for a soil with 2% of organic matter (268), at pH 7, is similar to Kow (219) in numeric size. Unfortunately, ù is not known for ioxynil but, for organic acids, it is often 0.01 to 0.001 (Schwarzenbach et al., 1993). Assuming that ù is 0.001. Kd(HA) is 500 times higher than Kd(HA.A-) at pH 7. Conversely, the tables show that the effective Kd(HA.A-) will change drastically at low pH but at pH above 7, it is more or less independent of pH. This means that an increase in pH as may be caused by photosynthesis, will have no effect on the partitioning of these pesticides. A decrease below a pH of 6 will, however, lead to a stronger sorption. |