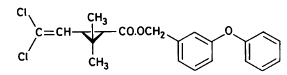|
Fate of Pyrethroids in Farmland Ponds 5 Materials and methods, part I
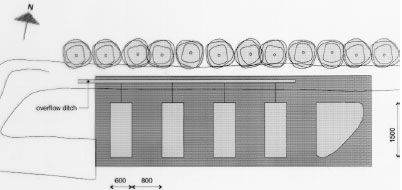
5.1 Establishing of experimental ponds, mesocosmsThe mesocosm facilities at NERI consist of four ponds with a bottom area of about 90 m2 and about 1 m's depth. The mesocosm facilities were established in November-December 1994. NERI is situated at the peninsula of Risø at Roskilde Fjord 8 km north of Roskilde, Sjælland, Denmark. The mesocosms are established in an area with heavy clay making it possible to retain water in the ponds without assistance of an artificial membrane. The excavation company Klingenberg in Roskilde carried out the excavation work. Consultant M.Sc. Lars Briggs, Amphiconsult, Fyn, gave advise on how to construct ponds and checked up on clay quality etc. The top soil was scraped into a bank 7 m south of the experimental area by the aid of a bulldozer revealing the clay. Excavators with caterpillar excavated 4 ponds and a larger reservoir. Size, cross section and mutual position of the ponds are shown in figures 5.1-5.2. A 1 m area along the four sides of each pond was left open. Outside this area the clay was piled to form banks around and between the ponds with an opening in the north bank for transport of equipment. Experimental ponds
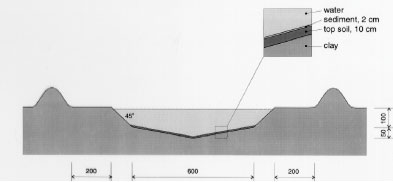
Figure 5.1 Oversigt over forsøgsarealet ved DMU, Roskilde, som viser de 4 vandhuller og reservoiret. Alle mål er i cm. Vandhullerne er nummereret fra 1 til 4 med vandhul 1 nærmest ved reservoiret. Skids were made in the short north sides of the embankments to facilitate transport of the sampling boat from one pond to another. A pipe from each pond leads to a ditch outside the bank. In case of surplus rainfall it is possible to adjust the water level in all ponds to 1.5 m by draining surplus water into the ditch. To initiate a sediment, the bottom of the ponds was carpeted with 10 cm of top soil. From a natural pond in a field near Svogerslev, in a landscape similar to Risø, natural sediment was sucked into a sludge tank and carried to the mesocosms. The sludge was uniformly sprayed unto the bottom of the ponds in a layer of about 2 cm. The natural sediment was introduced in order to initiate aquatic flora and fauna in the ponds.
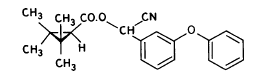
Figure 5.2 Tværsnit af et vandhul, som viser udgravningsprofilen. Alle mål er i cm. During the winter precipitation filled the ponds and during the summer a good variety of plants, crustaceans and insects developed creating an ecosystem resembling the ecosystem of a natural pond. The banks and the walking area was sown with grass to prevent soil erosion. A grid system was indicated with numbered sticks every 2 m along the banks. A reservoir was excavated east of the ponds. Water from the reservoir can be used for additional supply of water to the ponds and for mixing of water from the different ponds in order to retain comparable initial conditions in the ponds. Water can be pumped from the ponds to the reservoir and back into the ponds. The first spraying with pesticides took place in September 1995 5.2 Spraying methodSpraying boom A special equipment has been constructed to make it possible to spray pesticides uniformly upon the water surface. The company OB Teknik in Dalmose, Denmark constructed a 8 m long spraying boom made of stainless steel. A steel pressure bottle contains the pesticide solution. Compressed air from another pressure bottle drives pesticide solution into the spraying boom from both sides, which reduces problems with drop of pressure along the boom. Nozzles 1553-08 from Hardi International were used to disperse the pesticides. Two-three persons carry the spraying boom and the steel bottles during spraying. The amount of pesticide sprayed pr. ha. is depending on the air pressure and walking speed. At a pressure of 2.5 bar the amount of spraying liquid was 5 L per 50 seconds. 5.3 Choice of pesticidesAt each spraying event, four different pesticides were sprayed simultaneously or within an hour on the pond surface. This procedure does not mimic a normal agricultural practice. However, the objective of the experiment is primarily to study the general fate of the pesticides and to generate data for calibration of the distribution model, not to study effects to aquatic life. In order to improve the distribution model four pyrethroids were selected with a range of physical chemical properties. Properties of the pyrethroids are given in table 5.1 and structure formulas are given in figure 5.3. Table 5.1 Fysisk kemiske egenskaber af de udvalgte pyrethroider. Data er indsamlet fra The Pesticide Manual 1991(tal til venstre) henholdsvis 1997(tal til højre). (Worthing and Hance 1991 and Tomlin 1997).
It is difficult to measure the physical chemical properties accurately, which is illustrated by the discrepancies between data from 1991 and 1997 in table 5.1. Deltamethrin
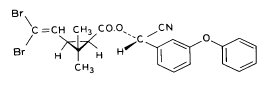
(S)-α-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dibromovinyl)-2,2dimethylcyclopropanecarboxylate Esfenvalerate
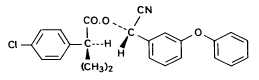
(S)- α-cyano-3-phenoxybenzyl (S)-2-(4-chlorophenyl)-3-methylbutyrate Fenpropathrin
(RS)-α -cyano-3-phenoxybenzyl 2,2,3,3-tetramethylcyclopropanecarboxylate Permethrin
3-phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl))-2,2-dimethylcyclopropanecarboxylate Figure 5.3 Strukturformler for de udvalgte pyrethroider: Deltamethrin, esfenvalerat, fenpropathrin og permethrin. (Worthing and Hance 1991) The spraying liquid was prepared from 3 formulated products. Active ingredients are given in brackets: Decis from Hoechst (deltamethrin), Sumirody 10 FW from Du Pont (fenpropathrin) and Sumi-Alpha 5 FW from Du Pont (esfenvalerate). A formulated product with permethrin was not available, so the active ingredient was dissolved in Decis prior to dilution of the product with water. Formulated products were preferred because additives like solvents and surfactants may influence the behaviour of the active ingredients in the aquatic system. 5.4 Sampling methodSampling took place from a small aluminium boat. The boat was moved from one site to another by pulling it with ropes in order not to stir the water unnecessarily. Surface microlayer Surface microlayer, SML, was collected by the method of Garrett and Duce (1980) with a steel screen 50 cm x 50 cm, mesh 1.25 mm The screen was lowered vertically into the water, turned 90°C and raised horizontally through the surface microlayer. The surface tension of the SML causes the SML to be caught in the mesh. The SML was collected in Duran bottles by tilting the screen. Water samples Water samples were collected at different depth. Two silicone tubes were fitted into the cap of a 1L Duran bottle and bent down with a ring. The bottle was lowered into the water and at the desired depth the ring was released, the tubes would then straighten and the water enter the bottle through one tube while the air from the bottle would escape through the other tube. This avoids turbulence at the inlet tube. Sediment samples Sediment samples were collected differently the two experimental years. The first year we used Kajak tubes consisting of a plexi glass pipe mounted on a stick. One end was closed with a one way valve allowing air and water to pass when the tube was lowered through the water and drilled into the sediment. The other end was open and the edge cut on the slant to facilitate the drilling. After the tube had been drilled into the sediment, the tube was lifted up, the valve would close and the sediment core was placed on a piston. The sediment core was pushed out of the tube with the piston and the upper 2 cm was cut off and collected in aluminium trays. The second year macrophytes had developed to have a more dense root web making it difficult to sample sediment with Kajak tubes. In stead a conventional half-sphere grab was used: Grab samples were placed in a box and the upper two cm collected into aluminium trays. 5.5 Semipermeable membrane devices (SPMDs)Passive integrative samplers were used to mimic the uptake of pesticide by aquatic organisms. We used semipermeable membrane devices (SPMDs) as described by Huckins et al., 1997. A standard SPMD consists of a LPDE (low density polyethylene) thin-walled, layflat tubing manufactured without additives. The membrane is sealed in both ends. A sequestration phase consisting of large molecular weight non polar liquids is placed as a thin film inside the tubing. Only the dissolved part of organic pollutants will diffuse into the membrane. Standard configuration is : 2.5 cm by 91.4 cm layflat LPDE-tubes (75-90 μm wall thickness) containing 1 ml (0.915 g) of triolein (sequestration phase) as a thin film. Standard semipermeable membranes (Huckins et al., 1997) were attached to two steel rods with metal clips. The rods with membranes were stuck in the pond bottom before spraying. The rods were placed so that the membranes were kept stretched between the rods. The lowest membrane was situated right above the sediment and the others in increasing distance from the bottom. After spraying of the ponds two membranes were attached to the rods above the water surface. The position of membranes in each pond is stated in the result chapter. 5.6 Experimental designEach year (1995 and 1996) two ponds were sprayed with pesticides and one pond served as a control. To obtain most information from the experiments (Liber et al., 1992) we wanted to spray two different concentrations of pesticides. However, in 1996 water snails had invaded one of the experimental ponds by thousands, causing a change in water conditions in that pond. In 1996 we therefore decided to spray the same concentration in both ponds and see if the different conditions would influence the fate of pesticides. Sampling time It was expected that changes in distribution and concentration would occur at a higher rate immediately after application. After spraying, samples were collected after 1, 2, 4, 6, 24 h and 2, 5, 8, 16 d. Samples of SML, water from20 cm's depth and sediment were collected from each quarter of a pond and pooled. Additional samples were collected from the middle of the pond, so called "gradient samples", from SML, 3 depth of water and sediment. Semipermeable mem-brane devices (SPMDs) For in situ measurement of bioavailable concentrations of pyrethroids were used semipermeable membrane devices (SPMDs) 5.7 Spraying schemeSampling schemes were intensive in the first week after spraying and it was logistically impossible to operate two simultaneously sprayed ponds. The spraying of the experimental ponds was therefore carried out with one weeks intervals. 1995 The first year pond 3 was sprayed 27 September. The pesticides were applied in two turns. Sumirody 10 FW (fenpropathrin) and Sumi-Alpha 5 FW (esfenvalerate) were applied at 11.30 a.m. and Decis (deltamethrin) with permethrin dissolved in it was applied at 12.00 a.m. Pressure was 2.5 bar, spraying time 50 s and 47 s. which corresponds to application of approximately 5 L of spraying liquid. Pond 4 was sprayed 4 October at 10.00 a.m. Fenvalerate, fenpropathrin, permethrin and deltamethrin were dissolved in Decis. All 4 pyrethroids were applied simultaneously. Pressure 2.5 bar, spraying time 49 s. corresponding to application of approximately 5 L of spraying liquid. 1996 The second year pond 3 and 4 were sprayed with the same dosage of pesticides and all four pyrethroids were sprayed simultaneously. Permethrin was dissolved in Decis, and this solution was mixed with Sumirody 10 FW and Sumi-Alpha 5 FW. Pond 4 was sprayed 12 June at 9.25 a.m. Pressure 2,5 bar. Spraying time 49s. corresponding to application of approximately 5 L of spraying liquid. Pond 3 was sprayed 19 June at 9.25 a.m. Pressure 2.5 bar. Spraying time 49s. corresponding to application of approximately 5 L of spraying liquid. Tables 5.2 and 5.3 show composition of the spraying liquid and the approximate application rate. Table 5.2 Koncentrationen af pyrethroider i sprøjtevæsken for hvert år og vandhul.
With a depth of about 75 cm, the size of the pond surface is about 7,5 times 17,5 m2, which is about 130 m2, and approximately 5 L of spraying liquid was applied. Table 5.3 Mængden af pyrethroid der er udsprøjtet på overfladen af vandhullerne, mg/m2
5.8 Handling and preservation of samplesIn the field Immediately after sampling the samples were placed in a thermo-box with freeze elements and stored there during fieldwork and transportation to the laboratory (2-4 hours). Preservation of samples in the laboratory After transfer of the samples to the laboratory they were all preserved according to the methods mentioned below. Sediment Sediment samples were frozen in the aluminium trays. SML and water samples SML and water samples for pesticide analysis: Recovery standard and isooctane were added to each bottle. The bottles were shaken and stored in a cold room at 4°C until time of analysis. Side parameters Samples for analysis of total-N and total-P were frozen. Samples for other side parameters were analysed the same day. SPMDs SPMDs were packed separately in aluminium foil and kept frozen until dialysis took place. 5.9 Analytical methods, pyrethroids5.9.1 ReagentsIsooctane p.a. (Merck, Germany), acetone glass distilled (Rathburn, UK), acetonitrile LiChrosolv 99.8% (Merck), dichloromethane HPLC glass distilled grade (Rathburn, UK), cyclohexane HPLC grade (Rathburn, UK), methanol LiChrosolv 99.9% (Merck), hydrochloric acid p.a. for activation of copper (Merck), carbon dioxide for supercritical extraction SFE/SFC grade (Air Products and Chemicals Inc.), copper granulate 0.2-0.6 mm 99.8% (Riedel de Haën, Germany), anhydrous sodium sulphate was cleaned by soxhlet extraction for 24 hours with dichloromethane, glass beads for the SFE cryo trap. Standards Lambda-cyhalothrin (ICI, UK), esfenvalerate (Pestanal, Riedel de Haën), cypermethrin (Pestanal, Riedel de Haën), fenpropathrin 97% (Riedel de Hahn), deltamethrin 99% (Pestanal, Riedel de Hahn), permethrin 97% (Pestanal, Riedel de Hahn). 5.9.2 ApparatusSupercritical fluid extraction (SFE) Suprex SFE Autoprep 44TM, Suprex, USA, equipped with a cryo trap, a modifier pump and an auto sampling system for collection of eluate from the trap. GC-system Gas chromatograph, HP 5890 (Hewlett Packard, USA) equipped with an electron capture detector and automatic sampler HP 7673A. HP Chemstation from Hewlett Packard was used for controlling the GC program, collection of data and data analysis. Column was 5% phenyl methyl fused silica capillary column from J & W Scientific. Length 60 m, diameter 0.25 mm, film thickness 0.1 μm. HPLC-system Waters pump model 510, Waters WISP 712 autosampler, HPLC gel permeation column Phenogel 10μ particle size, 100 Å pore size from Phenomenex, 300 x 21.2 mm, with a Phenogel pre column 10 μ, 50x7.8 mm. Column thermostat was produced in NERI's work shop. 5.9.3 Sample preparationSurface microlayer and water samples Surface microlayer and water samples were solvent extracted three times with approximately 120 ml/L isooctane each time. The extracts were dried by passing through sodium sulphate, concentrated by rotorevaporation and the volume adjusted to 1 ml after addition of lambda-cyhalothrin as an internal standard. Sediment Sediment samples were sieved at 2 mm, freeze-dried and extracted by super critical fluid extraction (SFE). 3 g of dry sediment was placed in an extraction cell, 100 μl 1000 ng/ml cypermethrin standard was spiked on top of the sediment as a recovery standard and 2 g of activated copper granules was added on top to desulphurize the extract. Extraction conditions were 10 min. static and 25 min. dynamic extraction, flow 1.5 ml/min, pressure 350atm., temperature 50°C, modifier 5% acetone. Trap material glass beads, trap temperature 50°C, restrictor temperature 50°C, eluting temperature 50°C, eluent acetonitrile 3.6 ml. The eluate was evaporated with a nitrogen flow and redissolved in isooctane after addition of internal standard. SPMDs Semipermeable membranes were dialysed twice in 200 ml cyclohexane. 200 μl of recovery standard cypermethrin, 1000 ng/ml, was added to the dialysates prior to evaporation to about 200 μl in a rotorvapor system. Possible residues of triolein, oleic acid or other were separated from the analytes by size exclusion HPLC. Injection volume 600 μl. Eluent 2% methanol in dichloromethane, 4 ml/min. Fraction collection from 13.5 to 21 minutes in 50 ml flask. The eluate was evaporated almost to dryness. The analytes were transferred to a 1 ml flask with isooctane, internal standard lambda cyhalothrin was added and the volume adjusted to 1 ml. 5.9.4 Detection by GC analysisGC-analysis All samples were detected by GC-analysis. GC conditions were: Splitless injection, purge time 3 min, injection volume 1 μl, carrier gas helium at flow rate 1-1.5 ml/min, injection temperature 325°C. Temperature program: Initial temperature 105°C increasing with 15°C/min to 250°C and then with 1°C/min to 280°C and 15°C/ min to 295°C. Detector temperature was 300°C. 5.9.5 Quality controlWater and surface micro layer Two pond water samples spiked with the four pyrethroids and one unspiked (blind) were analysed parallel to each batch of water and surface microlayer samples. Pond water was taken from an untreated pond. Sediment Sediment from an untreated pond was sieved and freeze dried and spiked with the four pyrethroids dissolved in isooctane. The sediment was carefully mixed by rotation in a rotorvapor system at atmospheric pressure. After this isooctane was evaporated. Two spiked sediment samples and one unspiked (blind) were analysed parallel to each batch of sediment samples. 5.10 Side parametersThe physical state of the ponds was controlled during the experiments by measuring a number of parameters parallel to the pesticide analyses. Chlorophyll A Content of Chlorophyll A in water is a measure of phytoplankton biomass. Chlorophyll A was measured by the Danish Standard DS 2201 (1986) method. Samples were filtered the day the were collected, and the filters were frozen and analysed within 14 days. Alkalinity Alkalinity was detected by the titrimetric method DS 253 (1977). Nitrogen and phosphorus Total nitrogen and phosphorus content was detected by the methods DS 221 and DS (1975) and DS 292 (1985). Samples were frozen the day of collection and stored at -20°C. The analyses were carried out in 1997 at the Department of Fresh Water Ecology in Silkeborg. Oxygen In 1995 temperature and oxygen saturation was measured in situ with a probe. During the experiment the oxygen electrode turned out to be unstable and some of the data are not reliable. Temperature In 1995 water temperature at various depth was measured together with oxygen. pH pH was detected in the laboratory the same day with a pH meter. Turbidity, conductivity, salinity, pH, oxygen, temperature In 1996 a new probe: HORIBA water quality checker U-10 (Horiba, Japan) was applied for determination of temperature, turbidity, conductivity, salinity, pH and oxygen concentration, corrected for salinity, in situ. Texture analysis The Danish Institute of Agricultural Sciences, Research Centre Foulum, carried out texture analysis of sediment.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||