|
[Front page] [Contents] [Previous] [Next] |
Toxicological Evaluation and Limit Values for Nonylphenol, Nonylphenol Ethoxylates, Tricresyl, Phosphates and Benzoic Acid
1. General description
1.1 Identity
1.2 Physical/chemical properties
1.3 Production and use
1.4 Environmental occurrence
1.5 Environmental fate
1.6 Human exposure
1. General description
1.1 Identity
Molecular formula: C7H6O2
Structural formula:
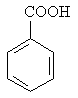
| Molecular weight: | 122.2 |
| CAS-no.: | 65-85-0 |
| Synonyms: | Benzenecarboxylic acid Benzeneformic acid Benzenemethanoic acid Benzoate Carboxybenzene Dracylic acid Phenylcarboxylic acid Phenylformic acid |
1.2 Physical / chemical properties
| Description: | Benzoic acid appears at room temperature as colourless feathery crystals or a white powder. The compound is odourless or with a faint, pleasant odour. |
| Purity: | > 99.5% for crystalline benzoic acid |
| Melting point: | 121.5-123.5° C, sublimes at 100° C |
| Boiling point: | 249-250° C |
| Density: | 1.321 g/cm3 (at 20° C) |
| Vapour pressure: | 8x10-4 - 4x10-3 mmHg (0.0011-0.0053 hPa) at 20° C |
| Vapour density: | 4.2 (air = 1) |
| Conversion factor: | 1 ppm = 5 mg/m3 20° C 1 mg/m3 = 0.20 ppm 1 atm |
| Flash point: | 121° C |
| Flammable limits: | No data have been found |
| Autoignition temp.: | 574° C at 1013 hPa |
| Solubility: | Water: 0.29 g/100ml (at 20 ° C) Soluble in alcohol (33 g/100ml) and chloroform (12.5 g/100ml) |
| logPoctanol/water: | 1.9 |
| Henry’s constant: | 0.0046-0.022 (Pa x m3/mol) at 20° C |
| pKa-value: | 4.21 at 20° C |
| Stability: | - |
| Incompatibilities: | - |
| Odour threshold, air: | - |
| References: | IUCLID (1996), FAO/WHO (1962), Kingsett (1966). |
1.3 Production and use
Benzoic acid is produced by air oxidation of toluene, hydrolysis of benzotrichloride, and decarboxylation of phthalic anhydride. (Merck Index 1996, HSDB 1998).
The major use of benzoic acid is as preservative in foodstuffs, beverages industrial products, medicines and cosmetics. Further, uses have been found for benzoic acid in resin preparations, in production of plasticisers, in dyestuffs, in synthetic fibres, as a chemical intermediate, as corrosion inhibitor in paints and as plugging agent in oil well applications. In 1991 about 120000 tonnes of benzoic acid were produced in US and in 1988 about 233000 tonnes produced in Western Europe. (Kirk-Othmer 1985, BUA 1995, HSDB 1998).
1.4 Environmental occurrence
Air
Benzoic acid may be released into the environment as emissions. It is formed in combustion processes and found in automobile exhaust, refuse combustion and tobacco smoke. It will largely appear as aerosols. (HSDB 1998). Only very sparse data are available concerning atmospheric concentrations of benzoic acid. Concentrations at 1-26 ppt (10 ppt mean; corresponding to 0.05 µg/m3) has been measured in city air (HSDB 1998) and 0-0.30 ppm (approx. 0- 1.5 mg/m3) was found in an industrial environment (Halvorson 1984). In side-stream smoke from tobacco a maximal emission of 0.027 mg benzoic acid/cigarette was measured. Due to dilution of the smoke no benzoic acid could be detected in indoor air by currently available analysis (Patty 1993).
Water
More commonly benzoic acid is released in wastewater during its production and use in manufacturing of other compounds. Benzoic acid has in a few cases been detected in drinking water and ground water. (BUA 1995, HSDB 1998).
Foodstuffs
Benzoic acid occurs in nature in free and combined forms, especially in berries and fruit, which may contain up to 30 mg/kg. Benzoic acid and its sodium salt have over many years been extensively used as a preservative in concentrations up to 0.1% in foodstuffs. The intakes from natural sources are low in comparison with potential intakes from food additive uses. (SCF 1994, Merck 1996).
1.5 Environmental fate
Air
In the atmosphere benzoic acid will largely appears as aerosols, be subject to gravitational settling and be scavenged by rain (HSDB 1998).
Water
If released in wastewater benzoic acid seems readily biodegradable (half-life 0.2-3.6 days). Adsorption to sediment and volatilisation seems not to be significant. (IUCLID 1996, HSDB 1998).
Soil
If released on land, benzoic acid will percolate into the ground due to its low soil adsorption and biodegrade. It is rapidly metabolised by micro-organisms to benzylaspartic acid, benzoylglucoside, salicylic acid and its glucoside, and other unknown compounds (HSDB 1998).
Bioaccumulation
Bioconcentration in fish and algae does not seem important because of the low logP value (HSDB 1998).
1.6 Human exposure
The general population is exposed to benzoic acid primarily through the ingestion of foods which contains benzoic acid naturally as well as food where benzoic acid is added as a preservative. Based on data regarding the amounts of benzoic acid and sodium benzoate produced in U.S. as food additives, a daily average exposure up to 4.4 mg benzoic acid /kg was calculated (IRIS 1997).
In addition to the oral route, minor exposure may result from inhalation via auto exhaust, tobacco smoke and other combustion sources.
Occupational exposure is primarily related to dermal contact or inhalation. The exposure may take place in pharmaceutical or chemical plants and the workers may be exposed to benzoic acid as dust or vapours.
|
[Front page] [Contents] [Previous] [Next] [Top] |