|
Arctic Mercury Releases InventoryContents
3 Sources and releases of mercury from Arctic countries
4 Discussion of major source categories
5 Reported landfills of special concern 6 Overview of existing action plan/strategy elements on mercury in the Arctic Countries
Appendices: Links to national responses to mercury questionnaire
PrefaceOver the past 10 years, the Arctic Monitoring and Assessment Programme (AMAP) have conducted assessments documenting the sources, levels, trends, and effects of a wide range of priority pollutants including mercury in the Arctic. The main conclusions of these assessments are that: “In comparison with most other areas of the world, the Arctic remains a clean environment. However, for some pollutants, combinations of different factors give rise to concern in certain ecosystems and for some human populations. These circumstances sometimes occur on a local scale, but in some cases may be regional or circumpolar in extent.” Mercury is a heavy metal of special concern. It is toxic to human and other living organisms and bioaccumulates in the Arctic marine food chain to reach levels that are a cause for concern especially for that part of the population of Canada and Greenland whose traditional diets include fish and marine mammals. In response to the AMAP findings, the Arctic Council decided in 2001 to implement a number of projects; among these a project on `Reduction of Atmospheric Mercury Emissions from Arctic States' as part of the 'Arctic Council Action Plan to Eliminate Pollution of the Arctic` (ACAP). The project objective is to contribute to a reduction of mercury releases from the Arctic countries, partly through supporting development of mercury release inventories and release reduction strategies, and partly by initiating actions to demonstrate release reduction options at one, or a few, specific sources located in an Arctic country. The sources are selected based on regional and national release inventories and a detailed evaluation of potential demonstration project sites. This Arctic Mercury Release Inventory has identified and quantified a number of sources of mercury releases. The sources are from a wide range of categories including intentional use of mercury in chemical industry and product manufacturing, as well as releases from mining and metallurgy, and energy production. Presently identified source categories include: coal fired power plants, waste incineration, chlor-alkali plants, and non-ferrous smelters. The Arctic countries are addressing these releases through a variety of strategies. Domestic initiatives are in place or being developed to meet the countries' objectives and to address international obligations. International agreements related to mercury and of relevance to Arctic countries include the UNECE Convention on Long-range Transboundary Air Pollution, OSPAR, HELCOM, the North American Regional Action Plan, regional agreements between USA and Canada, and EU legislation. These agreements cover multiples aspects of mercury (monitoring, emissions inventories, product controls, reduction of releases, etc.), having some aspects in common but also differences. Mercury specific reduction technologies may have evolved since the agreements were developed and will continue to evolve. The regional release inventory includes a listing of some current options to address mercury releases from the major source categories identified in the inventory. These options are reflected to varying degrees in the countries' existing strategies and in the international agreements. The listing provides Arctic countries with:
Further, the listing may provide valuable information for countries outside of the Arctic Council region that are seeking to develop national and regional mercury strategies. This review focused on the technical aspects of the inventory, including verification of the accuracy of reporting of country questionnaires, and options to address mercury releases. The review does not extend to endorsing, from a policy standpoint, any of the specific possible options related to source categories that are identified in the inventory. Therefore the possible options are not prescriptive to the countries but are intended to provide information and indicate possibilities for future consideration. The ACAP mercury project is co-ordinated by Denmark with COWI A/S as consultant. We would like to thank the members of the ACAP Steering Group and the reviewers contributing to the preparation of this report. Karsten Skov AcknowledgementProject Steering Group members:
Draft author This report was drafted by Jakob Maag, COWI A/S Reviewers In addition to the Steering Group members, the report has been reviewed by the following persons, among others: Frank Anscomb, USEPA (USA); Alexander McBride, USEPA (USA); David Kirchgessner, USEPA (USA); John Kinsey, USEPA (USA); Russell Bullock, USEPA (USA); Alexis Cain, USEPA (USA); Stephen Hoffman, USEPA (USA); Robert Stevens, USEPA (USA); Ann Pope, USEPA (USA); William Maxwell, USEPA (USA); Paul Almodovar, USEPA (USA); and, Ravi Srivastava, USEPA (USA); Jozef M Pacyna, Norwegian Institute for Air Research (Norway). Financial support The ACAP Mercury project was funded by Denmark, Canada, USA and Norway. Symbols, units and acronymsFor country-specific acronyms the country is indicated in brackets if it is not indicated in the name.
Units
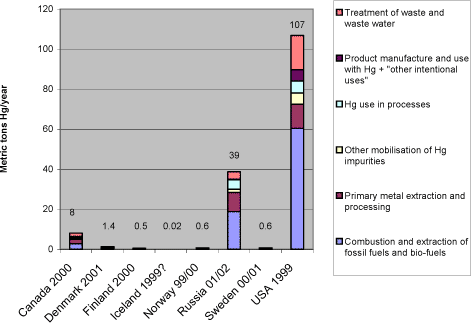
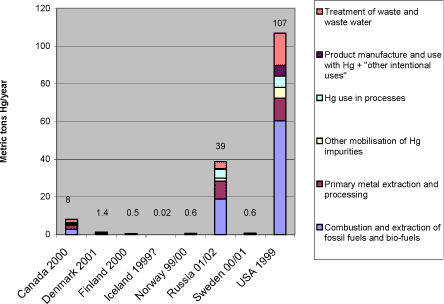
1 Executive summary1.1 This inventory report 1.1 This inventory reportThe AMAP Assessment reports (AMAP 1997, 1998, 2002) have evaluated the available information on selected pollutants and their effects in the vulnerable Arctic environment. In the `AMAP Report on Issues of Concern' update presented in 2000 (AMAP, 2000), mercury was highlighted as an `issue of concern'. This report confirmed the presence of significant levels of mercury in the Arctic environment, and identified a mechanism by which elemental mercury, previously thought to be of little biological significance, is converted in the Arctic to a reactive form which can accumulate in biota. It also reported studies in the Arctic that documented subtle effects on infant neurodevelopment and blood pressure from exposure to mercury during pregnancy, implying that infants may be at risk for similar effects in several parts of the Arctic. “Noting with concern that releases of mercury have harmful effects on human health and may damage ecosystems of environmental and economic importance, including in the Arctic” the Arctic Council Ministers, in 2000, “called upon the United Nations Environment Program to initiate a global assessment of mercury that could form the basis for appropriate international action in which the Arctic States would participate actively”. Furthermore, they agreed to take a lead in addressing this issue in the Arctic countries, by initiating a project aimed at reducing the anthropogenic releases of mercury from these countries. The project was implemented under the Arctic Council Action Plan to Eliminate Pollution of the Arctic (ACAP) with Denmark as project co-ordinator. This report is part of the ACAP Mercury project. The objective of the report is to present and analyse compiled data on mercury releases from the Arctic countries, summarise existing initiatives to reduce mercury releases, and propose options for further release reductions. The report has been prepared primarily on the basis of the Arctic countries' responses to a questionnaire on mercury releases, uses and wastes. It should be noted that the data from the Russian Federation were extracted from the report "Assessment of Mercury Releases from the Russian Federation", also prepared as part of this ACAP project (ACAP 2004), because official response to the mercury questionnaire from the Russian Federation has not yet been received. In addition, relevant information from various other recent national and international compilations and studies has been included in the report preparation. This document presents mercury data which for some countries have been newly updated, partly inspired by - and prepared for the needs of - this study. For other countries, the submitted mercury data have been prepared and reported in other, previous inventories. All in all, the Arctic countries have explored virtually all options for mercury release reductions during the last three decades or more. There is a solid basis for mutual inspiration between them, and in a wider perspective, to other countries of the world. The release reduction options presented in this document are based on this common data base of experiences. This also implies that strategies or actions to pursue these options are already on the way in many of the countries. Though mercury releases from the Arctic countries are still of significance, the data of this report should be seen in the light of the mentioned reductions achieved in many of the countries. Examples of reductions achieved so far are given for a couple of countries in the description of their respective mercury reduction efforts in section 6 of the report. The questionnaire methodology has provided a thorough basis for the responding countries to enhance comparability and transparency. Naturally, however, the countries have used their available estimates as background data, and the methods for estimation of these existing data have been quite different. The same is likely the case with the handling of data uncertainty. Thus, when comparing national release data, conclusions should be drawn with some caution. Newer the less, the compilation of data from the Arctic States does provide very interesting indications of which sources are the major ones across the Arctic countries, as well as of similarities and differences in the national mercury release source patterns. A general introduction to mercury release sources and a conceptual model illustrating its cycling in the biosphere is given in section 3.1. 1.2 Summary of inventory resultsSection 3 presents the results of this inventory on an overview level. According to the reported release data, USA has the responsibility of managing by far the largest amounts of mercury to the atmosphere among the Arctic countries. The Russian Federation comes second, while reported atmospheric releases from Canada and the smaller reporting countries are considerably lower, see figure 1-1. In this national perspective, it must be remembered that large parts of all the countries are situated outside the Arctic region it self. This means that only parts of their reported mercury releases have direct, local influence in the Arctic. Yet, according to the current understanding, mercury has a high residence time in the atmosphere (once emitted), and is therefore transported hemispherically or even globally (UNEP, 2002). This means that mercury releases originating outside the Arctic region it self may have significant influence on the mercury loads in the region, and releases from the whole area of all the Arctic countries are therefore relevant in the Arctic perspective. Figure 1-1 Reported national atmospheric releases in metric tons/year (data from questionnaires and ACAP, 2004 of this study).*1
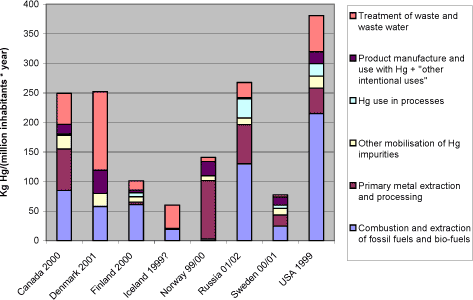
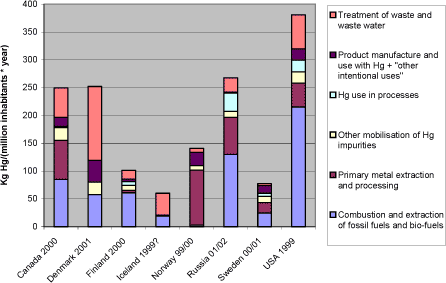
Notes: *1: Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Figure 1-2 shows, that in terms of atmospheric mercury releases per inhabitant, the countries are much more equal, yet some differences still appear. It should be noted that these figures may be quite vulnerable to uncertainties on estimates and differences in estimation methods between countries. Figure 1-2 Reported atmospheric releases in kg mercury/year per million inhabitants, by country (data from questionnaires and ACAP, 2004 of this study).*1
Notes: *1: Population and GDP data from CIA's World Fact Book (accessed in summer 2003 at: http://www.odci.gov/cia/publications/factbook/index.html). Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. The overall source categories contributing with largest atmospheric mercury releases are:
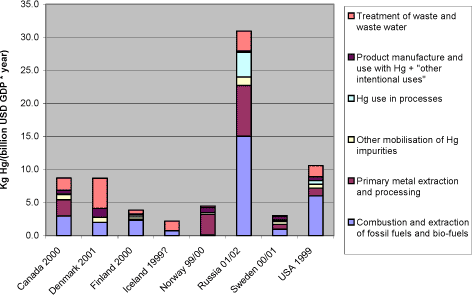
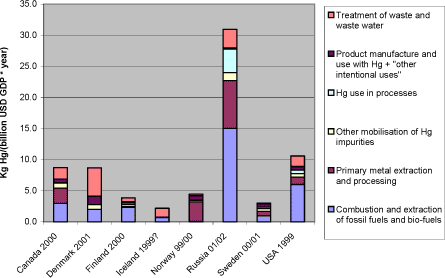
As an alternative normalisation basis, reported atmospheric mercury releases per GDP (Gross Domestic Product) in billion US Dollars are shown in figure 1-3. An important factor in this equation is of course the GDP per inhabitant ratios which are currently much lower in the Russian Federation than in the other Arctic countries, but are expected to rise in the near future. Figure 1-3 Reported atmospheric releases in kg mercury/year per GDP in billion US dollars, by country (data from questionnaires of this study).*1
Note: *1: Population and GDP data from CIA's World Fact Book (accessed in summer 2003 at: http://www.odci.gov/cia/publications/factbook/index.html). Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. 1.3 Discussion of major source typesIn section 4 the major source categories are discussed in more detail. For each category, the project data are analysed briefly, selected current release reduction initiatives are outlined, and possible options for further reductions are listed. Most of the options are well known in many of the Arctic countries, and are in many cases already under consideration, planning or implementation in one or more of the countries (see also section 6). This does, however, not make them less relevant in this presentation, as the results of this inventory confirms their relevance and stresses that the reduction measures should be pursued further, if mercury release reductions are wanted. Below, the suggested options for further mercury release reductions for each major source category are listed briefly. Here in the executive summary, it was chosen to focus on the suggested options; the background for the suggestions is given section 4. A broader overview of existing mercury release reduction plans/strategies in the Arctic Countries, national as well as regional, is given in section 6. 1.3.1 Combustion of coalAtmospheric emission reduction systems
Coal washing
Choice of energy sources
Reduction of energy consumption
1.3.2 Non-ferrous metal productionAtmospheric release reduction measures
Releases to other media, and releases from extraction residues
1.3.3 Waste treatmentThe following options could be considered as parts of an integrated approach:
1.4 Discussion of other selected sourcesMercury-based chlor-alkali production:
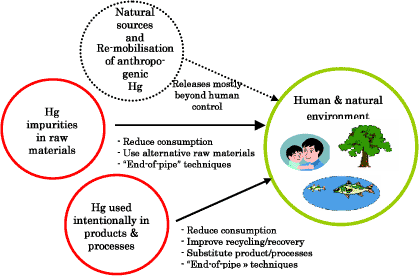
Mercury contamination from gold extraction in the Russian Federation Extensive mercury use through centuries and secondary gold extraction from tailings represent major environmental risks. Oil and gas extraction A significant source in some regions - warrants more attention in inventories and release control. Dental amalgam One of the last major product uses in the Arctic countries. Stronger incentives to consumers for choosing alternatives are needed. Laboratory chemicals An example of a mercury application where international co-operation could promote use of new mercury free standards. Development of a "Least essential uses" elimination procedure An option for enhancing substitution of uses where mercury-free alternatives are readily available. 2 Introduction2.1 Background 2.1 BackgroundMercury may be one of the best-documented hazardous substances utilised by man. Many of the world's countries have found the evidence of mercury's adverse effects substantial enough to take initiatives to guard against uncontrolled releases to the environment. International initiatives, however, may need to be strengthened in order to control the environmental effects of mercury. Mercury is a bio-accumulated and toxic metal that is of concern for both human and the environment. Mercury accumulates in biota such as fish and marine animals. This is of particular concern for the health of indigenous people in the Arctic who are highly dependent on food from the marine food web. Mercury is a volatile compound and emissions within and outside the Arctic can be sources for input of mercury to the Arctic states. For a summary of mercury's adverse effects on human health and the environment, see the Global Mercury Assessment (UNEP, 2002). The Arctic Monitoring and Assessment Program (AMAP) reports emphases the need for more knowledge about the sources, transport, fate and behaviour in the Arctic. Despite this need for more information the AMAP reports concludes that actions should be taken to reduce the anthropogenic input of mercury to the Arctic environment. Although sources for mercury pollution of the Arctic are not well known and important sources might be located outside the region, it is likely that sources within the Arctic states contribute significantly to the input of mercury to the Arctic environment. In terms of the ACAP criteria, the project is addressing a “common” (potential similarity in national problems) and “shared” problem (transboundary movement of heavy metals to the Arctic). The AMAP reports and other publications have drawn attention to the potential severity of mercury contamination in the Arctic and the linkages to sources within the Arctic as well as distant sources. There are clear and established concerns for human health, and this ACAP Mercury project is aimed at facilitating controls on mercury releases to the environment from Arctic countries. 2.2 Objectives and preparation2.2.1 Objectives of the ACAP mercury projectThe overall objective of the project is to contribute to reduce the atmospheric Mercury releases from Arctic states. The specific objective of the project is to identify important anthropogenic source categories for mercury emission within the Arctic region and to initiate cost effective reduction measures at one or a few specific sources or plants (in the Russian Federation) that could serve as pilot projects. 2.2.2 Questionnaire on mercury releases, wastes and usesA questionnaire on mercury releases, uses and wastes was prepared by Denmark and was submitted to the Arctic Countries' for their response. The questionnaire was designed to highlight all possible release pathways individually for each source category; including releases to the atmosphere, aquatic environments (water), terrestrial environments (soil), deposition in general or sector specific deposits, and outputs to marketed (by-) products. Though the atmospheric releases were in focus in the project, this was done because of a growing understanding that releases through other pathways may also be of significant importance, particularly in a longer time perspective. An important factor in the abatement of pollution with heavy metals and other highly persistent toxics is that once they are mobilised into the biosphere by humans, they are not degraded, but remain a threat to human health and the environment over long time spans. This is particularly the case for mercury, because of its potential to evaporate from wastes, land and materials, and thus be re-mobilised to potentially cause adverse impacts in the biosphere. The questionnaire can be seen in the appendices with submitted national responses. The mercury questionnaire was supplemented by a separate guideline for filling in the questionnaire. The guideline, or so-called introduction, gives additional definitions and advice on reporting national mercury releases into the questionnaire, in order to enhance comparability and transparency of the reported mercury data. The questionnaire introduction can be seen in the appendices to this report. 2.2.3 Preparation of this reportThis report is part of the ACAP Mercury project. The objective of the report is to present and analyse compiled data on mercury releases from the Arctic countries, summarise existing initiatives to reduce mercury releases, and propose options for further release reductions. This report has been prepared primarily on the basis of the Arctic countries' responses to the above mentioned questionnaire on mercury releases, uses and wastes. It should be noted that the mentioned data from the Russian Federation were extracted from the report "Assessment of Mercury Releases from the Russian Federation", also prepared as part of this ACAP project by ACAP, 2004, because official response to the mercury questionnaire from the Russian Federation has not yet been received. In addition, relevant information from various other recent national and international compilations and studies has been included in the report preparation. This document presents mercury data that for some countries have been newly updated, partly inspired by - and prepared for the needs of - this study. For other countries, the submitted mercury data have been prepared and reported in other, previous inventories. The data are analysed here in the light of the possibilities for comparison that a very detailed and specific data call allows, and in the perspective of the similarities, and in some cases clear differences, between the Arctic countries. All in all, the Arctic countries have explored virtually all options for mercury release reductions during the last three decades or more. There is a solid basis for mutual inspiration between them, and in a wider perspective, to other countries of the world. The release reduction options presented in this document are based on this common data base of experiences. This also implies that strategies or actions to pursue these options are already on the way in many of the countries. The questionnaire methodology has provided a thorough basis for the responding countries to enhance comparability and transparency, and the countries have known that this has been of high priority. Naturally, however, the countries have used their available estimates as background data, and the methods for estimation of these existing data have been quite different. The same is likely the case with the handling of data uncertainty. Only one country reported information on quantitative uncertainty on individual data - see the national overviews in section 3.3 and the questionnaire responses in appendices. Thus, when comparing national release data, conclusions should be drawn with some caution. Even though this report include perhaps some of the worlds best described countries as regards mercury flows and releases, it can not be ruled out that in some cases high reported mercury releases to some extend may reflect higher coverage of the release inventories made, rather than higher "true" values. One important factor may be whether releases from less well quantified sources are included (with high uncertainties) or simply not included in the inventories. For the figures presented in this report, an impression of possible significance of this factor can be made from studying the detailed reports given in the questionnaire responses in appendices to this report. The term "reported" data is used in this report to distinguish the available, reported data, from the "true" values of the data. This also includes the aspect that some releases may actually take place, but are not estimated or reported. Though this problem is inherent in all data inventories, it is often an important distinction for mercury, and one which is sometimes not mentioned in existing inventories. The numbers of significant digits in values presented in this report do not reflect the level of uncertainty on the presented values, except in cases where this has been incorporated by the countries themselves in their response to the mercury questionnaire. 3 Sources and releases of mercury from Arctic countries3.1 Introduction to releases and cycling of mercury 3.1 Introduction to releases and cycling of mercuryThe nature of mercury releases can be summarised as follows (extracted from the Global Mercury Assessment; UNEP, 2002). The releases of mercury to the biosphere can be grouped in four categories:
Figure 3.1 shows these release categories with main types of possible control mechanisms. Figure 3-1 Categorisation of sources of mercury releases to the environment and main control options.
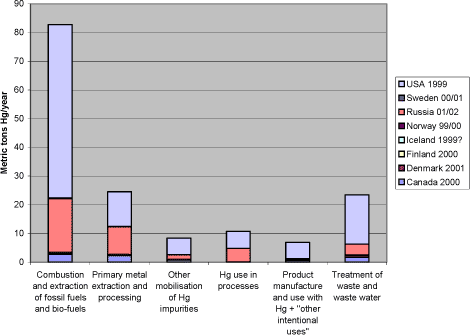
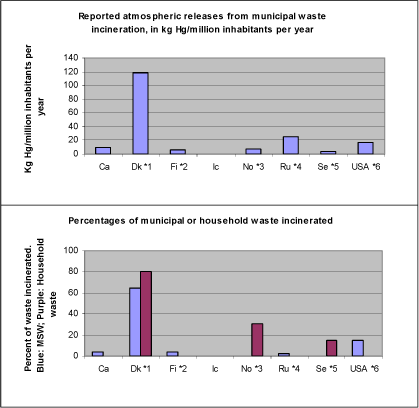
The recipients of mercury releases to the environment include the atmosphere, water environments (aquatic) and soil environments (terrestrial). There are continuing interactions – fluxes of mercury – between these compartments. Supplementary to figure 3.1, it can be mentioned that even for mercury releases already deposited in the environment, human activities such as water reservoir creation and land clearing, may result in enhanced bioavailability and re-mobilisation of the mercury present in the soil. Given the understanding of the global mercury cycle, current releases add to the global pool of mercury in the biosphere – mercury that is continuously mobilised, deposited on land and water surfaces, and re-mobilised. Being an element, mercury is persistent – it cannot be broken down to less toxic substances in the environment. The only long-term sinks for removal of mercury from the biosphere are deep-sea sediments and, to a certain extent, controlled landfills, in cases, where the mercury is physio-chemically immobilised and remains undisturbed by anthropogenic or natural activity (climatic and geological). This also implies that even as the anthropogenic releases of mercury are gradually eliminated, decreases in some mercury concentrations – and related environmental improvements – will occur only slowly, most likely over several decades or longer. However, improvements may occur more quickly in specific locations or regions that are largely impacted by local or regional sources." Figure 3.2 below shows a conceptual model of how mercury is cycling in the biosphere. The speciation of mercury, that is the chemical form mercury is present in, is an important factor in both the cycling and the adverse impacts of mercury. The species of mercury emitted to air vary among source types, and the further fate of the emitted mercury is also very dependent of the speciation. Mercury emitted as absorbed to particles, or in oxidised forms, will fall on land and water in the vicinity of the source (local to regional distances), while elemental mercury vapour can be transported with the air masses over hemispherical or global distances. These are important factors to consider when trying to establish links between releases and observed loads in the environment, for example when focusing on the impacts of specific sources to mercury pollution in a specific, vulnerable environment. Speciation is a key factor in the modelling of atmospheric transport and distribution of mercury emissions. The speciation of the mercury released is also a key factor in the efficiency of atmospheric emission reduction systems, mainly because particle-born and oxidised mercury is much easier to capture in these systems than elemental gaseous mercury. Also in the environment extensive transformation of mercury between the forms takes place. For example, while major parts of the mercury deposited on land may be oxidised, the re-emitted mercury from land is mainly in the elemental gaseous form. An important transformation process in aquatic environments (in water and wetlands) is the transformation in nature, of mercury released in elemental or oxidised forms, into methylmercury. Methylmercury is considered a main factor in normal human exposure, because it accumulates much easier in aquatic food webs than most other forms, and on top of this, it has stronger toxic impacts because it is more easily absorbed in vulnerable organs in humans and animals. Considerable research is ongoing regarding the transformation and cycling of mercury in the Polar Regions. Speciation plays an important part in the so-called polar "mercury sunrise", where large portions of the atmospheric mercury present is transformed, deposited and made bio-available within just a few months of the Polar sunrise. There are indications that this may perhaps result in and overall flux of atmospheric mercury to the Polar Regions, but evidence is still scarce. Figure 3-2 Conceptual model for the cycling of mercury in the biosphere. USA submitted speciated mercury release data for major mercury release point sources on their territory; see their response to the ACAP mercury questionnaire in appendices. For additional description of release and cycling principles, including mercury speciation in emissions from major release sectors and in the environment, see the Global Mercury Assessment (UNEP, 2002). 3.2 Releases to the atmosphere from the Arctic countriesWhile the quality and detail of the responses to the mercury questionnaire vary considerably, the reported atmospheric release data appear comprehensive and adequate for comparison between countries. 3.2.1 Breakdown of atmospheric releases by source category across countriesFigure 3-3 below shows a breakdown of the reported releases to the atmosphere on overview source categories across all the Arctic countries. The same data are presented in table 3-1. As regards direct releases to the atmosphere, the reported data confirm that combustion of carbon fuels, primarily coal, is the single largest mercury release source type in the region. The releases from this source category constitute about half (53%) of the total reported releases to the atmosphere from the Arctic countries. Coal combustion alone contributes with 44% (52.6 metric tons) of the total reported releases to the atmosphere from the eight Arctic countries(see table 3-4 below). This indicates that while some has already been done to reduce releases from coal combustion, it remains a potential for significant release reductions. The source of the mercury is natural mercury impurities in the coal. A breakdown of releases from this overall source group by individual source categories is given in table 3-4, section 3.2.3. The main source coal combustion is discussed further in section 4.1. The other two major overview source categories are primary metal extraction and waste treatment, respectively. The main contributions from the primary metal extraction sector are production of gold (mainly USA and Russia), zinc and zinc/copper (mainly Russia and Canada), and copper/nickel (mainly Russia) (based on: Questionnaires from this study; Environment Canada, 2002; ACAP 2004). The source of mercury inputs to this sector is natural mercury impurities in the used ore material. The same is the case for gold extraction, except for minor parts which originate from re-processing of tailings from previous gold extraction with the mercury amalgamation technology. Dedicated mercury mining does not take place anymore in any of the Arctic countries. The recorded releases from this sector do possibly not account for additional atmospheric releases from deposited extraction wastes, including flue gas cleaning residues (based on questionnaires in this study, Environment Canada (2002), and selected company release records at Canada's NPRI, 2003). The source category is discussed further in section 4.2. Regarding the reported releases from waste treatment activities, it is worth noting that the figures presented in figure 3-3 and table 3-1 mainly represent incineration of municipal waste and hazardous waste, while reported releases from recycling activities and "other waste treatment", including other sector specific industrial waste treatment, contribute only with minor releases in the reporting (see table 3.4). Disposal to waste treatment is generally the most important release pathway for products with intentional mercury use, such as dental fillings, thermometers, switches, light sources, measuring and control equipment. Even in countries like Denmark, which has a quite long history of reducing mercury inputs to waste, intentional mercury uses in products constitute a large part of the mercury inputs to municipal waste (UNEP, 2002; Skaarup et al., 2003). Some countries have reported small atmospheric releases from landfills/waste deposits, but the quantification of atmospheric releases from these sources should likely be considered under development and they may possibly be generally underestimated. As atmospheric emission reduction systems work by capturing mercury from exhaust air and transferring it to solid or liquid residues, significant amounts of mercury are continuously deposited with residues from these systems in all relevant sectors. Like mentioned for primary metal extraction, the presence of additional diffuse mercury releases from waste deposits can not be ruled out. The source category is discussed further in section 4.3. Figure 3-3 Breakdown of reported atmospheric mercury releases on overview source categories across the Arctic countries; metric tons Hg/year (data from questionnaires of this study and ACAP, 2004). *1.
Notes: *1: Note that the waste treatment category mainly represents incineration of municipal waste and hazardous waste. Industrial sector specific deposition is not included in this overview category. Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Table 3-1 Breakdown of atmospheric mercury releases on overview source categories across the Arctic countries; metric tons Hg/year. Figures and notes same as in figure 3-3.
3.2.2 Breakdown of atmospheric releases by countryWhen the data in table 3-1 are presented in a breakdown by country, the reported release pattern in figure 3-4 is displayed. As shown, USA represents by far the largest contributions of mercury in terms of national totals among the Arctic countries. The Russian Federation comes second, while reported atmospheric releases from Canada and the smaller reporting countries are considerably lower. In this national perspective, it must be remembered that large parts of all the countries are situated outside the Arctic region it self. This means that only parts of their reported mercury releases have direct, local influence in the Arctic. Yet, according to the current understanding, mercury has a high residence time in the atmosphere (once emitted), and is therefore transported hemispherically or even globally (UNEP, 2002). This means that mercury releases originating outside the Arctic region it self may have significant influence on the mercury loads in the region, and releases from the whole area of all the Arctic countries are therefore relevant in the Arctic perspective. For an overview presentation of mercury's global cycling, see the global Mercury Assessment (UNEP, 2002). Figure 3-4 Reported national atmospheric releases in metric tons/year (data from questionnaires of this study and ACAP, 2004).*1
Notes: *1: Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Figure 3-4 illustrates something about the shares of the atmospheric releases which can be controlled by individual countries. It does however not tell much about these countries' performance in management of mercury emissions. For a better understanding of this, figure 3-5 and table 3-2 show the reported atmospheric emissions as kg mercury released per million inhabitants. Figure 3-5 Reported atmospheric releases in kg mercury/year per million inhabitants, by country (data from questionnaires of this study).*1
Notes: *1: Population and GDP data from CIA's World Fact Book (accessed in summer 2003 at: http://www.odci.gov/cia/publications/factbook/index.html) . Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Figure 3-5 and table 3-2 show, that in terms of atmospheric mercury releases per inhabitant, the countries are much more equal, yet some differences still appear. It should be noted that these figures may be quite vulnerable to uncertainties on estimates and differences in estimation methods between countries. Combustion of carbon fuels is a large mercury release source in countries where coal plays a large role in power and heat production. A comparison between the countries' mercury releases from power production, national consumption of electricity, and the relative importance of coal in its production is given in section 4.1. There is a growing recognition that extraction of oil and natural gas is also a potentially significant source of mercury mobilisation in some regions of the world. In Russia for example, the quantifiable releases from processing and use of oil products is estimated at least 3.4 metric tons/year (see table 3-4), while the fate of most of the mercury mobilised by oil extraction (possibly about 40 metric tons/year) is unclear. The metal production sector is much more complex and diverse than coal combustion. Yet, the magnitude of reported atmospheric releases from primary metal extraction are quite equal for Canada, Norway, Russia and the USA (when seen on a per capita basis), but does of course cower differences in types and amounts of metals produced and mercury releases per product output. Again it is reflected that waste incineration plays a major part in reported mercury releases from waste treatment. The relation between mercury releases and dependence on incineration as a waste treatment option is discussed further in section 4.3. The source group "Direct releases from products and other intentional uses" include dental amalgam fillings, batteries, thermometers, manometers, blood pressure gauges and education, switches, relays and contacts, light sources and "other products and processes". This is the category in the questionnaire that includes all other releases from the turnover of products than releases from their treatment in waste systems. These other releases are diffuse and difficult to quantify, and the releases are often estimated with high uncertainties or simply omitted in inventories. Some of these uses still remain in trade, and even in countries were the sale of some of these products are prohibited or have decreased, they may remain in use and circulation in society for many years before they are disposed off. Being globally traded products indicating possible equal inputs per person on national markets except in cases of bans, the higher reported releases from this source group from Denmark and Norway could perhaps indicate that these sources were more thoroughly accounted fore in inventories from these countries. Again it should however be remembered that the major releases from these products happen in the waste treatment phase. Table 3-2 Reported atmospheric releases in kg mercury/year per million inhabitants, by country (data from questionnaires of this study and ACAP, 2004).*1
Note: 1*: Population and GDP data from CIA's World Fact Book (accessed in summer 2003 at: http://www.odci.gov/cia/publications/factbook/index.html) . Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. As an alternative normalisation basis, reported atmospheric mercury releases per GDP (Gross National Product) in billion US Dollars are shown in figure 3-6. The figure shows that in terms of mercury releases per national expenditure - partly a reflection of the material activity level - the Russian Federation lays substantially above the other Arctic countries. An important factor in this equation is of course the GDP per inhabitant ratios which are displayed in table 3-3. A firm interpretation of the figure is not easy, because the coupling of mercury turnover and releases, and an overall national economic summary figure like the GDP is not necessarily straight forward. But the figure could possibly indicate that the management of atmospheric mercury releases is less intensive in the Russian Federation than in the other Arctic countries. This may be useful to observe in the planning of mercury releases management, because the economic activity is expected to increase rapidly in the coming years. Figure 3-6 Reported atmospheric releases in kg mercury/year per GDP in billion US dollars, by country (data from questionnaires of this study).*1
Note: *1: Population and GDP data from CIA's World Fact Book (accessed in summer 2003 at: http://www.odci.gov/cia/publications/factbook/index.html). Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Table 3-3 Population and GDP data for Arctic Countries and related summary figures for reported atmospheric mercury releases.*1,2
Note: *1: Population and GDP data from CIA's World Fact Book (accessed in summer 2003 at: http://www.odci.gov/cia/publications/factbook/index.html). For definition of GDP, see the reference. *2: Reported mercury release data from questionnaires and (ACAP, 2004) of this study. 3.2.3 Reported atmospheric and aquatic releases - by source typesA detailed presentation of the reported national releases to the atmosphere is given in table 3-4 below. Selected main release contributions by country and source category are marked in bold. For details on data sources, uncertainties, background data etc. see the questionnaire responses in appendices and (ACAP, 2004). A detailed presentation of the reported national releases to aquatic environments is given in table 3-5 below. These releases are less well documented than atmospheric releases, and have therefore not been included in the analysis to the same degree. The table shows that reported mercury releases to aquatic environments are dominated by the releases from waste water treatment. This indicates that the main source is intentional mercury use in products and processes. For details on data sources, uncertainties, background data etc. see the questionnaire responses in appendices and (ACAP, 2004). For data on releases to other media, see the national overview tables in section 3.3 and questionnaire responses and (ACAP, 2004) in appendices. Table 3-4 Overleaf: Detailed presentation of the reported national releases to the atmosphere in the mentioned years *1; metric tons Hg/year. See the questionnaire responses in appendices and (ACAP, 2004) for information on data sources, estimate uncertainties etc.
Note to table 3-4: *1: Data from Norway on dental amalgam fillings and extraction and use of oil, gas and biofuels are from 1999. For Sweden all reported data with value other than cero are from 2001, other reported data (0's) are reported as for 2000. Data for Russia are for 2001/2002. *2: Releases from coal combustion in Iceland roughly estimated here based on consumption data submitted by Iceland (150,000 metric tons coal/year) and the emission factor for coal in Denmark (0.04 g Hg/ton coal; see table 4.3). Table 3-5 (Overleaf) Detailed presentation of the reported national releases to aquatic environments in the mentioned years; metric tons Hg/year. See the questionnaire responses in appendices and (ACAP, 2004) for information on data sources, estimate uncertainties etc.
3.3 National overviews of reported dataBelow, overviews of reported corresponding inputs to, and releases from, mercury source types present in the countries to all media are presented, including to air, water, soil, waste treatment/deposition, waste water treatment and by-products. Comprehensive reporting on all or most of these media were only submitted by Denmark, Finland and USA. Source types which are clearly not present in the country are not shown in the overviews. For details, data background and remarks, please see the questionnaire responses in appendices. For the Russian Federation, see (ACAP, 2004) also in appdices. Note that some countries have used other notations for data "not available", "not relevant", "not answered" and "existing but almost zero", than recommended in the introduction to the questionnaire. Not all such deviations seem clear in the questionnaire responses. "Inputs to the biosphere" is used here as a common designation for intentional consumption of mercury and mobilisation of mercury impurities. Table 3-6 Reported mercury inputs and outputs to all media in Denmark, 2001; metric tons mercury/year. For details on estimation and uncertainties, see the questionnaire response in the appendix. Table 3-7 Reported mercury inputs and outputs to all media in Finland, 2000; metric tons mercury/year. For details on estimation, see the questionnaire response in the appendix*1. Table 3-8 Reported mercury outputs to media, for which data were available, in USA, 1999(air)/ 2001(other); metric tons mercury/year. For a brief description of estimation methods, see appendices. Mercury consumption data for the USA in the mid 1990s are available in for example (USEPA, 1997) and (Sznopek and Goonan, 2000). Table 3-9 Reported mercury outputs to all media in Norway, 1999/2000; metric tons mercury/year. 1999 data on intentional mercury consumption in Norway can be found in (Maag et al. 2002). Some overview data on mercury in wastes, including metal extraction waste, can be found in (Huse et al. 2000). Table 3-10 Reported mercury outputs to all media in Canada, 2000; metric tons mercury/year. Table 3-11 Reported mercury outputs in Sweden, 2000/2001; metric tons mercury/year.
Data for Sweden's intentional mercury consumption can be found in (KEMI, 1998; summary also cited in UNEP, 2002). Some overview data on mercury in wastes, including metal extraction waste, can be found in Huse et al. 2000. Table 3-12 Reported mercury outputs to media, for which data were available, in the Russian Federation, 2001/2002; metric tons mercury/year. For description of estimation methods, see (ACAP, 2004), appendices.
Notes to table 3-12: *1: Mobilisation figure includes all coal use, that is, also on smaller plants, for coke production etc. 3.3.1 Submitted data from IcelandIceland submitted the data mentioned below. Estimated anthropogenic inputs to the environment from Iceland: No industry of concern because of Mercury in Iceland. The non-ferrous metals industry found is Al and FeSi industry, not of concern because of Mercury. The main potential sources of mercury in the aquatic environment are: Fossil fuel combustion; cement production; use of mineral phosphate fertiliser; sewage effluent; effluent from waste incinerators; releases from landfill. Atmospheric releases Ca. 570,000 metric tons of gasoline and diesel oil and ca. 150,000 metric tons of coal [1] and charcoal fossil fuel is used in Iceland annually. Air emission from Cement production (particle bound): 0.47 kg Hg / yr (250d /yr). Estimated emission from waste incinerators 11 kg Hg / yr (1999) Aquatic releases Estimated in sewage effluent is 31 kg /yr Hg (2001). Estimated release from landfill is < 0.11 kg Hg / yr (1999) Direct releases to land 2.400 metric tons of phosphate used in 1999, content of Hg is not known. Import of metal mercury Import of Mercury metal in 2001 was 8 kg and 180 kg of silveramalgam (Hg content ca. 90 kg Hg). 3.4 Mercury consumption and mobilisationReported information on mercury consumption and mobilisation in questionnaire responses is limited and of varying quality. Therefore, no individual presentation of this issue is given in this report. The issue is however dealt with as an integrated part of the discussion of source types in section 4, and consumption and mobilisation data, if reported, are summarised in the total reported releases overviews in section 3.3 above. Trade statistics of mercury and its compounds (which do not include mercury traded as part of products), and details on consumption and mobilisation data from the countries which reported them, are given in the questionnaire responses in appendices and in (ACAP, 2004). 4 Discussion of major source categories4.1 Combustion of carbon fuels In this section the major source categories are discussed in more detail. For each category, the project data are analysed briefly, selected current release reduction initiatives are outlined, and possible options for further reductions are listed. Most of the options are well known in many of the Arctic countries, and are in many cases already under consideration, planning or implementation in one or more of the countries. This does, however, not make them less relevant in this presentation, as the results of this inventory confirms their relevance and stresses that the reduction measures should be pursued further, if mercury release reductions are wanted. Note that a broader overview of existing mercury release reduction plans/strategies in the Arctic Countries is given in section 6. 4.1 Combustion of carbon fuels4.1.1 Analysis of mercury releases from combustion of carbon fuelsAs shown in table 3-4, coal combustion remains the largest single atmospheric mercury release source in the Arctic countries. Contributions from the use of carbon fuels are recalled in table 4-1. The magnitude of mercury release from this source category is closely related to the consumption of electricity, the role of coal as a dominating fuel type, and the suitability of applied emission reduction systems for mercury retention in the exhaust gases from power production and other major coal consuming sectors. The atmospheric releases from the 5 largest coal combustion point sources (power plants) reported from the Arctic countries amounted to The 5 coal combustion facilities (power plants) emitting most mercury in the Arctic countries released about 0.8 metric tons/ year each (on average). Together, the 5 largest point sources emit about 3% of the total reported atmospheric releases from the Arctic countries. The largest mercury releasing point sources in each country are reported in the mercury questionnaires; see the questionnaires in appendices for detailed information on reported point sources. Table 4-1 Reported atmospheric mercury releases from combustion and extraction of fossil fuels and bio-fuels (extracts from table 3-4); metric tons/year.
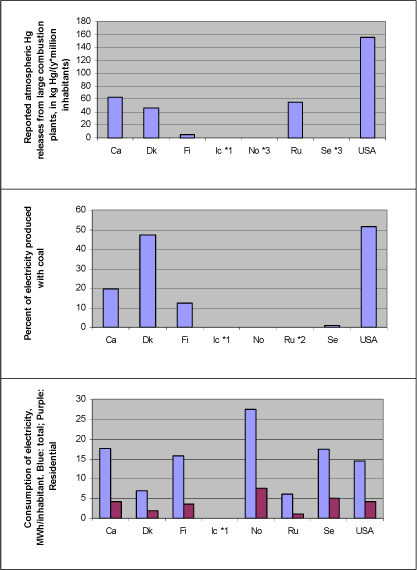
Electricity production with coal combustion Figure 4-1 illustrates reported atmospheric mercury releases from large power plants as related to percentage of produced electricity based on coal combustion. Additionally, consumption of electricity per inhabitant is shown, in total and for residential consumption only (the last thing as a possible indicator of personal use pattern, independent of industry use). The mercury release data are from the questionnaire responses and (ACAP, 2004), and all energy data are from the International Energy Agency's (IEA) reports "Energy Statistics of OECD Countries" and "Energy Statistics of Non-OECD Countries", both 2003 editions. The same data are presented in table 4-2. Note that all figures are subject to uncertainty and should be interpreted with caution. Uncertainties for release estimates from Denmark are presented in the Danish questionnaire response in appendices. No other countries reported quantitative uncertainties for submitted mercury data. Figure 4-1 Relations between reported atmospheric mercury releases from large power plants, dependence on coal for production of electricity, and consumption of electricity - total as well as residential only (Hg data from questionnaire responses and (ACAP, 2004); energy data from IEA, 2003 and 2003b)*4.
Notes: *1: Hg data from Iceland on coal does allow analysis of this aspect. *2: Data on percentage of electricity produced with coal from Yanin (2003). *3: Hg releases from small coal based power plants in Norway and Sweden were reported in another category, and are not included here (marginal releases) *4: Energy data for same year as reported Hg releases, except for USA for which reported atmospheric releases were from 1999, other releases for 2001, and energy data were for 2000. Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Table 4-2 Relations between reported atmospheric mercury releases from large power plants, dependence on coal for production of electricity, and consumption of electricity - total as well as residential only (Hg data from questionnaire responses and (ACAP, 2004); energy data from IEA, 2003a and 2003b)*4. Same data as in figure 4-1.
Notes: *1: Hg data from Iceland on coal did not allow analysis of this aspect. *2: Data on percentage of electricity produced with coal were not available for Russia. *3: Hg releases from small coal based power plants in Norway and Sweden were reported in another category, and are not included here (marginal releases). *4: Energy data for same year as reported Hg releases, except for USA for which reported atmospheric releases were from 1999, other releases for 2001, and energy data were for 2000 . Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Figure 4-1 and table 4-1 illustrate the following:
Of course any of the mentioned options for mercury release reductions have other adverse and positive effects that have to be taken into account. These include other types of environmental impacts, depletion of resources, and potential limitations on economic activity, among others. Other possible measures for mercury release reduction are mentioned below. National emission factors for coal use Another indicator for release reduction performance is the emission factor of mercury release per tonnage of coal used. Atmospheric mercury releases reported in the questionnaire responses are compared to national coal consumption data in table 4-3. For this comparison, mercury releases from large coal combustion plants (see definitions in the introduction to the ACAP mercury questionnaire in appendices) and other coal combustion/use were summed up (see table 4-3) and divided by the total consumption of coal of all types as reported by IEA (2003a; 2003b; for coal types included see table notes). For Denmark, Finland and USA, the same calculation was made for total reported mercury releases/outputs from coal use to all environmental media and by-products (includes Hg in deposition of residues and Hg in by-products for Denmark and Finland, but not for USA). The resulting calculated emission factors are shown in table 4-3. As shown, the calculated emission factors fall in the same range for all countries (except Norway) and are in line with emission factors for large power plants presented in the European EMEP/CORINAIR Emission Inventory Guidebook (2002) and by USEPA in AP 4-2 (1998). The calculated emission factors are, however, deemed too weakly documented here to allow strong comparisons between countries. The main confounding factor may be consumption of coal in sectors, for which mercury releases may not have been reported as assigned to coal use, even though coal contributed to the mercury outputs from these sectors. Coal related releases reported in this way do not contribute to the release category "other coal uses" included in the calculations, resulting in calculated emission factors which are lower than the true values. Probably, an important example of this could be coal (and coke) use in metallurgical production. This error could be of most significance for countries with relatively large consumption of coal in industries (this could be part of the reason for the deviation for Norway). Additionally, the reported releases may be associated with substantial uncertainties. For example for Denmark, the only country reporting uncertainties on estimates, the rounded "best estimate" value of 0.3 metric tons mercury released, represents estimated releases to the atmosphere of 0.19-0.31 metric tons/year (calculations were based on non-rounded mean of the range). The uncertainties on the presented calculated emission factors could be minimised by further analysis of existing data. Table 4-3 Reported releases from coal use, to the atmosphere and to all media, respectively, coal consumption data, and roughly calculated emission factors. See discussion in text (Hg data from questionnaire responses and ACAP, 2004; coal consumption data from IEA, 2003 and 2003b). Cross media transfers A part of the picture, which is not reflected in the atmospheric release figures, is the deposition of solid exhaust gas cleaning residues and marketing of by-products with contents of mercury, as well as minor direct releases to aquatic environments. As indicated by the data for Denmark, Finland and the USA in table 4-3, the total outputs of mercury from coal combustion quite likely add up to about the double of the direct atmospheric releases. Secondary atmospheric and aquatic releases from residue deposits and by-products most likely add to the direct releases to these media, though this is a poorly understood and possibly underestimated source type. The only way to reduce total mercury outputs to all media, is to reduce coal consumption by reductions of energy demands, or by substituting for coal with less polluting energy production methods or fuels. 4.1.2 State of mercury release reductions from coal combustion in Arctic countriesEmission reduction systems Currently, release reduction systems retaining parts of the mercury inputs to power plants, are in place on a number of the larger plants in the Arctic countries. These are dust (PM) controls in combination with flue gas desulphurisation ("FGD")/de-nitrification. Existing FGD/PM systems demonstrate very varying efficiency in mercury retention (0-90%), but average retention rates generally fall in the interval of 30-80% of the mercury input, depending principally on coal types fired and FGD systems applied (Pacyna and Pacyna, 2000). An important feature of these systems are their potential for reductions of atmospheric releases of a number of priority pollutants, most particularly acidic and eutrophisating gasses and other heavy metals than mercury. Still, many large power plants in the Arctic countries only have dust controls. For example, about 75% of the power plants in USA currently have PM retention only. Generally dust controls are not considered efficient for mercury retention because of mercury's volatility and its tendency to exist as elemental mercury vapour in the combustion gasses. In a few recent examples, highly efficient fabric filters and electrostatic precipitators have, however, exhibited good mercury retention under specific conditions favouring oxidation and adsorption of mercury on particulate material (USEPA, 2002). Coal washing prior to combustion is used some places in the USA, and in Russia prior to coke production. The washing reduces sulphur contents in the coal, and also removes some of the mercury contents (on average about 20%). This technology reduces mercury inputs to the combustion processes, but transfers the mercury to liquid or solid residues which have to be managed properly to avoid releases. Coal substitution and reduction of energy consumption As mentioned above, particularly Norway and Sweden have very limited mercury releases from coal combustion because they rely on hydropower and nuclear power. Denmark has shifted some of its electricity production from coal to natural gas and wind power during the last decades, resulting in a drop in releases of mercury and other pollutants (Skaarup et al, 2003; Danish Energy Authority, 2003). Additionally, Denmark, and possibly also other Arctic countries, has attempted to reduce, or at least stabilise, energy consumption by awareness raising activities, implementation of cleaner technology, introduction of CO2-taxes etc. These initiatives have had good results in Denmark, showing quite stabile energy consumption in spite of economical growth (Danish Energy Authority, 2003). Reductions of CO2 releases as aimed at in the Kyoto Protocol of the Framework Convention on Climate Change may also have reducing effects on mercury releases due to its direct links to consumption of carbon fuels, of which coal is a dominating part. As of November 2003, the Kyoto protocol has been ratified by Canada, Denmark, Finland, Norway and Sweden, whereas Iceland is in accession (per 25/05/2002) and Russia has only signed the protocol so far (UNFCC, 2003). The government of USA has announced that it does not intend to ratify the protocol. 4.1.3 Options for further release reductions for coal combustionFor the majority of the coal consumption in the Arctic countries, the atmospheric mercury releases are relatively well documented and monitored, and strategy development and implementation for release reduction is ongoing (UNEP, 2002; USEPA-ORD, 2000; Skårup et al, 2003). Here, the speed and degree of implementation appears to be largely a question of political and financial priorities. As regards the situation in Russia, documentation, strategies and implementation are among the issues of this ACAP project, as well as in other on-going projects (Munthe et al., 2003; Pacyna, 2003; and possibly others). Atmospheric emission reduction systems These technologies transfer input mercury from air emissions to deposition/landfilling and releases to other media. A possible first step is to implement flue gas desulphurization (FGD) on the facilities that are only equipped with particle filters today. Such a step would reduce releases of several priority pollutants, including some reduction of mercury releases to the atmosphere. For recommendations as regards types of technologies see (USEPA, 2002). To further reduce atmospheric mercury releases, implementation of flue gas cleaning systems optimised for mercury capture will be an option in the near future. Such systems (for utility boilers) with higher retention rates, are under development (USEPA, 2002 and USEPA-ORD, 2004). Such systems can involve injection in the flue gas of sorbents capturing mercury and/or oxidising agents, which convert elemental mercury to oxidised forms that are better captured in particle filters and FGD systems. Also the so-called selective catalytic reduction (SRC) processes used for NOx-reduction may enhance mercury oxidation for some coal types. See detailed recommendations and cost estimates for a range of mercury emission reduction techniques in (USEPA, 2002). Another evolving technique is the addition of fixed carbon filters downstream to other flue gas cleaning systems. For the situation in the USA, USEPA-ORD (2004) projects the potentials shown in table 4.4 for different mercury release reductions options for coal fired power plants. The potentials and the time frames are based on the assumption that aggressive research, development and demonstration will be implemented. Table 4-4 Research, development and demonstration (RD&D) goals in the USA for projected cost-effective mercury removal capability (% of Hg input to combustion plant) for key coal type/control technology combinations, as projected by USEPA-ORD (2004), for detailing on background see the reference.
Coal washing Coal washing also transfers input mercury from air emissions to deposition/landfilling and releases to other media. In principle, a wider implementation of coal washing could reduce the atmospheric mercury releases from coal combustion. Like for other emission reduction systems this technique requires careful management of washing water and residues to avoid secondary mercury releases. Choice of energy sources As mentioned above, switching to other energy such as natural gas or renewable energy sources could reduce the mercury releases to all media. This would also imply reduced expenses for management of solid and liquid residues, because the total input of mercury is reduced. Mercury concentrations in coal vary, both between seams and within the same seam. Therefore, in principle, it could perhaps in some cases be possible to reduce mercury emissions by selecting coal with low mercury contents. Besides some practical problems that such an approach might imply, a main concern in this scenario is however that cheaper high mercury coal could be attractive for countries with poorer emission reduction systems, potentially resulting in a worse situation locally and no improvement globally. Reduction of energy consumption Reduction of energy consumption would lead to direct cuts in mercury releases to all media. From other research fields it is indicated that there is large potential for energy savings by a combination of implementation of more energy efficient appliances (light sources, motors, electronics etc.) and awareness raising activities and other incentives. 4.2 Primary metal extraction4.2.1 Analysis of mercury releases from primary metal extractionNon-ferrous metal extraction mobilises significant amounts of mercury due to it's extensive turnover of materials, its high operating temperatures and the fact that several metals are primarily produced from sulphidic ore with naturally elevated mercury concentration (gold, zinc, lead and copper ore). The sector is not well described as regards mercury releases to other media than the atmosphere, and total mobilisation (input) of mercury. The 5 non-ferrous metal extraction facilities emitting most mercury in the Arctic countries released more than 1 metric ton/year each, while the single largest point source in this category releases more than 6 metric tons/year. Together, the 5 largest point sources emit about 7% of the total reported atmospheric releases from the Arctic countries. The largest mercury releasing point sources in each country are reported in the mercury questionnaires; see the questionnaires in appendices for detailed information on reported point sources. For the sector as such, most of the mobilised mercury is probably deposited on site or sold as by-product mercury. The major part of the recorded releases are emissions to the atmosphere, while minor releases to water and land are also recorded. Generally, mercury in extraction residues is not well unaccounted for in publicly available literature; some data for the Nordic countries are however given in (Huse et al., 1999), the questionnaire responses and (ACAP, 2004). As mentioned in section 3.2, the main contributions from the primary metal extraction sector in the Arctic countries are production of gold (mainly USA and Russia), zinc and zinc/copper (mainly Russia and Canada), and copper and copper/nickel (mainly Russia) (based on: Questionnaires from this study; Environment Canada, 2002; ACAP, 2004). As shown in table 3.4, the largest atmospheric mercury releases are reported for USA (12 metric tons/year), Russia (9.6 metric tons/year) and Canada (2.3 metric tons/year). Table 4-5 show, that as regards both atmospheric and total mercury releases in USA, gold extraction contribute with the largest releases, with copper and "other metals" (including zinc [3]) coming up next. Note particularly the reported releases from gold extraction to soil. To avoid misunderstandings, it should be mentioned that this mercury originates from mercury naturally present in the gold ore; the mercury amalgamation method used for artisanal (small scale) gold mining is not applied professionally in USA anymore. For Russia the largest contributions to atmospheric mercury releases are from nickel/copper extraction (5.3 metric tons/year) and zinc extraction (1.9 metric tons/year), while total mobilisation of mercury are roughly equal for zinc/lead extraction and nickel/copper extraction (estimated at app. 31 and 28 metric tons/year, respectively) (ACAP, 2004). It should be noted that these release estimates have not been confirmed with measurements. Gold extraction is also a significant mercury release source in Russia, and here a special factor is involved: The secondary gold extraction of older tailings containing large amounts of mercury from earlier extraction with the amalgamation method. This issue may warrant special attention. Gold extraction with the amalgamation method is illegal now in Russia, but may still take place (ACAP, 2004). For Canada, most of the atmospheric mercury releases from the sector are attributed to co-production of several non-ferrous metals in the questionnaire response (1.9 metric tons/year), while "other metals" contribute with 0.3 metric tons/year. A comprehensive report on emissions and pollution abatement in the sector describe that most of the atmospheric releases from this sector in Canada occur at a single combined copper/zinc smelter with less extensive emission reduction equipment on the copper production line (Environment Canada, 2002). Reported data on releases to other media and outputs are limited for this sector in the questionnaire response (and in the described report). Table 4-5 Reported mercury release data on primary non-ferrous metal production in USA (selection from table 3-8; data from questionnaire response from USA). Ferrous metal extraction Ferrous metal extraction also release mercury, but, as regards atmospheric emissions, generally in smaller amounts than from extraction of the dominant non-ferrous metals (Pacyna and Pacyna, 2000, ACAP, 2004). For Russia, the atmospheric releases from iron and steel production are estimated at 1.4 metric tons/year (ACAP, 2004). 4.2.2 State of release reductions in primary metal extraction in Arctic countriesFor non-ferrous metal extraction from sulphidic ore concentrates, the most important single factor influencing retention of atmospheric mercury releases is the presence of specific mercury removal steps in the exhaust gas lines. Sulphidic ore types are the most important virgin raw material for many of the non-ferrous metals (a major exception is aluminium). In the Arctic countries, such mercury removal steps appear to be present in most of the extraction facilities applying roasting, sintering and/or smelting of input ore (the process steps releasing most of the mercury present in the concentrates); (Environment Canada, 2002; European Commission, 2001; ACAP, 2004). The presence of a mercury removal step is likely partly driven by the technical need to purify the gases prior to the conversion of sulphur dioxide gases to sulphuric acid, and mercury removal is found in most extraction plants which are equipped with acid plants. The presence of a dedicated mercury removal step influences the distribution between output pathways considerably. Releases to the atmosphere are converted to marketed by-product outputs (mercury and its compounds) and releases to waste deposition, land and water. In case sulphuric acid is produced, mercury releases to sulphuric acid (a marketed by-product) will also be converted to other output pathways, if a mercury removal step is present. Most or all non-ferrous metal extraction plants applying heating in the initial process steps also have exhaust gas particle filters (cyclones, wet scrubbers, ESPs and/or fabric filters), which may also reduce atmospheric mercury releases somewhat and convert the retained parts of the mercury to solid, suspended and/or liquid residues. Particle filters generally only have limited retention efficiencies on mercury, because major parts exist as elemental mercury gas in the exhaust gasses. Some non-ferrous metal extraction plants in the Arctic countries employ so-called direct leach processes, in which the sulphur contents (and mercury with it) are not driven of the concentrates with high temperature processes prior to extraction in aquatic solutions/suspensions. With direct leaching, most of the mercury follows the wet extraction residues, of which some are treated to extract the marketable mercury, and which require careful handling to avoid further releases. Extraction plants employing direct leaching may have very limited atmospheric mercury releases, as is for example the case in Finland (Finnish response to questionnaire and Fugleberg, 1999). Waste waters from different process steps can contain mercury and must be treated carefully to avoid or minimise releases to aquatic environments. Release reductions in the Canadian base metal sector As an example, significant reductions of atmospheric mercury releases have taken place in Canada through reductions efforts over the last 15 years (or more). Atmospheric mercury releases dropped from 27 metric tons/year in 1988, to 10 metric tons in 1993 and 2 metric tons in 2000 (Environment Canada, 2002). Mercury releases from waste rock and tailings The waste rock and tailings from non-ferrous metal extraction may - just like the produced concentrates contain trace amounts of mercury. This material is much more susceptible to weathering due to the reduced particle sizes and higher accessibility for air and precipitation. For sulphicid ores, this weathering liberates and oxidizes the contained sulphur and produce sulphuric acid. The acid renders mercury and other constituents more soluble and thus increases leaching of the metal to the environment manifold as compared to the untouched mineral deposit. This process is called "acid rock drainage" (or ARD) and is considered a serious environment risk (European Commission, 2003). The questionnaire responses of this study give a few data on releases to soil which most likely relate to extraction residues. It is not known if reported releases to air and water include secondary releases from extraction residues. Otherwise, quantitative data on release of mercury from waste rock and mining tailings to air, water and land has not been identified in recent data compilations. This release source could potentially be significant, because even moderate mercury concentrations in the material may render substantial mercury amounts mobile because of the enormous amounts of materials handled in mining operations. 4.2.3 Options for further release reductions in the primary metal extraction sectorAtmospheric release reduction measures As primary metal extraction contributes with 25 out of a total of 157 metric tons of reported mercury releases to the atmosphere per year in the Arctic countries, further release reductions may be necessary in this sector if overall reductions are desired. As regards atmospheric releases, a general recommendation would be to raise the remaining facilities to the emission retention levels attained in many facilities today ("best practices"/best available technologies). Such actions would include, among others, establishing high efficiency mercury removal steps in all facilities, or convert production to the direct leach process. All such improvements could be based on existing, industrially mature technologies. Detailed recommendations are presented in for example the report "Multi-pollutant Emission Reduction Analysis Foundation (MERAF) for the Base Metal Smelting Sector" (includes also examples of economic estimates for reduction actions; Environment Canada, 2002), and in "Integrated pollution prevention and control (IPPC) - Reference document on best available techniques in the non ferrous metals industry" (European Commission, 2001). Both reports are available on the Internet (see the reference list for web-links). Releases to other media, and releases from extraction residues From publicly available literature, it could appear as if mercury releases from primary metal extraction to other media than the atmosphere may be less in focus as regards release reductions. If this is the case, it may be a field where further release reductions could be attained. This also includes management of extraction residues. It seams that secondary releases of mercury to all media - also the atmosphere - from deposition of extraction residues are often not accounted for or described with any detail. Secondary gold extraction from old amalgam tailings in Russia Because of the potentially large amounts of mercury involved and the risks of mobilising mercury while disturbing (excavating etc.) the tailings deposits, prudent precautions should made to avoid provoking substantial mercury releases to all media. A deeper analysis of this problem is not possible for this report. Improvement of mercury data base to enhance management possibilities Improvements to the data base on releases to other media than the atmosphere, as well as releases to all media from extraction residues, seams warranted in order to enhance possibilities for quantifying and managing these releases in a national and global perspective, as well as improving the basis for quantifying the relative importance of other mercury release sources for management purposes; preferably on a mass balance basis describing dependent inputs and outputs of mercury with all fluxes/pathways. Reduction of mercury inputs Mercury concentrations in non-ferrous metal ores and concentrates vary considerably. Therefore, in principle, it should be possible to reduce mercury emissions by selecting raw materials with low mercury contents. In practice however, it may be difficult, and there is also a risk of such a scenario, that cheaper high mercury concentrates are attractive for industry in countries with poorer emission reduction systems and poorer regulation, potentially resulting in a worse situation locally and no improvement globally. 4.3 Waste treatmentAs shown in table 3-4 in section 3.2.3, incineration of municipal waste and hazardous/medical wastes are large sources to atmospheric mercury releases within this category. As regards releases to other media, Denmark, Finland and USA has submitted the most comprehensive data sets. When considering reported data for all media (tables 3-5 to 3-7), waste water systems is also a major source in these countries. Other significant release categories, when considering reported data across all media, are the mixed categories "other waste treatment" (including in questionnaire responses for example "other incinerators", switches, electronics, contaminated soil) and "recycling of other materials" (including for example steel and non-ferrous metal recycling). As a whole, these source types include all or most of the mercury flow through society with products (consumer and industrial products); both products where mercury is used intentionally, and high volume products with trace concentrations of mercury. The 4 waste incineration facilities emitting most mercury in the Arctic countries released more than 0.7 metric ton/year each (on average). Together, the 4 largest point sources emit about 2% of the total reported atmospheric releases from the Arctic countries. The largest mercury releasing point sources in each country are reported in the mercury questionnaires; see the questionnaires in appendices for detailed information on reported point sources. Chlor-alkali production with mercury technology poses special problems as regards mercury containing waste; see section 4.4 on this issue. 4.3.1 Analysis of mercury releases from waste treatmentReported atmospheric mercury releases from municipal waste incineration per million inhabitants, and percent of general waste types incinerated are shown in figure 4-2 and table 4-6 below. Note that the percentage data have different basis according to available knowledge of waste types included. The figures show the expected relationship between dependency on waste incineration and mercury releases - high volumes of waste incinerated yield high mercury releases. Because of the modest efficiency for mercury retention of most emission reduction systems currently applied, this affect the atmospheric release figures directly. Denmark has rather high mercury releases per inhabitant from waste incineration in spite of decades of separate collection of mercury-containing waste. Reported mercury releases versus incinerated waste amounts are described further below. Table 4-6 also show reported releases from incineration of hazardous/medical waste, because this also describe aspects of how much mercury is flowing through society in products, and how this mercury is handled in the national waste treatment setups. Procedures of collection and treatment of hazardous waste influence the fate of mercury outputs from society's product usage quite strongly. As for the Danish situation for example, collection of waste products with high mercury contents (dental amalgam, thermometers, button cell batteries, manometers, blood pressure gauges etc.) has been going on for some decades, and the collected mercury waste has been recycled or deposited under special conditions. Yet, un-collected mercury-containing waste still follows other general waste to incineration due to the general waste handling priorities. In comparison, mercury releases from incineration of hazardous/medical waste seem to be relatively high in Canada and the USA. Figure 4-2 Reported atmospheric mercury releases from municipal waste incineration (in kg Hg/million inhabitants), and percent of general waste types incinerated. Note that the percentage data have different basis according to available knowledge of waste types included (see table notes below).
Notes: Please see identical notes to table 4-6. Table 4-6 Reported atmospheric mercury releases from municipal waste incineration (in kg Hg/million inhabitants), and percent of general waste types incinerated. Note that the percentage data have different basis according to available knowledge of waste types included (see table notes).*7.
Notes: *1: Percentage for MSW based on total waste amounts minus recycled construction wastes and composted gardening wastes (DEPA, 2003). Percentage for household wastes also from (DEPA, 2003). Uncertainties on atm. releases +/- 60% of mean (questionnaire response from DK). *2: Releases were unusually high in 2000; mean releases 1995-2001 was about 1/3. Release estimates are worst case and do not take effects of emission reduction systems into account (Questionnaire response from FI). *3: Percentage based on numbers for household wastes from 2002 (Sleire, 2003). *4: Based on (ACAP, 2004); uncertainty on waste definitions for percentage number. *5: Percentages are based on household waste data from (RFV, 2003). *6: Based on waste data from (Durkee, 2003) and waste definitions from www.epa.org/osw. *7: Comparisons among totals reported for the different countries should be made with caution, since the reported data for each country have differing levels of associated uncertainty, see section 2.2.3. Most of the remaining product wastes from the Arctic countries' societies are landfilled (Skaarup et al., 2003; Questionnaire response from FI; Sleire, 2003; ACAP, 2004; RFV, 2003; Durkee, 2003). Figures on reported current mercury releases from landfills are low and few (see table 3-4, and national overview tables in section 3.3). As mentioned for other waste deposition, quantification of releases from deposition of mercury-bearing wastes is not as advanced as for direct atmospheric releases. Also, releases from waste deposition happen slowly over decades, centuries and more; and through occurrences less predictable in time such as excavation activities or other disturbances of the waste deposits. However, waste deposition may constitute a delay of the releases of the mercury in wastes (and thereby sometimes lower current mercury concentrations in the local environment), when compared to waste incineration with today's atmospheric emission reduction systems. Many of the products intentionally containing mercury are internationally traded, and would therefore be expected to be relatively uniform as regards mercury in (western) market economies; any major differences would be due to national trade restrictions and possibly also to some degree consumer's/user's preferences. From the history of mercury use reductions in Denmark, including trade bans, special waste collection, public awareness activities etc., it is deemed highly unlikely that waste in Denmark contain more mercury than average on western markets. This could indicate that mercury amounts similar to those found in waste in Denmark (sum of all releases/deposition from all waste types) per inhabitant would be expected in the other Arctic countries as well, but here more of it is deposited (and therefore potentially more difficult to quantify). An exception could be Sweden, where the "detoxification of society", that is: eliminating old mercury wastes accumulated in society , has been more systematic and comprehensive than for example in Denmark (see von Rein and Hylander, 2000). Note that all figures are subject to uncertainty and should be interpreted with caution. Uncertainties for release estimates from Denmark are presented in the Danish questionnaire response in appendices. No other countries reported uncertainties on figures. National emission factors for waste incineration In table 4-7, calculated emission factors in g mercury released per metric ton of waste incinerated are presented for the countries which have reported the involved data. The calculated factors are very dependent on both the quality of the reported release data, and of which types of wastes are included under the "general/municipal waste" parameter. As can be seen from the table notes, it has not been possible with the available data to calculate all the countries' factors with the same waste definition basis. This naturally introduces a significant uncertainty in any comparison between the countries. The atmospheric emission factors do, however, appear relatively uniform, except for Norway and Sweden, for which the calculated factors are an order of magnitude lower than for Canada, Finland, Denmark and USA. Only Denmark reported the necessary data to calculate a total release factor per ton of waste incinerated; with the rounded "best estimates" presented in the table, the total releases from municipal waste incineration amounts to about five times the reported atmospheric releases. The uncertainties on the presented calculated emission factors could be minimised by further analysis of existing data. Table 4-7 Calculated emission factors for incineration of general/municipal waste. See table notes.
Notes: *1: Percentage for MSW based on total waste amounts minus recycled construction wastes and composted gardening wastes (DEPA, 2003). Percentage for household wastes also from (DEPA, 2003). Uncertainties on atm. releases +/- 60% of mean (questionnaire response from DK). *2: Releases were unusually high in 2000; mean releases 1995-2001 was about 1/3. Release estimates are worst case and do not take effects of emission reduction systems into account (Questionnaire response from FI). *3: Percentage based on figures for household wastes from 2002 (Sleire, 2003). *4: Based on (ACAP, 2004); uncertainty on waste definitions for percentage number. *5: Percentages are based on household waste data from (RFV, 2003). *6: Based on waste data from (Durkee, 2003) and waste definitions from www.epa.org/osw. *7: The differences and uncertainties in definitions of waste categories affect these calculations considerably. As marked in other notes, waste amounts are presented as "MSW" (= municipal solid waste) for some countries and as "household waste" for other countries, and even with this distinction, there may be differences in waste types included in the categories. Time trends in mercury disposal To evaluate the needs for mercury release reductions for society's waste treatment, it is useful to consider the trends in mercury consumption versus mercury in disposed off wastes. These data are shown for Denmark, as an example, in figure 4-3 and table 4-8. Similar consumption trends have been observed in USA, Sweden and Norway for example (Maag et al., 2002). Reduced mercury consumption has been seen generally in the West and the recent mercury release inventory for Russia of this project also shows a significant decrease (ACAP, 2004). In such a scenario, the corresponding decrease in mercury disposal is delayed a number of years due to product life time and time span from dysfunction of the product till it reaches the waste treatment facilities where it can be monitored (typically waste incineration and treatment facilities for hazardous waste). For Denmark, the current average delay for mercury in products is about seven years, as shown in figure 4-3. This delay varies strongly between products however, depending on their characteristics and users; for mercury containing alkaline button cell batteries it would only be a few years, while dental amalgam fillings blood pressure gauges, or fever thermometers used privately, may work for a decade or more. Furthermore, the mercury disposal curve would be expected to flatten out as the consumption approaches trace levels, because the last spent mercury-containing products are only found and disposed of slowly. The latest findings for Denmark indicate that perhaps this "detoxification" of taking old mercury products out of the cycling in society will continue longer than previously expected (Skaarup et al., 2003). Figure 4-3 Time trends in consumption and disposal of mercury with products that are disposed of as municipal and hazardous/medical waste in Denmark *3 (data from Skaarup et al, 2003; see notes under for table below).
Table 4-8 Time trends in consumption and disposal of mercury with products that are disposed of as municipal and hazardous/medical waste in Denmark (data from Skaarup et al, 2003)*3.
Notes: *1: Includes all consumption of intentional Hg uses + mobilisation of Hg impurities in "other materials in municipal waste" (packaging, food leftovers etc., estimated at 0.76-3.1 metric tons/year in 2000/2001). *2: Releases during use contribute with small amounts only, see table 3-6. *3: The true time gap between consumption and disposal may deviate slightly from the shown, because estimates of disposals are not completely independent of consumption figures, due to estimation procedures and lack of data to minimise uncertainties on cross-checking balances. 4.3.2 State of mercury release reductions in waste treatment in Arctic countriesEmission reduction systems As mentioned above, most emission reduction systems for exhaust gases from waste incineration are not optimised for mercury retention. Part of the mercury in the exhaust gas, especially gaseous elemental mercury, is not retained well by particle filters and acidic gas retention systems. Therefore, incineration currently result in an undesired enhanced spreading of the mercury in the waste, rather than preventing or delaying releases to the environment. This is well known, and has been one of the reasons for example Denmark's works on minimising the mercury input to waste by restricting trade of products with intentional mercury contents and enhancing separate waste collection. The recently initiated implementation of carbon injection/carbon filters in some Arctic countries on waste incinerators (mainly driven by dioxin reduction needs) have the potential for improving this situation. Though still significant, mercury releases from waste incineration has declined during the last decades as a result of various reduction efforts as described in this section. For example, most of the reductions in national mercury releases to the atmosphere in the USA since the early 1990's have been made in the waste incineration sector. As the case is for direct landfilling of waste practised in many countries, the part of the mercury that is retained from waste incineration with incineration residues still needs safe deposition and careful management for centuries to avoid unacceptable secondary releases from the residue deposits. Waste incineration residues are sometimes deposited in safer deposits than general waste due to the concentrated toxic constituents. The situation for waste water treatment (also considered waste treatment here) is in principle worse. Most of the mercury lead to waste water treatment ultimately ends up directly in the environment. Even modern waste water treatment plants only retain parts of mercury in the waste water (roughly about half in Denmark), that is: a significant part is released to aquatic environments after the treatment. The mercury that is retained ends up in the sewage sludge, of which much is applied directly on farm land as nutrition, and therefore also adds to the mercury pool that may affect humans and the environment (the remainder is incinerated or deposited). Mercury releases to waste water appear to be smaller than atmospheric releases in the countries which reported these data, but even the "basic" releases from dental clinics may be worth keeping under observation. The release estimates from Denmark indicate that this may be an underestimated release source in the other Arctic countries. Amalgam filters for dental clinics with very high retention rates exist and are widely used in Denmark and probably also in other Arctic countries, yet they are not used by all clinics as their use is not always mandatory (regulated locally in Denmark). Also for hazardous waste the situation as regards incinerated or deposited waste described above applies in principle. Waste products with high mercury contents can to some extent be collected separately to minimize their entry into the general waste treatment. This is done in many countries today. Generally however, such separate collection depends on consumers'/industry's own ability to identify these products, and their motivation to store and deliver them separately for waste treatment. Experience has proven that such collection schemes have some success, but that substantial parts of the disposed products are not collected, but end up in general waste (see for example Hansen and Hansen, 2003, and Skaarup et al., 2003). An additional aspect is the recycling of mercury from separately collected waste with high mercury contents, including virtually pure metallic mercury from spent manometers, barometers, switches etc. As mercury demand is falling, the desirability of mercury recycling will change due to risks of over-saturating the market, potentially resulting in increased releases to the environment (see discussion of this aspect in the Global Mercury Assessment: UNEP, 2002). Additionally, mercury recycling plants are also sources of direct releases to the environment. Sweden has taken the radical step of preventing collected waste mercury from being marketed and decisions for final disposal in a high safety deep rock mercury waste deposit is pending. Sales of mercury from US government stocks have been suspended for similar reasons since 1994. Reduction of mercury input to wastes In many of the Arctic countries efforts have been made to reduce consumption of many "non-essential" products with intentional mercury contents and some countries also have limits to allowable mercury contents in high volume materials such as packaging which also contribute to mercury inputs to waste systems. These efforts have produced results in the form of reduced mercury releases from waste treatment. Yet, the reported mercury releases from Arctic countries indicate that these reductions may still not be enough to reach environmentally sustainable levels. This may have several causes. The consumption of mercury for intentional use is still to high and can be lowered further (alternatives are available for most product uses). The delay in disposal of mercury-containing products is long, and even products which are not sold anymore may continue to be lead to waste treatment for a couple of decades. For brief, but good, overviews of trends in the release situation for waste treatment in Finland, see the "Release trends" sheet in the Finnish response to the questionnaire in appendices. 4.3.3 Options for future release reduction in waste treatmentReductions of mercury releases from waste treatment - that is: from mercury in products and materials - seems to be unavoidable if significant decreases in overall mercury releases to the atmosphere (or in total) are desired. Even in a scenario where mercury use in products was limited to the strictly essential, the disposal of older mercury-containing products would continue for some time - most likely at least two decades. And after that time, a certain mercury input to waste treatment would still take place due to trace level mercury impurities in high volume materials. Landfilling of waste with elevated mercury contents - including waste incineration residues - could impose a burden of continued landfill management and risks of adverse environmental impacts on future generations. But as mentioned above, provided the landfill is secured with lining to prevent ground water contamination, it does not invoke immediate long-range distribution of mercury from the waste, as waste incineration may do. The following options could be considered:
Denmark, Finland, Iceland, Norway and Sweden has earlier explored the possibilities for common strategies for - and handling of - mercury containing wastes in a process under the Nordic Council of Ministers (Endre et al., 1999). 4.4 Chlor-alkali productionChlor-alkali production with mercury technology range among the moderate size mercury release sources, as regards recorded atmospheric releases seen in an overview perspective for the eight Arctic countries (7 metric tons/year of total of 157 metric tons for atmospheric releases). These releases are however produced by relatively few plants with mercury technology, as many plants have converted to other technologies or closed down. This means that the individual plants are major point sources. The 5 chlor-alkali facilities emitting most mercury in the Arctic countries released about 0.6 metric ton/year each (on average). Together, the 5 largest point sources emit about 2% of the total reported atmospheric releases from the Arctic countries. The largest mercury releasing point sources in each country are reported in the mercury questionnaires; see the questionnaires in appendices for detailed information on reported point sources. The mercury balance for the remaining 4 mercury-based chlor-alkali production plants in Russia is presented in table 4-9 (from ACAP, 2004). Table 4-9 Mercury balance for chlor-alkali plants in the Russian Federation in 2002 (from ACAP, 2004).
Notes: * to water system (ponds-evaporators). *1 Purchased mercury amounts may differ from consumption in the year in same year due to internal mercury stock changes. Furthermore, a regularly encountered problem in assessing releases from these facilities is, that their material balances do not fit and substantial mercury amounts used cannot be accounted for by recorded emissions and disposals (UNEP, 2002; Sznopek and Goonan, 2000; ACAP, 2004). Parts of these releases may be accumulated in piping, equipment, building materials and in the ground under and around the production plant sites, and parts may possibly be emitted in ways that are not detected by the monitoring activities carried out. It could be feared that many old chlor-alkali plants (including shut down plants) are sites with severe contamination, posing great challenges and requiring large costs when they are decommissioned or clean-up is initiated for other reasons. These sites pose local mercury contamination risks and may possibly add to present and future regional releases due to evaporation of mercury. Data on a number of shut down chlor-alkali production sites in the Russian Federation are given in table 4-10. Table 4-10 Mercury in soils, waste dumps and water bodies by shot down enterprises in the Russian Federation (from ACAP, 2004).
Chlor-alkali production with mercury technology is considered an obsolete technology, even by the industry it self today, and besides its contamination problems, it drives substantial parts of the global mercury trade and recycling, and thereby increases risks also of other mercury releases in the cycle. The market shares for chloride produced with mercury technology have steadily been decreasing during the last years due to conversions and shut-downs. In line with OSPAR decisions, it could be considered to further stimulate conversion to available mercury-free technologies, and diligent clean-up on the contaminated sites. See the Global Mercury Assessment (UNEP, 2002) for further discussion of mercury use in chlor-alkali production. 4.5 Other selected release sourcesThis section gives some comments additional, selected source types. A detailed discussion of all source types is not given here. For description of other mercury release sources and options for their reduction, see the Global Mercury Assessment (UNEP, 2002). 4.5.1 Mercury contatination from gold extraction in RussiaThe following paragraphs are extracts from the text of Laperdina in (ACAP, 2004). For further description, see that report. Mercury contamination of traditional gold mining areas of Russia, as in all gold-mining areas of the world, is very urgent and poorly known problem. Scope of Hg contamination and its effect in different territories are not thoroughly investigated and require complex expensive research. However, it can be stated with certainty, that all traditional gold mining areas shown in Figure 3.3 have different extent of mercury contamination, which is not localized as a rule. With the introduction of the effective gold mining technologies , the same sites of rich placer deposits were repeatedly washed up again, with subsequent mixing of mercury-containing dredges and hydraulic monitors dumps with the washed-out rocks, which resulted in their distribution all-over the bigger territory. The point sources include abandoned and operating tailing dumps of extracting and concentrating plants, gold-receiving offices. The industrial and residential areas of old gold-mining enterprises are often either transferred from the worked-out territories or gradually destroyed. Restoration and conservation of the contaminated gold-mining sites have not been planned and carried out earlier, therefore the destroyed tailing dumps and exhaust schliches with high Hg content cause the severe environmental pollution. As the location of the old placer gold mining sites can not always be found based on historical records, the assessment of mercury contamination of the traditional gold mining areas requires conduction of the expensive field and desk studies. The local, but isolated from the gold-mining areas, sources of mercury contamination are the refining plants. In present, there are five main sources of mercury release from gold mining activities, quantitative characteristics of which depend on deposit type and gold reserves, duration and intensity of the deposit mining and mercury use in technological operations:
Re-processing of secondary industrial placers The extended re-processing of secondary industrial placers, as well as processing of tailings and schlich concentrates of ore and placer gold have lead to extraction of Hg buried in dumps, pits, , its conversion into the active migrating state and release to the environment with atmospheric emissions (thermal treatment of concentrates, mercury degassing from dumps etc.) and wastewater discharges. The licensing agreement on mining of such placers doesn't take into consideration a high industrial Hg content in the processed sands, and therefore the dissemination and extension of mercury contamination scope is not controlled. In spite of the currently developed and applied technologies of industrial feedstock processing with extraction of both gold and mercury, small scale enterprises with low revenues will likely to use cheaper technologies with only gold extraction, i.e. use burning of the amalgamated gold without Hg vapors condensation at the final phase. In case the environmental control over licensing and further mining of such gold- and mercury-bearing secondary industrial deposits is not strengthened, a half of mercury presently contained in dumps and wastes (3,000-6,000 t) is supposed to be released gradually to the atmosphere and water bodies. The scarce data available indicates, that a share of secondary industrial gold for various regions constitutes 1-5% of the total amount of the gold extracted. In general, a share of technogenic gold in Russia can be approximately estimated as 2-4 %, therefore the amount of secondary industrial gold extracted in 2001 may be equal to about 2,800-5,600 kg. Taking into account the average content of gold in the industrial wastes equal to 350 mg/m³ , the volume of re-processed industrial wastes can be estimated as the following: 2,800 kg : 350 mg/m³ = 8 million m³; 5,600 kg : 350 mg/m³ = 16 million m³. Given the amount of the re-processed industrial wastes as 8-16 mil. m³ and average Hg content as 0.2-0.5 g/m³, the total share of industrial mercury in this volume might make up from 2 to 8 t. About 15-20 % of this amount could have been utilized using modern technologies, however the basic amount of previously accumulated industrial mercury (approx. from 1.5 to 6.5 t) could be released in 2001 in the gold-mining sites and surrounding environments. 4.5.2 Oil and gas extractionA source type which has gained growing attention in the last years is the extraction of oil and gas. This source is generally poorly described as regards mercury releases. Mercury concentrations in oil and gas extraction fields vary quite strongly, and may be low in some countries. But for Russia, for example, the available data indicate that oil and gas extraction are major mercury release sources. As described by ACAP, 2004 the fate of this mercury is not clear. Oil and gas extraction activities may be worth more attention as regards mercury releases, and additional compilation of mercury mobilisation and release data on these sources may be warranted. 4.5.3 Dental amalgamDental amalgam is only large intentional use of mercury remaining in Denmark, and it constitutes significant release contributions to both waste water and waste treatment. Also in other Arctic countries, this may be a significant use of mercury, but it seems that it may possibly given less attention in some countries. The alternative filling materials have gained increased market shares, but in are still not deemed adequate for all filling types by the dental safety authorities in for example Denmark and Sweden. Both countries' environmental authorities are ready to promote full substitution when the health authorities find available alternatives adequate. In Sweden, to enhance the substitution, the public health subsidiary for amalgam fillings has been cancelled, whereas use of alternative fillings still gets financial support. In Denmark, the situation is that both filling types get the same subsidiary, and this enhances the use of amalgam fillings because they are cheaper (shorter dentist working time). As regards aquatic releases, high efficiency filters exist, which capture close to 100% of the mercury losses to the drain, but these filters are not compulsory in all countries. 4.5.4 Laboratory reagentsStill, a few types of laboratory standard analyses are involving the prescribed use of mercury compounds, COD analysis used to quantify organic substances (oxygen demand) in waste water is an example. Relevant alternative reagents and standard analyses are readily available, and international co-operation would enhance possibilities for a more expedite substitution, by involving the standardisation organisations, underlining the arguments for substitution, and stimulating substitution through changes in the regulation and practises of national and local environmental authorities. Nitrogen analysis with the Kjeldal method is also used in environmental control activities, and also for this analysis, the public environmental authorities themselves hold the keys to mercury substitution. 4.5.5 "Least essential uses" elimination procedureAs a possible activity of the Arctic countries' work with a mercury strategy framework, a process of identifying common positions on "least essential" mercury uses and developing concrete, non-binding recommendation on substitution could be initiated. Even though it is well known that mercury-free alternatives exist for many intentional mercury uses, a process of pointing out uses which could be eliminated, and proposing time schedules for their elimination according to the countries' priorities, could possibly stimulate substitution. Such detailed recommendations could be a concrete measure to enhance implementation of the already agreed goals of elimination/reduction of "priority hazardous substances" (OSPAR, HELCOM and EU goals). For some individual mercury uses, such recommendations have - for example - been developed under auspices of HELCOM (see http://www.helcom.fi/recommendations/reclist.html). Another example is the Danish mercury trade ban legislation which gives a general trade ban and detailed lists of uses which are exempted until specific dates, or for some uses until further notice. 4.5.6 Other mercury release sourcesA number of other mercury release sources exist and are reported from the countries to this project. A detailed description of every source can however not be given in this report. For listing of more source types please see the Global Mercury Assessment (UNEP, 2002) and the documents cited there (among many others). 5 Reported landfills of special concernInformation on mercury wastes and landfills of concern due to mercury wastes were requested as part of the ACAP project, because these represent potential current and future sources of mercury releases. Only USA has reported a selection of landfills of special concern. Site information for the Russian Federation was compiled by ACAP, 2004. The provided information is displayed in the questionnaire response from USA and in (ACAP, 2004) in appendices. 6 Overview of existing action plan/strategy elements on mercury in the Arctic Countries6.1 Common features of existing legislation in the Nordic countries 6.1 Common features of existing legislation in the Nordic countriesThe text of this section was extracted from the report "Mercury - a global pollutant requiring global initiatives" from the Nordic Council of Ministers (Maag et al., 2002). Descriptions of selected aspects of individual Nordic countries' legislation and initiatives are given in sections 7.1.2-7.1.5 below (except for Iceland). The following text gives an overview of common features of the existing legislation and regulation relating to mercury in the Nordic countries and in the European Union, of which Denmark, Finland and Sweden are members [4]. Other national measures, such as subsidies financing substitution efforts and voluntary agreements with industry or users of mercury, are not described here, though the efficiency of such measures may in some cases be significant. For examples of national regulation complexes with relevance to mercury, see the descriptions of Sweden and USA in sections 6.1.2 and 6.2, respectively. It should be noted that some variation within the presented themes does exist between the Nordic countries. Among other things, this applies to the types of products with sales prohibitions and policy regarding the final disposal of mercury wastes. The main features of such legislation – more restrictive than the general – are presented in detail below. The overall aim of the legislation on mercury is to prevent or reduce the release of mercury to the environment as well as direct and indirect impacts on human health. As shown, the existing types of legislation relate to most of the phases in the lifecycle of mercury products and processes (one of the exceptions is primary production of mercury). The legislation related to the production, marketing and use of mercury and mercury-containing products are specific to mercury in some cases, whereas the legislation and regulation on emissions and the disposal of wastes are usually more general, and often include other heavy metals and specific inorganic and organic pollutants. The legislation on mercury in the Nordic countries and the European Union in general lie within the following common categories: Mercury in products Legislation preventing certain products containing mercury from being marketed nationally (in some cases including exports) – for instance batteries, cosmetics and pesticides. For some products the actual use of the products is also prohibited (specific pesticide/biocide applications). More details about product legislation are given below. Mercury impurities in bulk materials Legislation setting limits to allowed contents of mercury present as impurities in bulk consumption materials. This follows, among others, the EU packaging directive (EU, 1994), which is aimed at enhancing packaging materials recycling/energy recovery and limiting the flow of heavy metals to waste treatment and resulting environmental releases (among other aims). Industrial point sources - control and "BAT" Legislation prescribing maximum allowable releases of mercury (and other pollutants) from industrial facilities to the environment (air, water and soil/groundwater). These sources of mercury to the environment are generally termed "point sources". Often, the releases from such point sources are regulated individually on a basis of national standards or guidelines. For certain types of potentially heavily polluting industries – for instance chlor-alkali industry – such legislation can also prescribe the use of specific less polluting production- and pollution prevention technologies (designated "best available techniques" - BAT, according to the so-called IPPC directive (EU, 1996). Separate waste collection Legislation prescribing separate collection and waste treatment of products and process waste containing mercury – for instance batteries, fluorescent light tubes and dental amalgam filter residues. The aim of such legislation is to prevent or minimise the diffuse spreading of mercury-containing products and prevent dumping of process waste in the environment, as well as limiting the amounts of mercury-containing waste in the general household waste stream (where it causes significant mercury emissions and increases waste treatment costs). Waste incineration emissions Legislation prescribing maximum allowable releases of a number of pollutants from incineration facilities for household and hazardous wastes, respectively, to the atmosphere and wastewater, as well as specification for the depositing of solid incineration residues. Indirectly, such legislation can dictate the use of a limited number of emission control technologies, which are capable of complying with the emission requirements. The Nordic countries have extensive exhaust gas filtering on almost all waste incineration facilities (hazardous, medical and household waste), holding back a major part of the otherwise emitted mercury. Use of solid incineration residues Legislation prescribing maximum allowable concentrations of mercury (and some other pollutants) in ashes and slag from waste incineration and fossil fuel combustion used for construction purposes (roads etc.), as well as in wastewater sludge used as fertiliser on agricultural land. Releases to wastewater system Legislation preventing or limiting the release of mercury from processes to the wastewater system in order to limit releases to the water recipient, to permit the use of sludge as fertiliser on agricultural land, and to reduce treatment costs. For instance mandatory use of amalgam separators in dentist clinics and cleaner technology or pre-treatment in industrial facilities. User safety Regulation prescribing aspects of user safety in the working environment and for private consumers (toys and certain chemical preparations). Information sources: Nyström (2001), Norwegian Pollution Control Authority (2001), Einarsson (2001), KEMI (1998), von Rein and Hylander (2000), Retsinformation (2001), OSPAR (2000c), European Commission (1998), EUR-Lex (2001). 6.1.2 Sweden(Selected aspects extracted from UNEP, 2002; for general features see section 6.1). Risk reduction of mercury has been an item of high priority in Sweden since the 1960's. In the early 1990's it was concluded that the substantial reduction of mercury releases achieved at point sources would not be sufficient to reduce the environmental load beyond critical levels. It was estimated that mercury content in fish in about 40,000 lakes (i.e. about half of the Swedish lakes) exceeded the limit value of 0.5 mg/kg recommended by the FAO/WHO Codex Alimentarius Commission. In the Government Bill 1990:91/90 “En god livsmiljö” (A living environment), a numerous set of legislative and voluntary actions were proposed, with the ultimate aim of a total phase-out of mercury use. Since then, the set of actions has several times been re-approved and strengthened in various government bills and parliament decisions. Table 9.2 presents some of the major initiatives on mercury use that have been implemented in Sweden as a result of the 1990 overall goal of total phase-out of mercury. It should be noted that Sweden is a Member State of the European Community since 1 January 1995, and is required to implement all Community legislation that applies to mercury, as described in the section on the European Community. However, some of the measures taken in Sweden exceed this legislation. Table 6-1 Major initiatives on mercury use in Sweden, as reported by Sweden.
Additional measures and initiatives under consideration in Sweden Chlor-alkali industry - There are two chlor-alkali plants in Sweden that still use the amalgam process. The more environmentally friendly membrane process is used at one site. In line with OSPAR Decision 90/3, the Swedish government has in several bills stated that the amalgam process should be out of use by 2010. To further assure the realisation of this object on the national level, the Swedish government is considering the inclusion of a ban in Ordinance 1998:944. Waste products - As far as waste disposal is concerned, there are separate collection systems and already existing efforts for the collection of batteries, fluorescent lamps, amalgam waste etc. Collected batteries are currently stored awaiting the decision on pre-treatment before it is put in a terminal storage facility for mercury. Dental amalgam – The overall goal to a complete phase out of mercury also includes dental amalgam. The consumption of mercury for dental use has decreased significantly after a policy decision by the Parliament in 1994 to phase out the use of dental amalgam. Up to now dental amalgam has been subject primarily to voluntary phasing out measures in Sweden. A voluntary agreement not to use amalgam fillings in the teeth of children and youth up to nineteen has resulted in an almost complete phase out. The Swedish Government is continously investigating further possibilities to reduce the use of dental amalgam. Laboratory chemicals - Mercury-containing chemicals for analysis and reagents are mainly used in the environmental control, by its use of mercury sulphate in COD (chemical oxygen consumption) analyses. Information activities have not been effective to phase-out this particular use. The Swedish government is therefore considering an amendment of Ordinance 1998:944, by which the use of mercury in chemicals for analysis and reagents would be banned from 1 January 2004. Lighting - There is at present no commercially available, mercury free alternative to linear fluorescent lamps and compacts fluorescent lamps. In order to minimise the environmental impacts from the use of mercury in these products, maximum permitted mercury contents should preferably be established. Such regulations will most likely be introduced in the coming EC Directive on Restrictions of Hazardous Substances in electric and electronic equipment. Collection of used products and goods - Recognising that mercury releases from products in use or forgotten "on the user's shelves" would continue for many years, the Government developed an action programme for a more effective and comprehensive collection of used products and goods containing mercury. The action programme included projects dealing with the collection of clinical thermometers, inventories and collection of mercury at different places, clearing out of mercury in schools, universities and colleges and providing information and raising awareness. In projects for the collection of mercury thermometers, economic incentives were used to invite household to turn in their mercury thermometers. Another project consisted of the identification of hidden “technical” mercury in technical goods and products within about 70 industries. The work involved tracking mercury with the world's first mercury dogs. A total of 10-11 metric tons of mercury have been identified through the action programme, 6-7 of which have been collected and 3.5-4 of which have been labelled for proper disposal once it is not in use anymore. The Government estimates that there are still a number of metric tons of mercury in industry (technical goods, stored metal mercury, etc.), in households (for example in thermometers, antique barometers, doorbells, etc.), in agriculture (old and stored pesticides) and in pipes in the sewage system, especially in pipes from old dental clinics. Final disposal of mercury - Mercury is a substance that remains a threat to human health and the environment in perpetuity, and for this reason it should not be recycled. Instead, mercury-containing waste must be dealt with permanently in a safe and environmentally acceptable way. In a report to the Government, the Swedish Environmental Protection Agency in 1997 proposed terminal storage of waste containing mercury in a deep rock facility. A governmental committee has recently submitted its final report on how to dispose waste containing more than 0.1 percent (by weight) of mercury. It is proposed that a mandatory requirement for permanent storage deep down in rock should be in force within five years. 6.1.3 DenmarkIn Denmark intentional mercury use has been reduced from an estimated 15-17 metric tons/year in 1982/83 to 6-7 tons in 1992/93 and 1.3-1.9 metric tons/year in 2000/2001 - about 10% of the 1982/83 level. In the same period atmospheric releases have been reduced from a reported 4.1-6.9 metric tons/year in 1982/83 to 0.8-2 tons/year in 2001 (Skaarup et al., 2003). (Below: Selected aspects extracted from Maag et al., 2002; for general features see section 6.1). Also in Denmark there has been a general ban on the sales of mercury and mercury-containing products since 1994. The legislation exceeds general EU legislation. As from 1998, the order explicitly includes exports also (Statutory order no. 692 of September 22, 1998). Some distinct uses are exempted with specific expiring dates or until further notice (respectively). The causes for the current exemptions are lack of adequate alternatives, avoidance of trade barriers within the European Union, measurement/analyses standards prescribing mercury-use (reactants or instruments), or use for calibration of non-mercury measurement instruments. The Danish statutory order includes banning of imported equipment with mercury-containing components. Dental amalgam is allowed only in molar teeth, where the filling is worn, until further notice. Only a few specified uses of mercury chemicals for analyses and catalyse are allowed. The efficiency of the Danish mercury ban has been evaluated thoroughly recently, and the annual 2000/2001 consumption with products has been reduced to about 25% of the consumption in 1992/1993, before the ban was brought into force (Skaarup et al., 2003). Batteries are regulated separately implementing the EU battery directive and its amendments. Also Denmark has decided to change the battery collection system, so that all batteries, irrespective of heavy metal content, will be collected in order to get higher collection rates for the environmentally harmful types. Earlier, only the particular types containing heavy metals were collected. The necessary changes in the collection set-up is currently under consideration. Denmark is ready to ban the remaining use of dental amalgam, whenever the Danish National Board of Health deems that the alternatives have the full substitution capacities (Danish E.P.A., 2001). 6.1.4 Finland(Selected aspects extracted from the "release trends" sheet of Finland's response to the mercury questionnaire of this study, for general features see section 6.1).
6.1.5 Norway(Selected aspects extracted from the appendix "Overview of existing and future national actions, including legislation, relevant to mercury" to UNEP, 2002; for general features see section 6.1). Air and water point sources - The Norwegian industrial plants have emission limits for mercury in their permits followed up by normal procedures for control by the authorities (textile, paper, chemical, cement and metal industry). New stringent emission limits for the most important point sources, waste-incinerators, ferromanganese and siliconmanganese smelters are in place. Chlor-alkali production – The Norwegian chlor-alkali plants have changed from mercury-cell technology to diaphragm and membrane technology. Crematoria - A separate regulation for crematoria is expected to be in place by year 2002. Dental sector - It is prohibited to release wastewater and waste containing amalgam from dental clinics. Dental clinics are obligated to have separate collection of amalgam waste and amalgam separators to prevent discharges of mercury from wastewater. The amalgam sludge and waste are to be delivered to an authorized facility for hazardous waste. Currently, Norway is developing a directive on the use of dental filling materials, which will encourage dentists to reduce the use of amalgam as much as possible. The directive is expected to take effect 1 January 2003. Ferromanganese production - To fulfil the obligations in their permits three ferromanganese plants in Norway have chosen to install mercury abatement facilities. The plants have chosen two different technologies. These are to our knowledge the first mercury treatment facilities for this industrial sector in the world. The mercury abatement facilities in the Ferromanganese smelters require good dust removal. This means that the plants also must add an improved dust filter and this will give a substantial reduction of other heavy metals as well. A new treatment unit for removal of mercury from the off-gas was installed at one of the plants in April 2000. The mercury content of the cleaned off-gas from the treatment unit is monitored continuously. Approximately 80 % treatment efficiency with respect to mercury was achieved during the first year of operation. Several technical problems were encountered over this period, but most of them have now been solved. It is anticipated that the treatment efficiency can be further improved when more experience is gained with the process. The other two plants use abatement facilities with another type of technology. The abatement facilities on these plants will be operating in September 2001 and the effectiveness of these facilities will be considered later on. The cost for all three plants is estimated to 75 mill NOK/8.3 mill USD. In addition there will be increased operational costs. Gas and petroleum processing - Offshore activities: Measures to reduce mercury releases from offshore activities are in progress through a project focusing on how to minimise the discharges of produced water. The project is based on collaboration between authorities and industry. Gold-mining - There is no gold mining in Norway. Sewage sludge - There are legislations prescribing maximum allowable concentrations of mercury in wastewater sludge used as fertiliser on agricultural land (3 mg/kg total residue) and on other areas (5 mg/kg total residue). It is not allowed to use wastewater sludge on agricultural land with soil containing more than 1 mg/kg total residue. Waste treatment including incineration - Generally hazardous waste containing mercury is disposed off in an authorized treatment plant. A smaller part of organic hazardous waste containing mercury is pre-treated at the plant before incinerated in an authorized incineration facility for hazardous wastes. Waste containing more than 0.25 % mercury are treated in accordance with the legislation for hazardous waste. The legislation prescribe separate collection and environmental sound waste treatment of products and process waste containing mercury – for instance batteries, electric articles, fluorescent light tubes and dental amalgam filter residues. The regulation on incineration of hazardous wastes restricts the concentration of mercury in air emissions not to exceed 0.05 mg/m³ from new facilities and 0.1 mg/m³ from existing facilities. The release of mercury from incineration plants for medical and household wastes is restricted by specified emission limits in their permits. All municipal incineration plants with permits newer than 1994 have 0.03 mg/Nm³ mercury as emission limit. This stringent limit will be in force from 01.01.03 for new facilities incinerating medical and hazardous wastes. Before 01.01.2006 this limit will be in force for all existing incinerators. The new limits are more stringent than in EU. Mercury is a priority substance in Norway because of its high levels in the environment and population in Norway and its severe properties. From 1.1.2003 ashes and slag will be on the new European waste list and be considered hazardous waste if it contains hazardous substances. For mercury the limit is as mentioned before 0.25 %. 6.2 United States of AmericaIn the USA intentional mercury use has been reduced by more than 70% since the 1980's. Reported atmospheric releases in the USA has been reduced from 191 metric tons/year in 1990 (210 short tons) to 107 metric tons/year in 1999. Please note that the description here of U.S. activities on mercury addresses only various major activities by the U.S. government. There are many other activities being conducted by the U.S. at all levels, i.e., other Federal agencies, state governments and tribal organizations. The United States has been actively addressing the risks posed by exposure to mercury for many years, both through implementation of regulatory activities and voluntary reduction programmes. For example, already in 1991 the USEPA initiated the "33/50 Program", a special programme to help reduce releases of mercury and 16 other toxic substances into the environment. The goal of the programme was to encourage companies to commit to voluntarily reduce their releases of some or all of these toxics by 33 percent by 1992, and 50 percent by 1995. As a result, between 1988 and 1991 environmental releases of mercury were reduced by 38 percent and transfers of mercury for off-site treatment or disposal were reduced by 30 percent (OECD, 1995). Understanding the characteristics and magnitude of mercury releases is critical to the design of effective risk management strategies. The Clean Air Act, as amended in 1990, required USEPA to prepare an assessment of the magnitude of USA mercury emissions by source, the health and environmental effects of the emissions, and the cost and availability of control technologies. The resulting report, Mercury Study Report to Congress, was published in December 1997. As the state-of-the-science for mercury is continuously and rapidly evolving, it represents a “snapshot” of current understanding of mercury in the USA. The report is a comprehensive document consisting of eight volumes. The USEPA's Office of Research and Development (ORD) in September 2000 published its Mercury Research Strategy, intended to guide the mercury research programme through 2005. The Strategy identifies the key scientific questions of greatest importance to the Agency, and then describes a research programme to answer those questions. The goal in addressing the questions is to reduce scientific uncertainties limiting USEPA's ability to assess and manage mercury and methylmercury risks. An integral part of the strategy involves study of the atmospheric mercury transport, transformation and fate. Mercury roadmap The USEPA is now preparing a Mercury Roadmap that will outline the Agency's strategy for addressing mercury over the next several years. Ongoing and planned actions to reduce mercury pollution in the United States The United States' approach to designing effective risk management strategies for mercury comprise both specific regulatory limits on releases and voluntary efforts with industry to reduce mercury use, implemented by a number of agencies at both federal and state levels. The most important are summarized below. Stockpiles of mercury – The United States government maintains a supply of mercury as part of the National Defence Stockpile, established at the end of World War I to maintain adequate supplies of materials deemed critical to national defense. The Defense Logistics Agency (DLA), a unit of the Department of Defense, manages the stockpile. The Strategic and Critical Materials Stockpile Act regulates mercury that the DLA sells from the national stockpile. In July 1994, DLA suspended future mercury sales pending analysis of the environmental consequences. An Environmental Impact Statement to determine the disposition of the stockpile was completed in April, 2004. In the meantime, a complete review of the four facilities across the USA currently storing its mercury and inspection of all the mercury containing flasks to ensure proper and safe storage is being undertaken. The US Department of Defence announced that it's “preferred option” is consolidated storage of its mercury at one location for at least a 40-year period. Water point sources - Mercury is listed as a toxic pollutant under the Clean Water Act. The Clean Water Act regulations specify technology-based effluent limits for mercury discharges from different industries, and describe the circumstances in which states may require effluent limits or monitoring requirements more stringent than technology-based standards. States must set water quality standards for pollutants including mercury. The Clean Water Act relies on a permit system, known as the National Pollutant Discharge Elimination System to regulate direct discharges to surface water bodies. Facilities are assigned a specific mercury discharge limit, and/or are required to monitor their discharge for mercury. Facilities report actual discharge levels in Discharge Monitoring Reports, which serve as the basis for determining compliance. A large number of industry point sources are covered, such as chlor-alkali, steam electric power generation, battery manufacturing etc. Air point sources - Mercury and mercury compounds are considered Hazardous Air Pollutants (HAPs) under the Clean Air Act. USEPA established National Emission Standards for Hazardous Air Pollutants (NESHAPs) for mercury emissions based on risk under the pre-1990 version of the Clean Air Act. Under the Clean Air Act Amendments of 1990 USEPA regulates Hazardous Air Pollutant Emissions by source categories using Maximum Achievable Control Technology (MACT) standards for each "major source" in any listed source category. The MACT floor for new sources is the level of HAP emissions control currently achieved by the best-controlled similar source. The MACT floor for existing sources is the average level of HAP emissions control achieved by the top 12 percent of the currently operating sources. Chlor-alkali industry - In August 2003, EPA promulgated a rule that limits mercury emissions from plants that produce chlorine using the mercury-cell method. The rule includes emissions limits based on maximum achievable control technology (MACT) and on stringent management practices. EPA estimates that this regulation will reduce stack emissions by 1,500 pounds, or 74 % from current levels, in addition to unquantifiable reductions in fugitive emissions expected as a result of improved work practice standards. This standard does not allow any new chlor-alkali mercury cell facilities to be built (http://www.epa.gov/ttn/atw/hgcellcl/hgcellclpg.html). The last USA mercury cell based factory was built in 1970. In addition, as a voluntary measure, the Chlorine Institute, on behalf of USA mercury cell chlor-alkali facilities, committed in 1997 to reduce mercury use 50 percent by 2005 and to report annually on progress. In July 2004, the Chlorine Institute provided its seventh annual report, which indicated that mercury consumption by US chlor-alkali factories has declined by 76 percent over an eight year period, or a 69 percent reduction after adjusting for shut down facilities. This is a decline from 160 tons per year (during a baseline period of 1990-1995) to 30 tons during 2001. Chlorine Institute progress reports to the USEPA may be found at: "http://www.epa.gov/Region5/air/mercury/reducing.html#heavy%20industry". Energy production – The largest anthropogenic source of mercury emissions in the USA is currently coal-fired power plants. Utility steam generating sources were subject to special study and required a determination by the U.S. EPA as to whether regulation is necessary. In December 2000, USEPA released its Regulatory Finding on the Emissions of Hazardous Air Pollutants from Electric Utility Steam Generating Units. The Agency concluded that regulation of HAPs from coal- and oil-fired electric (but not natural gas-fired) utility steam generating units is indeed necessary, and that mercury is the air toxic of most serious concern. On January 30, 2004, the U.S. EPA proposed alternative approaches to regulating mercury from coal-fired power plants. Under one approach, national emissions standards for hazardous air pollutants would be established under section 112 of the Clean Air Act. Under the other approach, EPA would withdraw its December 2000 determination and establish standards of performance for electric utility steam generating units based on a market-based cap-and-trade methodology. USEPA expects to issue a final rule by March 15, 2005. The proposed emissions standards would regulate mercury air emissions from new and existing coal-fired electric utility steam generating units, and nickel air emissions from new and existing oil-fired electric utility steam generating units. Waste treatment including incineration - Prior to 1995, municipal waste combustors and medical waste incinerators were the largest identifiable source of mercury emissions to the atmosphere. Regulations which have been finalized for municipal waste combustors and medical waste incinerators will, when fully implemented, reduce emissions from these source categories by an additional 90 percent over 1995 levels. As a voluntary measure, USEPA and the American Hospital Association in 1998 signed a memorandum of understanding committing to work together to significantly cut hospital wastes by 2005. The agreement envisions the virtual elimination of mercury-containing hospital wastes and a one-third reduction in total hospital wastes by 2005. In December 1995, the USEPA finalized New Source Performance Standards (NSPSs) and Emission Guidelines (EGs) applicable to municipal waste combustor (MWC) units with a capacity greater than 227 metric tons per day (i.e. large MWCs). The mercury air emissions standard for new and existing MWCs is 0.08 milligrams per day standard cubic meter (mg/dscm) at 7 percent oxygen (7 percent O2 ). All 167 large MWCs that are subject to the regulations that came into compliance by December 2000 and mercury emissions (based on year 2000 stack test compliance data) from this source category have been reduced by about 95 percent form 1990 levels. The typical performance level was 0.02 mg/dscm. A companion rule (NSPSs and EGs) for a small MWC unit (32 to 227 metric tons per day) was adopted in December 2000 with retrofit required by December 2005. The same mercury emissions limits apply and the same control technology is expected to be used. Since 1997, mercury emissions from medical waste incinerators have been limited by a USEPA regulation that sets strict standards for new sources and that requires existing sources to reduce emissions by 93 to 95 percent. The regulations also require training and qualification of operators, incorporate siting requirements, specify testing and monitoring requirements to demonstrate compliance with the emission limits, and establish reporting and record keeping requirements. Several states, including New York, California and Texas have adopted relatively stringent regulations in the past few years limiting emissions from medical waste incinerators. The implementation of these regulations has brought about very large reductions in emissions of mercury in those states. It has also significantly reshaped how medical waste is managed in those states. Many facilities have responded to state regulations by switching to other medical waste treatment and disposal options to avoid the cost of add-on pollution control equipment. The two most commonly chosen alternatives have been off-site contract disposal in larger commercial incinerators and on-site treatment by other means (e.g., steam autoclaving). Hazardous waste incinerators – On February 14, 2002, USEPA promulgated interim emission standards for hazardous waste incinerators, hazardous waste burning cement kilns, and hazardous waste burning lightweight aggregate kilns under joint authority of the Clean Air Act and Resource Conservation and Recovery Act (RCRA). The standards limit emissions of chlorinated dioxins and furans, other toxic organic compounds, toxic metals (including mercury), hydrochloric acid, chlorine gas, and particulate matter. USEPA will issue final standards for these three categories of hazardous waste burning facilities by 2005; in addition, the Agency will develop emission standards for hazardous waste burning industrial boilers and hydrochloric production facilities. Waste disposal – The RCRA regulations outline specific classification and disposal requirements for products and wastes that contain mercury. RCRA regulations are waste-specific, not source-specific, and thus may apply to any facility that generates mercury-containing wastes. RCRA regulations describe specific disposal requirements for individual wastes. All mercury-bearing wastes are subject to land disposal restrictions. That is, the mercury concentration in these wastes must be below the regulatory concentration level before the wastes may be land-disposed. For some types of waste, the regulations require a specific treatment, such as recovery of the mercury or incineration. In other cases, only a maximum mercury concentration is required, and any treatment method may be used. RCRA regulations also influence product disposal and recycling options for mercury containing products. Discarded products considered hazardous wastes are subject to storage, transportation, and permitting requirements. Currently, thermostats and fluorescent lamps are included in a "universal waste rule" that eases RCRA restrictions on hazardous waste management and enables states to set up special collection programmes. USEPA issued the universal waste rule (UWR) in 1995. It is designed to reduce the amount of hazardous waste in the municipal solid waste stream, encourage the recycling and proper disposal of some common hazardous wastes, and reduce the regulatory burden on businesses that generate these wastes. Universal wastes are items commonly thrown into the trash by households and small businesses. Although handlers of universal wastes must meet less stringent standards for storing, transporting, and collecting wastes, the waste must comply with full hazardous waste requirements for final recycling, treatment, or disposal. This management structure removes these wastes from municipal landfills and incinerators. In July 1999, USEPA added mercury-containing lamps to the UWR, which already covered batteries, thermostats, and pesticides. In 2002, EPA proposed adding other mercury-containing wastes to the universal waste rule. Recreational mining - There is no active mercury mining in the USA. There is also no use of mercury in large-scale gold mining in the USA. There has been minor recovery of mercury by recreational miners in California, but the mercury is recovered as elemental free mercury in stream bottoms as a by-product from historical use. The mercury is incidentally recovered on the sluices of recreational portable dredge operators. The USEPA and California are working on ways to set up collection points for waste mercury to ensure that recreational miners do not dump their waste mercury in streams. Foodstuffs – The Food and Drug Administration (FDA) regulates mercury in food, drugs, and cosmetics. FDA sets an action level of 1 ppm methylmercury in fish, shellfish and other aquatic animals, and may remove from commerce foods that violate this action level. FDA has advised women of childbearing age to limit their consumption of shark, swordfish, tilefish and king mackeral based on methylmercury content. States, tribes and territories are responsible for issuing fish consumption advise for locally-caught fish; many state health departments use 0.5 ppm methylmercury as a trigger for such advice. Some States also issue advice on limiting consumption of non-local commercial species (e.g. canned tuna). On March 19, 2004 the U.S. Food and Drug Administration (FDA) and the Environmental Protection Agency (EPA) announced their joint consumer advisory on methylmercury in fish and shellfish for reducing the exposure to high levels of mercury in women who may become pregnant, pregnant women, nursing mothers, and young children. The agencies believe that by following these recommendations for selecting and eating fish or shellfish, women will receive the benefits of eating fish and shellfish and be confident that they have reduced their exposure to the harmful effects of mercury. Additional information can be found at: www.cfsan.fda.gov or the EPA website at www.epa.gov/ost/fish Mercury in products - Mercury-containing products are regulated in several different ways. At a federal level, mercury product regulation has generally centered around health-based reasons to eliminate mercury from products, using the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and the Federal Food, Drug, and Cosmetic Act (FFDCA) regulations. In recent years, many states have taken a different approach. Restrictions on mercury-containing products, once used sparingly by the federal government, are increasing rapidly at the state level. Certain USA States have initiated a variety of initiatives aimed at reducing mercury releases from the use and disposal of products. These initiatives include notification and labeling requirements to gain information on the mercury content of particular products and inform purchasers that products contain mercury; prohibitions on the sale of a variety of products for which alternatives were deemed readily available such as fever thermometers, dairy manometers, novelty items (toys, shoes), switches in automobiles, and thermostats in residential and commercial applications; concentration limits on other products such as batteries and packaging; restrictions on product disposal so that the products must be segregated from the solid waste stream and ultimately recycled; and state-sponsored collection programmes for items such as fever thermometers, historic dental inventories, and products found in schools. Batteries – Between late 1989 and early 1991, all USA manufacturers converted production so that mercury content, except in button and "coin" cells, did not exceed 0.025 percent mercury by weight. A federal law called the Mercury-Containing and Rechargeable Battery Management Act went into effect May 13, 1996. The Act prohibits the sale of:
The Act contains labelling requirements and encourages voluntary industry programmes by eliminating barriers to funding the collection and recycling or proper disposal of used rechargeable batteries. The Act also grants states the authority to add other batteries to the recycling programme. This federal law followed the lead of several states that passed legislation in the early 1990's limiting the mercury content of batteries. Cosmetics – According to the Federal Food, Drug, and Cosmetic Act (FFDCA), mercury use as a preservative or anti-microbial is limited to eye-area cosmetics or ointments in concentrations below 60 ppm. Yellow mercuric oxide is not recognized as a safe and effective ophthalmic anti-infective ingredient. Dental amalgam – The Food and Drug Administration (FDA) also regulates dental amalgam under FFDCA. Dental mercury is classified as a Class I medical device, with extensive safety regulations on its use. Dental amalgam alloy is classified as a Class II device, subject to additional special controls. Lighting – Of the 500-600 million mercury-containing lamps sold in the United States annually, approximately 96 percent are fluorescent lamps. It is estimated that approximately the same number of lamps are disposed of on an annual basis. Mercury releases due to mercury-containing lamps are expected to decrease in the future for a number of reasons. One reason is that states are beginning to view recycling as a viable option to decrease mercury releases. In addition, there have been technological advances in the manufacture of fluorescent lamps. Since the mid-1980's, electrical manufacturers have reduced the average amount of mercury in each fluorescent lamp from an average of 48.2 mg to an average of 11.6 mg/lamp in 1999. A certain amount of mercury is needed, however, in order to maintain desirable properties. A recent survey by the National Electrical Manufacturers Association showed that the average 4-foot (1.22 m) lamp in 2001 contained 8.3 mg of mercury. Paints - As of May 1991, all registrations for mercury biocides used in paints were voluntarily canceled by the registrants, thus causing a drastic decrease in the use of mercury in paint. In addition to the paint industry reformulating its paints to eliminate mercury, USEPA banned the use of mercury in interior paint in 1990 and in exterior paint in 1991. Pesticides - The Federal Insecticide, Fungicide and Rodenticide Act (FIFRA) covers the sale and use of pesticides, including registration of chemicals that meet health and safety tests. Earlier, several mercury compounds were registered as pesticides, bactericides, and fungicides, however, registrations of the last mercury-based pesticides for use to control pink and grey snow mold were voluntarily cancelled by the manufacturer in November 1993. Thermometers – Voluntary efforts are underway jointly with appropriate industry and associations to reduce mercury in thermometers through mercury free substitutes. Several USA States have banned the use of mercury fever thermometers, and most major retailers no longer sell them. Thermostats - As a voluntary measure, the industry-funded Thermostat Recycling Corporation (TRC) launched a programme in 1997 to recycle mercury-switch thermostats in nine states (see www.nema.org/index_nema.cfm/664/). It has since been expanded to 48 states in the USA, and in 2001 collected 48,215 thermostats and 402 pounds of mercury, for a total of more than 120,000 thermostats and 1,000 pounds of mercury since the programme's inception. Recognizing that the capture rate for the TRC programme is relatively low, two USA States (Maine, Oregon) will prohibit the sale of new mercury thermostats for residential and commercial applications effective January 2006. Vaccines - Under the Food and Drug Administration Modernization Act of 1997, FDA is required to assess the risk of all mercury containing food and drugs. Under this provision, FDA asked vaccine manufacturers to provide information about thimerisol content of vaccines. Based on this information, the Public Health Service, the American Academy of Pediatrics, and vaccine manufacturers agreed that thimerisol-containing vaccines should be removed as soon as possible. Manufacturers have been asked for a clear commitment to eliminate mercury from vaccines, and FDA will do expedited reviews of resulting revisions to product license applications. Vehicles – In an effort to reduce mercury emitted from electric arc furnaces that consume scrap from recycled automobiles – which USEPA estimates emissions of about 8 - 12 tons per year of mercury – USEPA is pursuing multiple program efforts to encourage the removal of mercury switches from scrap automobiles prior to recycling. Nearly all obsolete automobiles in the U.S. are dismantled and shredded to recycle the metal. The scrap metal industry recycles approximately 10 to 12 million cars each year and sells the scrap to domestic and overseas consumers. Pollution prevention approaches to address these emissions must include not only the facilities that shred cars in preparation for steelmaking, but also those suppliers of end-of-life vehicles (ELVs) who dismantle and usually flatten them prior to delivery for shredding. Those facilities, known generally as auto dismantlers, have the best opportunity to recover the bullet-sized mercury switches that make up the bulk of the mercury present in ELVs. USEPA has initiated discussions with stakeholders on how to maximize the removal of mercury switches from ELVs, thereby reducing downstream mercury emissions from melting scrap steel in electric arc furnaces. USEPA has had discussions with the Partnership for Mercury Free Vehicles, a coalition made up of steel manufacturers, scrap recyclers, automobile dismantlers, and environmental groups; with automakers; and with state agencies that have studied or implemented programs to recover mercury switches. There have been several facilitated group discussions about possible solutions, beginning in August, involving representatives of all of these groups. USEPA's goal is to develop consensus for a comprehensive solution that can be put in place by next year. USEPA is also seeking to encourage reduced toxics use in automobile product design. USEPA will propose in 2005 an area source rule establishing performance standards based for hazardous air pollutants, including mercury, emitted by electric arc furnaces (EAFs). Because pollution prevention is likely the best approach for reducing EAF mercury emissions, approaches to remove switches can be incorporated into this proposed rule. USEPA hope to have collaborative solutions in place to recover mercury switches even prior to the EAF rule's effective date. At the same time, USEPA is considering other programs and authorities to use in this effort. For example, Best Management Practices used to control discharges of storm water under the Clean Water Act can be used to prevent potential mercury exposure or releases at auto dismantlers and scrap recyclers. Switches that are damaged, such as during crushing operations, and mercury-tainted scrap can contaminate storm waters. One effective management practice that can be used to prevent these discharges is to remove mercury switches from scrap autos before crushing or shredding. Raising awareness of this issue to auto dismantlers and scrap recyclers through federal and state storm water managers can increase chances for successful switch removal USEPA is also taking steps to include mercury applications, such as automotive switches, in the federal Universal Waste Rules, to lower the regulatory barriers for those removing switches. USEPA will then encourage those States that do not automatically incorporate such changes to do so, in the hopes of maximizing switch recovery. If USEPA can, through collaboration and the best use of incentives, regulations, barrier removal, and voluntary approaches, make switch removal more uniform, USEPA can help to avoid the export of scrap automobiles or shredded scrap containing mercury from un-removed switches, thereby avoiding mercury emissions in other countries and lessening any competitive disadvantage for domestic consumers of scrap USEPA is exploring other avenues, as well, such as voluntary waste minimization partnerships focusing on mercury applications, to reach our environmental goals quickly and efficiently without imposing unfair burdens on particular industries, small businesses, or other groups. Occupational safety and health - The Occupational Safety and Health Administration has responsibility for maintaining safe workplace conditions. OSHA sets permissible exposure levels for elemental mercury in workplace settings. Mercury is listed as a neurotoxin capable of causing behavioral changes, decreased motor function and other effects on the nervous system. OSHA mercury standards also recommend that skin contact should be avoided. Workplace standards may influence the types of processes used at a facility. For example, when OSHA tightens its standards for a particular substance, it may force users of that substance to modify their processes or eliminate use of that substance entirely in order to meet these new standards. Workplace air concentration levels for exposure to elemental mercury: Section 29 CFR 1910.1000 sets the permissible exposure limit (PEL) for an 8-hour time weighted average (TWA) of 0.1 mg/m³. Information and reporting requirements – Under the USA Toxics Release Inventory (TRI), starting with the 2000 reporting year, the reporting threshold for mercury and its compounds has been lowered to 5 kilograms per year (the previous threshold was 4,500 kilograms). Through this action, the United States will have a much more comprehensive picture of the amounts of mercury and its compounds that are released to the air, water, land, transferred off-site for disposal, transferred off-site for recycling or recycled on-site within industrial facilities. Transportation - The Department of Transportation regulates hazardous materials transport under the Hazardous Materials Transportation Act. Mercury and mercury compounds are hazardous substances subject to packaging, shipping and transportation rules for hazardous materials. Regional cooperation - In 1997, the United States and Canada signed the Great Lakes Binational Toxics Strategy. The goal of the strategy is to seek, by 2006, a 50 percent reduction in the deliberate use of mercury and a 50 percent reduction in the release of mercury caused by human activity. The goal applies to all mercury releases nationwide as well as all direct discharges to the Great Lakes Basin. The USA is co-operating with Mexico and Canada in the North American Regional Action Plan for mercury under the Commission for Environmental Cooperation's Sound Management of Chemicals Work Group. These regional initiatives are described in more detail later in section 6.5. 6.3 CanadaTotal reported atmospheric mercury releases decreased from 29.1 metric tons/year in 1990 to 8.9 metric tons/year in 1998 (Environment Canada, 2000). (Below: Selected aspects extracted from the appendix "Overview of existing and future national actions, including legislation, relevant to mercury" to UNEP, 2002). Air and water point sources Canadian-Wide Standards exist for the following mercury release sources:
For more information on Canada wide standards on mercury see the CCME website http://www.ccme.ca. Base metal smelting: Environmental source performance guidelines have been established for base metal smelters. For existing facilities, the guideline is 2 g Hg/tonne of finished metal, while for new and expanded facilities the performance guideline is 0.2 g Hg/tonne of finished zinc, nickel and lead, and 1 g Hg/tonne of finished copper. Chlor-alkali production The Chlor-Alkali Mercury Release Regulations under CEPA (1999) limit the release of mercury into ambient air from mercury cell chlor-alkali plants. The Regulations also include provisions with respect to reporting releases, malfunctions and breakdowns. The regulations prescribe the following release limits:
The Chlor-Alkali Mercury Liquid Effluent Regulations under the Fisheries Act limit the level of mercury contained in effluent from chlor-alkali plants. The regulations state that mercury deposited in effluent in any day must not exceed 0.00250 kilogram per tonne of chlorine times the reference production rate of the particular plant. The regulations include provisions with respect to sampling, testing and reporting. Energy production – A Canada Wide Standard is currently being developed for the coal-fired electricity generation sector. This standard is expected to be finalized in 2005. Waste treatment, including incineration: Emission limits have been established for incinerators. They are expressed as a concentration of mercury in the exhaust gas exiting the facility. Each government may choose the most appropriate measures to implement the standard within their jurisdiction. Time frames for existing facilities range from 2003 for hazardous waste to 2006 for municipal and medical waste incinerators.
Waste water effluent - National Guidelines on Physical-Chemical-Biological Treatment of Hazardous Wastes recommend maximum concentrations of mercury of 0.1 mg/L, 0.001 mg/L and 0.1 mg/L respectively in waste water effluent. Import/Export of Waste - Mercury and its compounds are subject to CEPA (1999) provisions for the movement of hazardous waste if they meet the Transport of Dangerous Goods Regulations hazard criteria. Transportation regulations also apply to import and export of toxic substances and wastes containing mercury. Provincial Acts, regulations and guidelines on mercury - In addition to federal regulations, a number of provinces have acts, regulations and guidelines covering emissions from industrial sources. Information on the 2001 status of provincial acts and regulations is given in the Canadian submission to UNEP's Global Mercury Assessment, available from www.chem.unep.ch/mercury. For more information on mercury in Canada, see Environment Canada's web page on mercury website, http://www.ec.gc.ca/mercury/en/index.cfm 6.4 Russian Federation(Extracted from ACAP, 2004). "Results of a previous mercury study in Russia The Committee on Ecology of the State Duma of Russia and the Government of the Russian Federation issued the Order for the State Committee on Environment Protection in 1998 to develop a National Report “On mercury pollution of the environment of the Russian Federation and its impact on population health”. The main purpose of this work was to analyse mercury pollution of the environment and to determine the main sources of mercury pollution. A background analysis for the program, namely “Analysis of mercury pollution state of the environment of the Russian Federation” was developed by Scientific Research Institute on Problems of Resource Saving and Wastes Management. The main purposes of the study were to determine the main sources of mercury pollution and make recommendations for potential development of the National Program. The main sources of Hg pollution in Russian Federation were acknowledged to be production and consumption wastes. It was not possible to make comprehensive and accurate assessment of the input from each of the sources, due to a lack of the public control over consumption and application of mercury and Hg-containing compounds. Additionally it was figured out the distinctive features and conditions in Russia, which should be taken into account during elaboration of the national program, such as:
Moreover the regulatory basis was reviewed. The legal basis for mercury pollution management was elaborated in 1970-80-ies. The existing regulations usually cover general issues and do not include specific requirements. One of the key causes of mercury pollution was acknowledged a lack of the Hg-containing wastes management system, i.e. collection, storage, transportation and neutralization. Collection, storage and transportation of Hg-containing wastes are the bottleneck in the existing system of Hg-containing wastes utilization and neutralization. Lack of the agreed documents and existence of contradictory requirements of various agencies and local authorities hamper the process of effective collection and delivery of Hg-containing wastes to the disposal site. The authors of the document concluded that the problem of mercury pollution in Russian Federation is strongly depends on the implementation on the “Wastes” Federal target Program. The following activities were recommended for the Program' implementation targeted on Hg wastes management:
Regulation of Mercury Releases The content of mercury in different media is regulated by maximum allowed concentrations. The concentrations were fixed in the regulatory documents developed and adopted by the Ministry of Health of the USSR. Table 6-2 Main regulatory documents on environment and population protection from potentially dangerous pollutants including mercury and its compounds
Table 6-3 Maximum allowed concentration (MAC) of mercury in different media and allowable residues of mercury in foodstuff
Regulation on collection, package, transportation and utilization of mercury-containing wastes is given in the Instruction of the Ministry of Non-ferrous Metallurgy of the USSR, adopted in October 27, 1966. Many statements of the Instruction are outdated, that is the reason why there were developed regional rules on mercury-containing wastes management in almost each region where a company dealing with collection and treatment of mercury-containing wastes is located." 6.5 Selected regional initiativesIn the following, four existing regional initiatives, each covering parts of the Arctic countries, are described briefly with a focus on their relevance to mercury. These are the Heavy Metals protocol of the LRTAP Convention, The North American Regional Action Plan on Mercury, the OSPAR Convention and the HELCOM Convention. The descriptions were extracted from the Global Mercury Assessment (UNEP, 2002). For a more detailed description of the coverage of mercury in these agreements, see the "Background document on mercury in existing agreements" prepared for this ACAP mercury project. 6.5.1 The Convention on Long-Range Transboundary Air Pollution and its 1998 Aarhus Protocol on Heavy Metals (LRTAP Convention)The objective of the Convention on Long-Range Transboundary Air Pollution is to protect man and his environment against air pollution and to endeavour to limit and, as far as possible, gradually reduce and prevent air pollution including long-range transboundary air pollution. The Convention sets up an institutional framework, bringing together policy and research components. It establishes a number of co-operative programmes for assessing and monitoring the effects of air pollution. The Convention requires Parties to develop policies and strategies that will serve as a means of combating the discharge of pollutants, by means of exchanges of information, consultation, research and monitoring. Parties are also required to co-operate in the conduct of research into and/or development of technologies for reducing emissions of major air pollutants, instrumentation and other techniques for monitoring and measuring emission rates and ambient concentrations of air pollutants, improved models for understanding the transmission of long-range transboundary air pollutants, the effects of major air pollutants on human health and the environment and education and training programmes related to the environmental aspects of pollution by major air pollutants. Implementation of the Convention has already contributed successfully in reducing sulphur emissions across Europe, and there has also been progress in reducing emissions of nitrogen oxides and volatile organic compounds. Geographic coverage and entry into force of the protocol The Convention and its protocols are open to member states of the United Nations Economic Commission for Europe (UNECE), as well as states having consultative status with the UNECE and regional economic integration organizations, constituted by sovereign states members of the UNECE. The UNECE has 55 member States, mainly from Central and Eastern Europe, but also includes Canada and the United States of America as members. (see http://www.unece.org/oes/eceintro.htm for the list of UNECE member States). The Convention entered into force on 16 March 1983 and had 49 Parties as of 1 October 2002. Since its entry into force, it has been extended by eight protocols, of these the 1998 Aarhus Protocol on Heavy Metals is especially relevant to mercury. The Aarhus Protocol entered into force in December 2003. Among the present Parties are Canada, Denmark, Finland, Norway, Sweden and the United States. The Russian Federation has not yet ratified the protocol. The Executive Secretary of the UNECE provides the Secretariat for the Executive Body of the Convention. It does so within the UNECE Environment and Human Settlements Division. The 1998 Aarhus Protocol on Heavy Metals, and its relevance to mercury The Executive Body of the Convention adopted the Protocol on Heavy Metals on 24 June 1998 in Aarhus, Denmark. It targets three particularly harmful metals: cadmium, lead and mercury, and requires Parties to the Protocol to reduce their releases for these three metals. It aims to cut emissions from industrial sources (iron and steel industry, non-ferrous metal industry), combustion processes (power generation, road transport) and waste incineration. It lays down stringent limit values for emissions from stationary sources and suggests best available techniques for these sources. The Protocol requires Parties to phase out leaded petrol and introduces measures to lower heavy metal releases from other products. Emission levels must be reported using as a minimum methodologies specified by the Steering Body of EMEP, the Cooperative Programme for Monitoring and Evaluation of Long-range Transmission of Air Pollutants in Europe. Article 3 describes the basic obligations set out in the Protocol, below is a summary of those especially relevant to mercury. A) Reduction of total annual emissions of mercury into the atmosphere, compared to the reference year for the Party (1990, or an alternative year between 1985 and 1995 set when becoming a Party), through application of best available techniques, product control measures or other emission reduction strategies. B) Use of best available techniques for stationary sources - for new plants within 2 years, for existing plants within 8 years. The standards for best available techniques are given as examples in Annex III to the Protocol, and include both cleaning technology and substitution of mercury based technology, for example in chlor/alkali plants. C) Application of limit values to control emissions from major stationary sources, both new and existing – Limit values for a number of sources are specified in Annex V of the Protocol, for example for particulate emissions from combustion plants, mercury emissions from chlor-alkali plants and mercury emissions from municipal, medical and hazardous waste incineration. D) Application of product control measures concerning mercury – The Protocol requires Parties to achieve specific mercury levels in alkaline manganese batteries within 5 years, or 10 years for Parties with economies in transition. Alkaline manganese button cells and batteries composed of button cells are exempted from this obligation. In addition, Parties should consider applying additional product control measures as described in Annex VII of the Protocol. Recommendations are given for mercury-containing products such as electric equipment, electrical components (thermostats, switches), measuring devices (thermometers, manometers, barometers), fluorescent lamps, dental amalgam, pesticides including seed dressings, paints and batteries other than alkaline manganese batteries, and include prohibition of specific products, voluntary agreements and recycling programmes. Monitoring and Evaluation of Long-Range Transmission of Air Pollutants in Europe Associated with the LRTAP-process, the main objective of the EMEP programme (Co-operative Programme for Monitoring and Evaluation of the Long-Range Transmission of Air pollutants in Europe) is to regularly provide Governments and subsidiary bodies under the LRTAP Convention with qualified scientific information to support the development and further evaluation of the international protocols on release reductions negotiated within the Convention. Initially, the EMEP programme focused on assessing the transboundary transport of acidification and eutrophication; later, the scope of the programme has widened to address other issues covered by the Convention, such as POPs, heavy metals, including mercury, and particulate matter. The EMEP programme relies on three main elements: (1) collection of emission data, (2) measurements of air and precipitation quality and (3) modelling of atmospheric transport and deposition of air pollution. Through the combination of these three elements, EMEP fulfils its required assessment and regularly reports on emissions, concentrations and/or depositions of air pollutants, the quantity and significance of transboundary fluxes and related exceedances to critical loads and threshold levels. The combination of these components provides also a good basis for the evaluation and qualification of the EMEP estimates. The EMEP programme is carried out in collaboration with a broad network of scientists and national experts that contribute to the systematic collection, analysis and reporting of emission data, measurement data and integrated assessment results. Three different Task Forces - on measurements and modelling, on emission inventories and projections and on integrated assessment modelling - provide for discussion and scientific exchange. Canada and USA are not part of the EMEP region and conduct their own comparable national activities. The coordination and intercalibration of chemical air quality and precipitation measurements are carried out at the Chemical Coordinating Centre (CCC). The storage and distribution of reliable information on emissions and emissions projections is the task of the Meteorological Synthesizing Centre–West in Oslo, Norway. The modelling development for heavy metals and POPs is the responsibility of the Meteorological Synthesizing Centre -East (MSC-E) in Moscow, Russian Federation. In 1999, the Executive Body of the Convention decided to include integrated assessment into the core activities of EMEP and to establish a Center for Integrated Assessment Modelling (CIAM) building on past modelling work, in particular the RAINS (Regional Acidification, Information and Simulation) model. Review of the Heavy Metals Protocol With the entry into force of the Heavy Metals Protocol, a review of its sufficiency and effectiveness will commence. Initial work related to this review will take place in the Heavy Metals Task Force. During 2005 the work plan for the Task Force is anticipated to include the following activities (according to UNECE, December 2004, at http://www.unece.org/env/documents/2004/eb/air/eb.air.2004.3.e.pdf):
6.5.2 North American Regional Action Plan on MercuryThe Commission for Environmental Cooperation (CEC) is an international organization created by Canada, Mexico and the United States of America under the North American Agreement on Environmental Cooperation (NAAEC). The CEC was established to address regional environmental concerns, help prevent potential trade and environmental conflicts, and to promote the effective enforcement of environmental law. The Agreement complements the environmental provisions of the North American Free Trade Agreement (NAFTA). The Council, the governing body of the CEC, is composed of the environment ministers (or the equivalent) of each country. It meets at least once a year to discuss CEC programmes and activities. The Commission provided the mechanism for the three member countries to negotiate an agreement, Council Regulation #95-5 on the Sound Management of Chemicals, which was agreed to on 13 October 1995. The resolution sets out a framework, together with specific commitments, to work collaboratively in addressing the sound management of chemicals in the region. A Working Group was established to work with the CEC to implement the decisions and commitments made in the resolution. Since then, four North American Regional Action Plans, on DDT, chlordane, PCBs and mercury, have been developed and are now at various stages of implementation. The ultimate goal of the Action Plan on Mercury is to achieve a reduction in the anthropogenic releases of mercury to the North American environment through appropriate national and international initiatives, to amounts that can be attributed to naturally occurring levels and fluxes. The Parties intent is to obtain this goal by seeking to reduce mercury releases from human activities, develop enhanced capacity to measure and manage mercury, asses impact and communicate concerns, establish an equitable implementation and compliance protocol and promote continued responsible mercury management initiatives on behalf of governments, industry and citizens through regulatory and voluntary/non-regulatory mercury management actions. The plan sets out detailed recommendations for reducing emissions and releases of mercury from a large number of sources and activities. Examples of some of the specific recommendations made in the Action Plan are:
Although the regional Action Plans under the Sound Management of Chemicals initiative are not legally binding upon any one or all of the Parties to the North American Agreement on Environmental Cooperation, there is a strong national commitment by each member country to ensure that the Action Plan on mercury results in significant reductions of mercury contamination to the environment. The implementation of the Action Plan will be ensured through the oversight of an Implementation Task Force. 6.5.3 The Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR Convention)The objectives of the 1992 OSPAR Convention for the Protection of the Marine Environment of the North-East Atlantic are to take all possible steps to prevent and eliminate pollution and take the necessary measures to protect the sea area against the adverse effects of human activities and to safeguard human health and to conserve marine ecosystems and, where practicable, to restore marine areas which have been adversely affected. The Convention contains annexes addressing different sources of pollution, such as prevention and elimination of pollution from land-based sources; prevention and elimination of pollution by dumping or incineration (which prohibits incineration); prevention and elimination of pollution from offshore sources; assessment of the quality of the marine environment and protection and conservation of the ecosystems and biological diversity of the maritime area. Geographic coverage and entry into force The OSPAR Convention is open to Parties to the “Oslo” and “Paris” Conventions (i.e., the Convention for the Prevention of Marine Pollution from Land-Based Sources and the Convention for the Prevention of Marine Pollution by Dumping from Ships and Aircraft), any other coastal state bordering the maritime area, any state located upstream on watercourses reaching the maritime area or any regional economic integration organisation having a member state that qualifies. The maritime area covers the north-east Atlantic including the North Sea and comprises the internal waters and the territorial sea of Parties, the sea beyond and adjacent to the territorial sea under the jurisdiction of the coastal state, and the high seas. Other States or regional economic organisations that do not satisfy the criteria may be invited unanimously by the Parties to accede to the Convention. The OSPAR Convention came into force on 25 March 1998. It replaced the Paris and Oslo Conventions. However, Decisions, Recommendations, and other agreements adopted under the two previous Conventions continue to be applicable, unaltered in their legal nature, unless they are terminated by measures adopted under the OSPAR Convention. The OSPAR Convention currently has 16 Parties (Belgium, Denmark, European Union, Finland, France, Germany, Iceland, Ireland, Luxembourg, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland and the United Kingdom of Great Britain and Northern Ireland). The OSPAR Commission, with representatives of each of the Parties, is the governing body of the Convention. The Commission meets annually, sometimes at ministerial level. The OSPAR Strategy with regard to Hazardous Substances, and its relevance to mercury In 1998 at Sintra, Portugal, the first ministerial meeting of the OSPAR Commission adopted, among others, a Strategy with regard to Hazardous Substances, with a view to the further implementation of the OSPAR Convention, which had just came into force. The objective of the Strategy is to prevent pollution of the maritime area by continuing to reduce discharges, emissions and losses of hazardous substances, with the ultimate aim of achieving concentrations in the marine environment near background values for naturally occurring substances and close to zero for man-made synthetic substances. The Strategy also includes a timeframe, setting out the basis for OSPAR's work for achieving the objective - every endeavour will be made to move towards the target of cessation of discharges, emissions and losses of hazardous substances by the year 2020. To this end, a process has been established to identify the OSPAR list of chemicals for priority action. This list was revised in 2001, and currently contains 42 substances or groups of substances, including mercury and organic mercury compounds. These chemicals are being addressed by preparing (for those in use in the OSPAR area) background documents for each substance or group specifying the sources of inputs of them to the marine environment, the threat posed and possible measures. Such measures are then considered. An OSPAR Background Document on Mercury and Organic Mercury Compounds (OSPAR Commission, 2000) was endorsed by OSPAR in 2000 and the actions recommended there are taken into account, as appropriate, in the work of OSPAR. There are several measures applicable under OSPAR to control mercury emissions, discharges and losses from specific sectors, e.g. the measures related to the chlor-alkali industry and PARCOM Decision 85/1 on Limit Values and Quality Objectives for Mercury Discharges by Sectors other than the Chlor-alkali Industry. Furthermore, OSPAR measures on Best Available Techniques (BATs) for various industrial installations and the offshore gas and oil installations will also help to limit discharges, emissions and losses of mercury. With regards to the chlor-alkali sector, there are a number of measures applicable as regards the control of mercury in discharges to water and emissions to air. In PARCOM Decision on New Chlor-Alkali Plants Using Mercury Cells, 1982 the Commission decided that authorisations for new chlor-alkali plants might be granted by Parties only if such authorisations were based on application of best technical means available for preventing discharges of mercury. Best technical means available at that time made it possible to limit discharges of mercury using the recycled-brine process to less than 0.5 g/metric ton of installed chlorine production capacity. Furthermore, the Commission agreed that when the construction of new plants was being considered, the use of mercury-free technology, in particular membrane cells should be encouraged whenever circumstances permitted. In PARCOM Decision 90/3 on Reducing Atmospheric Emissions from Existing Chlor-Alkali Plants, adopted on 14 June 1990, the Parties agreed that existing mercury based chlor-alkali plants would be required to meet by 31 December 1996 a standard of 2g Hg/t Cl2 capacity for emissions to the atmosphere, unless there was a firm commitment that the plant would be converted to mercury-free technology by the year 2000. It also agreed that mercury in hydrogen released to the atmosphere, or burnt, would be included in this standard. They also recommended that existing mercury cell chlor-alkali plants be phased out as soon as practicable and set the objective of complete phase-out by 2010. The chlor-alkali producers within the OSPAR area have met the emissions reduction requirements of PARCOM 90/3. In order to make progress towards the other recommendations within this decision they have presented six voluntary commitments with OSPAR. The details are provided in section 3.2.4 EUROPEAN COMMMUNITY in the description on chlor-alkali production. The main tools for controlling releases of mercury from products are the placing of restrictions on the marketing and use of the products, or the development of products containing non-hazardous substitutes for mercury. Mercury discharges from the dental sector - Several PARCOM Recommendations relating to the reduction of mercury discharges from dental sources are applicable under OSPAR. In 1981, the Paris Commission recommended the installation of special filters in dental surgeries and clinics to collect the residues of mercury amalgams. PARCOM Recommendation 89/3 on Programmes and Measures for Reducing Mercury Discharges from Various Sources urges that alternative materials to dental amalgams should be used where appropriate and where excessive cost can be avoided. Surplus or old amalgam should be trapped and separated efficiently, then sent for recovery of the mercury content. PARCOM Recommendation 93/2 on Further Restrictions on the Discharge of Mercury from Dentistry states that equipment should be installed to separate water and amalgam to enable collection of the amalgam as from 1 January 1997. Mercury in batteries - PARCOM Decision 90/2 on Programmes and Measures for Mercury and Cadmium-Containing Batteries lays down various measures dealing with the recovery, disposal and marketing and use of certain mercury and cadmium batteries. Pesticides containing mercury - PARCOM Recommendation 89/3 also proposed measures on restricting the use of biocides and pesticides containing mercury. Industrial, laboratory and medical control instruments and electrical equipment - PARCOM Recommendation 89/3 also proposes measures on recycling mercury used in such equipment and encouraged the use of equipment not containing mercury, whenever replacements become available at comparable costs. Some Parties have initiated actions for example to limit the use of mercury thermometers, to encourage the development of low-mercury lighting and to establish recycling and special collection schemes. 6.5.4 The Convention on the Protection of the Marine Environment of the Baltic Sea Area (Helsinki Convention)The objectives of the Helsinki Convention on the Protection of the Marine Environment of the Baltic Sea Area, adopted on 9 April 1992, are to take all appropriate measures, individually or by means of regional co-operation, to prevent and eliminate pollution in order to promote the ecological restoration of the Baltic Sea Area and the preservation of its ecological balance. The Convention establishes fundamental principles and obligations, as set out in Article 3, whereby Parties are obliged to:
Geographic coverage and entry into force The Helsinki Convention is restricted to the States and the European Community that participated in the 1992 Helsinki Conference and have ratified the Convention. Others can become a party upon invitation by all the Parties. The Convention covers the Baltic Sea and the entrance of the Baltic Sea and the drainage areas to these waters. Internal waters are included. The 1992 Helsinki Convention replaces the 1974 Convention on the Protection of the Marine Environment of the Baltic Sea Area. It entered into force on 17 January 2000. As of October 2002, the Helsinki Convention had 10 Parties (Denmark, Estonia, European Community, Finland, Germany, Latvia, Lithuania, Poland, Russia and Sweden). The governing body of the Convention is the Helsinki Commission - Baltic Marine Environment Protection Commission (HELCOM). HELCOM meets annually and, from time to time, meetings are held at ministerial level. The HELCOM strategy to implement its objective with regard to hazardous substances, and its relevance to mercury In 1998 HELCOM established an objective with regard to hazardous substances and a strategy to implement the objective, through the adoption of HELCOM Recommendation 19/5. The objective is to prevent pollution of the Convention Area by continuously reducing discharges, emissions and losses of hazardous substances towards the target of their cessation by the year 2020, with the ultimate aim of achieving concentrations in the environment near background values for naturally occurring substances and close to zero for man-made synthetic substances. A total of 42 chemicals have so far been selected by HELCOM for immediate priority action, including mercury and its compounds. HELCOM has adopted a number of recommendations specifically relating to mercury:
The HELCOM strategy on hazardous substances, including mercury, in many areas parallels the work implemented within the context of the OSPAR Convention. ReferencesACAP (2004): Assessment of mercury releases from the Russian Federation. Russian Federal Service for Environmental, Technological and Atomic Supervision and Danish Environment Protection Agency for Arctic Council. December 2004. AMAP (1998): Assessment report: Arctic Pollution Issues. Arctic Monitoring and Assessment Programme, Oslo, 1998. AMAP (1997): Arctic Pollution Issues: A State of the Arctic Environment Report. Arctic monitoring and Assessment Programme (AMAP), Oslo, Norway. AMAP (1998): AMAP Assessment Report: Arctic Pollution Issues. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway. AMAP (2000): AMAP Report on Issues of Concern: Updated Information on Human Health, Persistent Organic Pollutants, Radioactivity, and Mercury in the Arctic. September 2000. AMAP Report 2000:4 AMAP (2002): Arctic Pollution 2002 (Persistent Organic Pollutants, Heavy Metals, Radioactivity, Human Health, Changing Pathways). Arctic Monitoring and Assessment Programme (AMAP), Oslo. Danish Energy Authority (2003): Energy statistics 2002. Accessed December 2003 at http://www.ens.dk/graphics/Publikationer/Statistik/ Danish EPA (2001): Press release dated 25. January 2001. On www.mst.dk/presse/06011700.htm. Durkee S. (2003): Personal communication. USEPA, Washington DC, USA. EMEP/CORINAIR (2002): Emission Inventory Guidebook - 3rd edition, October 2002 UPDATE, EEA, Technical report No 30. Accessed Dec. 2003 on http://reports.eea.eu.int/EMEPCORINAIR3/en Endre, B., Einarsson, S., Nyström, M., Rahbek, L.W., von Rein, K. and Hansen, E. (secretary) (1999): Treatment and disposal of mercury waste – Strategic elements proposed by a Nordic expert group. TemaNord 1999:554, Nordic Council of Ministers, Copenhagen. Environment Canada (2000): The status of mercury in Canada, Report #2 - A background report to the Commission for Environmental Cooperation North American Task Force on Mercury. Transboundary Air Issues Branch, Environment Canada. Environment Canada, Minerals and Metals Division, National Office of Pollution Prevention (2002): Multi-pollutant emission reduction analysis foundation (MERAF) for the base metal smelter sector. Prepared for Environment Canada and The Canadian Council of Ministers of Environment (CCME), Canada. Available at http://www.ccme.ca/assets/pdf/bms_final_meraf_e.pdf (accessed October 2003). EU - European Union (1996): Directive 96/91/EU, "The integrated pollution prevention and control (IPPC) Directive". European Commission (2001): Integrated pollution prevention and control (IPPC) - Reference document on best available techniques in the non ferrous metals industry. Available at: http://eippcb.jrc.es/pages/Fmembers.htm (accessed October 2003). European Commission (2003): Draft reference document on best available techniques for management of tailings and waste rock in mining operations. Joint Research Centre, Seville, Spain. Available at: http://eippcb.jrc.es/pages/Fmembers.htm (accessed October 2003). Fugleberg S (1999): Finnish expert report on best available techniques in zinc production. The Finnish Environment series 315, Finnish Environment Institute, Helsinki. Available at http://www.vyh.fi/eng/orginfo/publica/electro/fe315/fe315.htm Hansen C. L. and Hansen E. (2003): Collection systems for batteries - existing experiences from Denmark and abroad. Environmental project no. 777, 2003, Danish Environmental Protection Agency (in Danish with summary in English). Available at www.mst.dk; publications. Huse A., Lindmark G.M., Sørensen P.L., Heie Å., Weholt Ø., Mroueh, U. and Wahlström M. (1999): Categories and Quantities of Mercury Waste, and Treatment Capacity in the Nordic Countries. Interconsult AS and VTT Chemical Technology, for the Nordic Council of Ministers. TemaNord 1999:545, Environment, 1999. International Energy Agency (2003): Energy statistics of non-OECD countries - 2000-2001. OECD/IEA, Paris, France. International Energy Agency (2003b): Energy statistics of OECD countries - 2000-2001. OECD/IEA, Paris, France. KEMI - National Chemicals Inspectorate (1998): Kvicksilveravveckling i Sverige - redovisning av ett regeringsuppdrag (Substitution of mercury in Sweden). KEMI, 5/98, The Chemicals Inspectorate, Solna, Sweden (in Swedish with English summary). Maag J., Hansen E. and Dall O. (2002): Mercury - a global pollutant requiring global initiatives. TemaNord 2002:516, Nordic Council of Ministers, Copenhagen. Available at www.norden.org. Munthe J; Wängberg, I.; Chugaeva, A.N.; Kiseleva, N.V.; Smigol I.N.; Bragina O.N., Anichkov, S.N.; Tumanovsky, A.G (2003): Emissions of mercury from coal fired power plants in Russia - preliminary estimated for ACAP. IVL Swedish Environmental Research Institute, Sweden and VTI All Russia Thermal Engineering Institute. (In English) OECD (1994): Mercury - Background and national experience with reducing risk. Risk reduction monograph no. 4. OECD, Paris, 1994 (web-version from http://www.oecd.org//ehs/risk.htm is dated 1995). Pacyna J (2003): Development of methods for trace metal emission evaluation and their implementation for flux estimates in the NIS territory, including economic aspects of flux reduction. INTAS No. 97-31581. Pacyna, J.M. and Pacyna, E.G. (2000): Assessment of emissions/discharges of mercury reaching the Arctic environment. The Norwegian Institute for Air Research, NILU Report OR 7/2000, Kjeller, Norway. Rentz O, Sasse H, Karl U, Schleff HJ, Dorn R (1996): Emission control at stationary sources in the Federal Republic of Germany - Volume II, Heavy metal emission control. French-German Institute for Environmental Research (DFIU), University of Karlsruhe, 1996 (submitted by Germany for the global Mercury Assessment). RVF (2003): Swedish Waste Management 2003. The Swedish Association of Waste Mangement (RVF), Malmö, Sweden. Skaarup S., Christensen C.L., Maag J and Jensen S.H (2003): Substance flow assessment for mercury. Environmental project, Danish Environmental Protection Agency, 2003 (in English). Available at www.mst.dk. Sleire B (2003): Personal communication. SFT, Oslo, Norway. Sznopek, J.L. and Goonan, T.G. (2000): The materials flow of mercury in the economies of the United States and the world. USA Geological Survey Circular 1197, vers. 1.0, USA Geological Survey, Nov. 2000, downloaded from http://greenwood.cr.usgs.gov/pub/circulars/c1197/ in January 2001. Available at http://minerals.usgs.gov/minerals/pubs/commodity/mercury/. UNFCCC (2003): Kyoto Protocol status of ratification. UNFCC, November 2003. Accessed in December 2003 at http://unfccc.int/resource/kpstat.pdf USEPA (1997): Mercury study report to congress. USEPA, Dec. 1997. Downloaded from http://www.epa.gov/airprogm/oar/mercury.html, January 2001. USEPA (1998): AP-42, Fifth Edition, Volume I, Chapter 1: External Combustion Sources. Accessed Dec. 2003 on http://www.epa.gov/ttn/chief/ap42/ch01/final/c01s01.pdf USEPA (2002): Control of Mercury Emissions from Coal-fired Electric Utility Boilers, Interim Report Including errata Data 3-21-02. EPA-600/R-01-109, National Risk Management Research Laboratory, Research Triangle Park, NC, April 2002. Available at http://www.epa.gov/appcdwww/aptb/EPA-600-R-01-109corrected.pdf. USEPA-ORD (2000): Mercury research strategy. Office of Research and Development. Available at http://www.epa.gov/ordntrnt/ORD/NRMRL/mercury/600R00073%20front.pdf USEPA-ORD (2004): Control of Mercury Emissions from Coal-fired Electric Utility Boilers. Triangle Park, North Carolina, January 2004. USGS (2003): Personal communication with Robert Seal and USGS colleagues in his network. United States Geological Surveys, 2003. von Rein, K. and Hylander, L. D. (2000): Experiences from phasing out the use of mercury in Sweden. Regional Environmental Change 1: 126-134. Appendices: Links to national responses to mercury questionnaireIntroduction to the ACAP mercury questionnaire The appendices will be available at www.mst.dk and can be accessed by pressing the links below. Introduction to the ACAP mercury questionnaireCanada, questionnaire responseDenmark, questionnaire responseFinland, questionnaire responseNorway, questionnaire responseSweden, questionnaire responseThe United States of America, questionnaire responseThe United States of America, memo on methods for release estimation for questionnaireAssessment of Mercury Releases from the Russian Federation (ACAP, 2004).Largest point sources identified in the Assessment of Mercury Releases from the Russian Federation (ACAP, 2004).Footnotes[1] Atmospheric releases from coal combustion in Iceland roughly estimated here at about 5 Kg/year based on consumption data submitted by Iceland (150.000 tons coal/year) and the emission factor for coal in Denmark (0.04 g Hg/ton coal; see table 4.3). No data on whether emission reduction systems are used were submitted from Iceland. The Danish emission factor reflects a situation where about half of the mercury input is captured in emission reduction systems. [2] Capacity values have been obtained from EMF controls available in “EPA's 2003 Clear Skies Act parsed file for 2010” available at http://www.epa.gov/airmarkets/epaipm/results2003.html. The capacity values have been rounded to the nearest whole number. [3] Because the U.S. used Standard Industrial Codes (SIC codes) and there is no code for primary zinc (Zn) smelting, emissions from this source category for the U.S. submission to this project are included in the “Other Primary Extraction of Metals” category in the ACAP mercury questionnaire. [4] Sweden, Finland and Denmark are EU members. Iceland and Norway are not EU members. They are part of the EFTA-agreement with the EU.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||