|
| Front page | | Contents | | Previous | | Next |
Survey, migration and health evaluation of chemical substances in toys and childcare products produced from foam plastic
3 Health assessment
Based on the results of the analysis of diisobutylphthalate (DIBP), di-n-butylphthalate (DBP), diisononylphthalate (DINP), monobutyltin (MBT) and dibutyltin (DBT) were selected for assessment. The toxicological profiles for the 5 chemical substances are described in the following.
3.1 Toxicological profiles
3.1.1 Diisobutylphthalate
3.1.1.1 Use
Is used as softener in plastic, paint, lacquer, glue and die (8).
3.1.1.2 Identification
| Chemical name |
Diisobutylphthalate |
| Synonym |
DIBP |
| CAS-No. |
84-69-5 |
| EINECS No. |
201-553-2 |
| Molecular formula |
C16-H22-O4 |
| Molecular Structure |
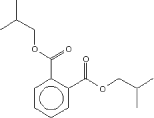 |
| Classification (9). |
Not classified
Based on new evaluations DK has suggested, a classification of Rep 2; R60-61 like DEHP |
| Limit value (10). |
3 mg/m³ |
| The list of unwanted substances (11). |
Not listed |
| ECB (European Chemicals Bureau) (12). |
The substance has not been risk assessed by the EU and is not on the priority list on assessment and control regarding risk and control of existing substances. |
| Synoptic document (13) |
Listed as an additive permitted in packaging for feedstuffs with a [2]group-R: 0.05 mg/kg bw[3]. |
Physical-chemical properties (14).
| Physical state |
Liquid |
| Mol weight (g/mol) |
278.35 |
| Melting point,°C |
- 64 |
| Boiling point,°C |
296.5 |
| Evaporation rate (Pa) |
0.89 at 25°C |
| Octanol-water partition coefficient |
Log Pw = 4.11 |
| Water solubility (mg/l) |
20.3 at 20°C |
| Acute toxicity |
Oral [4]LD50 = 15 g/kg body weight for rats (15).
Dermal LD50 = 10 g/kg bw for guinea pigs (15). |
| Irritation |
Testing for irritation of skin and eyes in rabbits according to the [5]OECD guideline 404 and 405 did not show signs of irritation on skin and eyes (16). |
| Allergy |
No data have been found on the skin sensitizing effect of DIBP. DIBP can be compared with linear DIBP, which has no sensitizing effect on skin and is thus not expected to cause skin sensibilization. (17). |
| Short-term exposure |
A NOEL[6] of 50 mg DIBP/kg bw based on liver effects was proposed. The NOEL was based on a test with female rats, which were administered orally 50 mg DIBP/kg bw for fourteen days. No data on other tested doses (17). |
| Long-term/ repeated exposure |
Rats administered 5% DIPB in the feed for several months showed no increased mortality. Total dose and number of months not indicated (8).
Dogs administered 2 g of DIBP/kg bw in the feed for several months (total not indicated) did not show increased mortality and no toxic effect was observed. (8). |
| Cancer |
No data were found on the carcinogenic properties of DIBP. Long-term studies of the carcinogenic properties of other phthalates with rodents demonstrate that tumors are mainly induced by an peroxisome proliferation mechanism. However, this mechanism is not found in humans, and indication of carcinogenic properties in rodents does not prove that it is carcinogenic to humans. |
| Mutagenicity |
DIBP showed a negative effect in the Salmonella typhimurium mutagenicity test (18) |
| Reproduction |
Groups of 5 gestated rats were administered DIBP corresponding to 0.390 0.779 or 1.299 ml/kg bw on day 4, 5, 10 and 15 of the period of gestation. In this study the highest dose was calculated to be one third of LD50 for intraperitoneal administration (3.9 g/kg bw). This value is lower than the oral LD50 found by DTC of 15 g/kg bw. The occurrence of resorption was 26.6% in the highest dosage group compared to 0% in the control group. The birth weight of all three dosage groups was lower than that of the control group. Abnormal skeletal formations were found in all three dosage groups (19).
Intake of 2% of DIPB through the feed for 7 days caused decreased zinc concentrations in the testicles of mouse and an increase of the relative weight of the testicles. The total dose is not indicated (20). |
| Critical effect |
A NOEL of 50 mg DIBP/kg bw based on effect of the liver was stated. No other dose levels are mentioned (17). |
The highest amount of DIBP measured in the 6 products (product 4 and 7 are not included as they were withdrawn from the Danish market) was 6.5 mg/kg in product 5 (corresponding to 0.00065%). The amount is markedly lower than the permitted limit for phthalates in toys intended for children between 0-3 years of 0.05% (3) and the substance is not further assessed.
3.1.2 Di-n-butylphthalate
3.1.2.1 Use
Is used as softener in plastic, paint, lacquer, glue and die (8).
3.1.2.2 Identification
| Chemical name |
Di-n-butylphthalate |
| Synonym |
DBP |
| CAS-No. |
84-74-2 |
| EINECS No. |
201-557-4 |
| Molecular formula |
C16-H22-O4 |
| Molecular Structure |
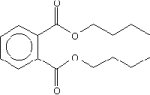 |
| Classification (9). |
Repr. Cat. 2; R61, Repr. Cat. 3; R62, N; R50 |
| Limit value (10). |
3 mg/m³ |
| The list of unwanted substances (11). |
Listed |
| ECB (European Chemicals Bureau). |
Risk assessed by EU in 2003. Addendum published in 2004 . |
| Synoptic document (13) |
Listed as an additive permitted in packaging for foodstuffs with a TDI[7] = 0.1 mg/kg bw. |
Physical chemical properties (14).
| Physical state |
Liquid |
| Molecular weight (g/mol) |
278.35 |
| Melting point,°C |
- 35 |
| Boiling point°C |
340 |
| Evaporation rate (Pa) |
0.0027 at 25°C |
| Octanol-water partition coefficient, |
Log Pow = 4.9 |
| Water solubility (mg/l) |
13 at 20°C |
| Acute toxicity |
Oral LD50 = 6,3-8,0 g/kg bw for rats (21).
Dermal LD50 > 20 g/kg bw for rabbits (21).
A 23-year old man, who accidentally ingested 10 g DBP, showed symptoms of nausea, vomiting and dizziness, and a few hours later lacrimation, photophobia and pain in the eyes. The cornea was severely damaged. Urinalysis showed
microhaematuria, oxalate crystals and pathological leucocyte counts. Recovery occurred within 14 days after treatment (21). |
| Irritation |
Test for irritation of skin and eyes with rabbits in accordance with OECD guideline 404 and 405 did not show any sign of irritation of skin and eyes (21). |
| Allergy |
DBP did not show skin sensitisation effects in two maximization tests in guinea pigs. (21) |
| Short-term exposure |
In a two-week dietary study with rats, a NOAEL[8] for induction of peroxisomally associated enzymes were estimated to be 200 mg/kg of the feed (corresponding to 19.9 mg/kg bw (21).
In a four-week dietary study with rats, a NOAEL value for increased enzyme activity was estimated at 104 mg/kg bw. However it should be mentioned that the liver weight of the animals significantly increased significantly at all dose levels: (51.5; 104; 515; 1,040 and 2,600 mg/kg bw (21). |
| Long-term /repeated exposure |
Based on a 3 months dietary study, an oral NOAEL of 152 mg/kg bw has been established (21).
A NOAEL of 19.9 mg/kg bw has been established for peroxisomal proliferation in rats (21).
In an inhalation study performed in accordance with OECD Guideline no. 412, rats were exposed for 6 hours/day, 5 days/week during 4 weeks at concentrations of 0; 1.18; 5.57; 49.3 or 509 mg DBP /m³. The exposure by repeated inhalation caused no systemic effects at the highest concentration, 509 mg DBP/m³. NOAEC[9] could not be established, as even the lowest exposure effected the upper respiratory tract. A LOAEC[10] for rats of 1.18 mg/m³ was established for local effects in the upper respiratory tract (21). |
| Carcinogenicity |
Sufficient data on DBP’s carcinogenic properties are not available.
Long-term studies of the carcinogenicity of other phthalates in rodents demonstrate that tumours are mainly provoked by an peroxisomal proliferation mechanism. However, This mechanism is not found in humans, and indication of carcinogenicity in rodents does not prove that it is carcinogenic in humans. |
| Mutagenicity |
DBP was positive in Salmonella typhimurium mutagenicy test (18).
Based on tests with other phthalates and in vitro/in vivo studies of DBP’s genotoxic effects the substance is assessed to be non-genotoxic by the EU (21) |
| Reproduction |
In an extensive 2-generation reproduction study in rats with continuous breeding and exposure of both female and male animals, the animals were administered 0; 0.1; 0.5; and 1.0% in the feed, 0.1% in the feed (corresponding to 52 mg/kg bw for males and 80 mg/kg bw for females) corresponds to the LOAEL[11] in the study.
Several determinations of NOAEL-values are shown in other studies, but as the above test has incorporated a number of more sensitive endpoints, it takes into account fx. endocrine disrupting effects. Therefore, the EU risk assessment uses the LOAEL-value of 52 mg/kg bw for further assessment (21). |
| Critical effect |
The critical effect is assessed to be reduced weight of
offspring in litter no. 2 corresponding to a LOAEL of 52 mg/kg bw (21).
A worst-case exposure of children of 0.81 µg/kg bw/day was estimated for DBP. The estimate was based on a maximal migration rate of 259 µg/dm²/day (measured in a Danish investigation) converted to a daily DBP dose, under the assumption of a child weighing 8 kg mouthing 10 cm² of the toy for 6 hours a day (21).
In the EU risk assessment of DBP, the assumed worst-case infant exposure of 0.81 µg/kg bw and the LOAEL value of 52 mg/kg bw/day was compared resulting in a MoS[12] of approx. 65,000, which is assumed to be sufficient for the protection of children (21). |
The highest amount of DIBP measured in the remaining 6 products was 11.9 mg/kg in product 6 (corresponding to 0.00119%) The amount is considerably lower than the permitted limit for phthalates in toys intended for children between 0-3 years of 0.05% (3) and the substance is not further assessed.
3.1.3 Diisononyl Phthalate
Use
Used as softener in plastic (8).
Identification
| Chemical name |
Diisononylphthalate |
| Synonym |
DINP |
| CAS-No. |
28553-12-0 |
| EINECS No. |
249-079-5 |
| Molecular formula |
C26-H42-O4 |
| Molecular Structure |
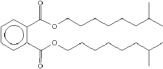 |
| Classification (9). |
Not classified |
| Limit value (10). |
3 mg/m³ |
| The list of unwanted substances (11). |
Not listed |
| ECB (European Chemicals Bureau)(12). |
Risk assessed by EU in 2003. Addendum published in 2004. |
| Synoptic document (13) |
Listed as an additive permitted in packaging for feedstuffs |
Physical-chemical properties (14), (22)
| Physical state |
Viscous liquid |
| Molecular weight (g/mol) |
418.62 |
| Melting point,°C |
-54 - -42 |
| Boiling point,°C |
244-274 |
| Evaporation rate(Pa) |
7.2 * 10 -5 at 25°C |
| Octanol-water partition coefficient |
Log Pow = 8.8 |
| Water solubility (mg/l) |
0.2 at 20°C |
| Acute Toxicity |
Intake of up to 40 g DINP/kg bw caused respiratory trouble but no deaths in rats. Oral LD50 > 40 g/kg bw for rats (22).
Dermal LD50 > 3.2 g/kg bw for rabbits (22). |
| Irritation |
DINP causes slight irritation to skin and eyes. Irritating effects cease shortly. (22). |
| Allergy |
A toy producer has reported five cases of allergy due to misuse of a toy produced from a material containing DINP. None of these cases, however, could be directly related to DINP.
A patch test with repeated human exposure showed not positive reactions. Totally, the various cases only indicate that DINP may cause allergy in humans (22).
Two tests of DINPs sensitizing properties in guinea pigs have shown that DINP is slightly sensitizing and not sensitizing respectively (22). |
| Short-term exposure |
Single-dose tests to determine a LD50 value have been performed, but not multiple-dose tests for a short period of time. The tests for determination of LD50 values have been performed before the guidelines were established for which reason DINP is assessed to have low toxicity at short-term exposure, however they are not consistent (22). |
| Long-term/repeated exposure |
Groups of rats were orally administered 500, 1,500; 6,000 or 12,000 mg/kg through the feed for 2 years. Based on this test, a NOAEL value of 88 mg/kg bw (corresponding to 1,500 mg/kg/feed) was proposed with respect to negative effects on both liver and kidneys (22). |
| Carcinogenicity |
Sufficient data on DINPs carcinogenic properties in humans have not been identified. Long-term studies of DINPs carcinogenic properties in rodents demonstrate that tumours are mainly induced by a peroxisome proliferation mechanism. However, This mechanism is not found in humans, and indication of carcinogenic properties in rodents does not prove that it is carcinogenic in humans. |
| Mutagenicity |
Based on in vitro and in vivo studies of DINPs genotoxic effects, EU has assessed the compound to be non-genotoxic.
(22). |
| Reproduction |
By repeated exposure of mice for 104 weeks by ingestion, DINP causes reduced testis weight. Due to the effects on testis weight, a NOAEL of 276 mg/kg/day was set in this study (22).
In a one generation study with rats, the dams were exposed to DINP by oral ingestion in the gestation period. A reduced number of live-born pups and congenital malformations were observed at doses, which were toxic for the dams. NOAEL for reduced number of live-born pups was 622 mg/kg bw/day, and NOAEL for congenital malformation was 500 mg/kg bw/day (22).
DINP demonstrates a slight estrogenic effect on cells in vitro.
In the EU risk assessment, the above does not justify a classification of DINP as toxic to reproduction (22). |
| Critical effect |
Based on animal studies, the critical organs by long-term DINP exposure are liver and kidneys. The NOAEL for liver and kidney effects has been established at 88 mg/kg bw/day for rats. This NOAEL protects from the toxic effects on reproduction mentioned above (22). |
The highest amount of DIBP measured in the 6 remaining products was 935 mg/kg in product 1 (corresponding 0.0935%.) The amount is higher than the permitted limit for phthalates in toys intended for children between 0-3 years of 0.05% (3) However, as the product is intended for children > 3 years, it has not been withdrawn from the Danish market. The distributor of product 1 has been informed of the content of DINP in the substance and is working on eliminating the substance from the product.
3.1.4 Monobutyltin
Use
Is used as catalyst in the production of PUR foam. Organic tin compounds are also used in other kinds of polymers (e.g. PVC), packaging for foodstuff, pesticides and paints (23).
Identification
| Chemical name |
Monobutyltin |
| Synonym |
- |
| CAS-No. |
78763-54-9 |
| EINECS No. |
- |
| Molecular formula |
C4-H9-Sn3+ |
| Molecular structure |
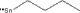 |
| Classification (9). |
Not classified |
| Limit value (10). |
0.1 mg/m3 (Tin compounds, organic calculated as Sn) |
| The list of unwanted substances (11). |
Not listed |
| ECB (European Chemicals Bureau) (12). |
Not assessed by ECB |
| Synoptic document (13) |
Not listed |
Physical-chemical properties
| Physical state |
- |
| Molecular weight (g/mol) |
175.83 |
| Melting point,°C |
- |
| Boiling point,°C |
- |
| Evaporation (Pa) |
- |
| Octanol-water partition coefficient, (log Pow) |
- |
| Water solubility (mg/l) |
- |
No data were found on the toxic effects of monobutyltin in animals and humans, by searching toxicological databases (Micromedex[13] and TOXNET[14]) and standard literature at DTC. The compound is assessed in section 3.1.7.
3.1.5 Dibutyltin
Use
Is used as catalyst in the production of PUR foam. Organic tin compounds are also used in other kinds of polymers (e.g. PVC), packaging for foodstuff, pesticides and paints (23).
Identification
| Chemical name |
Dibutyltin |
| Synonym |
- |
| CAS-No. |
1002-53-5 |
| EINECS No. |
- |
| Molecular formula |
C8-H20-Sn |
| Molecular structure |
- |
| Classification (9). |
Ikke klassificeret |
| Limit Value (10). |
0.1 mg/m3 (tin compounds organic, calculated as Sn) |
| List of unwanted substances (11). |
Not listed |
| ECB (European Chemicals Bureau) (12). |
Not assessed by ECB |
| Synoptic document (13) |
Not listed |
Physical-chemical properties
| Physical state |
- |
| Molecular weight (g/mol) |
234.97 |
| Melting point,°C |
- |
| Boiling point,°C |
- |
| Evaporation (Pa) |
- |
| Octanol-water partition coefficient, (log Pow) |
- |
| Water solubility (mg/l) |
- |
| Acute toxicity |
Oral LD50 = 800 mg/kg bw for rats (24). |
| NOAEL |
NOAEL = 2.5 mg/kg/day (teratogenicitet) (25).
NOAEL = 5.0 mg/kg/day (maternal toksicitet) (25). There is no description of or reference to the studies that the NOAELs are based on. |
Sufficient data has not been found on the toxic effects of DBT in animals and humans. Literature has been searched for in toxicological databases (Micromedex and TOXNET) and standard literature at DTC. The substance is assessed in section 3.1.7.
3.1.6 Tin compounds generally
Tin can combine with other chemicals to form compounds. Combinations with chemicals like chlorine, sulfur, or oxygen are called inorganic tin compounds. These are used in toothpaste, perfumes, soaps, food additives and dyes. Tin also can combine with carbon to form organic tin compounds like the compounds above, monobutyltin and dibutyltin (23).
Tin metal is used to line cans for food and beverages. Exposure to inorganic tin compounds can occur by eating food or drinking liquids from tin-lined cans (today greater than 90% of tin-lined cans used for food are protected with lacquer).
Metallic tin is not very toxic due to its poor gastrointestinal absorption. Human and animal studies show that ingestion of large amounts of inorganic tin compounds can cause stomachache, anemia, and liver and kidney problems (23).
Exposure to some organic tin compounds can occur by eating seafood from coastal waters (23) or from contact with consumer products i.e. toys produced from PVC or PUR, supports in shoes, biker’s pants, foot spray and silicon coated cooking paper (26). Inhalation of dust can also cause exposure to organic tin compounds from floor- and wall covers that releases organic tin compounds due to wear and tear. Levels of MBT and DBT have been found in dust from European households of respectively 2.8 and 1.3 mg/kg dust (27). Breathing or swallowing, or skin contact with some organotins, such as trimethyltin and triethyltin compounds, can interfere with the way the brain and nervous system work. In severe cases, it can cause death. Some organic tin compounds, have been shown to affect the immune system in animals and to affect the reproductive system. This has not been examined in people (23).
Inorganic or organic tin compounds placed on the skin or in the eyes can produce skin and eye irritation. There is no evidence that tin or tin compounds cause cancer in humans. Studies in animals have not shown evidence of carcinogenicity for inorganic tin. A study in rats and another in mice showed that a specific organic tin compound, triphenyltin hydroxide, could produce cancer in animals after long-term oral administration. There is conflicting evidence as to whether tin compounds can be transferred to offspring through breast milk (23). Data from a study of women in Zaire and Guatemala has shown tin levels in the breast milk of respectively 0.24 and 2.81 µg/l breast milk. There is no mention of whether the tin originates from inorganic or organic tin compounds (28).
Children under 6 months are mainly expected to ingest breast milk or milk substitute (29). If the measured concentrations of tin in breast milk originate from organic tin compounds, food can be a source of exposure for infants (28). Furthermore exposure from inhalation of dust containing organic tin compounds can also be a source of exposure (27).
Children of more 6 months are mainly exposed to tin through the feed, by eating contaminated soil, and by inhalation of dust containing organic tin compounds. A 7-day study of 1.75 – 2.2 year-old children illustrated that the daily intake of tin through feedstuff was 1.78 mg/kg bw and the daily exposure through intake of contaminated soil was 1-200 µg Sn (23). There is no specification of whether the measured tin levels originate from inorganic or organic tin compounds (23).
3.1.7 Assessment of organic tin compounds in product no. 6
Testing was performed for organic tin compounds in 2 products (4 and 6). As product 4 had been withdrawn from the Danish market, it was not included in the assessment. In product 6 (a fabric book with PUR-stuffing intended for children under 3 years) was measured 1.95 µg MBT/kg and 1.15 µg DBT/kg.
The exposure route of the fabric book with PUR-stuffing is mainly oral, as children are expected to mouth things. By contact with saliva, the external fabric layer may be soaked and possible chemical substances in the PUR-stuffing may be available for ingestion. Testing for release of organic tin compounds to sweat and saliva has not been performed and the below assessment is therefore based on a worst-case scenario.
Table 3.1 Concentration of MBT and DBT in product no. 6. The values are averages of duplicate determinations. CSTEE’s recommended TDI for MBT and DBT.
| Organotincompound |
Average concentration i no. 6 (µg/kg) |
Average Sn concentration i no. 6 (µg Sn/kg) |
Average amount Sn per book* (µg Sn) |
**Recommended TDI of CSTEE (30)
µg Sn/kg bw/day |
| MBT |
1.95 |
1.32 |
0.04 |
0.5 |
| DBT |
1.15 |
0.585 |
0.019 |
0.1 |
* Weight of product no. 6: 32 g
** There are no available data on the critical effects of MBT and the TDI is therefore very conservative. The DBT value is based on TDI for tributyltin, which is also a very conservative method. |
The average weight of a newborn child in Denmark is 3,450 g (5), and of a child of 1.5 years 9,850 g (6). It is assumed that the measured total amount of MBT and DBT is released in one day and that the substances have an additive effect. The following form is used to calculate the total effect of MBT and DBT. A fraction sum < 1 means that the total content of MBT and DBT in the book do not cause any health concern.
Daily exposure, tin, child:
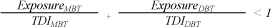
Daily exposure, tin, newborn:
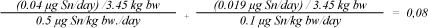
Daily exposure, tin, 1.5 years:
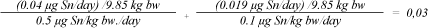
The above calculation shows that the content of MBT and DBT in the book does not cause reason for health concern.
The Margin of Safety (MoS)[15] for the exposure of the two organic tin compounds from the book is calculated by dividing NOAEL with the exposure (under the assumption that the entire amount of MBT (1.95 µg/kg book) and DBT (1.15 µg/kg book) is released in one day. A NOAEL for DBT of 2.5 mg/kg bw/day was used for both MBT and DBT.
MoS, MBT + DBT, child:
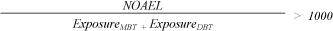
MoS, MBT + DBT, newborn:
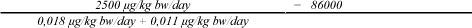
MoS, MBT + DBT, 1,5 year:
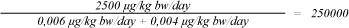
A MoS of 86000 and 250000 was calculated for a newborn child and a child of 1.5 years old, respectively. A high MoS means a good protection of the consumer. In this case it is recommended that the MoS is at least 1000, corresponding to 10 in order to account for sensitive individuals, 10 to account for difference between animals and humans, and 10 in order to account for quality and relevance of the data that NOAEL is based on. Furthermore the used NOAEL for MBT and the assumption of a release of the total content of the organic tin compounds in one day underestimates the MoS. DTC estimates that exposure to organic tin compounds from the book does not cause any health concerns. Because of the large amount of organic tin compounds found in dust from European households, and the potential to ingest organic tin compounds through breast milk and food and from a number of other consumer products, DTC recommends that the contribution from other sources is calculated. An estimation of how large an amount of TDI that could be allocated to consumer products is then possible.
Fodnoter
[2] Group: Quantitative restrictions for migration in feedstuffs
[3] bw: Body weight
[4] LD50: The lethal dose for 50% of a given population
[5] OECD: Organization for Economic Co-operation and Development
[6] NOEL: No Observed Effect Level
[7] TDI: Tolerable Daily Intake
[8] NOAEL: No Observed Adverse Effect Level
[9] NOAEC: No Observed Adverse Effect Concentration
[10] LOAEC: Lowest Observed Adverse Effect Concentration
[11] LOAEL: Lowest Observed Adverse Effect Level
[12] Margin of Safety
[13] Micromedex includes the following databases: MEDITEXT®, HAZARDTEXT®, CHRIS, Dolphin MSDS, HSDB®, IRIS, LOLI®, New Jersey Hazardous Substance Fact Sheets, NIOSH Pocket Guide (TM), OHM/TADS, RTECS®, REPROTEXT®, REPROTOX®, Shepard's Catalog of Teratogenic Agents, TERIS, - Teratogen Information System og Martindale.
[14] TOXNET includes the following databases: CCRIS, ChemIDplus Lite, ChemIDplus Advanced, DART/ETIC, DIRLINE, GENE-TOX, Haz-Map, Household Products, HSDB, IRIS, ITER, TOXLINE, TOXMAP, TRI.
[15] Margin of Safety (MoS) expresses the factor, that NOAEL is higher than the estimated exposure level. A high MoS means a smal risk.
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 March 2006, © Danish Environmental Protection Agency
|


