|
Survey of Chemical Substances in Consumer Products no. 51, 2004 Mapping and release of chemical substances from products made of chloropreneContents
7 Assessment of Health Effects
PrefaceThe project "Mapping and releasing of substances from products made of chloroprene" was carried out for the National Agency of Environmental Protection in the period from 22nd May to 8th December 2003. This report describes the results of the study. The project was carried out by the Danish Technological Institute, Materials division. Lic. scient. Nils H. Nilsson of the Danish Technological Institute was manager of this project and the liaison between the institute and the National Agency of Environmental Protection. Laboratory analyses and migration investigations were carried out in co-operation with laboratory managers Ivan Christensen and Paul Lyck Hansen, Chemistry- and Water Technology, and MSc Kenneth Brian Haugshøj, Microtechnology and Surfaceanalysis. Bjørn Malmgren-Hansen, lich. tech., MSc (engineering), Ole Christian Hansen, MSc, and Kirsten Pommer, BSc, were attached to the project as experts to screen and evaluate health impact (consumer exposure) and risks. An experienced sport diver took part in a full-scale migration test by diving under conditions very similar to "real-life" conditions. In addition to being the Technological Institute's expert in rubber materials and technology, the project manager was also responsible for the consumer research, the purchase of chloroprene products, interviews and information searching. The reference group consisted of Shima Dobel (chairman), the National Agency of Environmental Protection, and Nils H. Nilsson, the Danish Technological Institute. The purpose of the project was to focus on the problematic substances that appear in different consumer products of chloroprene, such as boots, waders, dive suits and supports available in retail stores in Denmark. The project comprised three phases. Firstly, mapping of consumption and consumption patterns regarding products made of chloroprene. Secondly, a screening phase for problematic substances as well as migration/exposure tests under conditions determined by "worst case" scenarios. Finally, health screening based on the results from the migration tests completed the project. SummaryOn behalf of the National Agency of Environmental Protection, the Danish Technological Institute has mapped the consumption and the consumption pattern of products made of chloroprene with which consumers get into contact. The investigation showed initially that the shops or other sales channels do not know the word chloroprene, but solely the word neoprene, which is the raw material company of DuPont Dow Elastomers' trademark for chloroprene rubber. The investigation has shown that a considerable amount of consumers gets into contact with products made from chloroprene. First of all products like support bandages, boots and waders contribute. Also the number of consumers who use wet-, semi wet -and dry suits for sport or exercise in the wet element is considerable. It has been difficult to get precise numbers for the consumption of chloroprene products because many consumers only practise sport at individual basis as exercise. It is judged that the amount of consumers who during a year is in contact with support bandages and boots is around 100.000. With regard to waders the number is judged to be 50.000 and for isolating suits around 25.000. The number of comsumers using gloves is estimated to 40.000, but the number is uncertain as gloves are used for different sports and leisure activities. Professionals, e.g. windows polishing people, also use gloves. Eight different types/brands of products made of chloroprene were selected for a screening analysis to detect any chemical substances contained in the products. The selection was based on the recognised consumer pattern and by taking contact times and the exposed skin area in consideration. The products selected comprised two types of supports, two different brands of dive gloves, a pair of dive socks, a dive hood, a pair of waders and a dive suit. The screening analyses included an element analysis using wavelength dispersive x-ray spectroscopy, gas chromatography with mass spectroscopic detection (GC/MS) of headspace, gas chromatography with mass spectroscopic detection of extracts of the products, and thin layer chromatography also of extracts of the products. Raw chloroprene rubber contains approx. 30 – 40 weight percentage chlorine, depending on type. For a current sample received from a Danish rubber factory the content was measured at 34% w/w. An amount of 30 – 50 weight parts of raw rubber will typically be used in a chloroprene recipe. It is, therefore, remarkable that two of the eight products purchased, i.e. the two dive glove brands, appeared to contain chlorine only at trace level which was demonstrated by the x-ray analysis. Two other products, waders and dive socks, contained slightly less than 2% chlorine by weight basis indicating that the chloroprene content in these two products is quite low. The dealers of the two products, which do not contain chloroprene, could not explain why this was so. However, both of them informed us that almost all of the rubber fabrics for neoprene products (chloroprene) are produced in the Far East and that it is impossible to receive precise information about the composition of the rubber fabrics from there. The gas chromatographic/mass spectroscopic screening through headspace and analysis of extracts of the products themselves demonstrated the presence of a fairly large number of different types of substances. The concentrations in the headspace analysis appeared to be quite low, even at 100°C. The level is typical from 0.1µg/g – 3µg/g. For waders, however, the toluene level appeared to be 21µg/g. Thin layer chromatographic screening was used to identify which types of anti-aging agents had been added to the products. At the same time, it appeared to be suited to demonstrate whether ETU (ethylene-thio-urea as accelerator) had been used or not. This information was needed partly due to the fact that this substance appears in all the standard recipes published in The Rubber Formulary (Peter A. Ciullo), and partly due to the fact that the substance appears on the list of harmful substances under T (toxic), R61(may cause harm to the unborn child) and R22 (harmful if swallowed). In HSDB (Hazardous Substances Data Base), EPA (Environmental Protection Agency) has assessed the substance in terms of carcinogenic properties and it is classified as a group "B" substance and may therefore be carcinogenic to humans. The thin layer chromatographic screening for anti-aging agents was consistent with the GC/MS analysis of the extracts of the products. On the other hand, the extracts was not found to contain ETU. This may be because ETU has not been used or has been transformed into the corresponding urea derivative when sulphur is released (Röthenmeyer). Five products were selected for the migration tests based on the results of the initial screening analyses. A knee bandage, a pair of dive gloves, a dive hood, a pair of waders and a dive suit were selected. The laboratory scale migration tests were based on "worst case" scenarios, with exposure times, contact media (artificial sweat and artificial sea water) and temperatures being as close to "real life" as possible. In addition to a laboratory scale migration test, the dive suit was also subjected to a realistic full-scale sea test, which involved an experienced sport diver. The suit was a semi-wet suit and after two dives and a break on shore, the water was drawn off the suit. The water was analysed by GC/MS for organic substances and by atom absorption using a graphite oven for nickel according to DS 2211. It was quite interesting that migration of N,N’- diethyl-thio-urea occurred in this final test as opposed to the laboratory test that involved contact with artificial sea water. Since it has been verified that the dive suit contains N,N`-diethyl urea, it has to be concluded that the full-scale test was justified and that it is difficult to simulate real life in laboratory test alone. The samples that contained from 7-14 %w/w chlorine all contained nickel in weight percentage concentrations ranging between 0.01 %w/w and 0.06 %w/w. At first, it was assumed that the presence of nickel could have been caused by wearing particles in the rubber mixing department but towards the end of the project, the Danish Technological Institute realised that the nickel salt from dimethyl dithio carbamic acid or dibutyl dithio carbamic acid could be included in rubber recipes as a very efficient antiozonant in order to prevent oxidative breakdown of the rubber (R. Kuschel). The nickel release was therefore measured in the full-scale test with diving using a semi-wet suit. The test demonstrated that nickel was released in a 0.2µg/kg concentration. Health assessmentThe chemical substances released in measurable concentrations in the migration tests and identified unambiguously by the chemical analysis were judged in details for possible consumer health impacts. The investigation of the selected chloroprene products revealed the release of a row of chemical substances. In total 46 chemical substances were identified and assessed for health effects in the screening phase. In the migration studies 7 “problematic” chemical substances were identified from the screening list for health effects. These chemical substances were selected for a closer assessment. The table below contains a summary of this assessment.
The following appears from the table:
Based on the products studied, it can be established that none of the substances found will directly give rise to health problems. ResuméTeknologisk Institut har på vegne af Miljøstyrelsen foretaget en kortlægning af forbruget og forbrugsmønstret af produkter fremstillet i chloropren som forbrugerne kommer i kontakt med. Undersøgelsen viste indledningsvis at butikkerne eller andre salgskanaler ikke kender begrebet chloropren, men udelukkende betegnelsen Neopren som er firmaet DuPont Dow Elastomers varemærke for chloropren. Undersøgelsen har vist at ganske mange forbrugere kommer i kontakt med produkter i chloropren. Det gælder først og fremmest støttebind, støvler og waders. Men også antallet af forbrugere der anvender våd-, semivåd og tørdragter i forbindelse med sport eller motion i det våde element er betydelig. Det har vist sig vanskeligt at få præcise tal for forbruget bl.a. fordi mange dyrker sport på motionsplan, men antallet af forbrugere der årligt er i kontakt med støttebind og støvler ligger skønsmæssigt på 100.000. For waders skønnes tallet at være ca. det halve og for isolerende dragter i chloropren en fjerdedel, dvs. ca. 25.000. Antallet af forbrugere, der anvender handsker er sat til 40.000, men tallet er usikkert da handsker finder anvendelse på tværs af sports- og fritidssysler. Handsker anvendes også af professionelle f.eks. vinduespudsere. Der er udvalgt 8 forskellige typer/fabrikater af produkter i chloropren til en screeningsanalyse for kemiske indholdsstoffer på baggrund af kortlægningen. Udvælgelsen er sket på baggrund af det fundne forbrugsmønster under hensyntagen til kontakttider og eksponeret hudareal. De produkter der blev udvalgt, omfattede to typer støttebind, to forskellige fabrikater af dykkerhandsker, en dykkerhætte, et sæt waders og en dykkerdragt. Screeningsanalyserne har omfattet grundstofanalyse ved bølgelængdedispersivt røntgenspektroskopi, gaschromatografi med massespektroskopisk detektion (GC/MS) på headspace, gaschromatografi med massespektroskopisk detektion på ekstrakt af produkterne og tyndtlagschromatografi ligeledes på ekstrakt af produkterne. Chloropren rågummi indeholder ca. 30-40 vægtprocent chlor afhængig af typen. For en aktuel prøve modtaget fra en dansk gummifabrik blev indholdet målt til 34 %w/w. I en chloroprenrecept vil rågummiet typisk indgå i en mængde på 30 til 50 %w/w. Det er derfor bemærkelsesværdigt at to ud af de 8 produkter der blev indkøbt, nemlig de to fabrikater af dykkerhandsker, ved røntgenanalysen viste sig kun at indeholde chlor på sporniveau. To andre produkter, nemlig waders og dykkersokker, indeholdt i underkanten af 2 % chlor på vægtbasis hvilket indikerer at indholdet af chloropren i disse to produktet er ganske lavt. Forhandlerne af de to produkter der ikke indeholder chloropren, kunne ikke give en forklaring på hvorfor dette var tilfældet. Begge oplyste dog at gummitekstilerne til Neoprenprodukter (chloropren) stort set udelukkende fremstilles i Fjernøsten, og at det ikke er muligt at få præcise oplysninger om gummitekstilernes sammensætning herfra. Ved den gaschromatografiske/massespektroskopiske screening ved headspace og ved analyse på ekstrakter af selve produkterne er der fundet ganske mange forskellige stoftyper. Koncentrationerne i headspaceanlysen viste sig ganske lave selv ved 100°C. Niveauet for afgasning af de enkelte stoffer ligger typisk på 0,1 µg/g til 3 µg/g. Dog fandtes der for waders et afgasningsniveau for toluen på 21 µg/g. Den tyndtlagschromatografiske screening blev anvendt til at konstatere hvilke typer antiældningsmidler der var tilsat produkterne. Samtidig viste den sig egnet til at konstatere om man havde anvendt ETU (ethylenthiourinstof) som accelerator. Behovet herfor skyldes dels at stoffet indgår i alle de standardrecepter der er offentliggjort i The Rubber Formulary (Peter A. Ciullo), dels at stoffet på listen over farlige stoffer er klassificeret som T (Giftig), R61 (Fosterskadende) og R22 (Farlig ved indtagelse). I HSDB (Hazardous Substances Data Base) er stoffet vurderet af US EPA (Environmental Protection Agency) med hensyn til kræftfremkaldende egenskaber, og det er mærket som et gruppe B-stof og dermed muligvis kræftfremkaldende for mennesker. Der var overensstemmelse mellem den tyndtlagschromatografiske screening for antiældningsmidler og GC/MS-analysen af ekstrakterne af produkterne. Det kunne konstateres at ekstrakterne ikke indeholdt ETU. Forklaringen er enten at man ikke har brugt ETU, eller at ETU er omdannet til det tilsvarende urinstofderivat ved afgivelse af svovl (Röthenmeyer). På baggrund af resultatet af de indledende screeningsanlyser blev fem produkter udvalgt til migrationsforsøg. De produkter der blev valgt ud, var en knæbandage, et sæt dykkerhandsker, en dykkerhætte, et sæt waders og en dykkerdragt. De scenarier der blev lagt til grund for migrationsforsøgene i laboratorieskala, tog udgangspunkt i ”worst case” og med eksponeringstider, kontaktmedier (kunstig sved og kunstig havvand) og temperaturer så tæt på virkelige forhold som muligt. I tilfældet med dykkerdragten blev der ud over et migrationsforsøg i laboratorieskala gennemført et realistisk fuldskalaforsøg i havet med erfaren fritidsdykker. Dragten var en semivåddragt, og efter to dyk med ophold i land blev dragten tappet for vand. Vandet blev analyseret ved GC/MS for organiske stoffer og ved atomabsorbtion med grafitovn for nikkel efter DS 2211. Interessant nok konstateredes der migration af N,N’-diethylthiourinstof i fuldskala forsøget i modsætning til laboratorieforsøget med kontakt til kunstig havvand. Da det er verificeret at dykkerdragten indeholder N,N`-diethylurinstof, må det konkluderes at fuldskalaforsøget har været berettiget, og at det er svært at simulere virkeligheden i laboratorieforsøg alene. De prøver der indeholdt chlor i mængder på 7-14 % w/w, indeholdt alle nikkelkoncentrationer på 0,01-0,06 % w/w. Det blev indledningsvis antaget at nikkel kunne stamme fra slidpartikler fra gummiblanderiet, men sidst i projektforløbet blev Teknologisk Institut opmærksom på at nikkelsaltet af dimethyldithiocarbamaminsyre eller dibutyldithiocarbaminsyre kunne indgå i gummirecepter til hindring af oxidativ nedbrydning af gummiet (R. Kuschel) som følge af ozonpåvirkning. Der blev derfor foretaget en måling af nikkelafgivelsen i fuldskalaforsøget med dykning i semivåddragt. Resultatet af undersøgelsen var at der finder frigivelse af nikkel sted i en koncentration på 0,2 µg/kg gummi. Sundhedsmæssig vurderingKun de kemiske stoffer som indgik i screeningen for sundhedseffekter og hvor der blev konstateret afgivelse af i målelige koncentrationer ved migrationsforsøgene, og som blev entydigt identificeret ved den kemiske analyse, blev vurderet mere detailleret for mulige sundhedsmæssige effekter for forbrugerne. I undersøgelserne af de udvalgte chloroprenprodukter er der fundet en række kemiske stoffer. Der er oplistet i alt 46 kemiske stoffer for hvilke der er gennemført en indledende screening. I migrationsforsøgene er der blevet identificeret 7 ”problematiske” kemiske forbindelser fra screeningslisten for sundhedseffekter, og disse er udvalgt til nærmere vurdering. Resultatet af denne vurdering er samlet i nedenstående tabel. De anførte ”Analyserede mængder” i tabellen angiver de mængder der potentielt kan optages pr. kg legemsvægt. Sammenfattende vurdering af de fundne stoffer ved migrationsforsøgene.
Af tabellen fremgår det at: Umiddelbart ligger de målte mængder langt under de grænser det har været muligt at finde for nul-effekt-niveauer for alle de 7 vurderede stoffer Ingen af de vurderede stoffer giver i de aktuelle koncentrationer anledning til hudirritationer Visse af stofferne har egenskaber der indebærer en potentiel risiko for kroniske effekter. Da de enkelte stoffer forekommer i meget små mængder, vurderes denne risiko dog som minimal Ser man på de undersøgte produkter, kan det konstateres at ingen af de fundne stoffer umiddelbart giver anledning til sundhedsmæssige problemer. 1 IntroductionChloroprene rubber is used in products such as boots, waders, surf and dive suits, gloves, socks and similar products for leisure activities. Neoprene is also used as a synonym for chloroprene. Neoprene is the raw material company of DuPont Dow Elastomers' trade mark for chloroprene (polychloroprene). The Danish consumption of chloroprene rubber for consumer products is unknown; however, on a global basis the consumption of chloroprene is estimated at approx. ½ million tons per year (IDA). The products can smell very strongly of "chemistry". Some chloroprene products are worn tightly against large surfaces of the skin. Consequently, there is a risk that volatile substances in chloroprene may be inhaled by the user or absorbed by the skin. Chloroprene rubber in finished products is vulcanised using zinc oxide. A complicated, chemical process occurring during vulcanisation of chloroprene rubber forms cross links between the rubber chain molecules that improve the elasticity of the rubber, remove its adhesiveness and make the rubber dimensionally stable. The chloroprene monomer differs from the isoprene monomer, which is the building stone in natural rubber, in that a methyl group is exchanged with a chlorine atom. This means that chloroprene rubber is far more weather resistant than natural rubber and that its resistance to oil, petrol and chemicals is also good. For most chloroprene products, this involves either fabric-reinforced or fabric-covered types. Nylon or polyester is typically used as fabric. For suits, gloves, supports, etc. based on chloroprene, the rubber is also constructed of closed cells to obtain good product insulation properties. On a global basis, raw polychloroprene rubber is manufactured by a total of five raw material suppliers: DuPont (Neoprene), Bayer (Baypren), Enichem (Butachlor), Denka Kagaku (Denka) and Tosoh (Skyprene). According to according to the literature (John S. Dicks, p. 133), DuPont accounts for more than 75% of the chloroprene production capacity. The raw material suppliers also produce several chloroprene rubber variants to provide the finished products with special properties. Special attention is paid to the ability of chloroprene to crystallise making the material rigid and inflexible. Thus, sulphur-modified polychloroprene types with improved storage and in-use properties are manufactured since they crystallise slowly. There are also mercaptan-modified types which crystallise either quickly or very slowly. Polychloroprene is also available as a copolymer with methacrylic acid in the form of latex. This variant is used for example to produce rubber gloves. 2 Mapping phaseIn this phase, the market has been mapped for chloroprene products with which consumers get into contact. 2.1 MethodInformation has been searched via:
A detailed description of mapping procedure and the result from the Internet search is to be found in annex A. 2.2 Results2.2.1 The InternetThe result of the search for chloroprene in relation to recipes, health and migration was disappointing since no information about recipes appeared in addition to what we already knew. However, a single, completely new monograph was identified as being of interest in terms of chloroprene formularies and it was acquired (R.N. Datta). Search on the combination of health and migration did not result in any usefull information. Search on chloroprene/neoprene and wetsuits gave a row of hits on which products based on chloroprene one can buy via the Internet. Search on web sites in relation to chains of sportsshops, health products and leisure activities gave no usefull information. On the web site for the Sports Confederation of Denmark (DIF) www.dif.dk it is possible to get information about the number of members of the different special national confederations. 2.2.2 Contact via phone2.2.2.1 Contact to federations and agenciesThe results of the direct contact via phone to the national federations under DIF appear from the table 2.1 below. In those case where it was impossible to contact a federation over the phone, the number of members was taken from the statistics available at www.dif.dk. Table 2.1 Number of organised sportsmen broken down by national federations
It should be pointed out that not all sportsmen are members of the Sports Confederation of Denmark and that quite a lot of consumers are simply engaged in the relevant sports activities to keep fit. On the other hand, it is to be expected that consumers who are members of the national federations under the Sports Confederation of Denmark are much more often in contact with chloroprene-based garments. The websites www.fd.dk (the Danish Directorate of Fisheries), www.lystfiskeren.dk, www.sportsfiskeren.dk and www.jaegerforbundet.dk have been visited to record the number of anglers and hunters in Denmark. Hunters and anglers are not considered as real sportsmen. Table 2.2 Number of hunters and anglers
2.2.3 Contact to wholesalers and retailersWe contacted wholesalers and retailers dealing in chloroprene-based consumer products for leisure activities to record the consumption of chloroprene-based garments. The results appear from Table 2.3 made anonymous. Table2.3 Various wholesalers and dealers
2.2.4 Personal interviewsThe results of interviewing participants in sports in which garments based on chloroprene are used are summarised below. The following sports are involved:
2.2.4.1 TriathlonTriathlon includes the sportive disciplines swimming, biking and running. A full ironman distance involves a 3.8 km swim. But triathlon also involves shorter swimming distances. We have been informed that participants typically swims about 2 km wearing wetsuits and that they swim for about 40 minutes and rest for about 20 minutes. According to the literature (Terje Nordberg), elite athletes typically swim 3km twice weekly in addition to running and cycling training. 2.2.4.2 Canoeing, kayaking and rowingChloroprene garments are primarily used in sea kayaking. In the sea kayak, a chloroprene cover is used around the cockpit and the paddler. Only a relatively few people are engaged in sea kayaking but the sport is gradually becoming more popular. Very few persons are considered to be using chloroprene garments for ordinary kayaking and rowing since these sports are typical summer sports. 2.2.4.3 Dinghy sailing, windsurfing and kitesurfingFor optimist dinghy sailing, we were informed that children who are very active may wear chloroprene suits for up to 10 hours at a time. The Danish Sailing Association estimates that the optimist sailors typically sail for two hours when training and 5-6 hours when competing. All sailors are wearing chloroprene gloves when it is cold. The Danish Sailing Association informed us that a suit used for sailing optimist dinghies lasts for max. one year as the seat of the suit is exposed to a high degree of wear due to the rough surface which the sailor sits on and which is necessary on account of the friction. According to information provided, active windsurfers and kite surfers can spend up to 3-4 hours a day all week windsurfing and even more in connection with competitions. They wear wetsuits very much all year since they often fall into the water. Surf suits made of chloroprene last longer than suits used for sailing dinghies. Typically 3-5 years. However, suits are known to last up to ten years if maintained well and if the user is experienced, knowing how to avoid tearing the suit. 2.2.4.4 DivingDivers can dive max. 30 minutes at a time at a depth of 20-25 m and based on interviews, it has to be concluded that a diver is wearing his diver's suit for at total of 2-4 hours at a time, and four hours has to be considered as the max. period of time. It is possible only to dive for a period of max. 1½ hours at a time at shallower depths before the cylinder is emptied of air so four hours correspond to diving twice and a break of one hour ashore. We were informed that the "old", 7mm thick types were able to last for up to 10 years (Scubatech) but that the tendency is towards greater use of 3mm and 5mm suits. Their service lives are estimated at 3-5 years. The difference between a wetsuit and semi-wet suit is that the latter has rubber seals on the inside that prevent significant water replacement. This means that you keep warm better in the semi-wet suit due to insignificant water replacement. 2.2.4.5 WaterskiingNo special information has been collected about waterskiing. There are very few waterskiers and they are assumed to wear their wetsuits for a limited period of time compared to wind- and kitesurfers. 2.2.4.6 Other sportsIn other sports, such as ball games, athletics, gymnastics, running and cycling, the participants can get into contact with primarily supports made of chloroprene. The contact time will typically be for the duration of a run or a competition/match. So the participant will rarely be in contact with chloroprene for more than a couple of hours at a time. 2.2.5 Visits to shopsThe following different types of shops were visited in the mapping phase:
The truss and bandage shop was interviewed about chloroprene supports. They do not recommend using chloroprene supports if they are to be worn all day since the skin cannot breathe through the material. So in that case the shop recommends its customers to buy alternative materials even though they do not provide as efficient support as chloroprene supports. Since this shop is a surgical appliance shop, its customers are not necessarily sportsmen but also people suffering from a physical impairment that requires support of a weak joint. These consumers are expected to use their supports for a longer period of time each day than is the case for sportsmen. At the Bandagist – Centret which was visited in the purchase phase for supports the firm was interviewed with regard to supports in chloroprene. Danish Technological Institute was informed that patients which had permanent problems with their junctions easily could be in direct skin contact with the support for more than twelve hours a day. The diving shop deals in a range of products used for diving activities. The shop has several different types of suits. The owner informed us that no customers had complained about chloroprene suits having caused inconvenience but in connection with a dry suit a customer had once had rashes caused by the wrist and neck seals manufactured from another rubber material and that this material had been replaced with chloroprene to remove the inconvenience. 2.2.6 LiteratureIn connection with the mapping, information has been gathered from two types of literature. One type discussed the various sports in which chloroprene garments are known to be used. The other type of literature contained information about chloroprene and the composition of chloroprene recipes. As for the latter, literature also comprised information collected from the websites of the raw material suppliers. 2.2.6.1 Sports literatureThe specialist books consulted on sports concerned sailing, windsurfing, diving and running. The books on sailing, windsurfing and diving were the only books that contained information about the clothing used for these sport activities, i.e. wetsuits used for all three sports and the dry suit for diving. The latter is used in the cold season of the year because of its thicker insulation (7mm). Besides the suit, gloves, socks and hoods are used in diving. In the warm season, windsurfers have bare feet. But when it gets colder, shoes with chloroprene uppers, etc. are used whereas, according to the book, chloroprene socks are unfit for use since they wear too soon. Chloroprene gloves are recommended for use only in the winter season because it is difficult to hold on to things properly when sailing. 2.2.6.2 Chloroprene literatureInformation has been collected about the availability of types of raw chloroprene rubber on the market as well as the additives which may be used in a chloroprene recipe. Details of the results of this search and the visits to the websites of three raw material suppliers (DuPont Dow Elastomers, Tosoh and Bayer) are available in Annex B. The next section points to the types of chemical substances and elements which the literature on chloroprene recipes gives rise to look into in the analysis phase. 2.3 Conclusion/Summary2.3.1 Consumption pattern and exposureThe results of the mapping phase regarding the number of consumers getting into contact with chloroprene products, exposure conditions and estimated duration of contact each week are summed up in the table 2.4. Despite the comprehensive search for information, the figures are to a high degree based on very rough estimates. For the number of consumers using waders, it was estimated that at least half of those active in inshore fishing and angling are wearing waders. For rubber boots, 108,000 Danish hunters are known to go hunting and they all wear rubber boots, however, not necessarily boots made of chloroprene. The reason why the figure is estimated to be higher than 100,000 is that consumers, who do not go hunting, are expected to wear chloroprene rubber boots to some extent because they are comfortable. As far as these two product groups are concerned, the consumer usually wears a suit underneath so direct contact with the skin does not occur. However, a certain degree of contact will occur if the user wears ankle socks or short trousers underneath. The number of consumers using wetsuits or semi-wet suits is based on information obtained from the national federations under the Sports Confederation of Denmark. All people who are active in diving, windsurfing, kitesurfing and triathlon are indicated as wearing chloroprene suits. For kayaking and rowing, it is assumed that only those active in sea kayaking wear wetsuits. For sailing, it is very difficult to give an exact figure. In this connection, approx. two-thirds of the 10,000 dinghy sailors are assumed to wear wetsuits and it is assumed that there is a similar number of windsurfers (windsurfers also belong under the Danish Sailing Association). The Danish Sailing Association informs that 3.000 – 4.000 wind- and kitesurfers are members of the association. The Association guess that further 5 – 7.000 consumers are active at exercise level, but it is a pure guess. The number of persons wearing dry suits is according to a telephone interview with the daily manager of the magazine “DYK” estimated to 5000. The suits are primarily worn in the cold autumn and winter periods. They are quite expensive. Clothing is always worn underneath the dry wetsuits. It should be added that the sport in the wet element reaches its peak in the summer season and that the use of chloroprene products above and below water culminates in that period, however, this does not include holidays under warmer skies in the winter season. In summer the use of wet suits dominates. The number of consumers wearing chloroprene gloves is considered to be about 40,000 since we learned that this type of glove is used widely among all sailors of whom 60,000 are members of the Danish Sailing Association alone. The figure was found by estimating that approx. 50% of the sailors wear chloroprene gloves, except windsurfers. This type of glove prevents windsurfers from holding properly on to the sail. For socks and boots made of chloroprene, the number of consumers using these products is estimated to be equal to the number of users of wet and semi-wet suits as it is assumed that all users of suits also use socks and boots. The daily manager of the magazine “DYK” informs that besides leisure divers organised in The Danish Sport Diving Federation further 4.500 divers is educated by PADI (Professional Association of Diving Instructors). The Manager estimates that the total amount of leisure divers in Denmark is around 10.000. Many leaves the sport after 3 years but newcomers balance the figure. It might be that the amount based on the figures from the retailers with regard to sales of diving suits is too low an estimate. However it is difficult to etimate how many diving suits are sold via the Internet. However many internet web sides have been recognised in the mapping phase. For supports, it is very difficult to determine how many consumers get into contact with supports made of chloroprene. In principle, any consumer risks having to wear a support; however, in the great majority of cases the consumer will have to wear it only for a short period of time until he/she has recovered from a sports injury. However, consumers suffering from weak joints may have to wear supports more or less permanently. This group is probably the one most severely exposed to nuisance due to repeated and daily contact all year. The contact time column is based on the most enthusiastic persons in the various sports. It is not unusual for an angler, who is on a fishing holiday for a week, to wear his waders for more the 10 hours each day. Wind- and kitesurfers also often wear their suits all day; they just strip off the upper part when on shore. For supports, the lower limit is estimated at 15 hours for normal sportsmen and the upper limit at 60 hours for consumers suffering from joint impairments. This is of course a rough estimate. The contact conditions are divided into direct and indirect contact. As previously mentioned, waders and chloroprene boots can to some extent be in contact with the user's legs when he is wearing ankle socks or short trousers. No temperature has been indicated for the contact, but since the body temperature is typically 37°C, a temperature of about 30°C does not seem unrealistic. The temperature underneath a support may be higher when running or playing ball due to the high activity level. Table 2.4 Consumption pattern for chloroprene products
2.3.2 Which environmentally problematic substances can appear in chloroprene productsA detailed review of slightly older as well as the most recent literature on chloroprene and chloroprene formularies indicates that it would be relevant to screen the products to be selected for the analysis phase. The chemical substances to screen are to be found within the following groups:
As for accelerators, ETU (ethylene-thio-urea/imidazoline-2-thione) will receive special attention since it is a constituent in the great majority of published chloroprene recipes and is included on the list of hazardous substances class 2; R 45 “Might cause cancer”". Attention should also be paid to thiuram compounds or their breakdown products since they can give rise to nitrosamine formation. Degradation products from the classes of chemical substances above should also be part of the screening study. In annex B a more detailed information is given with regard to chloroprene and recipes for chloroprene. 2.3.3 Which products should be included in the screening phaseBased on the exposure conditions expected for the different types of chloroprene products with which consumers get into contact, it was suggested to select the following product types for the initial screening study:
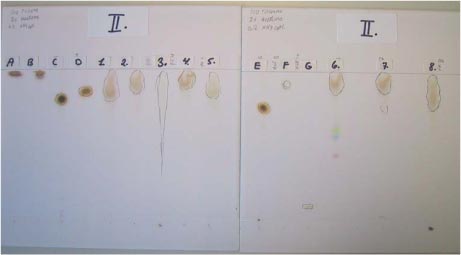
3 Samples purchased/acquired3.1 Chloroprene products purchasedMost of the consumer chloroprene product samples were acquired for the project through visits paid to surgical appliance shops or wet sport or hunting and angling shops in the Århus area. The dive suit was ordered over the phone from a diving shop in the Kolding area. The following eight products were subjected to investigations within the framework of this project: Table 3.1 Types and makes of samples
4 Screening analyses4.1 IntroductionBy experience the most suitable method for analysis of the liberation of volatile chemical substances from rubber is by headspace GC/MS methods (Rubber Fume). By this technique one gets soon a survey over the volatile or decomposition products, which might be liberated from the rubber. To detect less volatile chemical substances, e.g. anti-ageing agents and plasticisers an extraction followed by GC/MS analysis in combination with thin-layer chromatography is considered the most suitable method of analysis. The thin layer based method has the advantage that one by use of different spraying reagents can obtain different colours of the spots. This can give supplementary information regarding substance classes. Screening for heavy metals is most convenient carried out by X-ray analysis directly on the product. In the same analysis information is obtained regarding other chemical elements e.g. chlorine, sulfur, calcium, magnesium and alumina which might be present in the rubber recipy or as is the case chlorine in the chloroprene raw polymer. The chloroprene products purchased underwent screening analyses by x-ray analysing them for inorganic elements, including heavy metals, if any, thin layer chromatography for detection of anti-aging agents, a GC/MS analysis using headspace technique to detect volatile organic compounds, and a GC/MS analysis of a rubber extract in dichloromethane/isopropanol 90/10 vol.% in order also to determine the more slightly volatile compounds in the form of plasticisers. The analysis programme was stopped for one of the products that appeared not to be made of chloroprene, i.e. no. 3 dive gloves. The other pair of gloves, no. 7, that later appeared not to contain any chloroprene was included in the entire analysis programme. Having found that the gloves did not contain any chloroprene, the Danish Technological Institute contacted the importer and dealer of the product but they could not explain why the product did not contain any chloroprene. The product is marketed as titanium neoprene gloves. The supplier in England could not give a satisfactory explanation either. According to the importer, the manufacturer of the rubber-fabric composite is situated in the Far East. He did not believe that it would be possible to obtain any further details from the manufactor. 4.2 Description of analysis methods and test preparationThe analysis methods used in the screening phase are described briefly in the following. 4.2.1 X-ray analysisThe analysis was performed using a wavelength dispersive Phillips PV 2400 instrument. The analysis was performed directly on the cellular rubber without pretreatment. For some samples, an additional analysis of the rubber, as well as of the fabric and rubber, was performed. This involved samples containing so small amounts of chlorine that they could not be chloroprene products (despite the fact that they had been marketed as such). 4.2.2 Gas chromatography with mass spectrometric detection4.2.2.1 Headspace analysesApprox. ¼g of each rubber sample was cut into smaller pieces and placed in a 10ml vial with PTFE septum. The sample was heated for two hours at 100°C. An air sample was then taken using a gas-tight syringe and analysed by means of GC-MS. The content of volatile emission products was determined quantitively/semi-quantitively against selected compound standards heated in Pyrex bottles for evaporation and analysed in the same way as the rubber samples. The compounds used were carbon disulphide, diethylamine, toluene, aniline, phenol, DMF and 2-methoxyethanol. Equipment and parameters used:
4.2.2.2 Other GC/MS analysesThe samples were extracted using dichloromethane/isopropanol 90:10 vol.%.
4.2.3 Thin-layer chromatographic screening for anti-aging agents2g of rubber were weighed. The sample was placed in a 100 ml bulb and extracted using 50ml dichloromethane for an hour in a shaking bath. The solvent/extract was removed by decanting. An additional 10ml of dichloromethane was added and briefly shaken. Removal by decanting was then carried out again. The dichloromethane extract was evaporated using a rotary evaporator at max. 30°C to approx. 1ml. The concentrated extract was then transferred to a small test tube with a closely fitting cap. It was then rinsed with dichloromethane to achieve a total volume of 2ml. The thin layer chromatographic screening to identify the presence of anti-aging agents was carried out according to the principles of ISO 4645 (1995) ”Rubber and rubber products - Guide to the identification of antidegradants – Thin layer chromatographic methods”. Two different elution liquids were used. One of them (I) corresponds to method A in the standard: n-heptane:ethylacetate 90:10 (volume percentage). The other (II) elution liquid consisted of a toluene:acetone:ammonium hydroxide solution (item 4.3.13 of the standard) 100:20:0.2 (volume percentage). As for the latter method, it is possible to screen to identify the presence of ETU which is used, as already mentioned, as an accelerator in chloroprene recipes according to literature. The thin layer plates used for the analysis were Merck (article 1.11798) 20 x 20cm Silica gel 60 F 254 with concentration zone. Five micro litres were added of the solutions and standards, the production of which is described below. After eluation, the eluation liquid evaporated in a fume cupboard before visual assessment. The plates were first investigated under UV light and then after immersion in a chromatograpic chamber with iodine crystals. The results of the iodises plates were documented by photos. The reference substances used for the thin layer chromatographic screening are indicated in table 4.1 Table 4.1 References used for the TLC screening
The reference substances A, B, C, D, E and F were dissolved in dichloromethane as 1% solutions (0.1g in 10ml). Reference substance G (ETU) was dissolved in methanol (0.1g in 10ml) and thinned 1:4 in dichloromethane. Reference substance A (ODPA) was also produced in 2% and 5% strengths (0.2g and 0.5g in a 10ml measuring bulb) for the semi-quantitative assessment. 4.2.4 Estimated detection limitsThe detection limits for the various analysis techniques depend to a high degree of the compounds to be analysed for as well as interfering components and the method used. In table 4.2 typical detection limits based on experience are indicated for the analysis methods used. Table 4.2 Detection limits based on experience
As for the headspace analysis used for the screening for the more volatile constituents of the rubber, a semi-quantitative determination of the amount of selected individual components identified by the analysis at the exposure temperature of 100°C and a 2-hour period of exposure. The results are submitted and discussed under the result section. 4.3 Screening results4.3.1 X-ray analysisThe X-ray analysis gives a general view of which elements were included in the chloroprene rubber (from aluminium and upwards in terms of atom weight). The method is well-suited to screen for heavy metals in the rubber, such as nickel, but elements in environmentally problematic substances, such as tin in organotin compounds and arsen in arsen trioxide, would be identified by means of the analysis. Furthermore, it would be possible to get an indication of the content of chloroprene rubber in the product as well as other elements, such as sulphur, zinc, calcium, magnesium, aluminium and silicium, which are components typically found in the additives that form part of a chloroprene recipe. The results of the X-ray screening analysis are indicated in Table 4.3 and Table 4.4. Table 4.3 Results of X-ray analysis (weight percentage) Table 4.4 Results of x-ray analysis (weight percentage) It is remarkable that only two of the eight products purchased contained traces of the element chlorine. This concerned the two types of dive gloves nos. 3 and 7. This means that even though the products were purchased as neoprene products a different type of rubber was used to manufacture the gloves. As mentioned under section 3 "Samples purchased/acquired", the importer of one of these two types of gloves was not able to explain why the glove does not contain neoprene. It could have been foamed EPDM rubber since this type of rubber is extremely weather-resistant and widely used in the rubber industry. It should be pointed out that it was not demonstrated to be EPDM rubber. However, since both samples contained the basic elements, sulphur and zinc, which are substances characteristic of sulphur-vulcanised types of rubber, the presence of these elements in any case indicated that a type of rubber other than chloroprene was used in these gloves. The chlorine content for some of the other products was also remarkably low. This concerns the following products: dive socks (no. 5) and waders (no. 6). As both products contain the chemical elements sulphur and zinc, it is assumed that chloroprene is used in combination with another rubber. It should be mentioned that chloroprene is a medium prised rubber and money can be saved by use of cheaper rubbers like EPDM and SBR. It appears from the following considerations that are based on the fact that the sum formula of chloroprene is C4H5Cl if manufactured from polymerisation of 2-chlor-1.3-butadien alone. The content of chlorine in the polymer would then be 40.1% w/w, calculated on the basis of the sum formula. For sulphur-modified types, the chlorine content would be lower due to the integrated polysulphidic groups. The x-ray analysis of a chloroprene sample received from a Danish rubber factory showed a content of chlorine of 34% w/w. This could fit a sulphur-modified type even though the sulphur content was low. If we then look at the standard formulary mentioned in annex B, the content of chloroprene was 45.5% w/w. For a chloroprene type as the one used as reference (the sample from the rubber factory), this corresponded to a chlorine content in a dive suit of approx. 15.5%. For dive socks and waders (nos. 5 and 6, respectively), the x-ray analysis indicated a content of 1.9 and 1.8% w/w chlorine corresponding to a content of chloroprene raw rubber in the recipe of approx. 6-7 % w/w if based on the reference sample of 34% chlorine and the dive suit recipe. The chlorine content found in sample 2 (lower leg protector) and in sample 8 (dive suit) of 12 and 14% w/w, respectively, corresponded well in relation to that of a standard recipe. Sample 1 (knee bandage) and sample 4 (dive hood) have a chlorine content of 7.0 and 9.7% w/w, respectively, which still indicated that the content of chloroprene was fairly high and not atypical. With regard to the products with a low chlorine content Du Pont Dow Elastomers and Nordica Elastomers has been consulted. The result from the discussions with the latter (referred to by Du Pont Dow Elastomers) is that one was not able to give an example of a typical chlorine content in a chloroprene product as other rubber types could contain chlorine e.g. CSM ( chlorsulphonated polyethylene). However it was stressed that among divers Neoprene is equal to good quality and this might be the reason for the Neoprene labelling even in products without chlorine. Also the view was expressed that products with a low content of chlorine might be had a small addition of chloroprene to justify the Neoprene labelling. As to heavy metals, no lead was found in any of the products. Rather than zinc oxide, lead oxide is known to be used as an accelerator particularly for water-resistant types of chloroprene. However, smaller quantities of nickel were found in the 1, 2, 4 and 8 samples (knee bandage, lower leg protector, dive hood and dive suit). The levels ranged between 0.01% w/w and 0.06% w/w. It was characteristic that nickel was found in all the samples with a high content of chlorine (7 – 14 % w/w). There was no nickel in the reference raw chloroprene from the rubber factory. The source of contamination can be wearing parts from the mixer where the rubber compound is produced due to the intimate contact at this processing level between the chamber wall and the rotors which may have been made in a steel alloy with nickel. The nickel content in the rubber is so low that it is not likely that the limit value of 0.5 µg/cm² per week per cm² (Bekendtgørelse om nikkel, 2000)for nickel release would be exceeded in connection with contact attempts. Thus, based on the specific gravity of the foamed chloroprene of 0.11g/cm³, the content per cm² rubber of nickel was calculated to range between 1 - 6 µg for the total of the four samples where nickel was found. [5] In sample 3 (dive glove), the repeated analysis demonstrated an amount of 0.0067% w/w chromium on the surface of the fabric on the inside. As for the other gloves tested, chromium was also found, i.e. an amount of 0.0087% w/w for TI dive gloves and 0.026% w/w for Power neoprene gloves. This was localised to the fabric, not to the rubber. Apart from the magnesium content, there were no special remarks to be made regarding the other elements recognised since they might all originate from fillers added to the rubber, as mentioned initially. Magnesium forms part of a chloroprene rubber recipe in the form of oxide. This is to prevent untimely vulcanisation of the rubber as a consequence of the segregation of hydrogen chloride. The magnesium oxide thus functions as an acid catcher. It should be noted that the largest amount of magnesium is present in the products with a high content of chlorine, except for waders which tops the list with 1.4% w/w. The silicium content being high also for waders indicates that this product has been added magnesium silicate filler. Finally, it should be noted that no trace of the tin element was found in the products. So based on the x-ray screening, there was nothing to suggest that organotin compounds had been used as yeast and mould inhibitors in the products studied. 4.3.2 GC/MS headspace analysisThe headspace analysis by GC/MS was carried out at 100°C . This temperature is of cause much higher than the consumer exposure temperature, but is the most suitable test temperature to determine volatile chemical substances in the rubber. In all investigated samples a row of volatile chemical substances were revealed during the two-hour exposure time. Only one of the dive glove samples (no. 7) was tested because the Technological Institute left out glove sample no. 3 according to agreement with the National Agency of Environmental Protection since it contained chlorine only at trace level. The chlorine content in diving glove (sample no. 7) was also quite low, however the analysis had been performed when the above agreement was made. In Table 4.5 Results from GC/MS-headspace screening, we have tried to list in which samples the different substances appear in the volatile emission and in which groups of substances. Table 4.5 Results from GC/MS-headspace screening
Minor volatile hydrocarbon emission from all the samples was also seen. The volatile emission was most significant from sample 2 (lower leg protectors) and sample 4 (dive hood). The volatile emission from samples 1 (knee protector) and 2 (lower leg protector), and from samples 6 (waders) and 8 (dive suit) consisted primarily of hydrocarbons with 15-20 carbon atoms (sample 6, though, 12-20 carbon atoms); however, the samples differed to some extent as to the precise composition of the volatile hydrocarbon emission. The volatile emission from sample 4 (dive hood) and sample 5 (dive socks) was different and consisted of two fractions. One fraction was isomers of dodecyl benzene while the other fraction was isomers of butylated dodecyl benzene. The isomeric dodecyl benzenes seem primarily to be branched around the benzylic position. To supplement the ranking on a scale from 1-5 which was made on the basis of the number of counts per chromatographic peak, the volatile emission level was determined semi-quantitatively for a representative range of the substances detected in the screening analysis. The results appear from Table 4.6. Table 4.6 Volatile emission levels from rubber samples heated for 2 hours at 100°C [7]
Table 4.6 Volatile emission levels from rubber samples heated for 2 hours at 100°C [8]
The general volatile emission levels for other compounds are considered to be about 1µg/g (1ppm) or lower. However with regard to isophorone the level is about 3µg/g, glycols have a level around 3 – 6 µg/g and the level of toluen from waders (no.6) is 21µ g/g. The last result is without question much higher than for any other identified substance in the headspace. Otherwise, many types of compounds were found during the investigation of other types of rubber, primarily EPDM rubber. This concerns breakdown products from thiuram disulphides which are widely used in sulphur vulcanisation of rubber (but not chloroprene). Thiurams, however, are used to control the integration of sulphur in the production of sulphur-modified types of chloroprene. We know from literature (Rubber Fume, R. Badura) and well as from the Technological Institute's own studies that thermal breakdown of thiuram disulphides leads to the formation of carbonyl sulphide, carbon disulphide, secondary amines, ureas, thiourea substances, isothiocyanates and formamides. Similarly, it has to be expected that the isothiocyantes can be transformed into isocyanates when reacting with zinc oxide. Anti-aging agents can result in the formation of ketones. It is a fact that volatile emission of aniline can also occur from rubber vulkanisates. This may originate from anti-aging agents. The level of volatile emission does not exceed the level found by the Technological Institute for types of rubber other than chloroprene (primarily EPDM). 4.3.3 GC/MS analysis of extractsThe results of the GC/MS screening of the dichloromethane/isopropanol extracts from the chloroprene product samples studied are indicated below. In this case the GC/MS analysis has been performed directly on extract from the rubber products. Table 4.7 The results of the GC/MS screening of the dichloromethane/isopropanol extracts
The result of the GC/MS screening supported the detection of some of the breakdown products from accelerators and anti-aging agents which had already been identified by the headspace analysis, e.g. ethylisothiocyanate, dibutylformamide and BHT. The number of identified substance components, however, is significantly lower. The advantage of this method is that it provides us with additional information about the slightly more volatile components in the form of plasticisers and anti-aging agents. Thus, the presence of the DEHP phthalate plasticisers was also identified in the sample nos. 1 (knee bandage), no. 3 (dive gloves) and no. 7 (dive gloves). The amounts in 1 and 3 were at trace level whereas concentrations in no. 7 (dive gloves) were significantly above trace level. Dibutylphthalate was found in the sample nos. 1 (knee bandage), no. 3 (dive gloves) and no. 5 (dive socks). Sample 1 involved traces whereas the amounts in sample 3 (dive gloves) and no. 5 (dive socks) exceeded the trace level significantly. It was not possible to analyse the samples for chlorinated paraffines due to the content of plasticisers in the form of mineral oils. However, the very low content of chlorine in the x-ray screening indicated that chlorinated paraffines were not present in the products. Even in the few samples where the chlorine content approached the expected level for a typical chloroprene recipe, the content was not so high that the presence of chlorinated paraffines could be suspected. DOPA, which is an anti-aging agent, was found in all the samples, except in the dive glove sample no. 3. 4.3.4 TLC screeningsThe results of the TLC screenings are indicated in Table 4.8. This method was used to screen for content of anti-aging agents and ETU (ethylene thio urea). Table 4.8 Results of TLC screenings [9]
The thin layer chromatographic screening verified that anti-aging agents in the form of ODPA or bisdiphenylamine (references A and B) had been added. Based on the running of different concentrations of A, the concentration in the samples was estimated at approx. 0.5% w/w in the rubber. No ODPA/bisdiphenylamine was found in sample 3 (dive gloves) or in no. 5 (dive socks). Octylated diphenylamine was found in the GC/MS analysis but the amount was smaller than in the other samples. TLC screening will probably have to be performed for a more concentrated sample of no. 5 to obtain a positive screening result for this sample. A positive result was obtained for the presence of 6PPD in sample no. 3 (dive gloves). For sample no. 2 (lower leg protector), sample no. 7 (dive gloves) and sample no. 8 (dive suit) the IPPD result was positive. The concentration levels for these anti agening agents were significantly lower than for ODPA and hardly exceeds 0.1% w/w. ETU was not found in any of the samples during screening. It is known from literature that ETU is transformed during the vulcanisation process into its corresponding urea (without sulphur) (Röthenmeyer).
Photo 4.1 Example of TLC in eluation system II of reference substances and extracts of samples after development using iodine. 5 Migration investigations
5.1 Chloroprene products selected for the migration investigationAccording to agreement with the National Agency of Environmental Protection, the following products were selected for further migration investigation: Figure 5.1 Selected products
The chlorine content of the sample nos. 1, 4 and 8 was as expected when compared to published chloroprene formularies. The chlorine content of sample 6 was quite low in relation to standard formularies and sample 7 had, as previously mentioned, a chlorine content at trace level even though these dive gloves are marketed as neoprene and thus chloroprene-based gloves. 5.2 Method descriptions for the migration investigations5.2.1 Initial test to determine the final test planIt was decided to select two of the products for the initial tests since, based on the screening analyses, the concentrations of organic substances to be determined by short-term exposures were expected to be very low. Sample no. 1 (knee bandage) and sample no. 6 (waders) were selected. The knee bandage was selected because it represents a typical chloroprene product and because the temperature is expected to be relatively high when the user is engaged in sport activities. For sample 6, the headspace analysis had identified a relatively high content of toluene in relation to the other samples, and waders represented a product with low chlorine content. The following exposure conditions were selected for the two products: Figure 5.2 Exposure conditions
The GC/MS analysis conditions used on the chromatograph were the same as those used for the screening analysis of extracts. The artificial sweat was produced as prescribed by literature (DS/EN 1811). The exposure conditions and sample preparation were as follows: A piece of the product was cut and weighed (approx. 1.5g) for the analysis. The sample was placed in a 250ml glass with a teflon-covered screw cap and was in contact with 50ml artificial sweat. The rubber was held down by means of metal mesh wire. The cap was placed on the glass which was then placed in a ultrasonic bath at the exposure temperature for the time specified. Concentration was carried out by Solid Phase Micro Extraction (SPME) in the headspace which was desorbed at the analysis. The rubber was then removed from the contact medium after it had been exposed to a physical, pumping load ("massaging"). The purpose of this load was to pump the contact medium out of the rubber before the analysis. When the rubber was removed, the contact medium was extracted using dichloromethane. A direct GC/MS analysis of the extract was performed. The results of the analysis work showed that no additional information is obtained from SPME and it was therefore decided to continue the analysis work by analysing the dichloromethane extract alone. Results of the initial tests: Method A: SPME-GC/MSSample 6 (waders) was found to contain toluene. The content was quantisised against toluene-d8 and found to be 3.1µg/g. No significant content of volatile organic components could be measured in sample 1 (knee bandage). Method B: GC/MS of the dichloromethane extractsTwo different substances were identified in the migration liquids studied. The results of the analysis are indicated in Table 5.3. Table 5.3 Analysis results from method B [10]
Identification was carried out by searching in the NIST MS library. 5.2.2 Final migration testsThe actual migration analyses were initiated on the basis of the initial migration tests. According to agreement with the National Agency of Environmental Protection, the tests were carried out under different contact conditions. First, the test involved an analysis of migrating substances to the simulant either in the form of artificial sweat or artificial sea water. The artificial sea water was produced according to a recipe provided in literature (Standard Methods). Subsequently, the samples were analysed quantitatively for content of the migrants after extraction using dichloromethane. The gas chromatographic conditions are identical to those previously listed under the method description i 5.2.2. The results of tests and analyses are indicated in Table 5.4. Table 5.4 Analysis results
The following exposure conditions were used in the tests: No. 1 Knee bandage: 37°C, 2 hours, artificial sweat. In the table, the results are indicated as migrated amount per gram of sample and as migrated amount per exposed area. The results are indicated as the mean value of two determinations. Raw data are listed in Annex C. The table shows that the following substances migrated:
5.2.3 Quantitative analyses of selected substances in the productsIt was subsequently decided to analyse quantitatively for some of the substances in the products. Aniline was included to see to what extent the substance is present in relation to phenol. Table 5.5 Analyses of components in products
When comparing Table 5.4 with Table 5.5, the highest concentrations in the migration tests are consistent with those in the products. Thus, this applies to:
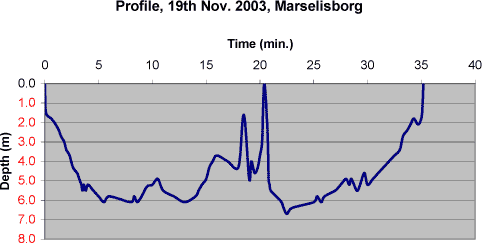
6 ”Real life” exposure test6.1 Test descriptionIn order to be able to assess the exposure in "real life" of potential migrants from consumer products made of chloroprene, a full-scale test with an experienced sport diver was carried out to complete the migration studies. The diving test was carried out near Marselisborg marina in Aarhus where the water is 6-7m deep near the shore. The diver dived twice on Wednesday, 19th November 2003. The conditions for one of the dives appear from the graph. The temperature of the sea water was 8°C and the temperature of the water drained from the suit was 18 – 20°C.
After the two-dive phase (65 minutes in total) and a break on shore (90 minutes), the dive suit was emptied of water on site by drawing it off from the sleeves and legs into a clean beaker. The water was then poured into a clean glass with a screw cap. The water from the dive suit was then to run off overnight so a clean beaker was placed below each of the legs of the suit. The water that ran off was combined with the water drawn off on site. The total amount of water was 155ml. A sample was taken of the sea water as reference for the migration analysis. Water emptied of the socks on the diving site was so muddy that it was decided to measure only for the nickel content in this water (the socks were not new).
Photo 6-1 Surfacing after the first dive. 6.2 Results from the migration investigationThe water samples from the "real life" experiment were analysed for nickel release and for release of organic substances. The results of the analyses are listed below in Table 6.1. Converted to skin area based on 20.000 cm² one can calculate the following concentrations of migrating chemical substances per cm² skin area: Isophoron 0,003 ng/cm², N,N-dibutylformamid 0,26 ng/cm², N,N-diethylthiourea 0,28 ng/cm² and nickel 0,74 ng/cm². If one compares the migration tests carried out in the laboratory to artificial seawater one realises that only in the “real life” experiment migration of N,N-diethylthiourea is detected. Further one realise that the measured migration levels of chemical substances calculated by area unit are less than 1 ng/cm² for all substances in the “real life” experiment. For two substances i.e. isophoron and N,N-dibutylformamide the results from the laboratory exposures were 11 ng/cm² and 20 ng/cm². This results are much higher and underline the importance of carriing out migration experiments as close to actual exposure conditions as possible. Table 6.1 Results of the analysis of liquid from the dive suit after the diving test
7 Assessment of Health Effects
7.1 Initial health screening of the identified substances in chloroprene rubberFor the identified substances in the screenings analysis an initial assessment has been carried out. In the initial assessment focus has been towards classification of the substances and potential health effects. The assessment was carried out by combining the CAS-numbers of the substances with information from the list of hazardous substances and other assessable sources. In cases where information was not available for the chemical substance were the properties judged from similar chemical substances. Further to a certain extent information from new investigations has been inclused in the assessment. In table 7.1 one has listed the classification of the substances which are included in the list of hazardous substances. For substances not classified comparison has been made to classified substances. In case this has been made it has been marked in Italics. Table 7.1 Screening of possible health effects
7.2 Assessment of health effects and risk based on migration analysisThe principles for the assessment of health risks are based on EU's Technical Guidance Document (TGD). The assessment is based on the exposure of an adult with a body weight of 70 kg. For calculation of the of the human uptake the following exposure areas are assumed:
The uptake is calculated by:
In the calculation is assumed the exposure happens no more than once a day in the amount of hours that are given for each product. Further, it is assumed that 100 % of the substance is absorbed. The results of the migration tests and the diving test are calculated to amount of the substance that might be present in the body after exposure. The calculations are based on data from table 5.1 and table 6.1. Tabel 7.2 Substances that potentialy can be uptaken in the body
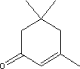
The possible uptake where 100 % of the substance is assumed absorbed is compared with information from the chemical substance under assessment in relation to NOAEL (No observed Adverse effect Level), LOAEL (Lowest Observed Adverse Effect Level) or other relevant sources available. 7.3 Assessment of selected substancesIn the following, a description of the substances potential health effects is described. In the assessment is focused on the properties, that are relevant for skin contact and exposure to skin (dermal uptake). Primarily data on skin penetration, dermal uptake and irritation is given. When is has been possible measured concentrations and the calculated amounts of uptake is compared to NOAEL, No Adverse Effect Levels. For substances, where evaporation is relevant, data for NOAEC, No Adverse Effect Concentrations, is included. 7.3.1 Isophorone7.3.1.1 Identity
Isophorone has a boiling point of 215°C and a melting point of 8.1°C (Lide, DR). The substance is soluble in ether, acetone and alcohol and is used as a solvent for substances that has a very low solubility in other solvents. The substance is soluble in water, 12 gram/litre (Kirk-Othmer). The ration between solubility in octanole and water for the substance, log KOW = 1,7, which means that the substance is more soluble in organic solvents than in water (Veith, 1980) Isophorone has a vapour pressure of 0.438 mm Hg at 25°C (Daubert, 1989). 7.3.1.2 Amount of the substance in the tested productsIsophorone is found in the two tests of diving suits. In the lab-test a concentration of 0.15 mg/litre was found equivalent to a potential uptake of 3 µg/kg based on 100% uptake. In the diving test an amount of 0.1 µg/kg was found based on 100% uptake. Both amounts are based on the earlier mentioned assumptions of contact time per day. 7.3.1.3 Function of the substanceIsophorone has a technical function as highboiling solvent for adhesive and ovendrying laquers. Technical applications include PVC joint sealants. It is judged that in relation to chloroprene based products it is used in relation to the use of adhesive or lamination. 7.3.1.4 ClassificationIsophorone is included in the list of hazardous substances and classified as EU index no. 201-126-0 (Listen over farlige stoffer, Miljøministeriet 2002):
7.3.1.5 Health effectsIsophorone is describes in IUCLID's dataset from 2000 (IUCLID, isophorone). From this comes the following:
Isophorone is included in Environmental Health Criteria 174. From this comes the following information:
7.3.1.6 AssessmentBecause the substance can be absorbed through the skin it is reasonable to assume 100 percent uptake. The potential uptake is 0.003 mg/kg and 0.001 mg/kg. The lowest found value for NOAEL is 150 mg/kg by intake. No information regarding NOAEL data for dermal uptake. LD50 for rabits is 1.200 mg/kg. It is judged that dermal uptake will not be a health risk. It is also assumed that the relative low observed concentrations at 0.15 mg pr. litre will cause no irritations. Based on the above described assessment it is concluded that the observed amounts of isophorone will cause no health effects. 7.3.2 Toluene7.3.2.1 Identity
Toluene has a boiling point of 110.6°C and a melting point of -94.9°C. The substance is miscible with most solvents. Toluene has solubility in water of 526 mg/litre. It has a log KOW of 2.73, which indicate that it is far more soluble in organic solvents than in water. Toluene has a vapour pressure of 28.4 mm Hg at 25°C. 7.3.2.2 Amount of the substance in the tested productsToluene was found in test of waders. The test showed a concentration of 0.12 µg/gram, which is equivalent to an uptake of 0.418 µg/kg, based on 100 percent uptake. Toluene was not found in the other tested products. 7.3.2.3 Function of the substanceToluene is a technical solvent. In relation to chloroprene products it is judged that toluene is used by gluing and lamination of rubber/textile. 7.3.2.4 ClassificationToluene is on the list of hazardous substances and classified under EU index nr. 601-021-00-3 (Listen over farlige stoffer, Miljøministeriet 2002):
A new classification has been proposed for toluene (EU's Risk Assessment report nr. 30). This upcomming classification is:
7.3.2.5 Health effectsIn the Risk Assessment report no. 30 from the EU (EU's Risk Assessment report no. 30). From this report the following information is found. Toluene is very rapidly absorbed by inhalation. The substance is able to penetrate through the skin and will be absorbed by skin contact. Toluene is distributed in the whole body and is primarily found in fatty tissue. Toluene has a low toxicity. Humans exposed to toluene at concentrations of 285 mg/m³ and higher will experience headache, dizziness and fatigue. A value for NOAEC of 150 mg/m³ is estimated based on these findings. Liquid toluene irritates the eyes and vapours in concentrations of around and above 150 mg/m³ causes eye irritations in humans. Based on this a NOAEC for eye irritations is estimated to 150 mg/m³. In relation to inhalation a value of NOAEC is estimated to 1,125 mg/m³. Long term exposure of high concentrations of toulene has caused serious brain damage. It has not been possible to estimate values for NOAEC or LOAEC for long tern exposure in respect to brain damage. There are very limited tests for oral intake and skin contact. In a 13-day test with rats a NOAEL of 625 mg/kg was estimated based on brain damage. In another test with mice a NOAEL of 625 mg/kg was estimated based on damage of the liver. It is stated an LOAEC 330 mg/m³ value with risk for spontaneous abortion and a NOAEC value of 2.250 mg/m³ for low birth weight and retardation. 7.3.2.6 AssessmentWhile toluene is able to penetrate the skin and be absorbed in the body it is assumed that 100 percent will be absorbed. A part of the toluene might evaporate, while the substance is volatile and will be absorbed by inhalation. If it is assumed that 100 percent is absorbed though the skin, the level in the body will be about 0.0004 mg/kg, which is considerable lower than the value of NOAEL of 625 mg/kg. Toluene is present in the product in concentration measured to 0,0046 µg/cm³. This result in a calculated amount in the product corresponding to 0,0029 mg. This amount can in theory evaporate as toluene is rather volatile. However the concentration in the air will be so low that no health risk exist. Although a part of the toluene will evaporate, at no time the concentration in the air will come close to the value of NOAEC at 150 mg/m³. Based on the above-described assessment it is concluded that the observed amounts of toluene will cause no health effects of significance. 7.3.3 Phenol7.3.3.1 Identity
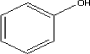
The boiling point of phenol is182°C and the melting point is 41°C. The substance has a vapour pressure of 0,35 mm Hg at 25°C. Phenol is soluble in most organic solvents. The water solubility is 66 gram per litre. At temperatures above 65°C the substance is 100 percent mixable with water. 7.3.3.2 Amount of the substance in the tested productsPhenol is found in dive gloves no. 7. An amount of 0.9 µg/gram was found equivalent to a potential uptake of 0.744 µg/kg. 7.3.3.3 Function of the substancePhenol is not used for technical reasons in chloroprene products. It is judged that phenol originate from inpurities in phenolic resins. Phenolic resins are used for priming of textile for better adhesion to the rubber. 7.3.3.4 ClassificationPhenol is on the list of hazardous chemical substances and classified under EU index no. 203-632-7 (Listen over farlige stoffer, Miljøministeriet 2002):
7.3.3.5 Heath effectsPhenol is toxic with a lethal dose of 50-500 mg/kg for humans. Some persons can be hyper sensitive with serious effects or death caused by exposure to even lower doses. Repeated exposure to drinking water contaminated by phenol during several weeks is reported. The estimated amount of uptake was around 10-240 mg/day and caused skin damage in the mouth, diarré and dark urine. There was no permanent effects six month after the exposure (IARC, 1999). Phenol is able to penetrate the skin and is absorbed quickly to the body. Effects on the body are damage to the CNS, the hart, blood, lungs and kidneys. Observed effects from short-term exposure can include chock, coma, delirium and death. Long term or repeated exposure can cause damage to the liver, kidneys and eyes. Changes in the pigmentation of the skin are reported. Inhalation can cause irritations and oedema. Phenol is included in the IUCLID database from 2000. From the data set comes the following information. Tests show that phenol is not a sensitiser. In a 28-days test with mice were shown that oral intake cause effects on red blood cells and on the level of antibodies in the blood. LOAEL was estimated to 1.8 mg/kg body weight. In a test with rats (Argus Research Laboratories, 1997) the effects on the development of the offspring was analysed. A NOAEL of 60 mg/kg per day was estimated. A benchmark dose of 93 mg/kg was calculated and by including a safety factor of 300 the reference dose was estimated to 0,1 mg/kg/day. Phenol is not recognised as a carcinogen (IARC, group 3) based on insufficient evidence for both humans and animals (IARC, 1999). 7.3.3.6 AssessmentWhile phenol is able to penetrate skin it is assumed that 100 percent of substance is absorbed. The reference dose of 100 µg/kg/day is substantially higher than the calculated amount of 0.7 µg/kg, which might be the daily possible uptake. The calculated amount 0,7 µg/kg is also in relation to the LOAEL of 1800 µg/kg on an acceptable level. Based on the above-described assessment it is concluded that the observed amount of phenol will cause no health effects of significance. 7.3.4 Dibutylformamide7.3.4.1 Identity
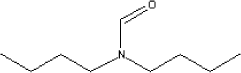
7.3.4.2 Amount of the substance in the tested productsThe substance dibutylformamide was found in knee bandages, diving hut and diving suit. From the knee bandages can be absorbed an amount of 1.3 µg/kg per day if 100 percent is absorbed. From the diving hut can be absorbed an amount of 0.4 µg/kg per day if 100 percent is absorbed. Tests for diving suits were carried out twice, - in the laboratorium and in practice. In the laboratorium an amount of 5.7 µg/kg per day was found and in practise an amount of 0.7 µg/kg per day. The value found in the practical experiment is judged to be the most reliable. 7.3.4.3 Function of the substanceDibutylformamide has no direct technical function in relation to chloroprene products. It is a decomposition product from the accelerators used for crosslinking. 7.3.4.4 ClassificationThe substance is not classified by EU (Listen over farlige stoffer, Miljøministeriet 2002). The substance dimethylformamid which has a similar chemical structure is classified by EU under index no. 616-001-00-X:
7.3.4.5 Health effectsThere is a very limited amount of information regarding the substance dibutylformamide. The substance is described in a few articles in the database TOXLINE, but these articles are primarily about analysis methods. In an article by Chang P-H et al. (1973) is dimethyl-, diethyl-, dipropyl- and dibutylformide briefly described. For both acute and long term toxicity it was shown that the toxicity was decreasing as the molecular weight was increasing. Dipropyl- and dibutylformamide caused liver damage, but did not cause damage to the testicles as the two other formamides did. After daily doses of the four formamides damages of the liver was observed as well as biochemical changes in blood and urine. The most serious effects was seen with lowest molecular weight. Stula og Krauss (1977) have conducted a test for reproductive effects. Both dibutyl- and dimethylformamide were reported causing damages to the foetuses in test with rats. In the following, it is decided to base further information on dimethylfor-mamide while dibutyl- and dimethylformamide in the two references are described as having similar effects or that dibutylfomamide has less health effects than dimethylformamide. In a test with rats lasting 104 days NOAEL was estimated to 210-235 mg/kg based on observations of liver damages (Becci et al, 1983). With respect to absorption through the skin is found a 60-day test with rats, where the animals 4 hours daily had their tails dripped with the substance. Observations were changes in the liver and damages on the CNS. NOAEL was estimated to 60 mg/kg (Medyankin, 1975). Reprotoxic effects have been studied for dimethylformamide. The experiments were all based on inhalation studies. LOAEC for rabbits was determined to 448 mg/m³. The effect of the dimethylformamide was a reduction of birth weight. In the same study NOAEC was found to 150 mg/m³ (Praetorius, W. 1989). In another study pregnant rats were exposed to dimethylformamide vapours in the period from 6. to 15. day of pregnancy. The effect of the inhalation of the substance was low birth weight and fewer new-born young ones. LOAEC was found to 900 mg/m³ and NOAEC to 90 mg/m³ (Bio/Dynamics, Inc. 1978). 7.3.4.6 AssessmentThe amounts found in the test are all lower than 0.002 mg/kg per day. The lowest NOAEL, which has been found for dimethylformamide, is based on skin absorption and estimated to 60 mg/kg per day. It is assumed that NOAEL for dibutylformamide wil have approximately the same value or higher. Even if a safety factor of 1000 is included the observed amounts will still be less than the no-effect-level. Dibutylformamide is like dimethylformamide toxic for reproduction. Data for NOAEC in tests with rabbits and rats where the animals had been exposed during pregnancy showed a lower birth weight for the exposed animals. The lowest NOAEC was determined to 90 mg/m³. It is judged that the amount (less than 0,002 mg/kg per day) which potentially can be inhaled will not give rise to damage to the unborn child. Based on the above describes assessment it is concluded that the observed amounts of dibutylformamide will cause no health effects. 7.3.5 Diethylthiourea7.3.5.1 Identity
The substance is solid and has a melting point of approximately 70°C. Diethylthiourea is soluble in water, methanol, ether, benzene and ethylacetate. The substance is insoluble in oil. The solubility in water is estimated to 4555 mg per litre. Log KOW is 0.57 (Govers H et al; 1986). 7.3.5.2 Amounts of the substance in the tested productsThe substance diethylthiourea was found in waders and in the test with the diving suit in practice (“real life”). In the test with waders a concentration of 1.8 µg/ml was observed equivalent to a potential uptake of 6.7 µg/kg. In the test with the diving suit a concentration of 0.36 µg/ml was observed equivalent to a potential uptake of 0.8 µg/kg. 7.3.5.3 Function of the substanceDiethylthiourea is used as accelerator by the vulcanisation of chloroprene rubber goods. 7.3.5.4 ClassificationThe substance is not included in the List of hazardous substances. It is included in the consultative list from the Danish EPA )Miljøstyrelsens vejledende liste til selvklassificering af farlige stoffer 2001). In this list diethylthiourea is found as EINICS no. 203-308-5 and classified as Xn; R22, Harmfull and harmfull by ingestion. 7.3.5.5 Health effectsIn Sax (1984) is described 2 short-term test based on oral intake. For rats LD50 was estimated to 316 mg/kg and for mice LD50 was estimated to 681 mg/kg. In a test for potential cancer effects diethylthiourea was given to mice and rats by ingestion over a period of 103 weeks. Rats were daily given 125 mg and 250 mg and mice were given 250 mg and 500 mg. Changes in cells in the thyroid were observed in rats. The test was negative for mice (Bioassay, 1979). IARC (2001) has classified the substance in group 3, not carcinogenic for humans. There is inadequate evidence in humans and only very limited test with animals. Tests show that dimethylthiourea can cause allergy and sensitivity. Dooms-Goossens (1998) describes a study of 4 patients, where 3 developed dermatitis. I Ugeskrift for læger (Medical journal, in Danish) did Buus and Andersen (2002) describes the substance as causing allergic contact dermatitis. It has not been possible to find any data regarding NOAEL. 7.3.5.6 AssessmentThe highest value that was observed for potential uptake was 6.7 µg/kg for waders. No data was possible to retrieve for skin adsorption. While the substance is both water soluble and soluble in fats, it is assumed that the substance can be absorbed. The very limited data regarding toxicity shows LD50 values by oral ingestion as low as 300 mg/kg. Including a safety factor of 1000 this correspond to an acceptable limit of 300 µg/kg. It has been shown that the substance causes a risk for allergic dermatitis, but no information is available of which amount, that causes this effect. Based on the above-described assessment it is concluded that diethylthiourea probably not is toxic in the observed amounts, but there can be a risk of allergic dermatitis. 7.3.6 N-Butylbenzenesulfonamide7.3.6.1 Identity
A very short data set from the UICLID database has been used. From this comes the following information. Butylbenzensulfonamides melting point is –30°C and the boiling point is higher than 250°C. The solubility in water is 1.02 at 20°C. There is no information about solubility in other media's as well as log KOW. 7.3.6.2 Amount of the substance in the tested productsButylbenzensulfonamid is found in knee bandages and in waders. For knee bandages the concentration was measured to 0.50 µg/ml equivalent to a potential uptake of 0.13 µg/kg. In waders the concentration was measured to 0.64 µg/ml equivalent to a potential uptake of 2.4 µg/kg. 7.3.6.3 Function of the substanceIt is judged that butylbenzenesulfonamide is a secundary decomposition product froman accelerator. Accelerators for rubber is e.g. sulfenamides, and formation of sulphoneamides can occur by oxidation of the divalent sulfur in the sulfenamide. 7.3.6.4 ClassificationThe substance is neither included in the List of hazardous substances nor on the consultative list from the Danish EPA. 7.3.6.5 Health effectsIn IUCLID two tests of short term toxicity is reported. The tests are tests on rats and oral ingestion. The reported results range from 1725 to 2050 mg/kg for LD50. For skin absorption is described two test with rabbits, where LD50 was estimated to be higher than 1150 mg/kg. A test with guinea pigs and skin irritation showed a negative result. In a test with rabbits irritations of the eyes were observed. No information regarding allergy is given. In a 28-days study with cats functional disorders were observed by oral ingestion of 57.5 mg/kg. No NOAEL was given. An Ames test with salmonella typhimurium gave a negative result. Besides this IUCLID has not included any further information regarding health aspects. Hashimoto et al (1991) did test the substance in mice to determine weather the substance is toxic of reproduction. Mice got the substance with the feed in doses of 500 mg/kg per day and 750 mg/kg per day. In both groups effects in terms of smaller foetuses were found. The test gave no explanation of weather the damage was caused directly to the foetuses or by effects from the mother. 7.3.6.6 AssessmentBased on the available data it is concluded that NOAEL will be lower 57.5 mg/kg. If it is assumed that NOAEL is about a level of 10 mg/kg and if a safety factor of 1000 included, amounts of 10 µg/kg will be acceptable. The observed values are around 2.4 µg/kg and less. N-butylbezensulfonamid will probably not cause any health effects based on the observed amounts, - but it has to be emphasised that the data is insufficient. Some data indicate that the substance can be toxic to reproduction. 7.3.7 NickelNickel is found in one of the tested products. It is assumed that dimethyl-dithiocarbamate, nickel salt is added to the chloroprene. The nickel ion is present as Ni 2+ and can migrate to the surroundings. In the following, the nickel ion is assessed based on information on nickel oxide and nickel sulphate. 7.3.7.1 Amount of the substance in the tested productsIn the test of the diving suit in practise a content of nickel is measured to 0.095 µg/ml equivalent to a potential uptake of 0.2 µg/kg. 7.3.7.2 Function of the substanceNickel salts from dithiocarbamic acid are effective to inhibit ageing of rubber goods due to exposure to ozone. 7.3.7.3 ClassificationNickeloxide has CAS-no. 1313-99-1 and EU index-n. 1313-99-1. The substance is classified:
Nickelsulfat has CAS-no. 7786-81-4 and EU index-no. 232-104-9. The substance is classified:
7.3.7.4 Health risksIn IUCLID’s data set for nickel sulphate the short-term toxicity in test with rats and oral ingestion showed an LD50 of 275-350 mg/kg. No data for cancerogenic and reproductive effects were found. In a two-generation test with rats (RTI, 1987) nickel chloride was given oral by drinking water. NOAEL was estimated to 30.5 mg/kg/day (250 ppm). Damage at high doses caused decreased birth weight and liver damage. Allergy caused by contact to nickel has been reported both among the general population and in the working environment (Environmental Health Criteria 1991). In IARC's assessment of nickel compounds (IARC, 1990) both nickel sulphate and nickel oxide are characterised as carcinogens, group 1. 7.3.7.5 AssessmentClassification of nickel oxide is irrelevant with respect to inhalation i this study. Based on a NOAEL of 30.5 mg/kg and a safety factor of 1000 a level of approximately 30 µg/kg will be an acceptable level. The observed amount in this study is 0,2 µg/kg and will therefore represent no concern. It should be mentioned that nickel ions can result in allergic reactions by contact with the skin. However it is not possible to judge if this effect can be expressed at the very low level 0,2 µg/kg. 7.4 ConclusionIn the study of the selected chloroprene products a number of chemical substances were found. The study includes identification of 46 chemical substances for which an initial screening was carried out. In the migration tests were identified 7 chemical substances and these are selected for a further assessment. The result of this assessment is presented in table 7.3. The "Analysed amounts" in table 7.3 are the amounts, which potentially can be adsorbed per kg body weight. Table 7.3 Summary of the assessment for substances identified in the migration test.
From table 7.3 can be seen that:
Overall it must be concluded that the found chemicalsubstances will not contribute to a health risk for the investigated products of chloroprene. 8 ReferencesArgus Research Laboratories, Inc. (1997). Oral (gavage) developmental toxicity study of phenol in rats. Horsham, PA. Protocol number: 916-011 Becci PJ, Voss KA, Johnson WD, Gallo MA, Babish JG (1983) Subcronic feeding study of N, N-dimethyl formamide in rats and mice Bekendtgørelse nr. 439 af 03/06/2002 om listen over farlige stoffer Bekendtgørelse om nikkel, 2000: Bekendtgørelse om forbud mod import og salg af visse nikkelholdige produkter, 25. januar 2000 Bio/Dynamics, Inc. 1978. A segment II teratology study in rats following inhalation exposure to DMF. Project No. 77-1960. EPA Document #86-890000245, submitted by Exxon Chemical Co. Bioassay of N,N'-diethylthiourea for Possible Carcinogenicity (1979) Technical Rpt Series No. 149 DHEW Pub No. (NIH) 79-1705, U.S. Department of Health Education and Welfare, National Cancer Institute, Bethesda, MD 20014 Chang P-H, Azum-Gelade MC, Nguyen-Van-Bac, Xuong N-D: Toxicological studies of the N-n-propyl and N-n-butyl derivatives of formamide TOXICOL APPL PHARMACOL; 26 (4). 1973 (RECD 1974) 596-605 Dansk Sportsdykker Forbund: Sportsdykkerhåndbog, 5. udgave Daubert, T.E., R.P. Danner. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, D.C.: Taylor and Francis, 1989. Dooms-Goossens A et al; Br J Dermatol 116 (4): 573-9 (1987) DS/EN 1811, 2. udgave (200-03-21)”Referenceprøvningsmetoder til bestemmelse af nikkelafgivelse fra produkter beregnet til at komme i direkte og længerevarende i kontakt med huden” Environmental Health Criteria 108: Nickel. pp. 17-22 (1991) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization Environmental Health Criteria 174 Isophorone. pp.1-22 (1995) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization EUs Risk Assessment report no. 29 for stoffet toluen, udført af Danmark, 2003 Govers H et al; Chemosphere 15: 383-93 (1986) Hashimoto R, Hatta T, Tatewaki R, Otani H, Tanaka O: Effects of the plasticizer N-butylbenzenesulphonamide on pregnant mice Teratology 1991 Dec;44(6):35B ; Department of Anatomy, Shimane Medical University, Izumo, Shimane, Japan IARC (1999) Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work).V71 762 IARC (2000) Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work).p. V49 410 IARC (2001) Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work).p. 79 655 IDA – Polymerteknisk Selskab, Møde om gummi, 20. maj 1998 IRIS, 2000: U.S. Environmental Protection Agency's Integrated Risk Information System (IRIS) on Isophorone (78-59-1) Available from: http://www.epa.gov/ngispgm3/iris on the Substance File List as of March 15, 2000 IUCLID dataset (2000) Isophorone. European Commission, European Chemical Bureau IUCLID dataset (2000) N-Butylbezenesulphonamide, European Commission, European Chemical Bureau. IUCLID dataset (2000) Nikkelsulfat. European Commission, European Chemical Bureau IUCLID dataset (2000) Phenol. European Commission, European Chemical Bureau J.C. Bament: Neoprene Synthetic Rubber: A guide to grades, compounding and processing, DuPont John S. Dick (Ed.): Rubber Technology Compounding and Testing for Performance, Editor, Hanser Verlag, München 2001 Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V21(1983) 385 Lide, DR (ed.). CRC Handbook of Chemistry and Physics. 81st Edition. CRC Press LLC, Boca Raton: FL 2000, p. 3-131 Medyankin AV (1975) Complex action of dimethyl formamide under condition of a long term experiment. Gig Saint 9 39-42 Miljøstyrelsens vejledende liste til selvklassificering af farlige stoffer, 2001 Peter A. Ciullo, Norman Hewitt: The Rubber Formulary, Plastics Design Library, 1999 Peter A. Ciullo, Norman Hewitt : The Rubber Formulary, Plastics Design Library, 1999 Praetorius, W. 1989. BASF Corporation. Letter to the Document Control Officer of the Information Management Division, Office of Toxic Substances, U.S. EPA. March 8. Subject: FYI Submission - Results of a prenatal inhalation study of dimethylformamide in rabbits R. Kuschel, G. Streit, Altautoverordnung und die Gummiindustrie-Gummi und Gefahrstoffe, GAK, 56.årgang, juni 2003 (nikkel) R.Badura, H. Linde, R.H. Schuster, Analysis of volatile products in vulcanisation fumes, DIK foredrag præsenteret på IRC 89 i Prag R.N. Datta: Rubber Curing Systems, , RAPRA Review Reports, Volume 12, No. 12, 2002 RTI (Research Triangle Institute). 1987. Two generation reproduction and fertility study of nickel chloride administered to CD rats in drinking water: Fertility and reproductive performance of the Po generation (Part II of III) and F1 generation (Part III of III). Final study report. Report submitted to Office of Solid Waste Management, U.S. EPA, Washington, DC Rubber Fume, Bryan Willoughby, 1994 Rapra technology Limited Rubber Handbook, SGF, 10. udgave Röthenmeyer/Sommer: Kautschuck Technologie, Werkstoffe, Verarbeitung, Produk, Hanser Verlag, 2001, ISBN 3-446-16169-4 Sanne Krogsbøll Buus & Klaus Ejner Andersen: Allergisk kontakdermatitis fra diethylthiourea i neoprengummi. Ugeskrift for Læger 2002; 164: 1511-2 Sax, N.I. Dangerous Properties of Industrial Materials. 6th ed. New York, NY: Van Nostrand Reinhold, 1984. 1030 Schill + Seilacher: Compounding Guide for the rubber industry, Struktol Standard Methods for the examination of water and wastewater, 18.udgave Ed. Arnold E. Greenberg, Lenore S. Clesceri, Andrew D. Eaton, side 8-11 Steve Sleicht, Komma’s sejlerhåndbog Aschehoug, 2000 Stula EF, Krauss WC: Embryotoxicity in rats and rabbits from cutaneous application of amide-type solvents and substituted ureas. Haskell Lab. Toxicol. Ind. Med., E.I. du Pont de Nemours Co. Inc., Wilmington, Del. 19898, USA Terje Nordberg: Politikkens løbebog, , 2002 The Vanderbilt Rubber Handbook, 13. udgave Veith GD et al; pp. 116-29 in Aquatic Toxicology. Easton JG et al, eds. Amer Soc Test Mat ASTM STP 707 (1980) Weast, R.C. (ed.) Handbook of Chemistry and Physics. 69th ed. Boca Raton, FL: CRC Press Inc., 1988-1989 Winkler, Reinhart: Bogen om brætsejlads, sejl og motors forlag Footnotes [1] The listed analysed amounts in the table are the amuounts which has a potential uptake per kg body weight by a 100 % dermal uptake. [2] NOAEL (No Observed Adverse Effect Level). [3] De anførte analyserede mængder i tabellen angiver de mængder der potentielt kan optages pr. kg legemsvægt ved en dermal optagelse på 100 %. [4] NOAEL (No Observed Adverse Effect Level) [5] Late in the project, the nickel salt of dimethyl dithiocarbamate or dibutyl dithiocarbamat was identified as being used as an efficient antiozonant (R. Kuschel, Rubber Handbook) in certain rubber recipes. In the "real life" diving experiment, the release of nickel was therefore measured based on this new knowledge. [6] The recognised organic compounds were detected only in the products where they had been given a mark ranging between 1 and 5. The numbers indicate the relative areas of the peaks in the chromatogram. The figure 1 indicates a high concentration in comparison with other peaks present. The figures 4 and 5 indicate that the concentration is rather low in the chromatogram. [7] The following remarks should be made to the volatile emission levels: The quantisising limit was established as the lower limit at which a "reasonable" quantitative value can be determined. Minus (-) means "not detected" 1): The content was determined quantitatively in relation to carbon disulfide. For carbonyl sulfide, this meant that the indicated values were probably maximum values wheras it was not directly possible to assess whether the values for ethylisothiocyanate were minimum or maximum values. 2): The content was determined quantitatively in relation to diethylamine. Since the response factors for a given type of compound are known, from experience, to increase as a function of the number of methylene groups (up to a certain limit), the level indicated must be considered the maximum value. The lower quantisising limit is due to the chromatography of dibutylamine being better than diethylamine. [8] The following remarks should be made to the volatile emission levels: The quantisising limit was established as the lower limit at which a "reasonable" quantitative value can be determined. Minus (-) means "not detected" 1): The content was determined quantitatively in relation to carbon disulfide. For carbonyl sulfide, this meant that the indicated values were probably maximum values wheras it was not directly possible to assess whether the values for ethylisothiocyanate were minimum or maximum values. 2): The content was determined quantitatively in relation to diethylamine. Since the response factors for a given type of compound are known, from experience, to increase as a function of the number of methylene groups (up to a certain limit), the level indicated must be considered the maximum value. The lower quantisising limit is due to the chromatography of dibutylamine being better than diethylamine. [9] The character "+" means "identified" and "–" means "not identified". A start (*) means that the TLC method cannot distinguish ODPA from bisdiphenylamine. When "+" is indicated in the ODPA column, it is because this is the substance component verified by GC/MS in the samples. [10] "-" means below the detection limit, 0.1µg/g.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||