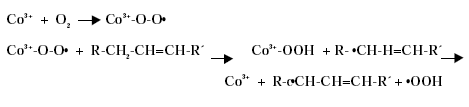|
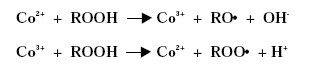
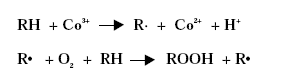
Substitution of Cobalt Driers and Methyl Ethyl Ketoxime 3 DriersThe drying rate of air-drying systems is, as already mentioned, slow and even for reacted oils as alkyds the drying is too slow for commercial applications. The drying time is therefore commonly reduced, by adding metal driers to the system, as these catalyse and hereby accelerate the drying process. The drying time can hereby be reduced from days to hours. The presence of efficient driers is therefore essential for the drying of air-drying coating systems. Different metal driers possess different drying properties as some metals have much more catalytic effect than others. Round 35 to 40 metals have been examined as possible driers, but less than twenty show worthwhile activity, /8/9/. Driers are commonly divided into two main classes according to their catalytic activity. Primary driers, which all possess some catalytic activity and secondary driers, which have no catalytic effect, when used on their own. Some make a further division of the driers splitting up the secondary driers into through-driers and auxiliary driers due to different effects of these driers. Primary driers can initiate and accelerate the oxidative drying process on their own, at least under certain conditions, but the strength of the catalytic activity varies within the group of conventional primary driers. At ambient conditions Co driers are the most active. As the secondary driers possess no catalytic effect, they will have no influence on the drying process if they are used on their own in air-drying systems, but combined with primary driers they become active enhancing the drying, especially the through-drying, and contribute to improved film properties as for instance gloss and film hardness. Primary driers give drying on their own, but as they primarily promote the surface drying they are usually combined with one or two secondary driers to obtain the right balance of surface and through-drying and to obtain the right film properties. Different metal driers are described shortly in the following paragraphs, /8/10/. All the presented metals are available as commercial driers, but some of them are used to a much larger extent than others. This especially accounts for cobalt, manganese, zirconium and calcium. 3.1 Chemical structureDriers, which are also known as siccatives, are a group of metallic soaps containing either alkaline-earth metals or heavy metals combined with mono-basic carboxylic acids. They have the general formula (RCOO)xM where R represents an aliphatic or alicyclic hydrocarbon and M represents a metal with valence x. The acid, which is the anionic part of the metallic soap, can be varied, /11/. The presence of the acid secures adequate distribution of the metal throughout the coating medium due to their solubility in organic solvents and binders, /11/ . Naphtenic acid or octoates, especially the synthetic acids 2-ethyl hexanoic acids are commonly used today, /7/11/. A drier product is besides the metallic salt also constituted of a solvent part. The drier component (the metallic salt) is dissolved or rather mixed into the solvent part, which acts as carrier medium. Today dearomatised hydrocarbons are typically used. Drier products with vegetable esters as carrier media have also become available. The fatty acid esters should have the advantage, besides being based on renewable resources, that it is able to cross-link with the coating film minimising the VOC contribution, /10/. Different types of drier products based on the same metal are available as several parameters can be changed. The metal can be reacted with different acids. The active drier compound can be mixed into different carrier media and the same metal drier type is usually available in different concentrations. The typical metal content in a drier product is between 5 and 20 %, lead driers being an exception typical having a higher content. Air-drying coatings usually contain a mixture of different driers and the coating manufacturers can either mix their own drier systems or they can purchase drier packages, which are commercial drier products combining two or more driers in one product. 3.2 Primary driersPrimary driers are also referred to as top driers, surface driers, oxidative driers or catalytic driers. Top driers and primary driers being the most used expressions. The main function of primary driers is to promote rapid surface drying of air-drying coatings. The driers do also, in varying degree, possess some through-drying properties. Primary driers are normally used in coatings in amounts varying from 0.005 to 0.2 % metal based on the solid binder or oil, /3/. Cobalt (Co), manganese (Mn), cerium (Ce), iron (Fe) and vanadium (V) are five metals used for commercial top driers. Driers based on cobalt and manganese are the most commonly used. The metal atom in the primary drier must be able to undergo oxidation from a lower state to a higher state with the fatty acid peroxides present in the system before the metal salt can act as a drier. In the case of cobalt the following mechanism has been proposed, /2/3/4/.
The driers have been shown to take up oxygen as follows, /2/:
Driers have also been shown to act as oxygen carriers to initiate the radical formation, /3/4/:
Primary driers catalyse the formation and/or decomposition of peroxides, which are formed by the reaction of oxygen with the air-drying binder or drying oil as described in a previous paragraph. Free radicals are formed and the formation of direct polymer to polymer cross-links (top drying) becomes possible. The reactions do also cause the formation of hydroxyl groups and carbonyl groups on the air-drying oil or binder, which are then available for the through-driers to form oxygen-metal-oxygen bridges (cross-links) between the polymer molecules, /10/. Primary driers can for several reasons loose some of their activity before the cross-linking takes part and it inevitably results in prolonged drying time for the air-drying coating. The phenomenon is called loss-of-dry. The presence of pigments in the coating system can lead to a loss-of-dry as the driers within time are adsorbed on the surface of the pigment particles. Adsorption of driers on the pigments will have substantial effects on the drying, as the adsorbed driers no longer can participate in accelerating the drying process. This is especially a problem during prolonged storage. The loss-off-dry effect is most apparent for pigments with very high surface areas, such as carbon black. In waterborne systems the driers can be hydrolysed during storage or the active driers might form complexes with certain coating ingredients present in the systems. Both things lead to a loss-of-dry. The loss-of-dry problems can usually be counteracted, at least to some extent, by using auxiliary driers or drying accelerators. Both types of compounds are described later. Primary driers can also loose activity simply by change of oxidation state and it is recommended to add very active driers, as cobalt driers, as late in the manufacture process as possible. 3.2.1 CobaltCobalt is far the most active of the five primary metal driers and therefore still the most important and widely used drier in air-drying coatings, solvent-borne as well as waterborne, despite the environmental and health drawbacks. Cobalt driers give a rapid surface drying. Used alone or in relatively large amounts it may cause surface wrinkling. If a surface dry too rapidly the oxygen uptake is prohibited beneath the surface of the coating film. This leaves the coating mobile and soft right under the surface due to a low degree of cross-linking. Movements of the coating beneath the dry surface result in wrinkles in the film. To avoid a too rapid surface drying and to provide uniform drying cobalt is commonly used in combination with other metal driers, such as manganese, zirconium, rare earth metals and calcium, /12/. Cobalt driers can be used on its own in the waterborne system, but are most often combined with a drying accelerator. Cobalt needs only to be added in very small amounts and does therefore tend to minimise discoloration compared to other drier metals. Cobalt does furthermore not discolour white coatings to the same extent as other driers since the deep blue colour of cobalt counteract the yellow of the oils and alkyd binders and thereby enhances the whiteness of the paint. 3.2.2 Manganese (traditional)Manganese driers are some of the most important metal driers next to cobalt driers. Traditional manganese driers are medium in activity having both oxidising and polymerising properties, for which reason they promote both surface and through-drying /11/13/. Manganese is, in its traditional carboxylate form, used extensively in air-drying products, most commonly in combination with Co driers to enhance the through-drying of a coating. Used alone manganese driers have a tendency to produce hard brittle films. Manganese has a relatively dark colour, which makes it most suitable for pigmented coatings as it tends to discolour light-coloured or clear coatings, /12/13/. Light-coloured manganese driers are though commercially available today, /10/. High atmospheric humidity may severely inhibit the efficiency of manganese, /13/. A new generation of manganese driers has become available which possess more catalytic effect than the traditional type making them more suitable alternatives to Co driers. These driers are described in paragraph 3.4. 3.2.3 VanadiumVanadium driers provide both surface drying and through-drying of the coating film. According to some drier manufacturers it can be used as a substitute for other top driers, especially cobalt driers, /13/. Improved through-drying can be obtained by combining it with strontium, zinc or zirconium based driers. Vanadium driers can be used in solvent-borne air-drying coatings and for high solids paints. In its emulsifiable form it can be used for water-borne systems as well, /13/. Vanadium driers can cause discolouration of the film. 3.2.4 IronIron is a primary drier which above all improves the through-drying. It exhibits very little drying at room temperature, for which reason the use in air-drying coatings is limited. It becomes a very efficient drier at high temperature and is therefore primarily used in stoving systems. Iron driers provide tough, durable, yet very flexible films with extremely good gloss. Iron driers are very dark in colour and have a severe tendency to yellow. Therefore they can only be used in dark coloured pigmented systems, /12/13/. If iron driers are used in air-drying systems, they are particularly useful in eliminating after tack common to oxide pigmented paints and unprocessed fish oil compositions. Iron driers can also function as pigment wetting agents and help to obtain quicker and better grinds when used with carbon black pigments. They can also act as adhesion promoters in anti-corrosion coatings, /12/13/. 3.2.5 CeriumCerium driers are far less active than cobalt and manganese. At low temperatures (below 0ºC) or at very high atmospheric humidity cerium driers do though show higher efficacy than the other primary driers. Especially combined with cobalt they retain good drying properties even at low temperatures, /13/. 3.3 Drying acceleratorsDrying accelerators or complexing agents are non-metallic compounds (organic ligands), which are able to increase the activity of primary drier metals causing a more rapid drying of the coating film. They function by complexing with the metal atoms by forming chelates, /10/14/. Two different types of drying accelerators are used extensively commercially. These are 2,2'-bipyridyl and 1,10-phenanthroline. They are used in solvent-borne as well as waterborne air-drying systems. In waterborne coatings, hydrolysis of the primary drier can lead to a loss-of-dry upon storage of the coating. Combining the primary driers with drying accelerators some protection from hydrolysis is obtained, /10/. Loss-of-dry due to adsorption of the metal drier on the pigment surface is also to some extent reduced by the use of drying accelerators, /10/. 3.4 Alternative driersNo non-metallic compounds with sufficient drying activity to substitute cobalt driers have to the best of our knowledge been identified so far and as the secondary driers possess no catalytic effect the alternatives to cobalt driers must necessarily be found within the group of primary driers. The substitution possibilities therefore seem quite limited. Cerium and iron based driers have not been included in the testing as these driers are not efficient at ambient temperature. Cerium is efficient at low temperature and high humidity, /13/, whereas iron only becomes efficient at elevated temperature, /12/13/. Vanadium driers provide both surface drying and through-drying of the coating film. Vanadium driers have been suggested to be useful substitutes for cobalt driers, /13/, and were therefore included in the evaluation. Manganese driers are well-known driers, which in their most common form as Mn carboxylates generally possess far less catalytic effect than Co driers /12/, but the ability of drying accelerators/complexing agents to enhance the activity of the primary drier metal has in the recent years been utilised in developing new types of manganese driers. These driers, which typically are complexes of Mn carboxylates with chelating ligands as for instance bipyridene or phenanthroline, possess far more catalytic effect than the conventional types, /14/. Several Mn complex based driers have been included in the evaluation and tested as alternatives to Co driers. Alternative cobalt free drier products based on both vanadium and manganese are commercial available. 3.5 Secondary driersThe primary driers, this accounts for both Co driers and their alternatives, need in most cases to be combined with secondary driers to obtain the right drying profile and film properties. Secondary driers are often divided further into through-driers and auxiliary driers according to the different effects of the driers. Secondary driers are not able to start any cross-linking reactions on their own and can therefore only function in combination with primary driers. They play no part in the oxidation/reduction cycle as the primary driers do, but once electron-donating groups are present through-driers assist in the polymerisation process by the formation of coordination compounds with a consequent increase in the drying rate, /3/. Through-driers are also called cross-linking driers, polymerising driers or coordination driers. Eight different metals are used for commercial through-driers: zirconium (Zr), lanthanum (La), neodymium (Nd), aluminium (Al), bismuth (Bi), strontium (Sr), lead (Pb) and barium (Ba), /10/. The other group of secondary driers are auxiliary driers, which are also referred to as promoters or coordination driers. Four metals are used for commercial auxiliary driers: potassium (K), lithium (Li), calcium (Ca) and zinc (Zn). The first three increase the rate of top drying, whereas zinc usually inhibits top drying, /10/. Auxiliary driers act as promoters for the primary driers and increase the rate of oxygen uptake in air-drying systems considerably, /3/. Besides promoting the through-drying they also improve the stability of drier systems by preventing loss-of-dry of the primary driers, /8/. Secondary driers are normally used in amounts varying from 0.05 to 0.5 % metal based on the air-drying binder. The most commonly used secondary driers in conventional air-drying coating are Zr, Ca, Ba and Zn. 3.5.1 ZirconiumZirconium driers strongly activate the primary driers thus promoting surface and through-drying. It is generally used in combination with cobalt, manganese and calcium and improves the through-drying primarily by the formation of coordination bonds, /12/13/. Zirconium driers have been known for a long time, but gained its real popularity when legislation restricted the use of lead driers in many countries. Compared with other secondary driers zirconium has better properties in terms of colour, yellowing and stability, /13/. In combination with cobalt it is particularly suitable for use in light-coloured air-drying coatings and stoving systems. It is furthermore recommended for eliminating tack of certain tall-oil alkyd resins, /12/13/. Zirconium driers are the most widely used substitutes for lead driers as zirconium is less toxic than both lead and barium, /10/. 3.5.2 AluminiumAluminium driers are effective through-driers, which promote the cross-linking. In combination with primary driers they provide enhanced through-drying, better pigment wetting and dispersion, greater water resistance, higher gloss retention and less discoloration of air-drying systems. A limitation to the use of aluminium driers is that they are more resin specific and tend to build viscosity in systems with high acid number and/or hydroxyl number, /12/. 3.5.3 Rare earth metal driersRare earth driers, containing high levels of lanthanum, neodymium or cerium, promote polymerisation and through-drying. Rare earth driers are especially effective at low temperatures and high humidity conditions. They also contribute to improved gloss, /12/. Rare earth driers are more active than lead or zirconium in oleoresinous and alkyd baking finishes, epoxy esters, styrenated alkyds and silicone formulations, /12/. Rare earths and aluminium are used in special formulations such as high solids paints, /12/. 3.5.4 BismuthBismuth driers have a strong activating effect on cobalt driers improving the through-drying properties especially in extreme weather conditions as for instance high humidity. When combined with cobalt driers it improves the drying properties of conventional alkyd coatings, /13/. 3.5.5 StrontiumStrontium driers are a cost effective alternative to zirconium driers providing superior drying performance in low temperatures and high humidity conditions, /15/. Strontium driers are classified as non-toxic, /16/. 3.5.6 LeadLead driers are effective through-driers, which are almost always used in combination with cobalt and manganese to promote a uniform through-drying of coating films. In contrast to the primary driers lead affects the drying of the film throughout the entire film thickness and due to its superior polymerising effect lead ensures a thoroughly hardened film. Lead driers also improve flexibility, toughness and resistance properties of the coating film, /11/13/. Lead is still a widely used drier even though its use becomes more and more restricted due to legislation. The use of lead driers is banned in Denmark due to its toxicity. 3.5.7 BariumBarium driers promote the through-drying and improve gloss. Furthermore, Barium acts as a wetting agent for pigments and extenders and therefore prevents the adsorption of primary driers at the surface of the pigments. The stability of the drier system is thus increased even during prolonged storage. It is used as a substitute for lead driers, but has a relatively high acute toxicity, which to some extent prohibits its use, /13/. In connection with toys the use of barium is totally banned. 3.5.8 LithiumLithium driers improve the through-drying and hardness of air-drying coatings and reduce their tendency to wrinkle. The best results are obtained when used in combination with cobalt. Lithium driers are especially efficient in systems based on low molecular weight binders and are therefore excellent driers for high-solids paints, but the whiteness of long-oil alkyd paints may be influenced, /11/13/. Lithium driers further improve the storage stability and through-drying of water reducible alkyd dispersions, /13/. 3.5.9 CalciumCalcium driers have a significant synergistic effect contributing to improving through-drying when combined with primary driers as cobalt and manganese, /13/. Calcium driers further help to improve hardness and gloss of the coating film. They also act as wetting agents and minimise loss-of-dry by being preferentially adsorbed at the surface of pigments, preventing the adsorption of primary driers. The drying stability of the system is hereby improved, even on prolonged storage, /11/. Salt formation might also be a contribution factor in the action of calcium as a stabilising drier. Calcium, which is a stronger base than other driers, would preferentially complex with acid groups, leaving the other driers free for catalytic activity in the system, /2/. 3.5.10 ZincThe primary function of zinc driers is to keep the film open by retarding the surface drying, thereby allowing easy access of oxygen throughout the entire coating film for a prolonged period of time. This results in a better through-drying, a harder film and it prevents surface wrinkling. The drying time may, however, be increased slightly, /11/13/. Zinc is the best wetting agent of all the metal driers and when incorporated in a formulation at an early stage, it greatly reduces the mixing and grinding time of the formulation. Zinc driers have extremely light colours for which reason they can be used in relatively large amounts without discolouring the film. Zinc also improves gloss and has the additional property of counteracting mildew formation, /11/. 3.6 Guidelines for use of driersThe combination of driers as well as the optimum concentrations of driers varies from coating system to coating system, but some general recommendation can though be given. The most commonly used drier system is a combination of Co, Zr and Ca as this system in most cases, at least for conventional solvent-borne alkyd coatings, will give reasonable drying times. High solids or coatings containing modified alkyds or non-alkyd binders often need other types of cobalt based drier system. The same accounts for waterborne coatings. Examples of cobalt based drier systems for different types of air-drying systems are given below. One example of a cobalt free drier system for high solids paints is given as well. The most commonly used cobalt drier system is presented in the first example. The concentrations given are metal concentrations on solid air-drying binder, /12/. Conventional solvent-based alkyd coatings, /12/: 0.06 % Co + 0.3 % Zr + 0.2 % Ca High solids, /12/: 0.04 % Co + 0.6 % Zr + 0.2 % drying accelerator or 0.04 % Co + 0.3 % Nd + 0.2 % drying accelerator or 0.04 % Co + 0.3 % Al Coatings with non-alkyd binders, /12/: 0.04 % Mn + 0.04 Co + 0.2 % drying accelerator or 0.04 % Mn + 0.2 % drying accelerator Waterborne coatings, /12/: 0.1 % Co + 0.2 % drying accelerator or 0.05 % Co + 0.05 % Mn + 0.2 % drying accelerator The optimum concentration will vary from system to system, for which reason the above-mentioned examples only should be taken as guidelines. Other combination than the ones mentioned in the examples might also work perfectly well. In table 3.1 ranges of commonly used concentration of selected driers are presented. The concentrations are given as metal content on solid air-drying binder in the coating system. The group of primary driers is placed at top of the table. The levels of used drier metals in the investigated alternative cobalt free drier systems are shown in chapter 8. Table 3.1
3.6.1 Driers for waterborne systemsIn solvent-borne air-drying systems the same cobalt based drier will be able to function satisfactorily in many different coating types. This is not the case in waterborne systems, as the drier system is strongly dependent on the nature of each individual coating product, /10/. This is partly due to the large diversity of water-reducible binders and partly due to the presence of certain ingredients in some waterborne systems that may affect the driers. The presence of large volumes of water changes the drying chemistry of air-drying binders. Water acts as a chain transfer agent in the free radical mechanism, which can slow the rate of the desired free radical reactions markedly, /10/. Therefore, large amounts of driers are needed in the waterborne systems. A cobalt content of 0.02-0.06% based on the solid binder is usually enough for most solvent-borne coatings to dry adequately, but in waterborne systems 0.1-0.15% cobalt are needed. Through-driers are less effective in waterborne coatings than in solvent-borne and in many systems no through-driers are needed at all. Manganese driers are though also effective in drying most alkyd emulsions, /10/. Compared to solvent-borne air-drying systems it can be much more challenging to combine a drier system that works properly in waterborne systems. The presence of certain ingredients in waterborne coatings may affect the drying time. Especially ammonia, amines or phosphates could lead to a loss-of-drying of the primary drier, as these ingredients complex with cobalt metal and thereby reduce its drying activity. Additives having a negative charge might also influence the drying time, /10/. Most of the mentioned problems can be counteracted, at least to some extent, by a proper choice of auxiliary drier and/or drying accelerator. Driers for specific use in waterborne systems are available, but driers intended for use in solvent-borne coatings can by proper dispersion often be used in waterborne systems as well.
|