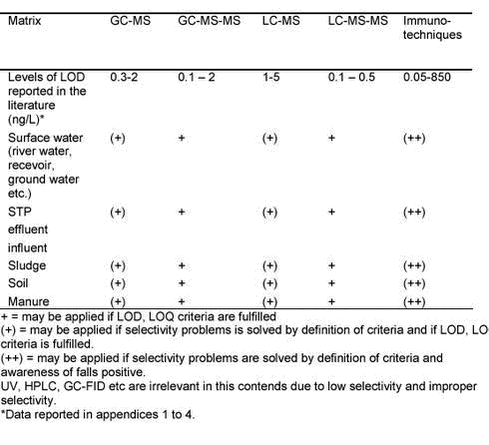
Evaluation of Analytical Chemical Methods for Detection of Estrogens in the Environment5 Recommendations, Perspectives and Conclusions5.1 Sample preservation, extraction and clean-up5.2 Analytical equipment 5.2.1 GC-MS or LC-MS versus GC-MS-MS or LC-MS-MS methods 5.2.2 Comparison of LC or GC methods 5.2.3 Immunochemical techniques 5.2.4 Comparison of methods 5.2.5 Future perspectives Analysis of steroid estrogens in environmental samples is a major challenge for analytical chemists and several aspects of this field are still considered unsolved. At present a myriad of instruments and techniques is available for the analysis of steroid estrogens. The selection of the instrument which is optimal for the purpose is problematic. It is important to realize that the selection of an optimal method is highly dependent on the type of matrix that is analyzed. Therefore, in selecting the correct method, a number of criteria should be considered. In a typical case the anticipated concentration level of estrogens in a given sample will force one to use a specific type of instrument and analytical method simply because of a required LOD, or LOQ. The choice of instruments and methods will again force one to define a number of specific validation criteria in order to validate the applied analytical method. Recall that in chapter 2 we gave an overview of the estrogen concentration level that can be expected in the different types of environmental matrices. In Table 5.1 we repeat the major figures. Table 5.1: 5.1 Sample preservation, extraction and clean-upAn effective sample clean-up procedure and pre-concentration technique may allow the use of less sensitive analytical methods and equipment. Table 5.1 outlines the different primary extraction techniques and clean-up procedures presented in literature for the discussed matrices. Matrices such as sludge, soil, manure and influent waste water require more effort in sample clean-up due to the possibility of matrix interference than do surface water or ground water. A comprehensive cleanup may thus allow the use of less sensitive detection methods if the criteria for variability and selectivity of the method are fulfilled (see below). Conversely, a reduction in sample preparation effort will require the use of analytical methods having a high selectivity. The choice of methods depends highly on the available expertise in a particular laboratory because sample extraction and clean-up is often expert work. 5.2 Analytical equipmentSeveral different analytical detection methods have been suggested in the literature for analysing estrogens in the environment. Table 5.2 gives some recommendations and limitations when using the different methods. UV, GC-FID and HPLC are not included in Table 5.2 as we generally do not recommend the use of these methods due to low sensitivity and selectivity. GC or LC hyphenated with single MS or the use of immunochemical techniques is the minimum necessary for providing sufficiently high quality results. Table 5.2: 5.2.1 GC-MS or LC-MS versus GC-MS-MS or LC-MS-MS methodsAs shown in Table 5.2 both the single MS techniques and triple quadrupole and ion trap MS may be used if appropriate validation is performed. An often asked question is therefore, what is gained, other than enhanced sensitivity, by using the MS-MS based techniques in place of single MS. MS-MS techniques also improve the selectivity. Ternes et al. (18) presented EI spectra (Figure 5.1) of EE2 and an unknown impurity illustrating the problem. Both compounds exhibited exactly the same retention time and both EI spectra showed the m/z values of 440 (molecular weight of silylated 17á-ethinylestradiol) and 425 (Mw minus CH3), however with a different ratio. Using MS-MS –detection (two lowest chromatograms on the figure) of the target ion m/z 425, a confirmation with regard to identification and quantification of EE2 can be carried out. Due to the fact that the MS-MS-spectra of the contraceptive and the unknown impurity are different, a precise quantification is possible using the product ions m/z 193 and m/z 231 of the precursor ion m/z 425. For the unknown compound these two precursor ions were not formed showing that this unknown compound was not 17á-ethinylestradiol, but an impurity that co-eluted. Using single MS detection it would not have been possible to distinguish between the two compounds.
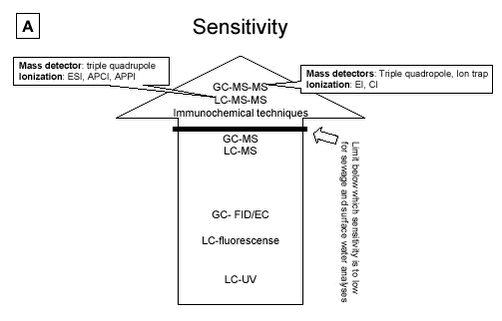
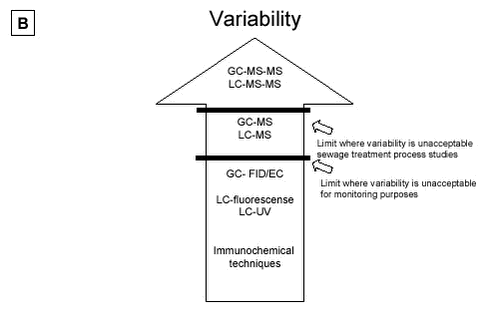
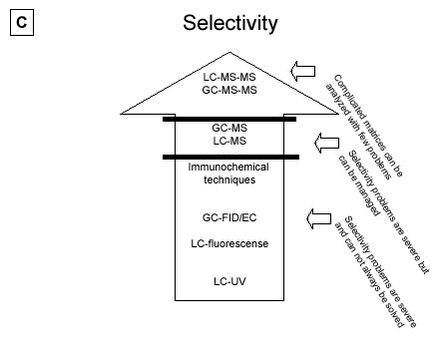
Figure 5.1: 5.2.2 Comparison of LC or GC methodsIn terms of accuracy and repeatability, LC-MS, GC-MS-MS and LC-MS-MS are in general satisfactory, although the derivatisation step used prior to gas chromatography, in addition to being time-consuming, can constitute a source of inaccuracy. An advantage of GC-MS compared with LC-MS, is the availability of external libraries of mass spectra which are useful for the identification of unknown peaks in estrogenically active fractions. The recent introduction of the tandem mass spectrometry detection, has substantially improved the performance of chromatographic methods by reducing detection limits and aiding analyte identification. Future development of equipment favours the choice of LC based methods as a strategy for analysing estrogens. The same situation that occurred in pesticide analysis may be repeated in this field. Five years ago many pesticide analyses were performed with GC based techniques, but the LC-MS-MS are now so sensitive that they rival the GC based techniques in selectivity and sensitivity. Also, new ionisation techniques such as the APPI and others have been developed for several types of highly sensitive triple quadrupole instruments. These developments may cause the LC-MS-MS to become the first choice analytical method due to increased sensitivity and higher selectivity. 5.2.3 Immunochemical techniquesImmunochemical methods provide very sensitive methods, especially for waste water and STP effluents, but the selectivity is poor compared with the triple quadrupole instruments. Highly polluted samples should generally be avoided due to adsorption to binding sites. These methods are also subject to problems with low selectivity and false positive samples. Variability is also a severe problem. Methods for the conjugated estrogens are also on the market, but suffer from the same problems as the methods available for parent estrogens. Immunochemical methods are not recommended as a stand alone “analytical tool”. Immunochemical methods have the potential to provide useful data when used in conjunction with chemical analysis. Today such strategies are not developed and research is needed to develop an appropriate strategy combining such methods with LC-MS-MS or GC-MS-MS or single MS. 5.2.4 Comparison of methodsFigure 5.2 A to D gives an overview that ranks the analytical methods according to the four most important parameters (A:sensitivity, B:variability, C:selectivity and D:investment costs for the purchase of analytical instruments) to be used in selecting adequate methods for analysing estrogens in matrices. These rankings anticipate that the highest possible clean-up is performed so matrix interference is reduced as much as possible. 5.2.4.1 Sensitivity From a sensitivity point of view the triple quadrupole instruments such as GC-MS-MS and LC-MS-MS are superior to all other instruments. Furthermore, new modes of ionisation and sample handling which are on the way to market, may improve the sensitivity even more. One example is the newly developed APPI ionisation technique (see section 3.4.2.3). But other ionisation techniques such as both ESI and APCI are also adequate for use. Research in sample preparation has shown promising new tools that may be tried in this field. But under all circumstances, if an LOD of less 0.1 ng/L is required further research is needed. LC-APPI-MS-MS seems superior to other ionisation methods (e.g. ESI or APCI), but it is still not very well documented. Single GC-MS and LC-MS methods are of limited value without extraordinary sample clean-up efforts, as LODs are often below the sensitivity level recommended for both effluent sewage and surface water analysis. Immunochemical methods are very sensitive, but care must be taken to recognize false positive samples. 5.2.4.2 Variability As with the parameter “sensitivity”, “variability” is much improved when the triple quadrupole instruments are used. In fact, a cut-off line has been drawn below the GC-MS-MS and LC-MS-MS instruments that excludes the other methods from use in sewage treatment process studies (see Figure 5.2B). Both single GC-MS and LC-MS may be applied from a variability point of view for monitoring purposes. GC-FID/EC, LC-fluorescence, LC-UV and immunochemical techniques are all inadequate from a variability viewpoint. 5.2.4.3 Selectivity Highest selectivity is obtained (see section 5.2.1) using the triple quadrupole Instruments, and these instruments are recommended for all analyses of estrogens in manure, soil and sludge. Figure 5.2C shows that single MS instruments are sufficiently selective if the previously discussed selective criteria are fulfilled for the applied method (see above). With single MS selectivity problems are severe but may be managed. Again, GC-FID/EC, LC-fluorescence, LC-UV and immunochemical techniques are inadequate if high selectivity is required. The immunochemical methods have selectivity problems that might not be solvable.
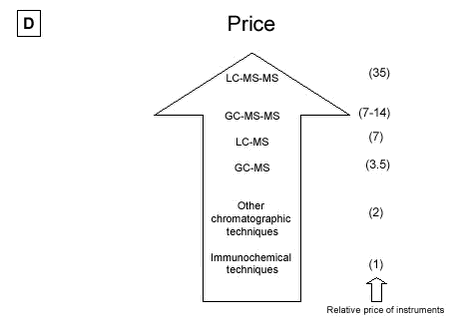
Figure 5.2 A to D: 5.2.4.4 Costs The purchase prices of the different instruments are ranked on Figure 5.2D. Price is often an important criterion when selecting an analysis strategy and must be taken into account when selecting the optimal method. One strategy is to place the costs on a high degree of sample clean-up; another is to purchase a sensitive, but often expensive instrument. The immunochemical techniques are the lowest in cost and are 35 times less expensive than the most advanced LC-MS-MS. GC-MS-MS is less expensive than LC-MS-MS. Single GC-MS is half the price of single LC-MS. Other chromatographic techniques which are often not recommend for use, are all lower in cost. Sample preparation is a crucial step and its cost must be considered. Sample preparation costs vary widely between the different matrices. The least expensive are surface water/ground water samples, whereas, sample preparation for STP influent, manure or soil will probably be a factor of 4 more expensive than the surface water/ground water samples. Sample preparation for effluents from STP will probably be a factor of 2 more expensive than the surface water/ground water samples. Table 5.3 attempts to recommend preferred analytical methods for estrogen analysis of effluent waste water or surface water, depending on the expected concentration levels in the samples. These recommendations are made taking into account the above discussion of selectivity, sensitivity, variability and cost. If required LOD levels are below 0.1 ng/L estrogen (E1, E2 or EE2) (e.g. LOEC concentrations), no method functions today without research. LODs at the level of 0.1 to 5 ng/L estrogen may be reached with all triple quadrupole and ion trap instruments. As indicated above, proper sample preparation techniques can raise the selectivity and sensitivity of single MS methods, allowing these methods to be used at levels much lower than the 20 ng/L listed in Table 5.3. Without using extra validation criteria, however, single MS instruments should be used with great care below 20 ng/L. Table 5.3: 5.2.5 Future perspectivesThe recent introduction of the tandem mass spectrometry detection instruments, LC-MS-MS or GC-MS-MS (first on the market and better documented), has substantially improved the performance of chromatographic methods by reducing detection limits and aiding analyte identification. The next few years will no doubt see the general application of these advanced techniques, integrated into completely automated, on-line systems. These integrated systems will improve analytical performance (analyse traceability, reliability, and repeatability), increase sample throughput, and reduce operating costs and contamination risks. Further advances in the form of new extraction techniques, such as those based on the use, on-line or off-line, of molecular-imprinting materials and immunoaffinity cartridges, which are currently under development can be expected in the near future. These advances promise to greatly simplify the detection and measurement of these important environmental pollutants in environmental matrices. The introduction of biosensors, most of which are still in the prototype phase, will provide another promising alternative to traditional methods for the field monitoring of estrogenic compounds.
|