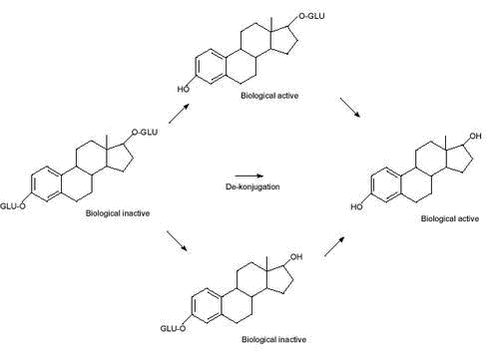
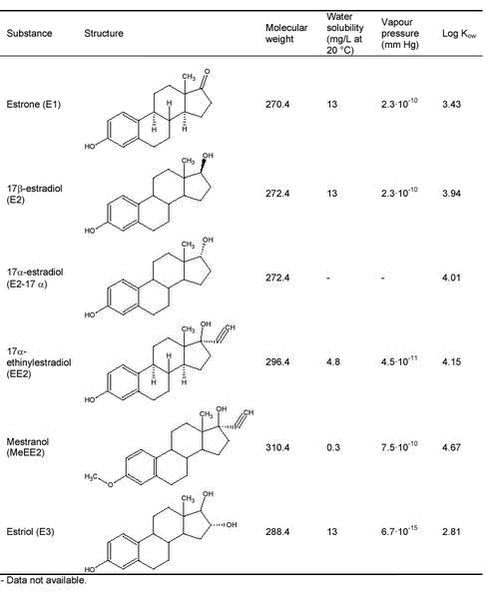
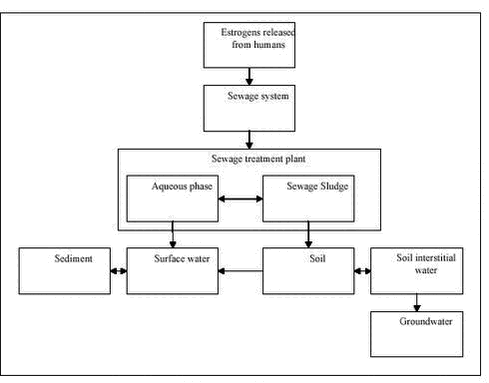
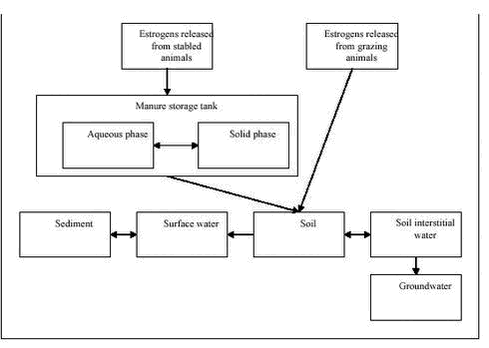
Evaluation of Analytical Chemical Methods for Detection of Estrogens in the Environment2 Environmental and chemical properties of estrogens with relevance for analysis2.1 Physicochemical properties2.2 Release of estrogens from humans and animals and transport to the environment 2.2.1 Metabolism and excretion of estrogens 2.2.2 Human release of estrogens 2.2.3 Release of estrogens from farm animals 2.3 Effect concentrations in the environment This section describes the properties of steroid estrogens that are important in analysing for these substances. A limited number of steroid estrogens are important environmental pollutants and this report will cover only these compounds. Therefore, in this report the terms steroid estrogens and estrogens will be used for the natural estrogens, E1, E2, E2-17α (excreted by animals) and E3 and the synthetic analogs, EE2 and MeEE2. These substances are listed in Table 2.1 along with their full names and physicochemical data. All the substances are released from humans and animals as the structures shown in Table 2.1 or in various conjugated forms (see section 2.2.1 for more details). The primary conjugates are glucuronic acids and sulphate and these will therefore be included in this report. Other types of conjugates will not be mentioned in this report. 2.1 Physicochemical propertiesThe water solubility of steroid estrogens is low and range from 0.3 to approximately 13 mg/L with the natural steroids having the highest solubility.The synthetic steroids have the highest octanol-water partitioning coefficients (log Kow). All steroids have very low vapour pressures and relatively high pKa-values (above 10). From these data it can be seen that the estrogens are non-volatile, highly lipophilic substances that can be expected to adsorb to solids in environmental matrices. The low water solubilities of the substances, can cause problems when preparing solutions for analytical purposes, e.g., standards. As the compounds readily dissolve in organic solvents (e.g, methanol), using these solvents will solve this problem. When estrogens are excreted from mammals, the primary route is via formation of glucuronic acid or sulphate conjugates (for more details see section 2.2.1). Conjugated steroids are a factor 10 to 50 more water soluble that the parent estrogens. 2.2 Release of estrogens from humans and animals and transport to the environmentThe purpose of section 2.2 is to identify the matrices and analytes that are relevant when estrogens pollute the environment. 2.2.1 Metabolism and excretion of estrogensThe mechanisms and kinetics of de-conjugation of estrogens are important factors to determine, in order to assess and predict the estrogenic potency of surface waters. To facilitate the excretion with urine the female body primarily excretes estrogens in a biological inactive form as sulphate- and glucoronide conjugates. Such conjugates may, depending on different factors, easily be cleaved, resulting in a re-activation of the estrogens to an active form (4) (see Figure 2.1). This reformation or de-conjugation of estrogens depends partially on the acid-base properties of the environmental matrix and on possible bacterial processes withinthe matrix. Knowledge of these factors allows one to predict whether there are sufficiently high concentrations of the free and active compounds to elicit an estrogenic response in an exposed environmental organism. Different glucuronid conjugates of estrogens are known (5). Conjugation of E2 and EE2 can occur in the C3 position, in the C17 position and in both the C3 and C17 positions. Estriol conjugation occurs in all the previous positions and can occur in the C18 position, as well. Sulphatation can also be expected in all the previously cited positions on the molecule. Conjugates possessing both glucuronidation and sulphatation also exist. Because the estrogen receptor is an unspecific receptor, a response will depend only on de-conjugation in the C3 position. In Figure 2.1 de-conjugation pathways are exemplified by E2 and the biological activity is given. Similar tentative pathways could be identified for the other estrogens. Figure 2.1: Preliminary experiments have shown that about 80 % of 17ß-estradiol glucoronide conjugates are detected as E2 and E1 in environmental matrices after 20-30 hours, and after 50 hours 10-20 % of the E1 and E2 was still not degraded (6). In other studies slower degradation of E2 and the primary degradation products E1 were observed. Here 88 % was degraded to E1 after 24 hours, and 95 % of the E1 was degraded after 14 days (7). In corresponding studies under anaerobic conditions E2 degraded considerably slower (50 % after 7 days), while E1 accumulated. Concerning the synthetic estrogen EE2 only 20 % was degraded after 24 hours under aerobic conditions. 2.2.2 Human release of estrogensEstrogens excreted by humans may occur in a range of matrices where analytical methods should be available. Human waste is released to the sewer system which leads to the sewage treatment plants (STPs) where the estrogens can either be removed by degradation or adsorption to the sludge. If no removal occurs the estrogens are released to the receiving waters (streams, rivers or coastal waters) where they can adsorb to sediment and other solids. The estrogens adsorbed to sewage sludge may re-enter the aqueous phase when the sludge is dewatered otherwise they may reach the agricultural fields if the sewage sludge is used for fertilizing the soil. An overview of the different compartments that determine how estrogens from humans may enter the environment is shown in Figure 2.2. Table 2.1: Figure 2.2: The understanding of the processes determining the exposure routes depicted in Figure 2.2 is complicated further by the fact that estrogens are excreted in conjugated forms. It is well known that microorganisms can re-activate estrogens by breaking down the conjugated substances (4;6;8). As the un-conjugated estrogens bind more strongly to solids (e.g. sewage sludge) than the relatively hydrophilic conjugated forms, it is important to evaluate whether the STPs act as chemical reactors producing free estrogens, or if they act in a positive role in removing free estrogens. Therefore monitored concentrations of the conjugated as well as the un-conjugated estrogens are crucial in order to understand the processes involved in the removal of estrogens in STPs. Several authors specify the amounts of estrogens released from humans (9-11). Generally the endogenous excretion of hormones by healthy pre-menopausal women is reported to range from 10 to 100 µg estrogens per day. The amount of E1 excreted is typically twice as high as of E2 and E3. After menopause, women only excrete between 5 to 10 µg estrogens daily. The values for normal men average from 2 to 25 µg per day (12). Pregnant women may excrete up to 30 mg per day, but average values are around 250 µg/day (11). Women using contraception-pills are assumed to excrete the whole daily dose of 25-50 µg. Based on data on the daily excretion of estrogens from humans, influent and effluent concentrations of steroid estrogens in sewage treatment plants (STPs) can be estimated using simple assumptions about dilution in sewage water, sorption to sewage sludge, dilution of sewage effluent, etc. Using such methods, the expected environmental concentrations have been calculated for a range of relevant compartments (10;13;14). With regard to the analyte concentrations for methods for detecting estrogens, these estimates are important as they represent maximum or worst-case levels of the concentration of estrogens in the analytical matrices. More realistic concentration levels of estrogen released from humans can be obtained from monitoring data reported in numerous publications (see e.g., (15-19)). Table 2.2 lists representative concentrations of estrogens in a range of relevant matrices. Both estimated concentrations and measured data from literature are shown. The purpose of presenting the data is to indicate the concentration levels of estrogens to be expected in the environment; therefore an extensive presentation of all references with measured environmental data as presented in several reviews is omitted (3;19). Table 2.2: 2.2.3 Release of estrogens from farm animalsThe emission of natural estrogens from farm animals (cattle, pigs, chicken, etc.) is potentially a major source of estrogen pollution in the environment. The major components are E1, E2, 17α-estradiol (E2-17α) and their conjugates. In contrast to humans, the release of E2-17α is significant (up to 56% (27)). After storage in the manure-tank, estrogens excreted from stabled animals may be released to the soil environment when manure is used for fertilization of soil. Alternatively the compounds may reach the soil, directly in urine and faeces from grazing animals. Sorption of the estrogens is a significant removal process in both the manure and in soil and the release to the aqueous environment (groundwater and surface water) may therefore be minimal. An overview of the different compartments that determines how estrogens from animals may enter the environment is shown in Figure 2.3. Quantities of estrogens vary among other things with animal species and with the types of animal production. Therefore, estimates of the total amounts released are encumbered with greater uncertainties than similar data for humans and further, the regional differences in animal farming practices are important issues to consider. Okkerman and co-workers (27) estimated the release of estrogens from animals in the Netherlands. According to their literature-study, the concentration of estrogens in faeces and urine from non-pregnant cows is around 30 µg/kg and 15 µg/L respectively. The average excretion during pregnancy is reported to be 1.3 mg/day/animal. Similarly the average excretion of estrogens in manure is 1.13 mg/kg from pregnant pigs. The release from non-pregnant pigs is estimated to be 100-200 times lower. The assumption that 3% of manure applied on fields in the Netherlands is leached to surface water results in estimated concentrations of approximately 1.3 µg/L in surface water (27). Due to the difficult analytical matrix, very few investigations report concentrations in manure and soil of estrogens of animal origin. In fact it is still a question whether the estrogens from animals reach surface waters. As a consequence it is not possible to give a general picture of the concentrations measured in manure; therefore Table 2.3 lists only examples from the few existing studies. Table 2.3: Figure 2.3: 2.3 Effect concentrations in the environmentA detailed overview of the effects that estrogens may cause in the environment is not the purpose of the current report. However, from an analytical perspective the effect concentrations expressed as NOEC (No Observed Effect Level) or LOEC (Lowest Observed Effect Level) are of interest because such data expresses the sensitivity needed for analytical methods. The vast amount of data on adverse effects on environmental organisms due to estrogens have hitherto been reported for various fish (3;33;34) species. A comparison of the LOEC’s obtained in different studies is presented in Table 2.4. Based on these data and considering that the occurrence of a pollutant in the environment is considered safe only if the concentration is orders of magnitude below the LOEC’s obtained in laboratory experiments (35) it is obvious that extremely sensitive analytical techniques (at least below 0.05 ng/L) are needed to detect estrogens at sub-effect concentrations in surface water. When sewage effluent is entering surface water the dilution obviously reduces the concentration of estrogens. It is common praxis to operate with a 10-fold dilution when the risk of the release of sewage is assessed. Thus, a concentration of estrogens in sewage effluent leading to LOEC’s in surface water is 10 times higher than the values indicated in Table 2.4. Table 2.4: The release of estrogens when manure is used for fertilizing agricultural soil, may lead to effects on soil organisms. Very few studies report such data. One example is a study showing that the growth of alfalfa (Medicago sativa) was significantly stimulated by concentrations of steroid estrogens of 10 ng/L (39). Due to the limited data available it is not possible to establish lowest observed effect concentrations for steroid estrogens in the soil environment.
|