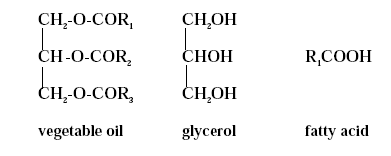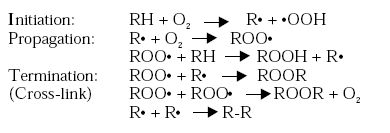|
Substitution of Cobalt Driers and Methyl Ethyl Ketoxime 2 Air-drying systemsCoatings, which are able to dry by oxidative cross-linking, are classified as air-drying or oxidative drying coatings. Air-drying coating systems contain binders such as oils, alkyds and epoxy esters, which are all based on vegetable oils or vegetable oil derivatives. On a volume basis alkyds are far the most important of the air-drying binders. 2.1 Vegetable oilsVegetable oil molecules are glycerides, which are constituted of glycerol backbones combined with different fatty acids. The majority of the molecules are triglycerides with only small proportions of mono- and diglycerides. The fatty acids present in vegetable oils have varying hydrocarbon chain lengths even within the same oil. The chain of a fatty acid does commonly contain an even number of carbon atoms ranging from 10 to 20 including the carbon atom in the acid group (-COOH). The chemical structures of vegetable oils, glycerol and fatty acids are schematically indicated in figure 2.1. The fatty acids combined with glycerol determine the specific properties of vegetable oils and as the fatty acid combination differs from one type of oil to another so do the properties.
Figure 2.1 . The fatty acids can either be saturated containing no double bonds or they can be unsaturated containing one or more double bonds. The presence of double bonds makes the oils reactive as the double bonds are able to polymerise (cross-link) when exposed to oxygen. This ability to cross-link makes unsaturated oils able to form a solid, coherent and adherent film when spread on a surface and exposed to oxygen in the air. The drying properties of oils depend on the degree of unsaturation. The more double bonds present in the oil the better the drying properties. Oils are usually classified as drying, semi-drying or non-drying oils according to their ability to dry when exposed to air. Over a period of time drying oils will form a tack free film, whereas semi-drying oils form films that will never become completely tack free. Non-drying oils are unable to react to form a cross-linked structure by oxidation as they mainly consist of saturated fatty acids, which have no drying properties. The non-drying oil types or derivatives of non-drying oils are therefore not being used for air-drying binders. Semi-drying oils, such as soybean oil, sunflower oil, tall oil or safflower oil contain acids with only one or two double bonds. Semi-drying oils cannot be used unmodified in coatings. They are typically used for the manufacture of air-drying binder such as alkyds and epoxy esters. Drying oils are oils with a high degree of unsaturation, as they consist of glycerides of fatty acids containing two or three double bonds. Linseed oil, tung oil, and oiticica oil are all classified as drying oils. Oils containing conjugated unsaturated acid show a much higher reactivity and better drying properties than oils only containing non-conjugated double bonds. Most of the oils are non-conjugated but tung oil and oiticica oil contain large amounts of fatty acids with conjugated double bonds. Drying oils, especially the refined ones, are able to form films in their unmodified form but only very slowly. In most cases they are therefore modified to increase molecular weight and viscosity before using them in coatings to improve as well drying time as the overall film forming properties. An increased initial molecular weight means that less cross-linking is necessary to obtain a coherent film and therefore the drying time is reduced. The oils can be modified in several ways either by thermal treatments, which polymerise the oil molecules, or by chemical reaction polymerising the oil molecules with other compounds. 2.2 Drying mechanismThe drying mechanism for air-drying systems is described in general terms only as the drying mechanism of the process is very complex. Although the principal reactions involved in the oxidative cross-linking are known, the total mechanism is still not fully established. It is however accepted that the first steps in oxidative drying involve hydroperoxide formation. This initial peroxide formation is followed by decomposition of peroxides to form free radicals, which then initiate polymerisation, /2/3/4/. The chemical mechanisms presented are suggested in the open literature and they are largely based on work with model compounds, which may not always be easily related to the more complex polymer systems used in practice, /3/. The simplest approach is to postulate oxygen attack at the site of the activated methylene, which is alpha to the double bond (C=C) involving the formation of allylic radicals obtained by hydrogen abstraction. This gives rise to peroxide formation. In the case of conjugated systems, such as tung oil, 1,4 cyclic peroxide is formed by oxygen addition, /2/3/4/. Once, a peroxide has been formed it dissociates into free radicals, which enable a series of further reactions to take place. The peroxides decompose by dissociation of the O-O bonds leading to a variety of reaction products including intermolecular linkage and a cross-linked film is obtained. The polymerisation mechanism for non-conjugated fatty acids is presented below, /2/3/. The reactions are chain reactions which once started generate more and more free radicals and peroxides leading to auto-oxidation, /5/. The overall effect of the reactions is that the molecular size of the drying oil molecules is increased.
The termination reaction favours formation of polyperoxides, which subsequently decompose to polyethers. The probability of chain termination is rather high for which reason the length of the polymerised chains is relatively short, /2/3/. The rate of cross-linking does furthermore slow down as the cross-linking structure is built, due to oxygen penetration of the coating film being increasingly inhibited /4/. The cross-linking reactions will though continue very slowly within the dry coating film even years after application. The process of oxidative polymerisation (cross-linking) is a rather slow process even for modified oil, as it normally takes from twelve to thirty-six hours to form a tack free film, /4/. Organic metal compounds, driers, can accelerate and modify these reactions. A coating that would take several days to dry will become tack free within a matter of hours when the proper driers are present in the coating systems. During oxidation, a great number of by-products are formed, notably ketones and aldehydes. These oxidative by-products are responsible for the odour of oil containing systems, especially those containing drying oils or drying oil derivatives. 2.3 Air-drying coating/binder typesTo give an impression of the diversity of air-drying binders, different vegetable oils and binders that are commonly used in air-drying coatings are described in brief in the following sections. 2.3.1 Vegetable oilsVegetable oils have traditionally been used in a lot in paints, varnishes and printing inks because of their ability to cross-link. The oils are though commonly modified before using them in coatings to improve their drying properties. The most extensive use of vegetable oils within the coating industry is the manufacture of alkyd resins, ink vehicle systems and other synthetic resins for air-drying coatings, /3/. 2.3.1.1 Refined oils 2.3.1.2 Polymerised and oxidised oils Isomerised oil is obtained by heating oil with an aqueous alkali solution hereby increasing the extent of conjugation in unsaturated oils and making them more reactive and thereby improving their ability to cross-link when exposed to oxygen in air. Oils polymerised by heating without the presence of accelerators are called heat-polymerised oils, heat-bodied oils or stand oils. Depending on the oil type the heating might be carried out in the presence of peroxides to improve the cross-linking. The heating is continued until the viscosity has increased to the desired value, /5/. In the case of highly conjugated oils the action of heat alone is sufficient to bring about a polymerisation. Even though the drying speed is increased, stand oils do still have a rather slow drying speed but their levelling properties are improved, which is also very important in many surface coating applications, /2/. Stand oils of drying oils can be used on their own in coatings or they can be used for further processing, for instance alkyd production. If the oils are heated and oxidised at the same time by blowing air through the oil they are called blown oils. The reaction may be catalysed by the addition of metal driers, /3/4/. Boiled oils are produced from linseed oil using one or more driers. They are traditionally processed by controlled oxidation of raw linseed oils where metallic driers are used to accelerate the cross-linking. The oils are called boiled oils even though the cooking temperature is below the boiling and decomposition point. By proper control of the reaction, boiled oils with a wide range of viscosities can be obtained. Boiled oils are usually used in oil paints, enamels and oil-based primers. Today boiled oils are though often a simple blend of stand oils and driers, /6/7/. 2.3.1.3 Linseed oil 2.3.1.4 Tung oil (wood oil) 2.3.1.5 Oiticica oil 2.3.1.6 Dehydrated castor oil 2.3.1.7 Soybean oil and sunflower oil 2.3.1.8 Safflower oil 2.3.1.9 Tall oil 2.3.2 Alkyd bindersAlkyd is one of the most used binder type within the European paint industry accounting for approximately 25 % of the total amount of consumed binders and at present they hold a majority share of the world market for non-aqueous binders, /1/3/. Alkyd resins are short branched polyester chains containing fatty acids. They are condensation products of polyols, polybasic acids and vegetable oils or fatty acids. The properties and nature of the final alkyd are dependant on the quantity, type and nature of the modifying oil, fatty acid or acid anhydride used as well as the processing conditions. The presence of the oil provides alkyd binders with good pigment wetting properties and when the oil is unsaturated good air-drying properties are provided as well. The polyester chain gives hardness and durability to the film and improved drying speed, /5/. Alkyds may be further modified by reacting urethane, styrene, vinyl toluene or silicone groups into the alkyd binder to provide specific properties. Most widely used for the production of air-drying alkyds are linseed oil, soybean oil, tall oil, tung oil, and safflower oil. Dehydrated castor oil, linoleic acid and linolenic acid are also used in the production of alkyds, /4/. Alkyds are classified as drying, semi-drying or non-drying dependent on the oil type used for manufacturing the alkyd. Alkyds containing more than 55 % w/w of oils are called long oil alkyds. Alkyds with oil content ranging from 45-55 % w/w are classified as medium oil alkyds whereas short oil alkyds contain less than 45 % w/w of vegetable oil, /3/4/7/. Short oil types dry fast by solvent evaporation but show limited cross-linking. Long oil alkyds dry slower, but their final durability is much better due to better cross-linking, /2/. Air-drying alkyds do therefore usually have an oil length greater than 45%, /5/. The molecular weight of an alkyd is considerably higher than that of a vegetable oil, which means that fewer cross-links are required before a coherent film is formed. Alkyd binders do therefore dry much more rapidly than the corresponding vegetable oils. Addition of driers is though still needed to obtain a drying time, which is acceptable for commercial coating systems. Alkyds are very versatile in use and can be used in several coating types such as paints, enamels, stains, varnishes, lacquers and printing inks. They can be utilised in a variety of applications both in decorative, industrial and speciality coatings. Oil inks are formulated almost exclusively from long oil alkyds, /3/. 2.3.2.1 High solids paints 2.3.2.2 Waterborne systems 2.3.2.3 Modified Alkyd Resins Modified alkyds are made by grafting vinyl monomers (styrene, vinyl toluene, methacrylates etc.), by radical mechanism onto unsaturated sites of the resin or by reacting free hydroxyl groups with silicone and isocyanates (urethane alkyds), /2/. Modified alkyds are widely used in applications where higher weather ability and durability, faster drying and higher gloss are desired than in conventional alkyd coatings, /1/. The higher average initial molecular weight in the modified alkyds means an improved speed of drying. This especially accounts the surface drying as the through-drying might take longer time due to the reduced levels of unsaturation in the alkyd, caused by the copolymerisation, /3/. The modified alkyds are primarily used in industrial coatings. Polyamide modified alkyd resins (thixotropic alkyds) are made by chemical reaction with specially designed polyamide resins. This results in a jelly-like structured material, which breaks down under shear to a free flowing liquid. Once the shear is removed the resin re-sets to a jelly. These resins form the basis of non-drip or thixotropic paints. They are frequently used as blends, with unmodified alkyds or urethane modified alkyds, to impart structure. They are used in air-drying decorative paints, where their rheological properties make them attractive to users of do-it-yourself products, /3/. 2.3.3 Epoxy esterThe majority of epoxy ester resins are reaction products of an epoxy resin and a vegetable fatty acid combining the ease of handling of alkyds with some of the film properties of epoxy paint. Like alkyds, epoxy esters are characterised by oil length and oil type. All vegetable oils and fatty acids common to alkyd manufacture are also used in epoxy ester manufacture. Both air-drying and stoving types of epoxy esters are used commercially, /7/. Although epoxy esters have similarities with conventional alkyds, they generally offer films with better colour, flexibility, adhesion and chemical resistance. Epoxy esters are less versatile in use than alkyds and more expensive, /7/.
|