Survey of azo-colorants in Denmark
Consumption, use, health and environmental aspects
Contents
2 Methodology
2.1 Mass flow analysis
2.1.1 The mass flow analysis paradigm
2.1.2 The parameters of the mass balance analysis
2.1.3 Evaluation of the method
2.2 Technical aspects of azo colorants
2.3 Human toxicity assessment
2.4 Environmental assessment
3 Technical Aspects of Azo Colorants
3.1 General chemistry
3.2 Technical properties of azo dyes
3.3 Technical properties of azo pigments
4 Mass Balance of Azo Colorants
4.1 Industrial uses - general aspects
4.1.1 World production and trade
4.1.2 Danish production and trade
4.1.3 The Product Register
4.2 Plastics
4.2.1 Production and trade
4.2.2 Mass flow analysis
4.3 Leather and leather products
4.3.1 Production and trade
4.3.2 Mass flow analysis
4.4 Textiles
4.4.1 Industrial uses in Denmark
4.4.2 Mass flow analysis
4.5 Paper
4.5.1 Supply and use in Denmark
4.5.2 Mass flow analysis
4.6 Printing
4.6.1 Colorants for printing
4.6.2 Mass flow analysis
4.7 Paints and lacquers
4.7.1 Technical uses
4.7.2 Mass flow analysis
4.8 Mass balance
5 Toxicity and Fate of Azo Dyes
5.1 Physico-chemical properties
5.2 Toxicity
5.2.1 Acute toxicity
5.2.2 Sensitisation
5.2.3 Toxicokinetic
5.2.4 Mutagenicity
5.2.5 Carcinogenicity
5.2.6 Molecular mechanism of carcinogenicity
5.2.7 Aromatic amines - structure activity relationship
5.2.8 Problems of impurities
5.2.9 Exposure
5.3 Environmental fate and exposure
5.3.1 Releases into the environment
5.3.2 Degradation
5.3.3 Distribution
5.3.4 Adsorption
5.3.5 Bioaccumulation
5.3.6 Aquatic compartment
5.3.7 Atmosphere
5.3.8 Terrestrial compartment
5.4 Ecotoxicity
5.4.1 Aquatic compartment
5.4.2 Atmosphere
5.4.3 Terrestrial compartment
5.4.4 Risk characterisation
6 Toxicity and Fate of Azo Pigments
6.1 Physico-chemical properties
6.2 Toxicity
6.2.1 Acute toxicity
6.2.2 Sensitisation
6.2.3 Toxicokinetic
6.2.4 Mutagenicity
6.2.5 Carcinogenicity
6.2.6 Problems of impurities
6.2.7 Exposure
6.3 Environmental fate and exposure
6.3.1 Releases to the environment
6.3.2 Degradation
6.3.3 Distribution
6.3.4 Bioaccumulation
6.3.5 Aquatic compartment
6.3.6 Atmosphere
6.3.7 Terrestrial compartment
6.4 Ecotoxicity
6.4.1 Aquatic compartment
6.4.2 Atmosphere
6.4.3 Terrestrial compartment
6.4.4 Risk characterisation
7 Conclusion and Recommendation
7.1 Conclusions on the individual elements of the survey
7.2 Recommended areas for future investigations
Appendices
2 Effect concentration of azo colorants used in Denmark
3 Effect concentration of the metabolites
4B QSAR derived physico-chemical properties and effect concentrations
5 Molecular structure of selected azo dyes
6 Molecular structure of selected azo pigments
Preface
The present report encompasses results of a survey of azo colorants in Denmark: Consumption, use, health and environmental aspects.
The objective of the survey is twofold:
- Establishment of an overview of the Danish consumption and use of azo colorants, including mass balances for dyes and pigments.
- Assessment of fate, health and environmental toxicity of dyes and pigments.
The survey is based on the position paper: "Status and perspectives of chemicals", published by the Danish Environmental Protection Agency (1996c). The survey is conducted for the Agency by the Danish Technological Institute, Department of Environment, 1997-1998.
The survey was followed by a steering group consisting of:
| Claus Henningsen | National Consumer Agency of Denmark |
| Jette Overgaard | The Danish Paintmakers Association |
| Kirsten Stær | The Danish Paintmakers Association |
| Lillian Petersen | Danish Working Environment Service |
| Tove L. Andersen | Federation of Danish Textile & Clothing |
| Elisabeth Paludan | Danish Environmental Protection Agency |
| Ivan Grønning | Danish Environmental Protection Agency |
| Lea Hansen | Danish Environmental Protection Agency |
In addition, several Danish and foreign experts representing governmental offices, trade organisations, companies, educational institutions, fellow consultants and colleagues have been consulted. They have all provided a very helpful assistance.
The report is prepared by Mss Henriette Øllgaard (project manager), M.Sc., Mrs Lydia Frost, M.Sc., Mr Johan Galster, B.Sc. and Mr Ole Christian Hansen, M.Sc.
November 1998.
Executive Summary
Background
The Danish Environmental Protection Agency (Danish EPA) has in 1996 published a position paper on their standpoint regarding the status and perspectives of chemicals (Miljøstyrelsen, 1996c). With reference to the position paper and in the light of the general international legislative development, a list of chemicals of concern, including azo colorants, has been proposed by the Danish EPA.
Objective
The objective of the survey has been to summarise present knowledge concerning toxicological and environmental properties of the azo colorants. Furthermore, the objective has been to establish an overview of consumption and use of azo colorants in Denmark, aiming at establishment of a preliminary mass balance.
Based on the overview of consumption and use, the survey also aims at, on a provisional and qualitative level, identifying and assessing the human and environmental risks.
Scope of the survey
The survey has been limited/confined to include the trades manufacturing azo colorants, i.e. the dye industry, and the primary users of colorants, the plastics processing industry, leather and leather products, textiles, pulp and paper, printing, paints and lacquers.
Azo colorants consumed and applied in the drug, cosmetic and food industries are omitted, because they are subject to legislation.
The overview of consumption and use does not include either intermediates or metabolites. However, the survey encompasses their toxicological and environmental properties.
Content
The survey covers:
| Technical aspects of azo colorants. | |
| Consumption and use in Denmark and mass balances for dyes and pigments, respectively. | |
| Physico-chemical properties, toxicity, environmental fate and ecotoxicity of azo dyes. | |
| Physico-chemical properties, toxicity, environmental fate and ecotoxicity of azo pigments. | |
| Conclusions and recommendations. |
Technical aspects
Azo colorants are the most numerous and widely manufactured group of synthetic colorants encompassing both azo dyes and azo pigments. The chemical organic synthesis of azo colorants is relatively simple and cheap.
Azo colorants have a chromophore group, the azo linkage. Although all the azo colorants share this group, they exhibit a great variety of physical, chemical and technological properties. Azo dyes may be further divided into ionic and non-ionic dyes.
The azo linkage of azo dyes easily undergoes enzymatic, thermal or photochemical breakdown, whereas the linkage of azo pigments is stable, except with regards to thermal breakdown. Cleavage of azo dyes results in free component aromatic amines.
The main difference between azo dyes and azo pigments, is that azo dyes are soluble in water and/or in substrate, whereas pigments are only sparingly soluble.
Impurities may be found in almost all commercial available formulations of azo colorants. They may be introduced during the manufacturing process and/or as a result of thermal or photochemical decomposition of the native colorants.
The industrial production and use of pigments, including azo pigments, are expanding world-wide. Today, most likely 50% of organic colorants applied within industrial processes are organic pigments.
Mass balance
Danish azo pigments are mainly used in the processing industries in: paints, lacquers, printing and printing inks and in plastics. Azo dyes are predominantly used in the colouring of textiles and to some extent in plastics and leather.
Production of pigments takes place in Denmark (approximately 18,000 tonnes/year), whereas all dyes are imported. Mixing of dye formulations is, however, carried out in Danish dye houses.
The total input is 2,400 tonnes of dyes and 22,600 tonnes of pigments annually.
Imported goods account for an important share of the mass flow of azo colorants in Denmark: 3/4 of the azo dyes and 1/5 of the azo pigments are imported in manufactured products, especially in textiles and in printing inks.
The exports of azo colorants are 1,400 tonnes and 17,400 tonnes for dyes and pigments, respectively.
The survey has revealed that the major importers and producers of azo colorants do not import and/or sell azo colorants, restricted abroad, in Denmark. However, registrations in the Product Register indicate that some of these colorants are in use. In addition, the restricted compounds may be present in textiles and leather products from Asia, Eastern Europe and South America. The imports from Asia alone account for 430 tonnes of azo dyes, primarily in textiles and 40 tonnes of azo pigments in leather products. Thus, at least 20% of the azo dyes associated with imported goods stem from regions where there may be a potential use of the restricted dyes.
About 70 tonnes of dyes and more than 10 tonnes of pigments may be released to waste water during processing of textiles and to a minor extent leather. Presumably most of this does not reach the municipal sewage treatment plants, as most of the industries concerned are submitted to restrictions with respect to their emissions.
Washing of textiles in the use-phase, on the other hand, may cause a release of about 70 tonnes of azo dyes and 10 tonnes of pigments which are emitted directly to the municipal sewage treatment plant.
Emissions to the atmosphere during production, processing and incineration are insignificant, approximately 0.
Most of the azo colorants are disposed by incineration, however, approximately 1,000 tonnes are landfilled and 50 tonnes of the azo pigments from the paper recycling are associated with sludge, applied on soil.
Physico-chemical properties
The azo colorants share some common physico-chemical properties like absorption maxima in the range of visible and UV-light and low vapour pressures. The non-ionic dyes and pigments are sparingly soluble in water and have, in general, high octanol-water partition coefficients (log Kow 3 to 8). In contrast hereto are the ionic dyes, which are characterised by being very soluble in water and having low partition coefficients (-3 to 2.5).
The physico-chemical properties of the metabolites vary within the same range as the colorants, except with respect to their absorption maxima, which are generally below the range of visible and UV-light.
Human toxicity
Azo colorants exhibit an extremely wide variety of toxicological properties. Certain azo colorants, all azo dyes, belong to the first organic compounds associated with human cancer, although many of the azo dyes are not carcinogenic.
The azo linkage of azo dyes, but not of azo pigments, may undergo metabolic cleavage resulting in free component aromatic amines. 22 of these amines are recognised as potential human carcinogens and/or several of them have shown carcinogenic potential in experimental animals. The toxicity (carcinogenicity) of azo dyes is therefore mainly based on the toxicity of the component amines.
Aromatic amines are one of the first classes of organic compounds in which the structural and molecular bases for carcinogenicity are well understood.
The apparent generality of the metabolic cleavage of azo linkage has raised concern about the potential hazards associated with exposure to azo colorants, inclusive azo pigments.
Extensive toxicological investigation on experimental animals have been carried out in the past decades. The investigations have mainly been related to carcinogenicity and the mechanism behind, whereas to the remaining toxicological end-points only very limited attention has been given.
Based on the experiences with azo dyes, the probable carcinogenicity of azo pigments has been of main concern. Although epidemiological studies have not revealed any risks, several carcinogenicity studies have been carried out on experimental animals. Azo pigments are, due to their very low solubility in water, in practice, not available for metabolic activity. Consequently, metabolic cleavage to the component aromatic amines has not been found for the pigments.
Although the metabolic cleavage of azo dyes is the main source of aromatic amines, aromatic amines may also be present as impurities in both azo dyes and azo pigments.
Despite a very broad field of application and exposure, sensitising properties of some groups of azo colorants have been identified in relatively few reports. The allergenic potential of azo colorants seems to be very low.
Due to a strong relationship between exposure to azo dyes and/or aromatic amines and evidence for human cancer and/or cancer in experimental animals, the aromatic amines account for the greatest hazard to health. Consequently, exposure to azo dyes based on aromatic amines, which are known or suspected human carcinogens, encompasses the greatest risk for health.
Azo pigments do not show carcinogenic potential neither in humans nor in experimental animals. However, the presence of aromatic amines as impurities in azo pigments may, depending on the actual exposure, constitute a risk for human health.
Environmental fate and ecotoxicity
Adsorption seems to be the major route of removal of azo colorants in the environment. This applies for the metabolites, as well.
Abiotic degradation (photolysis and hydrolysis) does not play a dominant role in the environmental fate of azo colorants or their metabolites.
In contrast, biotic degradation of the azo dyes may take place in an anaerobic environment. Biodegradation of azo dyes, in general, varies from hours to several months or more indicating that they are at least inherent biodegradable. The pigments, however, do not seem to be biodegradable, neither ready nor inherent. The metabolites are primarily biodegraded under aerobic conditions. Some of the metabolites are ready biodegradable and some are inherent biodegradable.
In general, it is indicated that the ionic dyes do not have any significant bioaccumulation potential. However, when looking at the log BCFs (bioconcentration factor) of the dyes encompassed in the survey, it is indicated that some may bioaccumulate in fish. The non-ionic dyes and pigments, on the other hand, have a potential risk for bioaccumulation. But for the pigments, experimentally assessed BCFs indicate that the immediate concern for bioaccumulation is very low.
The metabolites, generally, have a bioaccumulation potential.
Generally, it is indicated that the ecotoxicity of azo pigments to aquatic organisms, compared to the azo dyes, is lower.
Some of the ionic dyes, i.e. acid and basic, are acute toxic to aquatic organisms. Reactive dyes are not considered to be toxic to aquatic organisms.
Furthermore, it is indicated that the non-ionic dyes are toxic or potentially toxic. Solvent dyes may even be acute toxic to aquatic organisms. The mordant dyes may, according to the present findings, not be of immediate concern.
Short term studies imply that azo pigments, in general, do not give rise to immediate concern about aquatic toxicity, but e.g. Pigment Yellow 83 is potentially toxic.
In general, it is indicated that the effects of the metabolites to aquatic organisms, except for algae, are at levels where potential toxicity is re-cognised. This applies for all metabolites with moieties of: anilines, benzidines and toluidines. Anilines and benzidines are both acute toxic and toxic depending on the specific species. The findings of the toluidines indicate potential toxicity for various aquatic organisms.
The estimated PEC (Predicted Environmental Concentration) and PNEC (Predicted No Effect Concentration) and the subsequent ratios indicate that there is a need of additional information on the potential environ-mental risks for sewage treatment plant and for the aquatic compartment, except for sediment, in association with processing and use of dyes and with production of pigments, whereas sludge applied on soil does not present immediate concern.
Dansk Sammendrag
Baggrund
Miljøstyrelsen offentliggjorde i 1996 et debatoplæg om status og perspektiver for kemikalieområdet (Miljøstyrelsen, 1996c). Med udgangspunkt i debatoplægget og i lyset af den internationale udvikling på reguleringsområdet har Miljøstyrelsen foreslået en liste over uønskede stoffer. Azofarver er en af de stofgrupper, som er omfattet af listen.
Formål
Formålet med undersøgelsen har været at sammenfatte den eksisterende viden om sundheds- og miljømæssige egenskaber af azofarver. Målet har desuden været at skabe et overblik over forbrug og anvendelse af azofarver i Danmark med henblik på opstilling af en overordnet massebalance. Endvidere sigter undersøgelsen på at udpege eventuelle sundheds- og miljømæssige risici.
Afgrænsning af projektet
Undersøgelsen omfatter brancher, som fremstiller azofarver, farveindustrien, og de industrier, der anvender farver i produktionen. Det drejer sig om følgende industrier: plast, læder, tekstil, papir, grafisk og farve/lak. Azofarver, der anvendes i lægemiddel-, kosmetik- og fødevareindustrien er reguleret, hvorfor disse industrier ikke er medtaget i undersøgelsen.
Undersøgelsen omfatter ikke opstilling af en massebalance for azofarvernes intermediater og metabolitter, men undersøgelsen omfatter disses toksikologiske og miljømæssige egenskaber.
Indhold
Undersøgelsen omfatter:
| Tekniske aspekter ved azofarver. | |
| Forbrug og anvendelse af azofarver i Danmark og massebalance for henholdsvis farvestoffer og pigmenter. | |
| Fysisk-kemiske egenskaber, humantoksicitet, miljømæssig skæbne og økotoksicitet af azofarvestoffer. | |
| Fysisk-kemiske egenskaber, humantoksicitet, miljømæssig skæbne og økotoksicitet af azopigmenter. | |
| Konklusioner og anbefalinger. |
Tekniske aspekter
Azofarver, som omfatter såvel farvestoffer som pigmenter, tilhører den mest udbredte og antalsmæssigt største gruppe af industrielt fremstillede syntetiske organiske farver. Den kemiske syntese af azofarver er relativ simpel og billig.
Selvom alle azofarver har den samme chromofore gruppe, azobindingen, har azofarverne mange forskellige fysiske, kemiske og teknologiske egenskaber.
Azobindingen i farvestofferne kløves let enten enzymatisk, termisk eller fotokemisk, hvorimod bindingen i pigmenter er stabil undtagen i forhold til termisk nedbrydning. Kløvning af azofarvestofferne resulterer i frigivelse af frie (komponent) aromatiske aminer.
Hovedforskellen mellem azofarvestoffer og -pigmenter er, at farvestofferne er opløselige i vand eller substrat, hvorimod pigmenter kun er meget lidt opløselige.
Næsten alle kommercielt tilgængelige formuleringer af farver indeholder urenheder. Urenheder kan også blive introduceret under de industrielle processer, hvor farver indgår, og som følge af termisk eller fotokemisk nedbrydning af farverne.
Den industrielle fremstilling og anvendelse af pigmenter, herunder azopigmenter, er stigende på verdensplan. I dag udgør pigmenter omkring 50% af de industrielt anvendte organiske farver.
Massebalance
I Danmark bliver azopigmenter hovedsageligt anvendt i farve/lak industrien, i den grafiske industri samt i plastindustrien. Azofarvestoffer bliver primært anvendt i forbindelse med farvning af tekstiler og i nogen grad til farvning af plastik og læder.
Der fremstilles azopigmenter (ca. 18.000 tons/år) men ikke azofarvestoffer i Danmark. Blandinger af forskellige formuleringer af farvestoffer finder dog sted.
Det totale input af azofarver udgør på årsbasis 2.400 tons farvestoffer og 22.600 tons pigmenter.
Importerede varer udgør en vigtig del af masseflowet for azofarver i Danmark. 3/4 af azofarvestofferne og 1/5 af azopigmenterne bliver således importeret i hel- og halvfabrikata (produkter), specielt i tekstiler og trykfarver.
Eksporten af azofarver udgør 1.400 tons farvestoffer og 17.400 tons pigmenter på årsbasis.
Undersøgelsen har vist, at danske hovedimportører og producenter af azofarver ikke importerer og/eller sælger azofarver, som er underlagt restriktioner i udlandet. Produkt Registrets data tyder dog på, at nogle af disse farver bliver anvendt i Danmark. Endvidere kan disse farver være tilstede i tekstiler og læderprodukter fra Asien, Østeuropa og Sydameri-ka. Importen fra Asien udgør alene 430 tons af farvestofferne, hovedsa-geligt i tekstiler, og 40 tons af azopigmenterne i læderprodukter. Mindst 20% af farvestofferne indeholdt i importerede produkter stammer således fra områder, hvor der potentielt kan anvendes farvestoffer, som er underlagt restriktioner.
Ca. 70 tons farvestoffer og mere end 10 tons pigmenter vil kunne udledes i urenset spildevand ved farvning af tekstiler og i mindre omfang læder. Pga. udledningskrav til virksomheden finder en forbehandling af spildevand sted, derfor vil sandsynligvis kun en begrænset andel af denne mængde blive ledt til kommunale rensningsanlæg, idet de fleste virksomheder inden for tekstil- og læderbranchen er underlagt emissionsgrænser.
Det er derimod estimeret, at vask af tekstiler i brugsfasen kan betyde udledning af ca. 70 tons azofarvestoffer og 10 tons azopigmenter, som udledes direkte til det kommunale rensningsanlæg.
Emissioner til luft under fremstilling, produktion og forbrænding er ubetydelig, tilnærmelsesvis 0.
Den største del af azofarverne bliver bortskaffet ved forbrænding, men ca. 1.000 tons bliver bortskaffet ved deponi, og 50 tons pigmenter fra papirgenbrug (slam) bliver anvendt på landbrugsjord.
Fysisk-kemiske egenskaber
Azofarverne har nogle fælles fysisk-kemiske egenskaber, f.eks. absorptionsmaxima i det synlige område og lave damptryk. De non-ioniske farvestoffer og pigmenter er kun svagt opløselige i vand og har generelt høje oktanol-vand fordelingskoefficienter (log Kow 3 til 8). I modsætning hertil er de ioniske farvestoffer let opløselige i vand og har lave fordelingskoefficienter (log Kow -3 til 2,5).
Metabolitternes fysisk-kemiske egenskaber varierer på samme måde, undtagen i forhold til absorptionsmaxima som generelt ligger under det synlige lys.
Humantoksicitet
Azofarver har meget forskellige toksikologiske egenskaber. Selvom mange azofarvestoffer ikke er carcinogene, er bestemte azofarvestoffer blandt de første organiske stoffer, som er kædet sammen med human cancer.
Azobindingen i farvestoffer, men ikke pigmenter, kan undergå metabolisk kløvning, der resulterer i frie aromatiske aminer. 22 af disse aminer er potentielle/måske humane carcinogener og/eller flere af dem har vist potentiel carcinogenicitet i forsøgsdyr. Toksiciteten (carcinoge- niciteten) af azofarvestoffer er derfor hovedsageligt baseret på toksici- teten af de frie aromatiske aminer, der indgår som komponenter i stof- ferne.
De aromatiske aminer er en af de første grupper af organiske stoffer, hvor den strukturelle og molekylære basis for de kræftfremkaldende egenskaber er velkendt.
Den tilsyneladende almindelige udbredelse af metabolisk kløvning af azobindingen har rejst bekymring om potentielle risici i forbindelse med eksponering for azofarver, herunder pigmenter.
I de seneste årtier er omfattende toksikologiske undersøgelser med forsøgsdyr blevet gennemført. Undersøgelserne har hovedsageligt været relateret til carcinogenicitet og mekanismerne bag. Opmærksomheden har kun i begrænset omfang været rettet mod andre toksikologiske "end-points".
På grund af erfaringerne med azofarvestofferne har der også været bekymring om azopigmenternes mulige carcinogenicitet. Selvom epidemiologiske undersøgelser ikke har afsløret nogen risici, er flere undersøgelser af carcinogenicitet blevet gennemført med forsøgsdyr. I praksis er azopigmenter ikke tilgængelige for den konkrete metaboliske nedbrydning, fordi de er tungt opløselige i vand, og der er ikke fundet metabolisk kløvning til frie aromatiske aminer.
Metabolisk kløvning af azofarvestoffer anses for at være hovedkilden til de frie aromatiske aminer, men de aromatiske aminer kan også være tilstede som urenheder i både azofarvestoffer og -pigmenter.
På trods af azofarvernes brede anvendelsesområde og eksponering har kun relativt få undersøgelser identificeret sensitiserende egenskaber for nogle grupper af azofarver. Dette tyder på, at azofarvers allergene potentiale er lille.
På basis af en tydelig sammenhæng mellem azofarvestofeksponering og/eller aromatiske aminer og evidensen for human cancer og/eller cancer hos forsøgsdyr, anses de aromatiske aminer for at udgøre den største sundhedsmæssige risiko. Derfor vil eksponering for azofarvestoffer, som er baseret på aromatiske aminer, der er kendt eller mistænkt for at være kræftfremkaldende, udgøre den største sundhedsmæssige risiko.
Azopigmenter har ikke vist et kræftfremkaldende potentiale hverken i mennesker eller forsøgsdyr. Men tilstedeværelse af aromatiske aminer i form af urenheder kan, afhængig af den aktuelle eksponering, udgøre en vis sundhedsmæssig risiko.
Miljømæssig skæbne og økotoksicitet
Adsorption er den væsentligste fjernelsesmekanisme for azofarver i miljøet. Dette gælder også for metabolitterne.
Abiotisk nedbrydning (fotolyse og hydrolyse) spiller ikke nogen væsentlig rolle for den miljømæssige skæbne for azofarverne og deres metabolitter.
Bionedbrydning af azofarvestoffer finder sted i anaerobe miljøer. Bionedbrydningen varierer fra timer til flere måneder eller mere, hvilket indikerer, at farvestofferne i det mindste er langsomt nedbrydelige. I modsætning hertil viser undersøgelsen, at pigmenterne er ikke bionedbrydelige. Metabolitterne bliver hovedsageligt nedbrudt under aerobe forhold. Nogle af metabolitterne er hurtig nedbrydelige og nogle er langsomt nedbrydelige.
Generelt set indikerer undersøgelsen, at ioniske farvestoffer ikke har noget signifikant bioakkumuleringspotentiale. Men enkelte af de rappor- terede BCF’er (bioconcentration factor) for de ioniske farvestoffer antyder, at nogle kan bioakkumulere i fisk. For non-ioniske farvestoffer og pigmenter er der derimod et bioakkumuleringpotentiale. Men for pigmenter indikerer eksperimentelt fundne BCF’er, at der ikke er grund til umiddelbar bekymring. Metabolitterne har generelt et bioakkumuleringspotentiale.
Undersøgelsen antyder, at økotoksiciteten af azofarvestoffer er større end økotoksiciteten af azopigmenter for akvatiske organismer. Nogle af de ioniske farvestoffer, sure og basiske, er akut toksiske for akvatiske organismer. Reaktive farvestoffer bliver ikke anset for at være toksiske for akvatiske organismer. Endvidere indikerer undersøgelsen, at nonioniske farvestoffer er toksiske eller potentielt toksiske. Solvente farvestoffer kan endda være akut toksiske for akvatiske organismer. Mordant farvestoffer giver derimod ikke anledning til umiddelbar bekymring.
Korttidsstudier antyder, at azopigmenter ikke umiddelbart er toksiske, men f.eks. Pigment Yellow 83 er fundet potentielt toksisk.
For akvatiske organismer, undtagen alger, er økotoksiciteten af metabolitterne fundet til generelt at ligge på et niveau, hvor de kan grupperes som potentielt toksiske. Dette gælder for alle metabolitter, der indeholder aniliner, benzidiner eller toluidiner. Aniliner og benzidiner er akut toksiske for nogle organismer og toksiske for andre. Det er antydet, at toluidiner er potentielt toksiske for forskellige akvatiske organismer.
De estimerede PEC’er (Predicted Environmental Concentration) og PNEC’er (Predicted No Effect Concentration) og de deraf følgende ratioer indikerer, at der er behov for yderligere information om de potentielle miljømæssige risici for det akvatiske miljø, undtagen sediment, og rensningsanlæg i forhold til industriel anvendelse og i brugsfasen af farvestoffer samt i forhold til fremstilling af pigmenter. Anvendelse af slam indeholdende farver til landbrugsformål er derimod ikke umiddelbart miljømæssigt problematisk.
1. Introduction
Background
The Danish Environmental Protection Agency (Danish EPA) published in 1996 a position paper on the status and perspectives of chemicals (Miljøstyrelsen, 1996c). The Agency stated that there is a need for additional information, in particular, regarding toxicity for man and environment, but also regarding consumption and use of approximately 100 chemicals, among them azo colorants.
Azo colorants are both nationally and internationally regulated, especially for use in drugs, cosmetics, food and in connection with packaging of food. In France, the Netherlands, Austria and Germany restrictions on the use of azo colorants in textiles (leather and leather goods) have been or are being implemented. Some restrictions concern the individual azo colorants, like e.g. the Dutch restrictions. In Germany, however, the restrictions are related to the possible presence of intermediates/metabolites, i.e. the 22 potentially carcinogenic aromatic amines in the working environment (MAK Werte Liste) and in consumer’s goods.
The reason for the concern about the azo colorants is that during the phases of production, processing and consumption there is a risk of exposure for man and environment to potentially carcinogenic aromatic amines. The exposure may take place as a result of cleavage of the colorants to their metabolites or from impurities of the colorants.
With reference to the position paper and in the light of the general international legislative development, a list of undesirable chemicals, including azo colorants, has been proposed by the Danish EPA. On this back- ground a survey of consumption and use of azo colorants in Denmark as well as an evaluation of health and environmental properties/effects have been carried out.
Objective of the survey
The objective of the survey was to summarise present knowledge concerning toxicological and environmental properties of the azo colorants. Furthermore, the objective was to establish an overview of the consumption and the use of azo colorants in Denmark, aiming at establishment of a preliminary mass flow balance.
Based on the overview of consumption and use, the survey also aimed at, on a provisional and qualitative level, identifying and assessing the human and ecotoxicological risks associated with the actual use.
Scope of the survey
Azo colorants belong to the group of organic colorants and constitute the dominant part of these. There are more than 3,000 single azo colorants and more than 10,000 commercially available products (for colouring) containing azo colorants.
Azo colorants may be subdivided into two groups: the azo dyes and the azo pigments. In some aspects they have the same attributes but in general the two groups are very different with respect to the physico-chemical properties and thereby applications. Both groups are included in the present survey. Because of the major differences it is important to distinguish between them, and the two groups are treated separately.
The azo colorants are used for colouring of plastics, leather, textiles, cosmetics and food, for manufacturing of paints and lacquers, for printing purposes and in drugs. Thus, the azo colorants have a very broad application field and are used in a great variety of products, e.g. plastic bowls, T-shirts, hair dyes and ball pens.
Azo colorants consumed and applied in the drug, cosmetic and food industries have been omitted from the survey, because they are already subject to legislation.
The survey has further been limited/confined to include the trades, which manufacture colorants or are primary users of colorants, i.e. the dye industry, the industries for processing of plastics, leather and leather products, textiles, pulp and paper, printing, paints and lacquers. As a consequence end-users, i.e. users of colorants in application, e.g. the iron and steel industry’s use of azo pigments containing paints and lacquers for surface treatment, are not included.
The survey includes both imported, domestic manufactured and exported products and semi-finished goods within the encompassed trades.
The overview of consumption and use does not include the cleavage pro- ducts (metabolites/intermediates) - aromatic amines - of the colorants. However, the human health effects and the environmental toxicity of the cleavage products (metabolites) of the colorants, i.e. the 22 potentially carcinogenic aromatic amines, are included in the survey.
With regards to impurities associated with colorants, they encompass e.g. PCB, heavy metal, dioxins etc. The survey focuses on the aforementioned 22 aromatic amines, because the properties and the effects of the other compounds have been investigated elsewhere.
Content
The applied methodology of the survey is thoroughly presented and discussed in chapter 2.
In addition the survey includes a presentation of:
| Technical aspects of the azo colorants. | |
| Consumption and use in Denmark and mass flow balances for dyes and pigments, respectively. | |
| Physico-chemical properties, toxicity, environmental fate and toxicity of azo dyes. | |
| Physico-chemical properties, toxicity, environmental fate and toxicity of azo pigments. | |
| Overall conclusions and recommendations. |
Each chapter or main section ends with a summary/conclusion
Methodology
Mass flow analysis
The mass flow analysis paradigm
The mass flow analysis of the present survey on azo colorants is based on an evaluation of the individual parameters in the equation below:
Input + Production = Output + Accumulation
The individual parameters of the balance are defined as follows:
| Input data consist of data on imports of azo colorants and products containing the colorants. | |
| Production encompasses azo colorants produced in Denmark and products containing colorants. | |
| Output data are re-exports of azo colorants, exports of colorants in products, disposal of waste (azo colorants and products) and emissions to the environment (water, soil and air). | |
| Accumulation refers in the present survey to stock building. Accumulation is assumed to be zero. |
Principally the equation always balances, as matter cannot be formed nor destroyed.
The Danish EPA has made a paradigm for mass flow analysis (Miljøsty- relsen, 1993) which focuses on analysis of compounds or products. The present survey is based on this particular paradigm, which has also provided the basis for definition of the scope of the survey.
However, conducting a survey like the present on azo colorants in Denmark implies that several thousand compounds are of potential interest, due to the fact that the azo colorant consists of more than 3,000 compounds and that at least 120 compounds, which are restricted in some countries, are in focus. The survey is further complicated because most statistical records describe the compounds on an aggregated basis.
Therefore, the method of the mass flow analysis has been adjusted to match the available data.
Statistics
It should be noted that no available statistics or database records specifically address the comsumption and applications of azo colorants in Denmark.
Generally, statistics of foreign trade and statistics on total supply are of limited value for the present survey. Single groups, like the azo colorants, are only registered in connection with trade in colorants (dyes and pigments), whereas their presence as ingredients in other products are difficult to trace in the statistics, exclusively.
Method of the present mass flow analysis
Input and output of colorants are estimated on the basis of studies of the application in products. Therefore, based on studies of specific uses and product groups, the input may be calculated. Knowing the input of azo colorants to a specific product group (application) and how it is used, the fate of the azo colorant may be estimated.
Numbers
Results from calculations are shown with 2-3 digits in order to facilitate control.
The parameters of the mass balance analysis
Each parameter of the mass balance analysis is described below with special attention to the sources of information and data input. Furthermore, the general assumptions and background for the estimates in chapter 4 are presented and discussed.
Input
Input data have been gathered from three main sources:
Statistics on supply and foreign trade
The statistics on supply and foreign trade have been used when describing individual product groups and country of origin.
Statistics on total supply and foreign trade have been used extensively. Both references provide data in terms of weight and sales values for a detailed list of materials and products according to the customs tariff. The statistics of foreign trade specify country of origin and destination, and the latter includes the Danish production. None of these references specify azo colorants.
Database of the Product Register
Certain products with dangerous properties must be registered in the Product Register. Here information on use and quantities of dyes and pigments is registered, and if they are mixed with chemicals which have to be registered.
It is not possible to conduct a broad survey of azo colorants as such in the Product Register. Therefore, the first survey was carried out on 200 specific azo colorants, which according to the literature are commonly used. Later a survey was conducted on approximately 100 azo colorants which are restricted in Germany and the Netherlands.
The survey on the data from the Product Register only provided in-formation on whether a colorant is in use or not. The data on the volume in use are doubtful due to the structure of the database, as pigments/dyes in e.g. paints are normally registered in bulks with a fixed percentage of all goods, even though some of the paint may not be coloured at all. Consequently, data from the Product Register on quantities of colorants are not used directly in this survey.
Contact to major importers and manufacturers
In order to confirm and validate the input data, 12 major importers and manufacturers of colorants have been consulted on their trade in azo colorants. The gathered information on the sales volume cannot be published due to their confidential character. However, all the companies consulted answered that they do not import azo compounds subject to restrictions abroad.
In some cases the colorants are only present in a part of a product (e.g. colorants in shoes are only to be found in leather and not all shoes consist of leather). In these cases the product group (shoes) is divided into more homogenous groups (clogs, sandals), where the relative share of the colour containing the element (leather) can be estimated more precisely. The volumes in tonnes of the product groups are obtained from the statistics. This method is used to estimate colorants used in leather, textiles and printed matter.
Output
Output data have been described and estimated by using different sources:
| Reports from the Danish EPA and articles are the main references. In some cases data from these sources are considerably older than those in the above mentioned statistics and thus adding uncertainty to the analysis. |
| Experts from companies, organisations etc. have supplied with in-sight, comments and estimates in cases where objective evidence is missing (e.g. on manufacturing of coloured plastics): |
| Federation of Danish Textile & Clothing | |
| Association of Graphic Industries in Denmark | |
| The Danish Plastics Federation | |
| The Danish Paintmakers Association | |
| The Danish Paintmakers Association | |
| Makrodan, Kunsstofkemi, Wilson Color, Berendsen Miljø, Brdr. Hartmann, Store Dalum | |
| Institute for Product Development | |
| Danish Technological Institute - Textile | |
| The Graphic Arts Institute of Denmark |
| Through their trade organisation, the above manufacturers of paints and varnishes have supplied us with information on their use of specific colorants. |
| Foreign organisations and companies have supplied with data, articles and references on colorants: |
| ETAD (Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers, Switzerland) | |
| European Chemicals Bureau, ISPRA, Italy | |
| RPA (Risk and Policy Analysis Limited, Great Britain) | |
| Bundesministerium für Jugend, Familie und Umwelt, Austria |
Disposal and emissions
In the output analysis of disposal and emissions, some general assumptions have been made:
Predominantly disposal takes place through disposal of waste to landfills and incineration.
The relative distribution between landfills, incineration and recycling of household waste is assumed to be valid for industrial waste too, as precise data are unavailable. Due to the conditions in the specific uses, the distribution is modified as follows:
| Paper: 42% of all types of paper products and waste are recycled, thus the remaining 58% are distributed between landfills and incineration. |
| Plastic, textile, leather and paint: As there is little or no recycling, the recycling rate is assumed to be approximately 0. Thus, the disposal is distributed between landfills and incineration. |
The distribution is shown in Table 2.1.
| Table .1 Distribution
among disposal routes. |
|||
Distribution in % between |
|||
Landfills |
Incineration |
Recycling |
|
| Treatment of household waste1 | 20 |
58 |
20 |
| Printed matter and paper | 15 |
43 |
421 |
| Plastics, leather, textile, printing ink, paints and varnishes | 26 |
74 |
0 |
| 1 Ref.: Rendan (1996). | |||
The analysis only evaluates the amount of azo colorants deposited and not the amount of the decomposition products.
Emissions
Emissions comprise of: Emission to waste water, atmosphere and soil.
Emissions to waste water are calculated in total amounts before waste water treatment.
Emissions to the atmosphere during processing in the use phase are estimated to be zero.
Emissions of azo colorants to the atmosphere during incineration of waste are assumed to be negligible, as the azo colorants in question being organic molecules are decomposed by incineration at 800-1,200 o C.
Emissions to soil are generally estimated to be zero, except from disposal of de-inking sludge and application of sludge to agricultural soil (see chapter 4, section 4.5).
Share of azo colorants
No statistics exist on the share of azo dyes in relation to the total amount of dyes, but several references agree that azo dyes represent the majority: 70% (Brown & Anliker, 1988), 60 to 80% (RPA, 1997), 60 to 70 % (ETAD, 1997), and "the majority" (Eitel, 1988). If nothing else is stated, azo dyes are assumed to represent 70 % of all dyes.
Likewise 70% of the pigments are assumed to be azo colorants, if nothing else is stated. This is probably an overestimate, because the inorganic whitening pigment TiO2 is extensively used in the graphic trade and in the manufacture of paints and lacquers.
Evaluation of the method
Critique
The applied method is one-dimensional, because the output is more or less estimated on the basis of the input. Alternative ways for estimating the parameters of the balance have been established for validation:
| The data used have been cross-checked whenever it has been possible. |
| The method implies that there are no stocks (accumulation = 0) which presumably is rarely true. Accumulation is only of interest if the stocks are large or vary a lot from year to year. |
Accumulation of colorants in production of materials and finished goods may be estimated to be approximately 0, as companies avoid binding capital in stocks. Accumulation of consumer products takes place to some extent, but it is assumed that stock piling is limited.
Accumulation of non-degraded colorants may take place in landfills and in soils, where sludge from waste water treatment is deposited. The mass flow analysis does not evaluate this process.
Validation
The results based on the above mentioned method are very dependent on the quality of the assumptions made. Apart from cross-checking whenever possible, the steering committee and independent experts have been consulted.
Technical aspects of azo colorants
The information on the technical aspects of azo colorants is mainly obtained from handbooks like Ullmann and Kirk-Orthmer, if no other author is stated.
Human toxicity assessment
Assessment of the human toxicity of azo colorants has been based on information from databases, namely CISDOC, ECDIN, NIOSHIC and HSDB (cf. references). Detailed information was provided by the published literature, including monographs published by IARC, the International Agency for Research on Cancer.
Due to the epidemiological evidence of carcinogenicity of azo dyes, extensive toxicological investigations were mainly related to carcinogeni- city and the mechanism behind. Some information from the clinic was available regarding some groups of azo colorants and skin sensitisation. To the remaining toxicological end-points, limited attention was given, because they are predominantly related obsolete colorants of today.
On this background the toxicity profile in chapters 5 and 6 reflects the relevant information available on azo colorants and therefore does not fulfil the whole spectrum of toxicological end-points.
Environmental assessment
Assessment of environmental fate and ecotoxicity of azo colorants are based on information from the databases ECDIN, AQUIRE, IUCLID and HSDB. Detailed information was provided by the published literature, including monographs published by MITI and NPIRI.
The assessment of persistence, accumulation and potential bioaccumulation as well as the ecotoxicity of azo colorants are based on the internationally accepted technical guidance documents of the EU Commission (TGD 1996).
Furthermore, the general lack of data on the above mentioned parameters implied that a serie of QSARs (Quantitative Structure Activity Relationship) had to be performed in order to obtain an estimated indication of, among other things, the partition coefficient and ecotoxicity. The applied QSAR methods are based on EPIWIN and TGD (1996).
The predicted environmental concentration (PEC) is estimated, based on a standard model of municipal sewage treatment plants accepted by the EU (TGD 1996). Predicted no effect concentration (PNEC) is estimated according to the OECD guidelines. Due to the limited availability of monitoring data, i.e. when no data from Denmark exist, worst case scenarios are presented.
Due to the epidemiological evidence of carcinogenicity of azo dyes in humans, studies have been performed to establish degradation in the environment and to a less extent the bioconcentration and the ecotoxicity of the dyes. The azo pigments are very poorly studied. Therefore, the survey is turned towards dyes. In general, no data on long-term exposure to azo colorants have been obtained.
Subsequently, the toxicity profile provided in chapters 5 and 6 reflects susceptibility and toxicity in short term studies, and therefore the effects of long-term exposure remain speculative.
Technical Aspects of Azo Colorants
General chemistry
Azo colorants
Azo colorants encompass substances, which have one or more chromophoric groups in their chemical structure and therefore are capable of colouring diverse substances by selective reflection or by transmission of daylight. Azo colorants include both azo dyes and azo pigments.
Azo colorants range in shade from greenish yellow to orange, red, violet and brown. The colours depend largely on the chemical constitution, whereas different shades rather depend on the physical properties. However, the important disadvantage limiting their commercial application is that most of them are red and none are green.
Azo group
The part of an azo colorant molecule which produces colour, the chromophore group, is a double bonded azo linkage. The chromophoric group of azo colorants alters colour of a substrate, either by selective absorption or by scattering of visible light, i.e. light with wavelengths of approximately 400-750 nm.
The azo linkage consists of two nitrogen atoms, which are also linked to carbon atoms. At least one of these carbon atoms belongs to an aromatic carbocycle, an aryl moiety, usually benzene or naphthalene derivatives or a heterocycle, e.g. pyrazolone, thiazole. The second carbon adjoining the azo group may also be part of an aliphatic derivative, e.g. acetoacetic acid.
In general, an azo colorant molecule can be summarised as follows:
aryl - N = N - R,
where R can be an aryl, heteroaryl or -CH = C(OH) - alkyl derivatives.
Stability of azo linkage
The azo linkage is considered the most labile portion of an azo dye. The linkage easily undergoes enzymatic breakdown, but thermal or photochemical breakdown may also take place. The breakdown results in cleavage of the molecule and in release of the component amines. However, the azo linkage of azo pigments is, due to very low solubility in water not available for intracellular enzymatic breakdown.
The component amines which may be released from azo dyes are mostly aromatic amines (compounds where an amine group or amine-generating group(s) are connected to an aryl moiety). In general, aromatic amines known as carcinogenic may be grouped into five groups (Clayson & Garner, 1976).
| Anilines, e.g. o-toluidine. | |
| Extended anilines, e.g. benzidine. | |
| Fused ring amines, e.g. 2-naphthylamine. | |
| Aminoazo and other azo compounds, e.g. 4-(phenylazo)aniline. | |
| Heterocyclic amines. |
The aromatic amines containing moieties of anilines, extended anilines and fused ring amines are components of the majority of the industrially important azo dyes.
Azo dyes
Azo dyes are, due to their relative simple synthesis and almost unlimited numbers of substituents, the most numerous group of synthetic dyes. Azo dyes do not occur naturally.
Azo dyes may have one or more azo groups. Azo dyes with one azo group are called mono azo dyes, with two azo groups, diazo dyes, followed by triazo and polyazo dyes. Azo dyes with more than three azo linkages are designated polyazo dyes. The most commercial important are mono- and diazo dyes, triazo dyes, whereas polyazo are much less important.
Nomenclature
Due to the complexity of the chemical names, azo colorants are only rarely referred to using the IUPAC or CAS nomenclatures. Technical literature has adopted the classification of azo colorants either by the chemical constitution or by the colour.
All commercial important azo colorants are identified by the Colour Index system. Each colorant is given a generic name, e.g. Direct Brown, which briefly gives information on application and colour. In addition to the generic name, a five-digit number is allocated which unambiguously identifies the chemical structure of the colorant.
In the Colour Index system, the azo colorants are provided with numbers ranging from 11,000 to 39,999 in correspondence with the Chemical Class shown in Table 3.1:
Table .1
Colour Index classification of azo colorants.
Klassificering af azofarvestoffer i henhold til Colour Index systemet.
| Chemical Class | CI constitution no. |
| Mono azo | 11,000-19,999 |
| Diazo | 20,000-29,999 |
| Triazo | 30,000-34,999 |
| Polyazo | 35,000-36,999 |
| Azoic | 37,000-39,999 |
Azo pigments
Azo pigments constitute the largest group of organic pigments due to the relatively easy synthesis and the good technical performance.
In principle, the chemical structure of azo pigments is identical to the chemical structure of azo dyes where the azo linkage is the chromophore group. The necessary low solubility is achieved by avoiding solubilising groups or by incorporating groups reducing solubility, e.g. amide groups, or by forming insoluble salts (lake formation) of carboxylic or sulfonic acids.
Azo pigments are particulate solids, which are almost insoluble in water or other media in which they may be dispersed for application. They colour other substances by being physically attached to or incorporated into it. Furthermore, they are physically and chemically unaffected by the substrates, which they are intended to colour.
Technical properties of azo dyes
Azo dyes represent the largest, in number, group of synthetic dyes and the most widely, in tonnage, manufactured. These dyes are, compared to natural dyes, better capable of meeting requirements regarding technical properties, e.g. fastness to light.
The chemical diversity of azo dyes permits a wide spectrum of shades, mainly within the scale of red. A disadvantage limiting their application is, however, that none of the azo dyes are green.
The great majority of azo dyes are water soluble and they colour different substrates by becoming physically attached. The attachment may be due to adsorption, absorption or mechanical adherence.
Azo dyes have a broad industrial application field. They are used for colouring of synthetic and natural textile fibres, plastics, leather, paper, mineral oils and waxes. Their abilities of keeping an intense colour and fastness to light are quite good in most cellulose fabrics but are relatively poor in colouring of cotton and wool.
A number of azo dyes are used as food colorants in cosmetics and as drugs for treatment of bacterial infections.
Most of the commercial available azo dyes are in fact formulations of several components in order to improve the technical properties of the dyeing process. The content of a specific dye lies in the range of 10 to 98%.
The grouping of dyes, including azo dyes, often reflects a strict defined concept of application. The majority of industrial important azo dyes belongs to the following groups:
| Acid dyes | |
| Basic dyes | |
| Direct dyes | |
| Disperse dyes | |
| Mordant dyes | |
| Reactive dyes | |
| Solvent dyes |
The acid, basic, direct and reactive azo dyes are ionic, whereas disperse, mordant and solvent azo dyes are non-ionic dyes.
Acid dyes
Acid dyes are the most widely used azo dye in Europe. The dyes are manufactured and employed as water-soluble sodium salts of the sulfonic or carboxylic acid groups.
Acid dyes, which are anionic, are used in the textile industry for dyeing of all natural fibres, e.g. wool, cotton, silk and synthetics, e.g. polyesters, acrylic and rayon. To a less extent they are used in a variety of application fields such as in paints, inks, plastics and leather.
Basic dyes
Basic dyes include water-soluble cationic azo dyes, characterised by positive charge(s) introduced to the molecule.
Basic azo dyes belong to the oldest known class of synthetic dyes. Their first application was in colouring of natural fibres, e.g. cotton, silk and wool. Later, they were applied for the colouring of synthetics, like e.g. polyesters, acrylics and rayon. Azo dyes with several cationic charges are important dyes for polyacrylonitril fibres.
Some of the basic azo dyes are used in medicine for treatment of bacterial infections.
Direct dyes
Direct dyes include water-soluble anionic azo dyes, which require the presence of electrolytes for the dyeing process. Most of the direct dyes are benzidine-based. They are classified as direct dyes, because they may be applied directly to celluloid fibres. Furthermore, they are used for co- louring of rayon, paper, leather and to a less extent nylon.
Disperse dyes
Disperse dyes encompass azo dyes, which are sparingly soluble in water and mainly used for dyeing of synthetic (hydrophobic) fibres. The disperse dyes are clearly the dominating group within azo dyes used world-wide. The fibres shall be in an organic medium, in which the dye is more soluble than in water. The disperse dyes have been used for cellulose acetate fibres, but now they are used in large quantities for dyeing of polyester, polyamide and acrylic fibres.
Mordant dyes
Mordant dyes include azo dyes, which are converted into their final, insoluble form on the fibres. A mordant is a metal, most commonly chromium, aluminium, copper or iron. The dye forms together with a mordant, an insoluble metal-dye complex and precipitates on the natural fibre. The application area is limited to the colouring of wool, leather, furs and anodised aluminium.
Reactive dyes
Reactive dyes encompass azo dyes, which form covalent bonds with the fibres they colour, e.g. cotton, rayon, cotton, wool silk and nylon. The dye molecule contains specific functional groups, which can undergo addition or substitution reactions with the -OH, -SH and -NH2 groups present in the fibres. Due to very good fastness of the substrate, the reactive dyes are one of the most important group of dyes for colouring of textiles.
Solvent dyes are used on a large scale in many industrial sectors. They are dissoluted in the substrate they colour. The small fastness to light of these dyes depends heavily on the substrates being coloured. They are used for coloration of inks, plastics (mainly for polystyrene and resins of polymethacrylate), wax and fat products and mineral oil products (gasoline, fuels lubricants and greases).
Solvent dyes
Technical properties of azo pigments
Pigments are widely used. The most important area of use is in the graphic printing inks, where approximately 50% of all pigments are used. 25% of the pigments are used in paints and coatings and less than 20% in plastics and fibres. The remaining application fields are e.g. textile printing, office articles, wood, paper, cosmetics and food and feed colouring.
The industrial production and use of pigments, including azo pigments, are expanding world-wide. Most probably 50% of the organic colorants applied within industrial processes are today organic pigments (Ullmann, 5th Edition).
Physical properties like size and shape of pigment particles, crystal geometry and presence of impurities are responsible for the efficacy of the colouring process. The maximum particle size of most of the commercial pigments is less than 1 µm and often even smaller than 0.3 to 0.5 µm. The smallest particles may be one to more than two orders of magnitude smaller. The small particles tend to agglomerate and form crystallites, and this tendency increases with decreasing particle size. Organic pigments, as powders, will therefore comprise of a mixture of such crystallites and single crystals.
Pigment particles may assume a variety of shapes, such as cubes, platelets and needles as well as a number of irregular shapes in combination.
Commercial pigments are available as powdered crystalline solids or already dispersed forms. Dispersion is performed by the manufacturer and may contain carrier material and dispersing agent. The efficiency of dispersion is very important for the process of colouring. After dispersion of the pigment, particles may be stabilised in order to avoid flocculation. This is particularly important for the application of pigments in thermoplastic materials, e.g. polyvinylchloride.
Technical properties of azo pigments always refer to the complete pigment system, which beside a pigment constitutes of e.g. solvents and binders etc. Of particular interest are migration, thermal stability, fastness to light and weather resistance. In solvent-based printing inks, pigments must be extremely resistant to the solvent used in the ink.
The rough grouping of azo pigments may be based on the numbers of azo groups and/or the type of coupling component. Azo pigments may be allocated to the following groups (Ullmann, 5th Edition):
| Benzimidazolone pigments | |
| Diazo pigments | |
| Disazo condensation pigments | |
| Monoazo Yellow and Orange pigments | |
| Naphthol AS pigments | |
| b -Naphthol pigments | |
| Azo pigment lakes |
Benzimidazolone pigments
Benzimidazolone pigments provide a range of colours ranging from greenish yellow to orange, medium red to carmine, bordeaux and brown shades. The technical performance is excellent. Benzimidazolone pigments are used for exterior-use paints of a high quality, e.g. car finishes. Furthermore they are used for colour plastics and for high grade printing inks.
Diazo pigments
Disazo pigments may be characterised by a double azo and/or by double coupling components. Diazo pigments provide colours in the range from very greenish yellow to reddish yellow and orange and red. In comparison with the yellow and orange pigments of monoazo, the diazo pigments provide better solvent and migration fastness, but poorer fastness to light and weather resistance. These pigments are economically very important, particularly in the production of printing inks. The main use encompasses printing inks and plastics.
Disazo condensation pigments
Condensation of two monoazo pigments provides a pigment "double in size". The final colours range from greenish yellow to orange, red and brown. Due to their large molecular size, they are of very good technical properties, particularly very good migration fastness and thermal stability. These properties make disazo condensation pigments suitable for colouring of plastics and paints.
Monoazo Yellow and
Orange pigments
Monoazo pigments provide a range of colours from yellow to orange. The yellow pigments were introduced 80 years ago and they are relatively cheap and very light fast. Therefore they are still very widely used, mainly in coating materials and especially in air-drying and emulsion paints. They are also used in the printing industry.
Naphthol AS pigments
Naphthol AS pigments, so-called naphthol reds, are all red, providing a range of colours from yellowish and medium deeply red to brown and violet. The technical properties vary. In general Naphthol AS pigments have a good fastness to light and are weather resistant, but they tend to migrate. The main area of use is in printing inks and interior paints.
b -Naphthol pigments
b -Naphthol pigments belong to the oldest known synthetic colorants. They are characterised by good fastness to light and weather resistance. On the other hand they have a poor migration fastness. Today only a few b -naphthol pigments are in use, mainly for colouring of inexpensive coating materials.
Azo pigment lakes
Azo pigment lakes are synthesised from monoazo dyes, which are converted to an insoluble form by formation of salt with metals. Azo pigment lakes provide colours from yellow to red. The red pigments have a brilliant shade and are of great industrial importance. The technical properties of azo pigment lakes vary, but they have a good fastness to light, weather resistance and a high thermal stability, whereas some tend to migrate. They are used in almost all printing sectors and for colouring of plastics.
Mass Balance of Azo Colorants
In this chapter, the results of the mass flow analysis are presented and discussed. The presentation is opened by a description of general aspects of industrial applications, production and sales of azo colorants on a world scale and in Denmark.
The following sections encompass the results of the mass flow analysis in the individual trades included in the present survey.
Finally, the total mass flows of azo dyes and azo pigments in Denmark are presented, together with conclusive remarks on the results of the survey.
Industrial uses - general aspects
Colorants, i.e. dyes and pigments, are imported to Denmark either as pure colorants or as ingredients in products. There is one Danish manufacturer of pigments but no domestic production of dyes. Colorants being sold in Denmark or abroad are mixed in a few dye houses.
In Denmark the colorants are used for colouring of plastics, leather and textiles, for manufacturing of paints and lacquers and for printing purposes. Other uses, which are not in focus in this report, are in cosmetics, food and drugs. Furthermore, there are considerable flows of colorants in imported textiles, paper and painted goods.
Thus, azo colorants have a broad application field and are used in a large variety of products, e.g. plastic bowls, T-shirts, hair-dyes and ball pens.
In some trades or fields of applications, pigments are used almost exclusively in e.g. paints and printing inks. In colouring of textiles, dyes are predominant.
The available data on the Danish consumption of dyes and pigments indicate that the dominant use is in paints and lacquers with the iron and steel manufacturing companies as the main end-users.
Considerable large amounts of products, e.g. textiles, are imported. Se-veral sources point out that azo colorants, which are known to cleave off potentially carcinogenic aromatic amines, may be present in imported goods (Mensink et al., 1997; Miljøstyrelsen, 1997).
World production and trade
Azo dyes
The world market for all dyes was 668,000 tonnes in 1991, see Table 4.1.
When excluding indigo, sulphur and vat dyes, which are not azo dyes, 527,000 tonnes of dyes still remain. However, the remaining dyes do not all belong to the azo group either (cf. section 2.1.2.: Share of azo colorants).
| Table .1 Total sale of dyes including azo dyes. 1991. Samlet salg af farvestoffer, inklusive azofarver. 1991. |
|||||||||
North and South Ame- |
China |
India |
Ja- |
Other Asiatic coun- |
East- |
West- |
World |
World |
|
1,000 ton- |
1,000 ton- |
1,000 ton- |
1,000 ton- |
1,000 ton- |
1,000 ton- |
1,000 ton- |
1,000 ton- |
% |
|
| Acid/mordant | 16 |
9 |
3 |
6 |
15 |
22 |
24 |
100 |
15 |
| Azoic | 3 |
9 |
5 |
3 |
11 |
9 |
2 |
48 |
7 |
| Basic | 6 |
9 |
1 |
3 |
7 |
5 |
8 |
44 |
7 |
| Direct | 14 |
5 |
6 |
1 |
12 |
13 |
9 |
64 |
10 |
| Disperse | 29 |
27 |
6 |
12 |
41 |
13 |
22 |
157 |
24 |
| Reactive | 29 |
8 |
7 |
13 |
29 |
9 |
13 |
114 |
17 |
| Sulphur | 8 |
54 |
4 |
2 |
10 |
15 |
3 |
101 |
15 |
| Vat1 | 6 |
5 |
2 |
2 |
4 |
2 |
3 |
27 |
4 |
| Indigo1 | 5 |
2 |
0 |
0 |
2 |
1 |
1 |
13 |
2 |
| Sum | 116 |
34 |
42 |
131 |
89 |
85 |
|||
| Relative share % | 17 |
19 |
5 |
6 |
20 |
13 |
13 |
||
| 1 None of these
types include azo dyes. 2 Africa and countries in the Pacific Ocean are included in the totals. Ref.: SRI (1993). |
|||||||||
Azo dyes and products containing azo dyes which are restricted in Germany, the Netherlands etc. are in some cases found in imported goods from the Asiatic countries, Eastern Europe and South America. Sales volume, relative importance of the different countries and dye types are shown in Table 4.1. Asia, South America and Eastern Europe account for 68% of the world sale of dyes. It is assumed that the total sale approximately equals the production.
Azo pigments
The world production of pigments is approximately of the same volume as the total dye production and the consumption of pigments is increasing (Ullmann, 5th Edition). The main part of the trade in pigments is carbon black and titaniumdioxide, which are inorganic and non-azo pigments.
Recent data on the world production of pigments are not available (pers. comm.: E. Clarke, ETAD, 1998).
Danish production and trade
The Danish imports and exports of dyes and pigments are shown in Table 4.2. Pigments dominate the imports and exports of colorants. Due to a Danish production of pigments, there is a net export of pigments. Only a minor fraction is sold at the home market. The volume of exports is known, but neither the Danish production nor the share of azo pigments are known. The Danish production of azo pigments is estimated to be 18,000 tonnes, and the exports of azo pigments are estimated to be 16,000 tonnes (70% of 23,000 tonnes).
The imports of dyes are 2,890 tonnes constituting 35% of the total imports of colorants. The exports of dyes origin from sales by regional sales offices of international manufacturers and from re-exportation from Danish dye houses.
Table .2
Imports and exports of organic dyes and pigments including azo colorants in Denmark. 1997.
Dansk import og eksport af organiske farvestoffer og pigmenter inklusive azofarver. 1997.
Imports |
Exports |
Est. Danish production |
|
tonnes |
tonnes |
tonnes |
|
| Dyes: Acid |
216 |
270 |
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total dyes | 2,890 |
1,721 |
0 |
| Pigments | 5,430 |
22,946 |
(25,000) |
| Total | 8,320 |
24,667 |
(25,000) |
Ref.: Danmarks Statistik (1997a).
The Product Register, trade organisations and industrial contacts have supplied this survey with information on azo dyes and pigments in actual use in Denmark. The individual colorants are listed in Appendix 1.
A questionnaire sent to importers and manufacturers of colorants has shown that none of the restricted azo colorants are marketed in Denmark.
The Product Register
Based on a search on 300 azo colorants in the database of the Product Register, 111 were identified as being used in Denmark. 50% of these colorants are pigments, cf. Appendix 1.
Of the colorants restricted abroad or colorants with possible toxicological effects, the data from the Product Register indicate that 21 colorants are actually used in Denmark, Table 4.3. Most of them seem to be used in small or negligible amounts, but Acid Red 73 is used in considerable amounts (15 tonnes, but presumably this figure overestimates the actual volume).
Table .3
Azo colorants restricted abroad and/or colorants with possible toxicological effects in use in Denmark.
Dansk anvendelse af azofarver reguleret i udlandet og/eller med mulig toksisk virkning.
| CI-Name | CI No. |
CAS No. |
| Acid blue 113 | 26360 |
3351-05-1 |
| Acid red 26 | 16150 |
3761-53-3 |
| Acid red 73 | 27290 |
5413-75-2 |
| Acid red 114 | 23635 |
6459-94-5 |
| Azoic Dia. Comp 12 | 37105 |
99-55-8 |
| Azoic Dia. Comp 48 | 37235 |
20282-70-6 |
| Azoic Dia. Comp 112 | 37225 |
92-87-5 |
| Azoic Dia. Comp 113 | 37230 |
119-93-7 |
| Direct blue 1 | 24410 |
2610-05-1 |
| Direct blue 14 | 23850 |
72-57-1 |
| Direct blue 53 | 23860 |
314-13-6 |
| Direct red 28 | 22120 |
573-58-0 |
| Disperse blue 1 | 64500 |
2475-45-8 |
| Disperse yellow 23 | 26070 |
6250-23-3 |
| Pigment red 8 | 12335 |
6410-30-6 |
| Pigment red 22 | 12315 |
6448-95-9 |
| Solvent red 1 | 12150 |
1229-55-6 |
| Solvent red 24 | 26105 |
85-83-6 |
| Solvent yellow 1 | 11000 |
60-09-3 |
| Solvent yellow 2 | 11020 |
60-11-7 |
| Solvent yellow 3 | 11160 |
97-56-3 |
Ref.: Produktregisteret, 1997/1998.
Colorants for plastics are subdivided into dyes and pigments. Generally, pigments are preferred for plastics, because they have a higher fastness to light and are more stable against migration than dyes. World-wide colorants for plastics are dominated by two non-azo pigments: titanium oxide (60-65%) and carbon black (20%). Only 2% are organic dyes (Kirk-Orthmer, 1978). The remaining approximately 15% may be a variety of different pigments and among these azo pigments.
Colorants for plastics are usually delivered in master batches, which are a mixture of colorants and dispersion agents.
In Denmark there are several importers (5-10) of colorants for plastics, and 4 companies mix colours according to the customer’s specifications.
Pigments in imported plastic products are difficult to assess as no data on the amount of imported plastic products exist.
Production and trade
As plastics are used for a wide range of products and can be substituted by other materials, it is difficult to identify the end-products in the statistical records. Therefore, the Danish consumption of coloured plastic products may be estimated from the import of different types of polymer resins. In Denmark polymers are not produced. Some polymer types can be omitted, as they are used for products, which are never or rarely coloured.
Imports and exports of plastics and products containing plastic are assumed to be in the same order of magnitude, because it is almost impossible to identify the plastic component of the involved product types in the statistics on foreign trade.
Mass flow analysis
Input
The information on the use of colorants has been collected by personal communication, because the statistical material is weak.
Generally, manufacturers of master batches and plastic products avoid diarylic pigments, subsequently, the market share for these pigments is decreasing (pers. comm.: Ole Hansen, Wilson Color A/S, 1998).
In Table 4.4 estimates for input of polymer resin are listed together with estimates of the ratio of colouring. In Table 4.5 the weight of azo colorants is calculated. The total input of azo dyes and pigments is estimated to be 100 and approximately 200 tonnes, respectively.
| Table .4 Input of polymers to be coloured. Input af polymer til farvning. |
|||
Weight1 |
Ratio coloured2 |
Comments2 |
|
tonnes |
% |
|
|
| PVC | 50,000 |
50 |
Pigments and dyes |
| PE for extruding | 20,000 |
50 |
Only pigments |
| HDP + PET | 20,000 |
25 |
Only pigments |
| Injection moulding | 100,000 |
100 |
95-99 % pigments |
| PS | 35,000 |
100 |
100 % dyes |
| 1: Jan Schäfer, Makrodan A/S (1998). 2: Webber (1979) and pers. comm. Frede Søndergaard, Kunststofkemi A/S (1998). |
|||
| Table .5 Estimate of input of azo colorants for plastics. Estimat af input af azofarver i plastikprodukter. |
|||
Coloured plastics |
Input of organic colorants |
Input of azo colorants |
|
tonnes |
tonnes |
tonnes |
|
| Pigments | 127,500 |
382 |
191 |
| Dyes | 47,500 |
142 |
100 |
The estimates in Table 4.5 are based on the following assumptions:
In a master batch the colorant constitutes 10 to 60% of the weight. On average the weight percentage is 20 to 25 including inorganic pigments and carbon black (pers. comm.: Frede Søndergaard, Kunststofkemi, 1998). When estimating the amount of azo colorants, it is assumed that a master batch contains 10% organic colorant on average.
The master batch constitutes 2-5% of the weight of the final plastic product (pers. comm.: Frede Søndergaard, Kunststofkemi, 1998). Thus, 3% are used in the calculation.
Approximately half of all the pigments used are azo pigments (pers. comm.: Frede Søndergaard, Kunststofkemi, 1998). The share of dyes being azo dyes is unknown, but estimated to be 70%.
Output
Disposal of plastic products depends on the end-use:
| Products containing plastic components may follow many different routes (e.g. iron products with minor plastic parts may be melted down or landfilled). | |
| Products like packaging for industrial use may be collected separately for incineration. | |
| Plastic products for consumer’s use may typically be disposed in the household waste. |
At present only a few possibilities of recycling are available.
Table 4.6 and Table 4.7 show the distribution of emissions of azo colorants to the different environmental compartments. Release from handling of colorants and processing of plastics are estimated to be approximately 0 (negligible amounts). This is due to recycling of most of the waste and because of efforts to minimise the waste.
It shall be noted that the landfill figures only represent the volume, which is deposited in landfills. They do not show where the colorants may end up, when the polymer matrix is degraded.
By incineration of plastics, the colorants will decompose, making the final emission of azo compounds to the atmosphere approximately 0 (negligible amount).
| Table .6 Output of azo dyes from plastic products in Denmark. Output af azofarvestoffer fra plastikprodukter i Danmark. |
|||||
Emissions to |
Disposal to |
Recycling | |||
Wastewater |
Atmosphere |
Landfill |
Incineration |
||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Processing | n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
| Use | n.a. |
n.a. |
26 |
74 |
n.a. |
n.a. = negligible amount.
| Table .7 Output of azo pigments from plastic products in Denmark. Output af azopigmenter fra plastikprodukter i Danmark. |
|||||
Emissions to |
Disposal to |
Recycling | |||
| Wastewater | Atmosphere | Landfill | Incineration | ||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Processing | n.a |
n.a. |
n.a. |
n.a. |
n.a. |
| Use | n.a. |
n.a. |
50 |
141 |
n.a. |
n.a. = negligible amount.
Leather and leather products
The Danish leather dyeing industry comprises of a single factory, and most of the dyed leather is imported. Products manufactured of leather include shoes, different kind of bags and suitcases and garments, of which there are a considerable trade.
Dyes are used for colouring while pigments are used for giving the product a protective layer and colour, i.e. finish.
Production and trade
It is estimated that the Danish production is 800 tonnes of dyed leather. Most of the production, estimated to be 90%, is exported. Data on the consumption of azo dyes are not available. However, it is assumed that due to restrictions on the main export markets, none of the restricted azo dyes are in use (pers. comm.: Stefan Rydin, DTI, 1998).
The net imports of leather were approximately 300 tonnes and the domestic consumption was approximately 80 tonnes in 1997, see Table 4.8. 195 tonnes of the leather originated from Asia.
In 1997, the total consumption of leather products was approximately 7,500 tonnes, of which half was production of shoes.
The content of leather in leather products varies between 10 and 100%. Therefore, individual product groups have been evaluated, e.g. suitcases are estimated to be 50% leather, belts and garment 100%, shoes 50% but clogs only 10%. Saddles are excluded as they are generally not dyed. On this basis, the actual leather consumption can be estimated to be approximately 4,000 tonnes, of which 3,000 tonnes are of Asiatic origin, see Table 4.9.
| Table .8 Dyed leather, 1997. Farvet læder, 1997 |
||||||
Domestic |
Imports |
Exports |
Total Consump. = |
Imported |
||
Prod.
Consump. |
B | C | A + B - C | from Asia | ||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Leather | 800 |
80 |
1,778 |
1440 |
418 |
195 |
Ref.: Danmarks Statistik (1997a)
Pers. comm. : W. Frendrup, DTI (1998).
| Table .9. Leather products, 1997. Læderprodukter, 1997. |
|||||
Domestic |
Imports |
Exports |
Total |
Imported |
|
Production |
Consump. |
from Asia |
|||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Products of leather | 1,549 |
9,275 |
3,310 |
7,514 |
4,566 |
| Calculated as 100% leather | 726 |
5,706 |
2,058 |
3,990 |
2,926 |
Ref.: Danmarks Statistik (1997a).
Danmarks Statistik (1997b).
Pers. comm. : W. Frendrup, DTI (1998).
Mass flow analysis
Imported leather and leather, which is not exported, are used for manufacturing of leather products. Afterwards, these products are either exported or consumed in Denmark. Therefore, hides of leather "consumed" in Denmark (80 tonnes) are accounted for in the final leather products in Table 4.9.
Some of the dyes for leather, the aniline dyes, are azo dyes. As azo dyes represent "the majority of the dyes" in the leather dying process (Eitel, 1988), it is assumed that the ratio of azo dyes used in leather is equal to their world-wide ratio of 70%. In the dyeing process 5 to 10% of the dye is not fixated (= release factor) and is emitted to the waste water of the company (Buljan et al., 1997; Motschi, 1994). The dyestuff content in leather can be estimated to be 2 weight percent (Buljan et al., 1997).
Pigments are used extensively in order to give the leather a finish. The content of pigments in leather is 1 to 2 weight percent. Most of this are inorganic substances and pigments, approximately 90% on average (pers. comm.: W. Frendrup, DTI, 1998). Consequently, the content of azo pigments may not exceed 0.1 to 0.2% of the total weight, and presumably it is less than this percentage. A release factor of 10% is assumed.
Input
In Table 4.10, the amount of imported and exported azo dyes and pigments in leather products are shown.
| Table .10 Content of azo colorants in imported and exported leather products. Indhold af azofarver i importerede og eksporterede læderprodukter. |
||
Dyes |
Pigments |
|
tonnes |
tonnes |
|
| Imported leather products | 80 |
8 |
| Exported leather products | 29 |
3 |
Dyeing of 800 tonnes of leather may cause a release to waste water of 1 tonne of azo dyes and 0.1 tonnes of azo pigments at a maximum, i.e. amounts close to 0 (negligible amounts).
Output
| Table .11 Output in Denmark of azo dyes from leather and leather products. Output af azofarvestoffer fra læder og læderprodukter i Danmark. |
||||||
Emissions to |
Disposal to |
Recycling | ||||
Wastewater |
Atmosphere |
Landfill |
Incineration |
|||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
||
| Processing of leather | 1 |
n.a. |
n.a. |
n.a. |
n.a. |
|
| Use | n.a |
n.a. |
9 |
47 |
n.a. |
|
| The division of disposal is described in chapter 2:
Methodology. n.a. = negligible amount. |
||||||
Based on the above assumptions, disposal of 4,000 tonnes of leather contained in leather products, results in disposal of 56 tonnes of azo dyes and 8 tonnes of azo pigments annually.
Annually 9 tonnes of azo dyes may be deposited at landfills and 1 tonne may be emitted through waste water, see Table 4.11. 40 tonnes of the azo dye contents stem from products of Asiatic origin and 6 tonnes of these end in landfills.
| Table .12 Output in Denmark of azo pigments from leather and leather products. Output af azopigmenter fra læder og læderprodukter i Danmark. |
|||||
Emissions to |
Disposal to |
Recycling |
|||
Wastewater |
Atmosphere |
Landfill |
Incineration |
||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Processing of leather | n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
| Use | n.a. |
n.a. |
1 |
7 |
n.a. |
| The division of disposal is described in chapter 2:
Methodology. n.a. = negligible amount. |
|||||
Disposed pigments in leather products are mainly incinerated, see Table 4.12.
Recycling of leather is estimated to be approximately 0 (negligible amount).
In 1997, approximately 40 companies carried out wet treatment of textiles in Denmark. The production figures for the single sectors are summarised in Table 4.13. The total textile dyeing production may be estimated to be 50,000 tonnes.
Table .13
Production in the Danish textile dyeing industry in 1992.
Dansk textilfarveindustris produktion i 1992.
| Sector | No. of companies | Production in 1992 |
tonnes |
||
| Yarn dyeing Knitwear dyeing Dyeing of woven goods Garment dyeing Carpet dyeing Textile printing |
5 |
9,000 |
1 Assuming an average weight of 300 g per meter fabric.
Ref.: Miljøstyrelsen (1994a).
An important part of the consumed textile in Denmark is dyed abroad.
If nothing else is stated, the data presented below are based on the results of a survey of resource management in treatment of wet textiles (Miljøstyrelsen, 1994a).
Industrial uses in Denmark
Yarn dyeing
Only disperse dyes are used, and 50 % of these are azo dyes.
Knitwear dyeing
15% of the cotton knitwear is treated with optical white and sold as white fabric. Of the remaining 85%, the main part is pre-bleached and dyed in light colours (50%), and the rest is dyed in dark colours (35%).
The colorants used for pure cotton can be divided into four groups, see Table 4.14.
Table .14
Colorants used for cotton.
Anvendelse af farver til bomuld.
| Type | Used for cotton |
Degree of fixation |
% |
% |
|
| Reactive dyes | 94 |
60-75 |
| Direct dyes | 5 |
90-95 |
| Sulphur dyes1 | 1 |
80 |
| Vat dyes1 | 0,5 |
70 |
1
Non-azo dyes.Ref.: Miljøstyrelsen (1994a).
For cotton polyester blends, reactive and disperse dyes are often used to dye the cellulose part of the blends, because they produce a good colour fastness. To a small extent, sulphur dyes, vat dyes, direct dyes, naphtol AS dyeing and pigments are used.
In 1992, the total amount of colorants used for woven goods was approximately 50.4 tonnes. These colorants are distributed between a number of different groups, see Table 4.15.
Woven goods
Table .15
Relative distribution of colorant types for woven goods.
Procentvis fordeling af farvetyper til vævede produkter.
| Group | Share in % |
| Reactive dyes | 22 |
| Acid dyes | 8 |
| Disperse dyes | 15 |
| Metal complex dyes | 1 |
| Sulphur dyes1 | 10 |
| Pigments for printing | 24 |
| Vat dyes1 | 20 |
1
Non-azo dyes.Ref.: Miljøstyrelsen (1994a).
Garment dyeing
Cotton garment is dyed with either reactive dyes (92%, of which 75% are fixated) or sulphur dyes (8%, of which 85% are fixated).
Carpet dyeing
Annually, 4-5 mill. m2 of carpets are dyed in Denmark. They are primarily made of polyamide, cotton and polyamide/cotton blends.
The dyes used are acid dyes (anthraquinone and azo dyes), metal complex dyes and vat dyes (anthraquinone). Yearly, approximately 25 tonnes of colorants are used for carpet dyeing.
Textile printing
Colorants for printing are first and foremost pigments and reactive dyes, but also small amounts of disperse and vat dyes are used.
The annual consumption of colorants for textile printing is approximately 65 tonnes.
Mass flow analysis
Dyeing in Denmark
There is no domestic production of dyes for textiles, and it has not been possible to obtain data on the Danish production of pigments for textile colouring. None of the restricted azo dyes are imported for textile use, according to the importers.
Knowing the output of dyed textiles, the relative amount of colour types and their fixation rates, the consumption of colorants may be calculated. On this basis, the volume of azo colorants can be estimated. The estimates are shown in Table 4.16 and Table 4.17.
| Table .16 Distribution of azo colorants (dyes and pigments) on different uses in the Danish textile dyeing industry. Fordeling af azofarver (farvestoffer og pigmenter) til forskellig anvendelse i dansk tekstil farveindustri. |
||
| Sector | Total colorants |
Azo colorants |
tonnes |
tonnes |
|
| Yarn dyeing Knitwear dyeing Dyeing of woven goods Garment dyeing Carpet dyeing Textile printing |
270 65 |
135 155 24 24 18 46 |
| Total | 672 |
402 |
| Table .17 Input and output of colorants distributed at different colorants per year. Årlig input og output af farver fordelt på forskellige farver. |
||||
| Type | Use of colorant |
Total azo input |
Fixated azo |
Release of azo to untreated effluent |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Reactive dyes | 263 |
184 |
126 |
58 |
| Acid dyes | 29 |
20 |
18 |
2 |
| Disperse dyes | 8 |
5 |
5 |
0 |
| Pigments | 71 |
49 |
48 |
2 |
| Direct dyes | 11 |
8 |
7 |
1 |
| Unspecified | 291 |
135 |
128 |
7 |
| Total | 672 |
402 |
332 |
70 |
Table 4.17 shows that azo compounds represent 60% of the input of colorants for textile colouring, and further approximately 10% of the compounds are disposed or emitted.
Azo pigments represent approximately 14% of the fixated azo colorants. It is estimated that azo pigments only represent 5-10% of the trade in azo colorants for textiles. On this basis, 10% of the azo pigments will be assumed in the calculations of disposal.
Reactive dyes dominate the textile dyeing. This is because knitwear dyeing use one third of all azo dyes and is almost solely based on reactive dyes.
Assuming that azo dyes represent 70% of the consumed dyes (except from yarn dyeing: 50%), the dyeing industry uses approximately 350 tonnes of the azo dyes per year. 70 tonnes may be emitted to the waste water.
Pigments are mainly used in textile printing. They account for approximately 50 tonnes of azo compounds, of which 2 tonnes are not fixated.
It shall be noted that emissions from dyeing houses to the waste water are regulated by the authorities, and that treatment is obligatory. Consequently, most of the emission may not enter the municipal waste water treatment plants.
Textile imports
For some applications the total volume of colorants is known, but in some cases only the volume of dyed textile is available, cf. section 4.4.2. In these cases, the use is assumed to be 1 kg dyestuff per 100 kg textile. This is based on the fact that dyed textiles contain 0.05 to 3.0% dyestuff after the dyeing process (Kemi, 1997 and pers. comm., H.H. Knudsen, IPU, 1998).
| Table .18 Danish imports and exports of textiles. Dansk import og eksport af tekstiler. |
|||||
Textile |
Dyes |
Azo colorants |
Azo dyes |
Azo pigments |
|
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Imports, total | 279,000 |
2,800 |
2,000 |
1,800 |
200 |
| Imports from Asia | 55,400 |
600 |
400 |
360 |
40 |
| Exports | 212,000 |
2,100 |
1,500 |
1,350 |
150 |
| Net imports | 67,000 |
700 |
500 |
450 |
50 |
| Ref.: Danmarks Statistik (1997a). | |||||
Table 4.18 shows that the annual net imports of azo dyes are approximately 450 tonnes. 20% of the imported textile products come from Asiatic countries, which may use restricted azo colorants. However, it shall be noted that due to re-exportation, a percentage of the 450 tonnes will not be used in Denmark.
Release in dyeing
There may be a release of 70 tonnes of azo dyestuff from the dyeing process to untreated waste water, cf. Table 4.17. The main part of this release, 48 tonnes, comes from the large volume of colorants for knitwear dyeing, which mainly uses reactive dyes and has poor fixation rates (Heinfling et al, 1997; Miljøstyrelsen, 1994a). If knitwear dyeing is not taken into account, the release factor is approximately 9%.
A release factor of approximately 10% of the dyestuff is considered to be normal (Brown & Anliker, 1988). For this reason the above calculated loss of 70 tonnes may be a fairly realistic estimate.
Emissions in and after use
The total supply of textiles for the Danish market may be estimated to be 117,000 tonnes (net imports 67,000 tonnes, see Table 4.18, and the Danish production of approximately 50,000 tonnes, see Table 4.13). Assuming that there is no accumulation of textiles, this amount is disposed of per year. With an average content of 1% colorant per tonne and assuming that 70% of the colorants are azo colorants, 1,170 tonnes of colorants are disposed of annually. 82 tonnes of these are pigments and 734 tonnes are azo dyes.
Approximately 212 tonnes of azo colorants may end up in landfills. The distribution between dyes and pigments is 190 tonnes and 20 tonnes, respectively, cf. chapter 2: Methodology.
The emissions from washing of textiles during the use phase are esti- mated as follows: With the above assumptions (117,000 tonnes of textile, 1% of colour content, 70% of azo compounds) and a lifetime loss of colour of 10%, there may be an annual loss of approximately 80 tonnes of azo colorants (72 tonnes dyes, Table 4.19, and 8 tonnes of pigments, Table 4.20) to the household waste water.
| Table .19 Emissions of azo dyes. Emissioner af azofarvestoffer. |
||||||
Emissions to |
Disposal to |
Recycling |
||||
Wastewater |
Atmosphere |
Landfill |
Incineration |
|||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
||
| Dyeing | 70 |
n.a. |
n.a. |
n.a. |
n.a. |
|
| Use | 72 |
n.a. |
191 |
545 |
n.a. |
|
n.a. = negligible amount.
| Table .20 Emissions of azo pigments. Emissioner af azopigmenter. |
|||||
Emissions to |
Disposal to |
Recycling |
|||
Wastewater |
Atmosphere |
Landfill |
Incineration |
||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Dyeing | 2 |
n.a. |
n.a. |
n.a. |
n.a. |
| Use | 8 |
n.a. |
21 |
60 |
n.a. |
n.a. = negligible amount.
In Denmark five factories manufacture paper and two factories produce pulp. Four of the factories mainly use recycled paper as raw material (pers. comm.: L. Hjelm Jensen, Store Dalum A/S, 1998).
Paper recycling is well organised and accounts for 42% of the total paper consumption in Denmark. For some products, the content of colour is insignificant, but for other products the colorants have to be removed by a de-inking process. This process is carried out at two factories.
Supply and use in Denmark
The net imports of paper are 145,000 tonnes of which most are not coloured, except from whitening agents. In the Danish production of 450,000 tonnes of papers, colours are not used with the exception of whitening agents (pers. comm.: H.H. Knudsen, IPU, 1998).
Mass flow analysis
Input
The ratio of coloured paper in the total paper import is estimated to be less than 5%. The content of colorants varies from 4.5 to 5.0 weight percent for dark colouring and 0.5 weight percent for bright colours (pers. comm.: H.H. Knudsen, IPU, 1998; Motschi, 1994).
Table .21
Input of colorants in paper to Denmark.
Dansk input af farver i papir.
In total |
coloured paper |
Colour content |
Colorants |
Azo pigments |
|
tonnes |
% |
weight % |
tonnes |
tonnes |
|
| Import | 145,000 |
5 (est.) |
2 (est.) |
145 |
100 |
| Domestic production | 450,000 |
0 |
0 |
0 |
0 |
| Ref.: Danmarks Statistik (1997a). Miljøstyrelsen (1994b). Pers. comm.: H.H. Knudsen, IPU, 1998. |
|||||
The total amount of colorants in paper is approximately 150 tonnes, see Table 4.21. In the output analysis, this volume is assumed to be a maximum value, as an important share is constituted by inorganic pigments. The share of azo compounds in the 150 tonnes of colorants is unknown but is estimated to be 70%, corresponding to 100 tonnes.
Output
42% of all paper products are recycled. 15% are disposed of to landfills and 43% incinerated. Consequently, 15 tonnes of the colorants in waste paper may end up in landfills. The amount of azo compounds in waste paper is unknown.
42 tonnes of colorants may be found in paper for recycling. When recycling paper products, colorants are removed or decomposed by different processes and trapped in a sludge, which is landfilled (21%), used as a filler in concrete (63%) or released to sludge for application in agriculture (16%) (Miljøstyrelsen, 1994b).
Presumably, all azo pigments in sludge used for manufacturing of concrete are decomposed in the production process. The remaining 37% of the colorants may contain some azo colorants, maximum 15 tonnes (100 tonnes ´ 42% ´ 37%).
| Table .22 Maximum emissions of azo colorants (dyes and pigments) from coloured paper. Maksimal emission af azofarver (farvestoffer og pigmenter) fra farvet papir. |
|||||||
Emissions to |
Disposal to |
Recycling |
|||||
Wastewater |
Atmosphere |
Landfill |
Soil |
Incineration |
|||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
||
| Use | n.a. |
n.a. |
15 |
n.a. |
43 |
42 |
|
| Recycling | n.a. |
n.a. |
9 |
7 |
27 |
n.a. |
|
n.a. = negligible amount.
Printing inks are used for printed matter, e.g.:
| newspapers, mostly printed on paper. | |
| printing on packaging materials like e.g. plastic foils or corrugated boards. |
About 900 companies operate in the printing business or related trades. Imports and exports of printed matter of all kinds represent an important part of the total trade (Danmarks Statistik, 1997c).
In Table 4.23 an estimate is given for the use of printing inks and for imports and exports. The total production of printing inks is 10-11,000 tonnes and the total use is approximately 13,000 tonnes, see Table 4.23 (Miljøstyrelsen 1996a).
Black colours are obtained with carbon black, which is not an azo compound. Therefore, the following analysis concentrates on non-black colours, which represent 77% of the total use of printing inks, corresponding to 8,600 tonnes.
Almost 100% of the colorants in use are pigments.
It shall be noted that the 5,317 tonnes of non-black inks for flexography, ref. Table 4.23, may be overestimated (pers. comm.: Håkan Wallin, Arbejdsmiljøinstituttet, 1998). The overestimation may be 50% due to extensive use of a white non-azo colorant, TiO2 (pers. comm.: E. Silberberg, Den Grafiske Højskole, 1998). Assuming that this overestimation of 50% is correct, the total use of non-black printing inks is approximately 6,000 tonnes or 70% of the total use.
Table .23
Danish imports, exports and total use of printing ink.
Dansk import, eksport og samlet forbrug af trykfarver.
Imports1 |
Exports1 |
Use2 |
Use corrected3 |
||
tonnes |
tonnes |
tonnes |
tonnes |
||
| Black | Newspaper offset Gravure printing Lithography Others |
1,015 |
1,120 |
2,304 107 175 3 |
2,304 107 175 3 |
| Non-black | Newspaper offset Gravure printing Lithography Flexography Screen printing Other printing inks |
6,613 |
4,208 |
1,308 596 969 5,317 74 369 |
1,308 596 969 2,658 74 369 |
| % of non-black printing inks | 77 % |
70 % |
|||
| Ref.: 1 Danmarks Statistik (1997a). 2 Miljøstyrelsen (1991). 3 As footnote 2 above, but non-black flexographic inks have been halved (pers. comm. E. Silberberg, Den Grafiske Højskole, 1998). |
|||||
Colorants for printing
Letterpress printing
Letterpress printing is today almost exclusively performed with offset printing inks. When necessary, the inks are slightly modified with printing auxiliaries.
Offset printing and lithography
These techniques are used for brochures, calendars, posters, business papers and packaging (carton and soft packaging). In typical inks the content of organic pigments is in the range 15-20 weight percent.
Flexographic printing
The primary application for flexographic printing is printing on paper for everyday use, such as paper sacks, shopping bags, wraps and polyolefin films for shopping bags and other packaging.
The content of organic pigments in flexographic inks for paper and film printing is 12-15 weight percent. As noted above TiO2 accounts for half of the ink used.
Screen printing
This technique is used for printing on many kinds of materials, e.g. plastic items, textiles and electrical printed circuits.
Gravure printing
The inks for publication gravure printing contain 8-15 weight percent of pigments. Only a few different pigments are used in publication gravure because this usually involves a four-colour process printing with standard colours.
Ink for packaging gravure printing on e.g. aluminium foils, rolls of paper, plastic films and laminated stock, is made of almost all types of organic pigments as in offset printing.
Azo pigments in inks
In offset printing inks, pigments are predominant. For a four-colour printing, a rather limited number of pigments is used, as most colours can be made from only three colours, red, yellow and blue. As black cannot be created by mixing of colours, the black colour is included in the four-colour system (Ullmann, 5th Edition).
The black colour is normally carbon black. The blue colour may often be based on cyan, e.g. Pigment Blue 15:3. None of these are azo pigments.
The red colour, magenta, is usually made from an azo pigment, Pigment Red 57:1.
For obtaining a yellow colour, Pigment Yellow 12 and 13 are used.
Disazo resins are used as coatings for offset printing plates, as they are rather insensitive to changes in temperature and humidity. The disazo compound, most commonly used for negative plates, is a condensation product of 4-diazodiphenylamine salt with formaldehyde (Kirk-Orthmer, 1978).
Mass flow analysis
As black colour is mostly produced with the non-azo pigment carbon black, the following mass flow analysis only relates to non-black colours.
Table .24
Total of non-black pigments for printing.
Mængde af ikke-sorte pigmenter i trykfarver.
Printing ink 1 |
Average concentration of pigment2 |
Pigment |
|
tonnes |
weight percentage |
tonnes |
|
| Newspaper offset | 1,308 |
13 |
170 |
| Gravure printing | 596 |
7 |
42 |
| Lithography | 969 |
7 |
68 |
| Flexography | 2,658 |
10 |
266 |
| Screen printing | 74 |
7 |
5 |
| Other printing inks | 369 |
15 |
55 |
| Sum | 5,974 |
Ref.: 1 Miljøstyrelsen (1991).
2 Miljøstyrelsen (1995).
In Table 4.24 a total use of 606 tonnes of pigments is calculated using process data as an
entry.
Table .25.
Input
Total supply of non-black pigments.
Total forsyning af ikke-sorte pigmenter.
Volume |
Non-black pigments |
Azo pigments |
|
tonnes |
tonnes |
tonnes |
|
| Domestic production of non-black ink | 6,000 1 |
606 |
424 |
| Import of non-black ink | 6,600 2 |
660 |
462 |
| Export of non-black ink | 4,200 2 |
420 |
294 |
| Net consumption | 846 |
592 |
Ref.: 1 Table 4.24
2 Danmarks Statistik (1997a).
As shown in Table 4.25, the Danish net consumption of non-black azo pigments in printing inks is estimated to be 592 tonnes. It is assumed that 70% of the ink is non-black (cf. Table 4.23), 10% of the ink is pigments, and 70% of the pigments are of the azo-type. In Table 4.24, the content of pigments is calculated for the individual uses on the basis of recipes for ink.
Table .26
Total supply of pigments via all printed matter.
Total forsyning af pigmenter fra alle arter af trykte emner.
Total supply |
Danish end-use of pigments |
Azo pigments |
|
tonnes |
tonnes |
tonnes |
|
| Domestic production of printed matter | 114,0000 |
958 |
671 |
| Import of printed matter | 82,000 |
71 |
50 |
| Export of printed matter | 615,000 |
171 |
120 |
| Net consumption | 858 |
601 |
Ref.: Danmarks Statistik (1997b).
Focusing on trade and product types (books, newspapers, cards etc.) an input of pigments in printed matter is estimated to be approximately 858 tonnes, see Table 4.26. This is based on statistical data on the actual production, imports and exports of different categories of printed matter. The use of non-black ink and the content of pigment for different products are esti- mated, based on data reported in the studies of cleaner technology in the graphic sector in Denmark (Miljøstyrelsen, 1991 and 1995).
The 592 tonnes of azo pigments in Table 4.25 should be identical with the use of pigments for domestic production in Table 4.26 (671 tonnes) and in Table 4.24 (424 tonnes). The discrepancy depends on the assumptions and the quality of the statistics, e.g.: Newspaper printing accounts for 75% of the production printed on paper, and it is assumed that non-black pigments account for 5% of the used pigments. If it is doubled to 10%, the total consumption of pigments is increased from 601 to 966 tonnes. Conclusion: The figures only indicate the size of the net consumtion of non-black azo pigments.
On this basis, the net consumption of azo pigments in the Danish printing indistries may be estimated to 600 tonnes per year, and the net content of azo pigments in printed matter, consumed and disposed in Denmark, is max. 600 tonnes.
Azo pigments in imported printed matter from Asia are negligible, as Asia accounts for 1% of the imports of printed matter.
Output
Table 4.27 shows the distribution of the output of azo pigments from the Danish printing industries and from disposal of printed matter. Most of the pigments are disposed from the end-use to incineration or paper recycling. The latter gives rise to de-inking sludge, which may be landfilled or used for application in soil or concrete, cf. section 4.5.2 on dyes in paper.
Using 37% of the de-inking sludge for landfilling or for application in agricultural soil results in a release of 183 tonnes of azo pigments to these compartments.
| Table .27 Distribution of azo pigments from printing ink and from use. Fordeling af azopigmenter fra trykfarver og forbrug. |
||||||||
Emissions to |
Disposal to |
Waste |
Special |
|||||
Waste water |
Atmos- |
Landfill |
Soil |
Incineration |
from recycling |
waste treatment |
||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
||
| Printing | n.a. |
n.a. |
51 |
n.a. |
21 |
n.a. |
49 2 |
|
| Use | n.a. |
n.a. |
90 |
n.a. |
258 |
2523 |
n.a. |
|
| Recycling | n.a. |
n.a. |
53 |
40 |
159 |
n.a. |
n.a. |
|
1
Wasted ink from cleaning.2 Unused ink and wasted ink on recycled cotton pads.
3 252 tonnes of pigments in de-inking sludge from recycled paper are divided into land- filling, soil improvement, and incineration in the next row of the table.
n.a. = negligible amount.
The waste water from offset printing houses may contain azo pigments, as especially the small printing companies remove ink from the dampening rollers with tap water and solvents. Data on the number of companies using this method as well as data on the release or concentration of pigments in the waste water are not available. For large plants other techniques are used.
The ratio of unused ink to input weight is approximated to 6% (Miljø-styrelsen, 1995) which adds up to 29 tonnes of azo pigments. Presumably, most of the pigments are disposed of in normal or special waste collection, thus, only a minor part of this may end up at landfills.
Approximately 200 tonnes of ink are lost in the cleaning process. Half of the ink is collected in a subsequent cleaning process for the cotton pads, and thereby the ink is destroyed. The other half is disposed with the waste (pers. comm.: Brian Lynggård, Berendsen Miljø, 1998). Thus, 7 tonnes of azo pigments are disposed in incineration (5 tonnes) or landfilling (2 tonnes).
A total of approximately 140 tonnes of azo pigments may end up at landfills and 40 tonnes of azo pigments are disposed in sludge for application in agriculture.
Accumulation of printed matter is estimated to be approximately 0 as the stocks of books etc. are assumed to be constant.
Paints and lacquers
Production and use of lacquers and paints account for the main part of the pigment consumption in Denmark. There is about 25 companies produ- cing paints etc.
Technical uses
The production of lacquers and paints was 130,000 tonnes in 1994 (Miljøstyrelsen, 1996a). According to the search conducted in the Product Register, 65% of the total consumption of colorants is used for production of paints. 10,000 tonnes of printing ink is accounted for in section 4.5.
The bulk of pigments constitute non-azo compounds: carbon black and titaniumdioxide. From interviewing the Danish manufacturers on their consumption of azo colorants, 56 azo colorants have been identified as being used in Denmark, 39 of these are pigments and 11 solvent dyes (see App. 1). It was not possible to establish a detailed picture of the quantities used.
Mass flow analysis
Input
Table .28
Net consumption of azo colorants in paint..
Nettoforbrug af azofarver i maling.
Paint etc. |
Azo pigments |
|
tonnes |
tonnes |
|
| Danish production | 130,000 |
4,252 |
| Export | 57,890 |
1,893 (est.) |
| Import | 23,680 |
774 (est.) |
| Net consumption | 96,000 |
3,000 |
Ref.: Miljøstyrelsen (1996a), the Product Register and Danmarks Statistik (1997a).
In paints and lacquers 3,000 tonnes of azo colorants, almost solely pigments, are consumed per year.
It has not been possible to estimate the imports or exports of pigment on painted goods as it depends on the trade of goods, the type of product and surface area and the thickness of the paint layer.
Output
The actual application of the paint has great importance for the fate of the pigments:
| Outdoor paint may peel off due to wind and weather. | |
| Paint on wood may end at landfills or presumably be incinerated. | |
| Paint on walls may normally not be incinerated but rather end up at landfills. | |
| Paint on metal may end up in a melting oven, an incinerator or at a landfill. | |
| Paint on wall paper may be disposed of with common household waste. |
Thus, it is difficult to present a qualified estimate of the fate of paint when disposed of. An estimate of the distribution of the disposal is shown in Table 4.29. This estimate is very likely to overestimate the actual amount, because an important part of the pigments used are carbon black and titaniumdioxide.
| Table .29 Distribution of azo pigments from paints and lacquers. Fordeling af azopigmenter fra maling og lak. |
|||||
Emissions to |
Disposal to |
Recycling |
|||
Waste water |
Atmosphere |
Landfill |
Incineration |
||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Production | >0 |
n.a. |
n.a. |
n.a. |
n.a. |
| Use | n.a. |
n.a. |
800 |
2,300 |
n.a. |
n.a. = negligible amount.
The fraction which is incinerated or melted down may not cause any release of azo pigments to the environment.
Mass balance
Based on the findings for the individual trades, the total mass balances for azo dyes and azo pigments are presented below.
Input
The production of azo dyes and pigments is shown in Table 4.30.
| Table .30 Production of azo colorants in Denmark. Fremstilling af azofarver i Danmark. |
|||
Azo dyes |
Azo pigments |
Total |
|
tonnes |
tonnes |
tonnes |
|
| Production | 0 |
18,000 |
18,000 |
The total use of azo colorants and their distribution among trades are shown in Table 4.31 and Table 4.32.
| Table .31 Consumption of azo colorants for manufacturing in Denmark. Forbrug af azofarver i dansk produktion. |
|||
Azo dyes |
Azo pigments |
Total |
|
tonnes |
tonnes |
tonnes |
|
| Plastics | 100 |
191 |
291 |
| Leather | 11 |
1 |
23 |
| Textile | 353 |
49 |
402 |
| Paper | 0 |
0 |
0 |
| Printing and printing ink | 0 |
424 |
424 |
| Paints and lacquers | 3,100 |
3,1001 |
|
| Total, approximately | 500 |
3,700 |
4,200 |
1
This figure is likely to be overestimated.| Table .32 Danish imports of azo colorants in manufactured products. Dansk import af azofarver i produkter. |
|||
Azo dyes |
Azo pigments |
Total |
|
tonnes |
tonnes |
tonnes |
|
| Plastics | ? |
? |
? |
| Leather | 80 |
8 |
88 |
| Textile | 1,757 |
195 |
1,953 |
| Paper | 0 |
100 |
100 |
| Printing and printing ink | 0 |
512 |
512 |
| Paints and lacquers | 0 |
? |
? |
| Total, approximately | 1,900 |
900 |
2,800 |
Output
The exports of azo colorants from the Danish production are approximately 16,000 tonnes.
| Table .33 Exports of azo colorants produced in Denmark. Eksport af azofarver fremstillet i Danmark. |
|||
Azo dyes |
Azo pigments |
Total |
|
tonnes |
tonnes |
tonnes |
|
| Production | 0 |
16,000 |
16,000 |
Exports of azo colorants in products are shown in Table 4.34.
| Table .34 Exports of azo colorants (colours and coloured products). Eksport af azofarver (farver og farvede produkter). |
|||
Azo dyes |
Azo pigments |
Total |
|
tonnes |
tonnes |
tornnes |
|
| Plastics | ? |
? |
? |
| Leather | 29 |
3 |
32 |
| Textile | 1,350 |
150 |
1,500 |
| Paper | 0 |
0 |
0 |
| Printing and printing ink | 0 |
414 |
414 |
| Paints and lacquers | 0 |
774 |
774 |
| Total, approximately | 1,400 |
1,400 |
2,700 |
The emissions to the environment and the disposal of waste are shown in Table 4.35 and Table 4.36.
| Table .35 Output of azo dyes to the environment. Output af azofarvestoffer til miljøet. |
|||||
Emissions to |
Disposal to |
Recycling |
|||
Wastewater |
Atmosphere |
Landfill |
Incineration |
||
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Plastics | n.a. |
n.a. |
26 |
74 |
n.a. |
| Leather | 3 |
n.a. |
9 |
47 |
n.a. |
| Textile | 142 |
n.a. |
191 |
545 |
n.a. |
| Paper | n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
| Printing ink | n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
| Paints and lacquers | n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
| Total, approximately | 100 |
n.a. |
200 |
700 |
n.a. |
n.a. = negligible amount.
| Table .36 Output of azo pigments to the environment. Output af azopigmenter til miljøet. |
||||||
Emissions to |
Disposal to |
Recyc- |
||||
Wastewater |
Atmos-phere |
Landfill |
Soil |
Incineration |
ling |
|
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
tonnes |
|
| Plastics | n.a. |
n.a. |
50 |
n.a. |
141 |
n.a. |
| Leather | >0 |
n.a. |
1 |
n.a. |
7 |
n.a. |
| Textile | 10 |
n.a. |
21 |
n.a. |
60 |
n.a. |
| Paper | n.a. |
n.a. |
24 |
7 |
70 |
421 |
| Printing ink | >0 |
n.a. |
148 |
40 |
416 |
2521 |
| Paints and lacquers | >0 |
n.a. |
8002 |
n.a. |
2,3002 |
3,1002 |
| Production | 1803 |
n.a. |
n.a. |
n.a. |
0 |
n.a. |
| Total, approximately | 200 |
n.a. |
1,000 |
50 |
3,000 |
4,000 |
1
The amount recycled is included in "Disposal to landfill, soil improvement or incineration".2 These figures are likely to be overestimated
3 Based on an estimate at 1% loss during production (Clarke & Anliker, 1980).
n.a. = negligible amount.
Total mass flow of azo dyes
The total flow of dyes is shown in Figure 4.1.
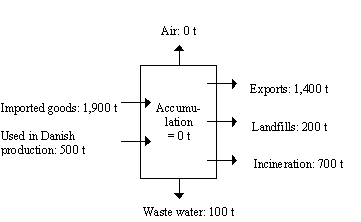
Figure .1
Mass flow of azo dyes in Denmark.
Massestrøm af azofarvestoffer i Danmark.
Total mass flow of azo pigments
The total flow of pigments is shown in Figure 4.2
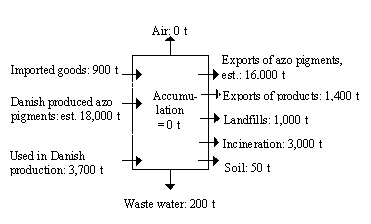
Figure .2
Mass flow of azo pigments in Denmark.
Massestrøm af azopigmenter i Danmark.
Summary
The mass balance of azo colorants is established and the balance may indicate the order of magnitude of the mass flow, but not the exact amounts.
Azo pigments represent the main use of colorants in the processing industry in Denmark, mainly in paints, lacquers, printing and printing inks and plastics. Dyes are predominantly used for colouring of textiles and to some extent in plastics and leather.
Production of azo pigments takes place in Denmark (est. 18,000 tonnes), whereas all dyes are imported. However, mixing of dye formulations is carried out in Danish dye houses.
The total input are 2,400 tonnes of azo dyes and 22,600 tonnes of azo pigments annually.
Imported goods account for an important share of the mass flow of azo colorants in Denmark. 75% of the azo dyes and 20% of the azo pigments are imported in manufactured products, especially in textiles and printing inks.
The exports of azo colorants are 1,400 tonnes and 17,400 tonnes for dyes and pigments, respectively.
The survey has revealed that the major importers and manufacturers of azo colorants do not import and sell the azo colorants, restricted abroad. However, registrations in the Product Register indicate that some of these colorants are in use. In addition, the restricted compounds may be present in textiles and leather products from Asia, Eastern Europe and South America. The imports from Asia account for 430 tonnes of azo dyes, primarily in textiles, and 40 tonnes of azo pigments in leather products. Thus, at least 20% of the azo dyes associated with imported goods stem from regions, where there may be a potential use of the restricted dyes.
About 70 tonnes of dyes and more than 10 tonnes of pigments may be released to waste water during the processing of textiles and to a small extent leather. Presumably, most of the dyes do not reach the municipal sewage treatment plants, because most of the concerned industries are submitted to restrictions with respect to their emissions. For further details please, cf. chapter 5, section 5.3.5.
On the other hand, washing of textiles in the use phase may cause a release of about 70 tonnes of azo dyes and 10 tonnes of pigments. These are emitted directly to the municipal waste water treatment plants.
Emissions to the atmosphere during production, processing and incineration are insignificant.
Most of the azo colorants are disposed of by incineration. However, approximately 1,000 tonnes are landfilled and 50 tonnes of azo pigments from paper recycling are applied to soil following sludge application.
Toxicity and Fate of Azo Dyes
Physico-chemical properties
The molecular weight for the azo dyes included in the present survey lies within the range of 197 to 996 g/mol. The ranges and mean values for the different chemical classes are listed in Table 5.1.
Table .1
Molar weight for azo dyes used in Denmark.
Molvægt for azofarvestoffer anvendt i Danmark.
Number of observations |
Mean |
Range |
|
| Acid | 11 |
582 |
351-830 |
| Basic | 2 |
- |
248-260 |
| Direct | 8 |
807 |
698-996 |
| Disperse | 1 |
625 |
- |
| Mordant | n.o.1 |
n.o.1 |
n.o.1 |
| Solvent | 8 |
273 |
197-379 |
| Reactive | n.o.1 |
n.o.1 |
n.o.1 |
1 No observations.
General aspects
As described in chapter 3, section 3.2 the dyes may be divided into water soluble cationic and anionic dyes and water insoluble dyes - non-ionic dyes.
The basic dyes are cationic. The acidic, direct and reactive, dyes are anionic. The disperse, mordant and solvent dyes have a low water solubility. These dyes are basically characterised as non-ionic or neutral dyes, and thereby hydrophobic in character.
The electron-withdrawal character of azo-groups generates electron deficiency. Thus it makes the compounds less susceptible to oxidative catabolism, and as a consequence many of these chemicals tend to persist under aerobic environmental conditions (Knackmuss, 1996)
Furthermore, dyes must have a high degree of chemical and photolytic stability in order to be useful. It is thus unlikely that they, in general, will give positive results in short-term tests for aerobic biodegradability (e.g. OECD), (Brown & Anliker, 1988). Stability against microbial attack is also a required feature of azo dyes (Pagga & Brown, 1986), because it may prolong the lifetime of the products, in which azo dyes are applicable.
Subsequently, photolysis is not considered to be an important degradation pathway for azo dyes. Even though, all the azo dyes have absorption maxima in the range of visible and UV-light.
Vapour pressure data are not available for most of the azo dyes. In Table 5.2, a few examples are listed. They clearly indicate that the vapour pressure, in general, is very low.
Table .2
Examples of vapour pressures.
Eksempler på damptryk.
| Compound | Vapour pressure (mmHg)2 |
| Acid Yellow 10 |
|
| Solvent Yellow 2*1 |
|
| Disperse Red 9 |
|
| Disperse Red 1 |
|
Ref.: Baughman & Perenich (1988b).
1 See footnote.
2 It is not stated, if the vapour pressure is measured or estimated.
Ionic azo dyes
In general the ionic azo dyes will be almost completely or partly dissociated in an aqueous solution. Solubility in the range 100 mg/l to 80,000 mg/l has been reported for the ionic azo dyes ( HSDB, 1998). In addition, they would be expected to have a high to a moderate mobility in soil, sediment and particular matter, indicated by the low Koc values. However, due to their ionic nature, they adsorb as a result of ion-exchange processes.
In addition, ionic compounds are not considered to be able to volatilize neither from moist nor dry surfaces, and the vapour pressures for these dyes are very low, e.g. Acid Yellow 10.
Only the reactive dyes show a high degree of hydrolysation. Reactive dyes form covalent bonds to the textile. The fixation competes with the reaction of the leaving group of the reactive dye with water (hydrolysis). Therefore the non-fixed dye in a dye bath is the hydrolysed derivative, which has no more the characteristics of the reactive substance. One of the characteristics of these reactive dyes, with a few exceptions, is that the aromatic moieties carry sulfonic groups. Chemical or enzymatic reduction leads to the formation of amino sulfonic acids (ETAD, 1991).
Estimated Kow values for the ionic dyes are generally very low, e.g. 2.75 x 10-5 for Acid Orange 10* and 100 for Direct Black 38*.
Non-ionic azo dyes
The solubility in water is in the range of 0.2 mg/l to 34.3 mg/l for the solvent dyes included in the present survey (HSDB, 1998; Baughman & Perenich, 1988a).
As stated above, vapour pressures are not available for most of the azo dyes, but they are generally low, as shown in Table 5.2. However, some of the disperse dyes have vapour pressures high enough for application from the vapour phase. Furthermore, disperse dyes are believed to dye fabrics by the same mechanism by which hydrophobic pollutants adsorb onto sediments, and the equilibrium can be described by a partition coefficient (Baughman & Perenich, 1988b).
Disperse dyes are the main group of hydrophobic dyes, thus they have a significant potential to adsorb sediments and bioconcentrate (Yen et al., 1991). Disperse dyes are further more highly lipophilic (Anliker, 1986). Solvent dyes are, like disperse dyes, neutral hydrophobic dyes (Baughman & Perenich, 1988b).
The solvent dyes are large, complex molecules, that can be expected to have lower vapour pressures than disperse dyes (Baughman & Perenich, 1988b).
The partition coefficients (Kow) are very high for the non-ionic dyes. In the range of 420 for Solvent Yellow 1* to 11,220 for Solvent Yellow 2. The disperse dye Disperse Blue 79* has a Kow of 3,630. The values are all based on estimates.
Metabolites
Generally, the physico-chemical parameters vary within the following 4 groups: aniline, toluidine, benzidine and naphthalene. These are potenti- ally carcinogenic aromatic amines, which are among the cleavage products and impurities of the azo dyes.
The solubility in water varies. Some are almost insoluble (e.g.
4,4´-methylenebis [2-chloroaniline] and 3,3´-dimethoxybenzidine), whereas others are highly soluble, up to 16.8 g/l (o-toluidine).
The absorption maxima are generally in the range of 240 to 300 nm, i.e. below the range of visible and UV-light.
The vapour pressures are in the range of 7.5 ´ 10-7 to 0.32 mmHg.
The estimated partition coefficients (Kow) lay within the range of 21 for benzidine to 8,300 for 4-o-tolylazo-o-toluidine.
Summary
The azo dyes may be subdivided into two groups: the ionic and non-ionic dyes. They have some common features, though. Their absorption maxima is in the range of visible and UV-light and the vapour pressures, if available, are very low in the range of 2.5 ´ 10-20 to 3.6 ´ 10-8 mmHg. The hydrolysation is, except for the reactive dyes, very low.
However, the two groups also exhibit major differences. In general, the ionic azo dyes will be almost completely or partly dissociated in an aqueous solution. The non-ionic dyes, on the other hand, are only sparingly soluble (<100 mg/l). The estimated Kow values for the ionic dyes are generally very low e.g. -2.75 ´ 10-5 for Acid Orange 10* and 100 for Direct Black 38*. However, the non-ionic dyes have very high partition coefficients (Kow ), e.g. 3,630 Disperse Blue 79* and 11,220 for Solvent Yellow 2.
The solubility of the metabolites varies similarly from almost insoluble to very soluble. The absorption maxima are generally below the range of 240 to 300 nm. The vapour pressures are in the range of 7.5 ´ 10-7 to 0.32 mmHg.
The estimated partition coefficients (Kow) lay within the range of 21 for benzidine to 8,300 for 4-o-tolylazo-o-toluidine.
Acute toxicity
The acute toxicity of azo dyes, as defined by the EU criteria for classification of dangerous substances, is rather low. Information about acute oral toxicity, including skin and eye irritation, is in form of material safety data sheets available for many commercial azo dyes. Only a few azo dyes showed LD50 values below 250 mg/kg body weight, whereas a majority showed LD50 values between 250-2,000 mg/kg body weight (Clarke & Anliker, 1980). Remazol Black Bâ (Reactive Black 5) represents an important group of newer azo dyes, namely the reactive dyes. For this dye a comprehensive study on acute toxicity was carried out. The study showed that LD50 exceed 14,000 mg/kg body weight, and that the dye was neither irritant to skin nor to eye (Hunger & Jung, 1991).
Exposure to aromatic amines may cause methemoglobinemia. The amines oxidise the heme iron of haemoglobin from Fe(II) to Fe(III), blocking the oxygen binding. This results in characteristic symptoms like cyanosis of lips and nose, weakness and dizziness. The extent of which various aromatic amines can cause methemoglobinemia varies, however, widely (Ullmann, 5th Edition).
Occupational sensitisation to azo dyes has been seen in the textile industry since 1930. The first observations were made in 1930 when 20% of the workers dyeing cotton with red azoic dyes, developed occupational eczema (Foussereau et al., 1982).
Attributing an allergy to a particular azo dye is a complex and difficult process, due to the following reasons:
| a great number of azo dyes, approximately 2,000. | |
| each azo dye is marketed under several different names. | |
| azo dyes very often contain impurities. |
This may be the reason why, in rather rare cases, exposure to azo dyes has led to recognition of a possible relationship between skin sensitisation and a particular azo dye.
The majority of sensitising dyes, present in clothes, practically all belong to the group of disperse dyes, which has been developed for use on synthetic fibres. The explanation is probably that the attachment of molecules from disperse dyes is weak, as they are more easily available for skin contact.
In clinical patch tests the following azo dyes have shown sensitising properties (Cronin, 1980):
| Disperse Red 1, 17. | |
| Disperse Orange 1, 3, 76. | |
| Disperse Yellow 3, 4. | |
| Disperse Blue 124. | |
| Disperse Black 1, 2. |
In Germany, disperse azo dyes like Disperse Blue 1, 35, 106 and 124, Disperse Yellow 3, Disperse Orange 3, 37, 76 and Disperse Red 1 have been associated with contact dermatitis, resulting from exposure to textiles coloured with these dyes. In most cases the dermatitis resolved, once the sensitising "textile" had been discarded. These dyes are no longer recommended for colouring of textiles, which come into contact with the skin (Platzek, 1995).
Non disperse azo dyes, used for colouring of natural fibres were investigated in 1,814 patients attending the clinic patch tests (Seidenari et al, 1995). 0.88% of the patients reacted positive to the following dyes: Direct Orange 34 (8 patients), Acid Yellow 61 (5 patients), Acid Red 359 (2 patients) and Acid Red 118 (1 patient).
Remazol Black Bâ (Reactive Black 5) was investigated for sensitisation potential in experimental animals and was found to be negative. However, a few cases of allergic reactions have been observed in man.
Despite a very broad application field and exposure, sensitising azo dyes have been identified in relatively few reports (Cronin, 1980).
Only limited information is available regarding absorption, distribution, and excretion of azo dyes, whereas the metabolism after administration of oral consumption has been investigated extensively. Absorption of azo dyes through the skin is doubtful, as intact azo dyes may not penetrate the skin (NIOSH, 1980).
A distribution study conducted with a 14C-biphenyl ring, labelled Direct Blue 15* and Direct Red 2, in rats showed that liver, kidney and lung accumulated and retained higher levels of 14C than other tissues, 72 hours after administration of a single oral consumption (HSELINE, 1998).
The azo linkage is the most labile portion of an azo dye molecule and may easily undergo enzymatic breakdown in mammalian organisms, including man. The azo linkage may be reduced and cleaved, resulting in the splitting of the molecule in two parts (Brown & DeVito, 1993).
The anaerobic environment of the lower gastrointestinal tract of mammals is well suited for azo-reduction. Several anaerobic intestinal bacteria are capable of reducing the azo linkage. The majority of these bacteria belong to the genera Clostridium and Eubacterium. They contain an enzyme associated with the cytochrome P 450, also termed azo-reductase. It is a non-specific enzyme, found in various micro-organisms and in all tested mammals (NIOSH, 1980).
In mammalian organisms azo-reductases are, with different activities, present in various organs like liver, kidney, lung, heart, brain, spleen and muscle tissues. The azo-reductase of the liver, followed by the azo-reductase of the kidneys possess the greatest enzymatic activity.
Although reduction and cleavage of the azo-linkage is the major metabolic pathway of azo dyes in mammals, other metabolic pathways may take place. Major routes of detoxifying metabolism of azo dyes and aromatic amines are ring hydroxylation and glucuronide conjugation.
After cleavage of the azo-linkage, the component aromatic amines are absorbed in the intestine and excreted in the urine (Brown & DeVito, 1993). However, the polarity of azo dyes influences the metabolism and consequently the excretion. Sulphonation of azo dyes appears to decrease toxicity by enhancing urinary excretion of the dye and its metabolites. Sulphonated dyes, mainly mono-, di- and trisulphonated compounds are world-wide permitted for use in foods, cosmetics and as drugs for oral application.
Highly sulphonated azo dyes are poorly absorbed from the intestine after oral intake. Practically a complete cleavage of the azo linkage takes place in the gastrointestinal tract. This results in sulphonic acids rather than aromatic amines. These acids are rapidly absorbed, modified by the liver and excreted in the bile and urine. Sulphonated, fat soluble azo dyes are not reduced by the gut micro-organism but absorbed from the intestine and metabolised to the more polar N- or O-glucuronide and excreted as glucuronide conjugates (Parkinson & Brown, 1981).
The aromatic component amines of azo dyes may be absorbed into the body through the lungs, the gastrointestinal tract or the skin (ECDIN, 1993).
In general, the correlation between results of mutagenicity tests and carcinogenicity shown in animal experiments of azo dyes, is poor. The lack of correlation is probably due to the rather complex metabolic pathways, which azo dyes undergo in mammalian organisms (Brown & DeVito, 1993).
The majority of azo dyes requires metabolic activation, namely reduction and cleavage of the azo linkage to the component aromatic amines to show mutagenicity in vitro test systems. Therefore the majority of azo dyes, if highly purified, will, at least without metabolic activation, be negative in such tests (Arcos & Argus, 1994).
Many of the commercial available azo dyes may, however, due to impurities, e.g. contamination with aromatic amines, show mutagenic activity in vitro.
Since the mid-nineteenth century the growth of the synthetic dye industry and in particular the azo dye industry has been based on aromatic amines and consequently contributed to a serious occupational exposure.
Correlation between exposure of aromatic amines and human cancer was reported as early as 1895 by Rehn. He reported four cases of bladder cancer, named as "aniline cancer", out of several hundreds of workers engaged in the manufacture of fuchsin from crude aromatic amines for 15-29 years.
Between 1921 and 1951 Case computed a number of bladder cancer deaths for men manufacturing azo dyes and compared this to the expected incidence of bladder cancer in England. Four bladder cancer deaths were expected, whereas 127 deaths were found. Approximately 25% of all workers being exposed to aromatic amines, including 2-naphthyl- amine and benzidine, developed bladder cancer. The workers, who were only exposed to benzidine, had fewer tumours (15%) than those being exposed to 2-naphthylamine (50%). A few workers, who distilled 2-naphthylamine, all died of bladder cancer (Cartwright, 1983).
Besides the historical evidence, case-control studies have later been carried out on several occupational groups, including machinists, cooks, hairdressers, coal miners, carpenters etc. In several occupational groups a low to an elevated risk of bladder cancer was seen (Miller & Miller, 1983).
For decades there has been a strong human evidence for the association of bladder and renal pelvis cancers with specific aromatic amines. In addition, there has been an evidence, although weaker, that stomach and lung cancers are also associated with exposure to these amines. Aromatic amines do not induce tumours in humans at the exposure site, e.g. lungs and skin, but usually at a site as the urinary bladder.
The latency period, namely the period between the first exposure and the diagnosis of bladder cancer, ranged from 5 to 63 years. The average latency period was approximately 20 years, but cases of cancer after a few months of exposure have also been described (Cartwright, 1983).
Association between aromatic amines and bladder cancer in humans lead to extensive examination of the possibility for induction of bladder cancer in experimental animals.
In experimental animals, aromatic amines induced tumours in liver, intestine or urinary bladder. Furthermore, tumours in mammary gland and the skin were observed in rats (Sontag, 1981).
The carcinogenicity of aromatic amines is species specific. In experimental animals, benzidine was carcinogenic after administration of oral consumption and subcutaneous injections, producing liver tumours in rats, mice and hamsters, whereas bladder cancers were only seen in dogs.
2-Naphthylamine was a potent bladder carcinogen in dogs, but it was non-carcinogenic in rats and rabbits. After treatment with substituted benzenediamine, the incidence of bladder cancer in treated rats was only slightly elevated, but in addition, kidney tumours were observed (Clayson & Garner, 1976).
Although the latency period for human bladder cancer is relatively long, this period may be very short for animal carcinogenesis. Dyes based on benzidine, namely Direct Black 38*, Direct Blue 6* and Direct Brown 95* were investigated in a 13 week subchronic feeding study in rats. All these dyes induced a high incidence of pathological changes (neoplastic nodules) and/or liver cancer within 5 weeks. This is most probably the shortest latency period known for any chemical study with carcinogenic properties (Clayson & Garner, 1976).
Molecular mechanism of carcinogenicity
There is a strong evidence that aromatic amines require metabolic activation for carcinogenicity. The first step involves N-hydroxylation and N-acetylation, and the second step involves O-acylation yielding acyloxy amines. These compounds can degrade to form highly reactive nitrenium and carbonium ions. These electrophilic reactants may readily bind covalently to genetic material, namely cellular DNA and RNA (Brown & DeVito, 1993).
This process may induce mutations, and it is recognised that mutations can lead to formation of tumours.
Although the primary acute hazard associated with exposure to aromatic amines is carcinogenesis, methemoglobinemia is attributed to the same mechanism of metabolic activation.
Aromatic amines - structure activity relationship
For this class of organic compounds, the structure activity relationship between aromatic amines and carcinogenic potential has been reviewed in details (Milman & Weisburger, 1994).
Carcinogenic potential of aromatic amines varies considerably with the molecular structures, although the mechanism of metabolic activation, resulting in formation of electrophilic reactants, seems to be common. General trends are obvious and may outline a structure-activity relationship as follows:
| Aromatic amines consisting of two or more conjugated or fused aromatic rings are associated with a high carcinogenicity potential. Single aromatic or non-conjugated ring amines may be carcinogenic too, but the potential is lower. |
| An aryl or alkyl group attached to the amino nitrogen can modify the carcinogenic potential by the interference of N-hydroxylation. |
| Certain substitution of the aryl ring has a fairly constant influence on carcinogenic potential. Aromatic rings substituted in para-position to the amino group are generally more carcinogenic than those non-substituted. Substitution with a methyl or a methoxy group in para- position to the aromatic amine group often enhances the carcinogenic potential, whereas sulphonic acid derivatives do not show mutagenic and carcinogenic potential. |
Carcinogenic aromatic amines, which are common in industrial important azo dyes, are containing the moiety of:
| aniline | |
| toluene | |
| benzidine | |
| naphthalene |
Correlation between exposure to aromatic amines containing the moieties mentioned above, and cancer in humans and/or in experimental animals has also lead to severe restriction or prohibition regarding manu- facture and use of these compounds.
Manufacture and use of azo dyes based on any of the 22 aromatic amines, presented in Table 5.3, have been restricted in several countries (Specht & Platzek, 1995). In Germany these amines are on a list encompassing hazardous substances in the working environment, see Table 5.3.
Table 5.3
Aromatic amines restricted according to the MAK- und BAT Werte Liste, 1998.
Regulerede aromatiske aminer i henhold til MAK- und BAT Werte Liste, 1998.
| Moiety | Synonym |
CAS |
| Aniline | ||
| 4-chloroaniline | 4-chloro-benzenamine | 106-47-8 |
| 2,4,5-trimethylaniline | 2,4,5-trimethyl-benzenamine | 137-17-7 |
| 4,4´-methylenebis[ 2-chloro- aniline] | - | 101-14-4 |
| 4,4´-methylenedianiline | 4,4´-diaminodiphenylmethane | 101-77-9 |
| 4,4´-oxydianiline | di (4-aminophenyl) ether | 101-80-4 |
| 4-methoxy-m-phenylenediamine | 4-methoxy-1,3-benzenediamine 2,4-diaminoanisol1 |
615-05-4 |
| 4-aminoazobenzene | 4-(phenylazo)- benzeneamine | 60-09-3 |
| o-anisidine | 2-methoxy-benzenamine | 90-04-0 |
| Toluene | ||
| o-toluidine | 2-methyl-benzenamine | 95-53-4 |
| 4-chloro-o-toluidine | 4-chloro-2-methyl-benzenamine 4-chlor-o-toluidin1 |
95-69-2 |
| 5-nitro-o-toluidine | 2-methyl-5-nitro-benzenamine 2-amino-4-nitrotoluidin1 |
99-55-8 |
| 6-methoxy-m-toluidine | 2-methoxy-5-methyl-benzenamine p-kresidin1 |
120-71-8 |
| 4-o-tolylazo-o-toluidine | 4-amino-2´,3 dimethylazobenzene o-aminoazotolulol1 |
97-56-3 |
| 4,4´-bi-toluidine | 3,3´-dimethylbenzidine | 119-93-7 |
| 4-methyl-m-phenylenediamine | 4-methyl-1,3-benzendiamine 2,4-toluylendiamin1 |
95-80-7 |
| 4,4´-methylenedi-o-toluidine | 3,3`-dimethyl-4,4´-diamino- diphenylmethan1 |
838-88-0 |
| Benzidine | ||
| benzidine | 4,4´-diaminobiphenyl | 92-87-5 |
| 4,4´-thiodianiline | di(4-aminophenyl)sulphide | 139-65-1 |
| 3,3´-dichlorobenzidine | - | 91-94-1 |
| 3,3´-dimethoxybenzidine | o-dianisidine | 119-90-4 |
| biphenyl-4-ylamine | 4-aminobiphenyl | 92-67-1 |
| Naphthalene | ||
| 2-naphthylamine | - | 91-59-8 |
1
German synonym.Problems of impurities
Several impurities may be found in almost all commercial available azo dyes. Impurities may be introduced during the manufacturing processes or during the storage.
Azo dyes, based on aromatic amines, may contain these amines as impurities introduced during the manufacturing process. For example, azo dyes based on benzidine or o-toluidine may contain residues of benzidine or o-toluidine, respectively, used as intermediates in the manufacturing process.
Aromatic amines may also be present as a result of thermal or photochemical degradation of azo dyes. It is known, that sunlight may cause release of 1-aminonaphthalene formed azo dyes based on this amine (Brown & DeVito, 1993).
Exposure to azo dyes also entails exposure to the component aromatic amines due to:
| breakdown of azo dyes. | |
| presence of aromatic amines as impurities (their intermediates or breakdown products). |
Exposure to aromatic amines is of great concern, as many of them are characterised by having serious long-term effects.
Exposure to azo dyes may take place through inhalation and accidental ingestion. Absorption of azo dyes through the skin is rather doubtful, whereas the aromatic amines may be absorbed.
In Denmark, occupational exposure to azo dyes may take place within colouring of textiles, leather and plastics.
Non-occupational exposure to azo dyes may take place by the wearing of coloured textiles and by playing with coloured toys which not conform to requirements and standards harmonised at the European level by the Council Directive concerning safety of toys.
Inhalation of cigarette smoke represents the greatest non-occupational exposure, as the smoke contain aromatic amines along with many other hazardous compounds. It is known that inhaled cigarette smoke enhance the incidence of bladder cancer, and heavy cigarette smoking doubles the risk of getting bladder cancer (Cartwright, 1983).
Summary
The acute toxicity of azo dyes is low. However, potential health effects are recognised, i.e. LD50 values between 250 and 2,000 mg/kg body weight.
Despite a very broad field of application and exposure, sensitising properties of azo dyes have been identified in relatively few reports. Red azoic dyes have been linked to allergic contact dermatitis in heavily exposed workers. Furthermore, textiles coloured with disperse azo dyes have caused allergic dermatitis in a few cases.
The azo linkage of the azo dyes may undergo metabolic cleavage which results in free component aromatic amines. After cleavage of the azo linkage, the component aromatic amines are absorbed in the intestine and excreted in the urine. 22 of the component amines are recognised as potential human carcinogens, and/or several of them have shown carcinogenic potential on experimental animals. Sulphonation of the dye reduces the toxicity by enhancement of the excretion.
Although the metabolic cleavage of azo dyes is the main source of aromatic amines, aromatic amines may also be present as impurities in commercial available azo dyes.
Due to a strong relationship between exposure to azo dyes and/or aromatic amines and evidence of human cancer, aromatic amines are the greatest hazard to health. Consequently, exposure to azo dyes based on aromatic amines, which are known or suspected human carcinogens, encompasses the greatest risk to health.
There is a small but possible risk of exposure to potential carcinogenic aromatic amines from dyes and coloured products in Denmark. Occupational exposure to azo dyes may take place in association with the colouring of textiles, leather and plastics. Non-occupational exposure may take place by wearing textiles, playing with toys and by inhalation of cigarette smoke. The exposure may take place as a result of a break- down of the dyes or due to impurities of the dyes.
Environmental fate and exposure
Releases into the environment
Dyes
Measured data concerning the emissions of azo dyes to the environment in Denmark are not available. This applies both for the production (processing) and the use phases.
The major route of release during the production phase is through waste water effluent from the processing industries, mainly from textile and to a smaller extent from leather. In the present survey it is assumed that releases from the remaining trades: paper mills, printing, plastics and paint industries are negligible and approximately 0. In addition, it shall be noted that no manufacture of dyes takes place in Denmark.
There is a potential release of dyes to the waste water during the consumption (use phase) of the end-products (paints, varnishes, textiles etc.) from industries as well as private households. However, the predominant potential release route from end-use is from waste deposited in landfills.
The potential atmospheric release route may be through particulate matter from soils which are treated with sludge, from waste deposits (land-fills), from incineration of waste and from emissions of the processing industry. It is estimated that the atmospheric release route is insignificant and approximately 0.
Agricultural soil fertilised with sludge may give rise to releases of dyes to soil/groundwater. In addition, landfill deposit of dyes contained in products may cause release of dyes to soil/groundwater, too.
The estimated Danish releases are shown in Table 5.4. The preconditions for the estimates are given in chapter 4. It should be noted, that the release to landfills is assumed to be associated, exclusively with the consumption of end-products (use phase).
Table 5.4
Estimated environmental releases of azo dyes in Denmark.
Estimeret frigivelse af azofarvestoffer til miljøet i Danmark.
| Processing | Waste water (Influent, stp1) |
Landfills |
||
| industry | Processing |
Use |
Use |
|
tonnes |
tonnes |
tonnes |
||
| Leather | 1 |
n.a. |
9 |
|
| Paint | n.a. |
n.a. |
n.a. |
|
| Paper | n.a. |
n.a. |
n.a. |
|
| Plastic | n.a. |
n.a. |
26 |
|
n.a. |
n.a. |
n.a. |
||
| Textile | 70 |
72 |
191 |
|
1
stp = Sewage Treatment Plantn.a. = negligible amount.
Metabolites
Impurities of the dyes as well as decomposition by reductive cleavage of the azo dyes may result in transformation of the azo dyes to the degradation products/metabolites (aromatic amines), of which some are potentially carcinogenic. Estimation of the decomposition of azo dyes in the environment may be derived from knowledge of the structural and molecular composition of the azo dyes and of a stoichiometric equation.
The environmental exposure routes of the aromatic amines are essentially the same as the ones described for the dyes.
Abiotic degradation
An important natural abiotic degradation mechanism is photolysis and hydrolysis as a function of pH in the range of pH 4-9 (ETAD, 1992a).
The evidence of the role of hydrolysis in degradation of azo dyes is not conclusive. Hydrolysis is by Baughman and Perenich (1988b) not considered to be important. If the dye is not broken during rigors of biological waste treatment, it is unlikely to degrade rapidly in the less severe conditions of the environment. This is supported by Clarke and Anliker (1980), who states that the reductive cleavage of the azo-bond is the major degradation pathway for azo dyes.
For the reactive dyes the abiotic half-life due to hydrolysis is approximately 2 days (IUCLID).
Photo-reduction of azo dyes to hydrazines and amines is possible, but it is likely to be very slow, except in oxygen-poor water. The stability of the dyes to visible and UV-light is very high, and therefore only slow degradation has been shown (Clarke & Anliker, 1980).
The photo-stability of azo dyestuffs is high in pure water but in the pre- sence of natural humic materials, the photo decomposition is strongly accelerated, probably through oxidation by single oxygen or oxy-radicals (Brown & Anliker, 1988).
Shu et al. (1994) demonstrated photo-oxidation (UV/H2O2- -photo chemical reactor) of two non-biodegradable azo dyes in waste water (Acid Red 1* and Acid Yellow 23*). It was observed that the decomposition of both azo dyes was pseudo-first order reactions with respect to azo dye concentrations. The reaction rates were dependent on the pH, the initial dye concentration and the hydrogen peroxide dosage, e.g. with high concentrations of H2O2 (18.95 mM) the half-life was 6 minutes for Acid Red 1* (20 ppm).
Other advanced oxidation processes include Fenton’s reagent and TiO2 photo-oxidation (Shu et al., 1994). A feasibility study by Dieckmann et al. (1994) indicates that azo dyes (Solvent red 1 and 4-hydroxyazoben-zene) can be degraded via sensitised photocatalysis on a surface of TiO2.
Shu and Huang (1995) investigated 8 acidic azo dyes for degradation of UV/Ozone. They found that the degradation rate were of the first order with respect to both azo dyes and ozone concentrations. UV-light did not significantly enhance the degradation ability. The half-lives were in the range of 1.2 to 2.6 minutes.
It is assumed that the main abiotic removal mechanism for dyestuffs in wastewater treatment plants is adsorption of sludge. However, other effects like sedimentation, precipitation or flocculation may also play a role (Pagga & Taeger, 1994).
Anionic dyes may be expected to react with Ca, Mg etc. to form highly insoluble salts (i.e. pigments) and thereby reduce the concentration, which is available for other reactions or biological effects (Baughman & Perenich, 1988b).
Other physico-chemical processes are flocculation, flotation, membrane filtration, electrokinetic coagulation, electrochemical destruction, ion-exchange, chemical oxidation and different sorption techniques. A review of the different treatment technologies and techniques and their efficiency towards degradation of xenobiotics has been given by Matsumoto et al. (1995). However as Banat et al. (1996) conclude, not one specific treatment process seems to be able to handle decolourisation of all textile waste waters. Generally, a customised process, which probably involves a combination of different methods, will be more applicable. Ozonation has achieved the greatest practical importance for removal of colours, but also precipitation and flocculation procedures have given good results. Decolourisation with reductive agents such as hydrosulphite is a workable proposition (Clarke & Anliker, 1980).
Metabolites
Some of the aromatic amines may be susceptible to photolysis, e.g.
4-methyl-m-phenylamine (HSDB, 1998).
Hydrolysis is, generally, not an important route of degradation of the aromatic amines (HSDB, 1998).
Summary
Even though the dyes have absorption maxima in the range of visible and UV-light, photo-reduction does not play a dominant role in the environmental fate of dyes, although its contribution to the total mineralisation of widely dispersed trace amounts may be underestimated. Furthermore, hydrolysis seems not to be an important degradation pathway either, except for reactive dyes, which are hydrolysed rapidly in aqueous solution.
For the metabolites, photolysis may be of some importance, whereas hydrolysis not seems to be an important degradation route.
Biodegradation
Razo-Flores et al. (1997a) estimate that due to the recalcitrance of azo dyes in aerobic environments, the azo dyes eventually end up in anaerobic sediments, shallow aquifers and in groundwater.
Extensive tests indicate that dyes are generally adsorbed to the extent of 40-80% by the biomass and are thus partly removed from the water phase in sewage treatment plants. They are, however, not biodegraded at this stage to any significant extent (Clarke & Anliker, 1980).
Dyes to be useful must possess a high degree of chemical and photolytic stability which implies that removal of dyes from effluents is difficult. Stability against microbial attack is also a required feature of azo dyes (Pagga & Brown, 1986). Subsequently, they are less amenable to biodegradation (Banat et al., 1996). It is thus unlikely that they, in general, will give positive results in short-term tests (e.g. OECD) for aerobic biodegradability (Brown & Anliker, 1988).
Furthermore, the electron-withdrawal character of azo-groups generates electron deficiency and thus makes the compounds less susceptible to oxidative catabolism. As a consequence, many of these chemicals tend to persist under aerobic environmental conditions (Knackmuss, 1996).
Biodegradation of azo dyes can occur in both aerobic and anaerobic environments. In both cases, the initial step in the biodegradation is the reductive cleavage of the azo-bond. Under aerobic conditions the initial step of cleavage of the azo-bond is typically followed by hydroxylation and ring opening of the aromatic intermediates (Zissi & Lyberatos, 1996).
Permeability through the cell wall has often been found to be the rate-limiting step in the reduction process. The microbial reduction of azo dyestuffs are either by reduction of living cells or by cellular extracts (Brown & Anliker, 1988).
Anaerobic and aerobic metabolic activities are a prerequisite for the complete biodegradation of recalcitrant aromatic pollutants, which contain electron-withdrawal substituents, such as azo dyes. Therefore, the recalcitrant nature of azo dyes can be overcome by utilising anaerobic-aerobic co-cultures (Field et al., 1995). This is supported by Clarke and Anliker (1980), who furthermore state that physical and chemical treatment is required as well. With the possible exception of basic dyes, the biological treatment processes (activated sludge) have in most cases proved to be insufficient for removal of dyestuffs from waste waters (Clarke & Anliker, 1980).
Bacteria - anaerobic
Brown and Laboureur (1983b) investigated the primary biodegradation of 13 azo dyes in an anaerobic sludge inoculum. The dyes were selected as commercially significant and represented both monoazo, diazo and polyazo dyes. The monoazo dyes were Mordant Blue 13, Mordant Black 9, Basic Red 18, Acid Yellow 151 and the diazos Direct Red 7, Acid Red 114*, Direct Blue 15*, Direct Yellow 12, Reactive Black 5 and Acid Blue 113*. All of these were substantially biodegraded (75-94%), whereas the polyazos Direct Black 19 and Direct Black 22 were only decolourised between 51-61% in a time period of 0 to 42 days.
Later results by Brown and Hamburger (1987) have confirmed that azo dyes are likely to undergo primary biodegradation in an anaerobic environment. The decolourisation was more than 90% in the time range of 0 to 56 days. The dyes tested were Acid Orange 7*, Acid Yellow 25*, Acid Yellow 36*, Acid Yellow 151, Acid Red 114*, Acid Black 24, Direct Red 7, Direct Blue 14*, Direct Blue 15*, Direct Yellow 12, Direct Yellow 50*, Mordant Black 9 and Mordant Black 11. This was also confirmed by Boethling et al. (1989) for Direct Red 28*.
Shaul et al. (1991) also found evidence of biodegradation of Acid Orange 7*, Acid Orange 8 and Acid Red 88. In 24 hours, 81 to 86% were degraded. The presence of sulfo groups on the aromatic component of some azo dyes seemed to inhibit the biodegradability significantly.
Direct dyes (Direct Red 28*, Direct Blue 1* and Direct Blue 14*) are degraded with more than 90% in anaerobic sediment-water systems with half-lives ranging from 2 to 16 days. The degradation is inhibited when the dyes are strongly bound to the sediment (Weber, 1991)
In sediments, Yen et al. (1991) showed that the degradation of two disperse azo dyes (Disperse Red 1 and Disperse Red 5) had half-lives within hours when the concentrations were kept below 10 ppm in the sediment. The reduction of nitro groups to amino groups and/or cleavage of the azo groups to give nitroanlines were found to be major pathways.
Zissi and Lyberatos (1996) demonstrated that Bacillus subtilis is, at least partly, able to degrade the disperse azo dye p-aminobenzene under anoxic conditions growing in a batch-reactor. The results proved that Bacillus subtilis co-metabolises p-aminobenzene under denitrifying conditions in the presence of glucose as a carbon source, producing aniline and p-phenyldiamine, as the N=N double bond is broken.
Other authors have reported degradation of disperse dyes with half-lives in order of minutes as well, e.g. Disperse Blue 79* (Weber & Adams, 1995; Freeman et al., 1996) and Disperse Red 1 with a half-life of less than 8 hours (Baughman & Weber, 1994).
The non-ionic dye Solvent Red 1 has been reported to have a half-life of 2.2 to 4 days (Baughman & Weber, 1994).
The reduction of benzidine azo dyes to free benzidine by soil bacteria has been reported for four aminobenzene azo dyes. The soil bacteria are Pseudomonas cepacia and Pseudomonas sp.. The initial reaction was azo reduction and cleavage, followed by acetylation and aromatic ring hydroxylation. The azo dyes were reduced with 42 to 91% at an aqueous concentration of 5 to 30 ppm during 24 hours of incubation. Similarly, a Plesiomonas bacterial species isolated from textile waste water has shown to degrade 5 different azo dyes under anaerobic conditions. Mixtures of sewage and soil bacteria (e.g. Pseudomonas aeuginosa) may also effectively degrade azo dyes. The dyes undergo azo-bond cleavage followed by carboxylation, hydroxylation and acetylation metabolism of the initial aromatic amine azo-reduction metabolites (Brown & DeVito, 1993).
Examples of removal of dyes in use in Denmark under anaerobic conditions are summarised in Table 5.5 below.
Table 5.5
Removal of azo dyes used in Denmark under anaerobic conditions.
Fjernelse af azofarver anvendt i Danmark under anaerobe forhold.
| Chemical class | Period |
Degree of removal |
days |
% |
|
| Acid Blue 113 | 0-421 |
94 |
| Acid Orange 7 | 282 13 |
972 813 |
| Acid Red 114 | 72 0-421 |
1002 621 |
| Acid Yellow 25 | 562 |
572 |
| Acid Yellow 36 | 72 |
972 |
| Direct Black 19 | 0-421 |
511 |
| Direct Blue 1 | 164 |
504 |
| Direct Blue 14 | 72 34 |
>902 504 |
| Direct Blue 15 | 0-421 |
831 |
| Direct Red 28 | 44 |
504 |
| Direct Yellow 50 | 352 |
1002 |
| Solvent Yellow 1 | 135 |
895 |
| Solvent Yellow 2 | 75 |
1005 |
| 1 Brown and Laboureur (1983b). 2 Brown and Hamburger (1987). 3 Shaul et al. (1991). 4 Weber (1991). 5 HSDB (1998). |
||
Bacteria - aerobic
Like dyes in general, the hydrolysed dyes are practically not biodegraded in the short retention time of the aerobic treatment processes. Most dyes are degraded under anaerobic conditions. Such conditions are met in the anaerobic digestion process at sewage treatment plants, and in sediments and soils (ETAD, 1991).
Pagga & Brown (1986) tested 87 dyes in a short-term aerobic biodegradation based on the OECD Guideline for a static test method with activated sludge. They found no significant biodegradation, but substantial colour removal was observed which was attributed to the elimination of the dyes by adsorption. The tested dyes represented all the ionic characters and chemical types.
A study by Zhang et al. (1995) revealed that Acid Orange 7* and Acid Orange 8 can be degraded aerobically in a rotating drum biofilm reactor. The more complex Acid Orange 10* and Acid Red 14*, however, were not aerobically degraded. However, the authors demonstrated that cleavage of the azo bond occurred easily under anaerobic/anoxic biofilm conditions.
Knackmuss (1996) suggests that a total biodegradation of azo dyes may be accomplished by bacteria, harbouring a highly efficient uptake and an azo reductase system which are used in a two-step anaerobic/aerobic process, at least with regards to biodegradation of sulphonated naphthalenes.
Fungi
Microbial degradation of lignin-containing pulp and paper waste water has been demonstrated by several authors, especially with the white-rot Basidiomycete fungus: Phanerochaete chrysoporium. The mechanism of colour removal involves lignin peroxidase and Mn-dependent peroxidase or laccase enzymes. The degradation of azo dyes is apparently dependent on the availability of nitrogen. If there is a high concentration of N the degradation rate decreases. Banat et al. (1996) have reviewed the literature and found out that azo dyes may be degraded by the fungus between 23 and 90% in a time span of 3 to 21 days with different concentrations. A wide variety of dyes has been tested, among them Acid Red 114*, Acid Red 88, Direct Blue 15*, Disperse Yellow 3, Disperse Orange 3 and Solvent Yellow 14* (Spadaro et al., 1992). In addition, other anionic dyes, like Reactive Orange 96, Reactive Yellow 5 and Reactive Black 5, have been demonstrated to be biodegraded by the white-rot fungus Phanerochaete chrysosporium, too (Heinfling et al., 1997).
The actinomycete strains, mainly streptomycetes, isolated from soil samples have been demonstrated to decolourise effluents containing different types of reactive dyes. In a study carried out by Zhou and Zimmermann (1993) it was concluded that the decolourisation of Reactive Red 147 was due to adsorption rather than biodegradation. Banat et al. (1996) has reviewed the studies of other fungal biodegradation of azo dyes and several (7 in total) other species have shown to decolourise but mainly by way of adsorption.
There are conflicting evidence of the influence of the substituents on the aromatic ring with regards to the effect on biodegradability by Phanerochaete chrysoporium. Paszczynski et al. (1992) found that substitution with sulfo groups on the aromatic component of some azo dyes not seemed to affect the biodegradability of the anionic azo dyes significantly. Pasti-Grigsby et al. (1992), however, found that significant degradation of the azobenzene derivative dyes and naphtol-derivative dyes (e.g. Acid yellow 9 and Acid Orange 12 (anionic)) occurred solely when the hydroxy group was in a specific position relative to the azo linkage. Spadaro et al. (1992) showed that when the aromatic rings of the neutral dyes (Solvent Yellow 14*, Disperse Orange 3 and Disperse Yellow 3) had substituted hydroxyl, amino, acetamido or nitro groups, the mineralisation was greater than by those with unsubstituted rings.
Algae
Decolourising with algal cultures has been found by Jinqi and Houtian (1992). The reduction of algae resembles that of the bacteria. The azo reductase of the algae Chlorella and Oscillatoria is responsible for degrading azo dyes into aromatic amines. The aromatic amine is then subject to further degradation by the algae. As for bacteria, azo compounds with a hydroxy or an amino group are most likely to be readily degraded than those with a methyl, methoxy, sulfo or a nitro group.
Mineralisation
FitzGerald and Bishop (1995) found an almost total decolourisation in the first stage of an anaerobic/aerobic treatment of sulphonated azo dyes (Acid Orange 10*, Acid Red 14* and Acid Red 189). Analyses of the intermediates at the first and second stages (aerobic) showed virtually no concentration of intermediates, which may indicate a total anaerobic mineralisation. In contrast, Seshadri et al. (1994) found that the aromatic amines remained undegraded in an anaerobic fluidised bed reactor.
Razo-Flores et al. (1997b) have demonstrated that Mordant Orange 1 may be completely degraded (mineralised) in a continuous upward-flow anaerobic sludge bed reactor in the presence of co-substrates.
Razo-Flores et al. (1997a) have further demonstrated that the azo dye, azodisalicylate is completely biodegradable in the absence of oxygen. The dye is mineralised in an adapted methanogenic consortium to CH4 and NH3 in both batch assays and continuous bioreactors.
Degradation of metabolites
Free aromatic amines are generally susceptible to environmental degradation (Brown & DeVito, 1993). Zerbinati et al. (1997) have found that naphthalenesulfonates can undergo oxidative degradation under physico-chemical conditions similar to those occurring in a river. However, other studies have shown that, e.g. benzidine is bound with the humic acid fraction of the soil (Weber, 1991).
Brown and Laboureur (1983a) showed in aerobic biodegradation tests that the four aromatic amines: aniline, p-anisidine, p-phenetidine and
o-toluidine are ready biodegradable and that both o-anisidine and
3,3´-dichlorobenzidine are inherent biodegradable in accordance with the OECD test guidelines. Brown and Hamburger (1987) confirmed these results for the lipophilic aromatic primary amines, but depending on their precise structure, some sulphonated aromatic amines may not be degradable.
Under aerobic conditions another type of recalcitrance can be recognised, namely, the tendency of certain compounds, susceptible to free radical reactions, to undergo oxidative coupling. These coupling reactions can result in the formation of recalcitrant humic-like polymers or in irreversible covalent binding of the pollutant into the soil humus. Aromatic amines and nitroaromatics are susceptible to these polymerisation reactions. Formation of azo compounds by oxidative coupling has been demonstrated in aerobic enrichment cultures from the aromatic amines (Field et al., 1995)
The metabolites of aromatic primary amines are not rapidly degraded under anaerobic conditions (Brown & Hamburger, 1987). Electron donating amino groups are expected to pose a serious problem to further reductive biotransformations by anaerobes. However, there is evidence for anaerobic aniline biodegradation by sulphate reducing bacteria and in mixed cultures under denitrifying conditions. Aniline degradation by a methanogenic consortium has also been claimed. Aromatic amines with carboxy, hydroxy and methoxy substituents are potentially mineralisable under methanogenic conditions (Field et al., 1995). Another example is o-toluidine, which is not degraded under anaerobic conditions (HSDB, 1998).
Summary
Various microbial species, i.e. fungi, bacteria and algae may be able to biodegrade azo dyes in an anaerobic environment. Total mineralisation or further degradation of the metabolites may predominantly take place in an aerobic environment.
The universal degradation route seems to be initial reductive cleavage of the azo bond followed by e.g. acetylation, carboxylation and aromatic ring hydrolysation.
The rate limiting step for bacterial degradation is the uptake across cell membranes for intracellular reduction, whereas some fungi may degrade the dyes extracellularly.
The substituents and the substitutional pattern may also significantly influence the biodegradability. The reported effects are contradicting, but the ionic azo dyes with hydroxy or amino groups are most likely to be readily biodegraded, compared to those with methyl, methoxy, sulfo or nitro groups. For the non-ionic dyes (disperse, solvent and mordant) an enhanced biodegradation is observed with hydroxyl, amino, acetamido or nitro groups compared to unsubstituted rings.
It is difficult to generalise about degradation rates and the degree of removal for specific azo dyes or for the different chemical classes based on the findings in the literature, because the experimental conditions vary.
However, biodegradation of azo dyes varies, in general, from hours to several months or more depending on, among other things, the physico-chemical properties of the dyes. The molecular size of the azo dyes, especially solvent and disperse dyes, may reduce the rate and probability of biodegradation. This is due to limited uptake possibilities, and the substituents may also influence the degradation rate.
The metabolites are primarily biodegraded under aerobic conditions. Some of the metabolites are ready biodegradable, and some of the sulphonated aromatic amines may not be degradable.
Volatilisation
Data concerning the volatilisation of azo dyes from aqueous surfaces are not available. With respect to volatilisation it is prerequisite to distinguish between the ionic dyes and non-ionic dyes, because ionic compounds are generally non-volatile. (Brown & Hamburger, 1987). Therefore, volatilisation will not be important for acid, direct, basic and reactive dyes. In principle, the solvent and disperse dyes have the potential to be volatile, but as they are large, complex molecules they can be expected to have low vapour pressures. Another reason for volatilisation to be unlikely for the uncharged dyes is that the escaping tendency or fugacity, which drives volatilisation, is also the driving force for both sorption and bioconcentration (Baughman & Perenich, 1988b).
Baughman and Perenich (1988a) calculated Henry’s law constants from solubility and vapour pressure. The values show that the disperse dyes will be entirely vapour-phase controlled in the environment in their rate of volatilisation from water and that this process is extremely slow. The vapour pressures lie in the range of 2 ´ 10-14 to 1 ´ 10-6 mmHg and the solubility in the range of 2 ´ 10-9 to 4.5 ´ 10-6 mol/l. The Henry law constant is on average 10-10 atm ´ m3/mol for disperse dyes.
Metabolites
In general, the metabolites, i.e. the 22 potentially carcinogenic aromatic amines, show moderate to low volatilisation with Henry’s law constants in the range of 4.7 ´ 10-11 (3,3´-dimethoxybenzidine) to 2.0 ´ 10-6 atm ´ m3/mol (o-toluidine).
Summary
Due to the chemical characteristics of the azo dyes, volatilisation from surfaces of either water or soil (wet or dry) is considered to be insignificant for both ionic and neutral (non-ionic dyes). This applies for the metabolites, too.
Because of dyestuffs inherent high affinity to substrates, they are adsorbed onto the sludge during sewage treatment and are thus removed from the final treated effluent (Anliker, 1986). But due to the chemical composition of some of the dyes, they may pass the sewage treatment unaffected and thus end up in the aquatic environment. Extensive testing indicates that dyestuffs are generally adsorbed to the extent of 40-80% by the biomass and are thus partially removed in sewage treatment plants (Clarke & Anliker, 1980). However, due to their relatively low affinity to substrates, the removal of the hydrolysed dyes (e.g. Reactive dyes) by adsorption onto the sewage sludge is only in the range of 0-30% (ETAD, 1991).
In the practical concentration range of 10 to 50 mg dye/l, there is an almost linear relationship between the concentration in solution and the amount adsorbed. The adsorptive capacity of activated sludge for dyes investigated was, in neutral media, in the range 0.01 to 4% of dyestuff on dry weight sludge (Clarke & Anliker, 1980).
The chemical properties and substitutional pattern of the chemical structure of the dyes and the composition of the waste water influences the degree of adsorption. The adsorption depends on the pH, salinity and the concentration and nature of organic contents.
Based on the properties of sediments, cation exchange is anticipated to be extensive and rapid for the basic dyes. A similar situation should exist for the anionic acid and direct dye, but the equilibrium constants would probably be much smaller (Baughman & Perenich, 1988b).
Shaul et al. (1991) investigated the partitioning of water-soluble azo dyes in the activated sludge process. A total of 18 dyes were tested and categorised according to their behaviour in the tests (Table 5.6). For Group 1 it was concluded that the high degree of sulphonation enhanced their water solubility and limited their ability to adsorb onto the biomass. Although the dyes in Group 2 were highly sulphonated, their greater molecular size was thought to account for their greater degree of adsorption.
Table 5.6
Fate of water-soluble dyes in activated sludge.
Vandopløselige farvestoffers skæbne i aktivt slam.
| Group 1: | Group 2: | Group 3: |
| Dyes passing through essentially unaffected | Dyes removed by adsorption | Dyes showing evidence of biodegradation |
| Acid Black 1* | Acid Blue 113* | Acid Orange 7 |
| Acid Orange 10* | Acid Red 151 | Acid Orange 8 |
| Acid Red 1* | Direct Violet 9 | Acid Red 88 |
| Acid Red 14* | Direct Yellow 28 | |
| Acid Red 18 | ||
| Acid Red 337 | ||
| Acid Yellow 17* | ||
| Acid Yellow 23* | ||
| Acid Yellow 49 | ||
| Acid Yellow 151 | ||
| Direct Yellow 4 | ||
| Ref.: Shaul et al. (1991). | ||
Weber (1991) has demonstrated that the sorption of several weakly basic benzidine-based dyes (Direct Red 28* (disulphonated) and Direct Blue 14* (tetrasulfonated)) strongly depend on the pH and the nature and concentration of inorganic salt in solution in an anaerobic sediment-water system. Sorption is strongly favoured with decreasing pH and increasing salt concentration. The sorption was enhanced especially for Direct Red 28*, which was less substituted.
Pagga and Taeger (1994) have found that the colour elimination of acid and disperse azo dyes (Acid Orange 7*, Acid red 88, Disperse Orange 29 and Disperse Yellow 5) depends on the hardness of the water. A high concentration of calcium ions favours adsorption as well as flocculation or precipitation processes or a better settling of the sludge and less turbidity.
In a study by Yen et al. (1990), it has been shown that newer disperse dyes show a higher degree of partitioning into the sediment than older disperse dyes based on calculated sediment concentrations.
Metabolites
The metabolites adsorb, except for 4-methyl-m-phenylenediamine, mode- rately to strongly onto sediments and soil. 4-methyl-m-phenylenediamine
does not adsorb to any significant degree (HSDB, 1998).
Summary
The removal of various dyes from different classes has been studied and the removal pattern may be summarised as shown in Table 5.7.
Table 5.7
Removal patterns of various classes of dyes.
Fjernelsesmønster for forskellige farvestoftyper.
| Classes | Removal pattern |
| Acid | High solubility leads to low adsorption, which appears to depend on the degree of sulphonation. |
| Basic | Typically high levels of adsorption. |
| Direct | High degree of adsorption, apparently unrelated to the number of sulphonic acid groups. |
| Disperse | Adsorption in the high-to-medium range. |
| Reactive | Very low degree of adsorption, apparently unaffected by the degree of sulphonation or ease of hydrolysis. |
Extensive adsorption onto soil and sediment has been demonstrated in several experiments. It is concluded that adsorption is the major route of removal of dyes in the environment. Adsorption is an important removal pathway for the metabolites, as well.
A high degree of solubility and sulphonation reduces adsorption, whereas increasing molecular size, hardness of the water and salinity favour sorption. This applies for a decreasing pH, as well.
The obtained data on bioaccumulation are primarily derived from fish-
tests.
The uptake rates are influenced by the partition coefficient (log Kow) (Erickson & McKim, 1990). Other factors may be of primary importance for the uptake as well, e.g. diffusional resistance, molecular size, respiratory volume and gill perfusion (Niimi et al., 1989).
The elimination rates for hydrophobic chemicals are low. For hydrophobic chemicals it has often been shown that uptake and clearance between fish and water is a first-order exchange process (Van Hoogen & Opperhuizen, 1988).
Anliker et al. (1981) have presented, estimated and experimentally assessed the log Kow and have experimentally assessed the log BCF (bio- concentration factor) in fish (MITI standard) for 50 azo dyes, representing both the ionic forms and the neutral forms. The average values for the different dyes are presented in Table 5.8 below.
Table 5.8
Partition coefficients and the measured bioaccumulation factors for 50 azo dyes allocated on 5 chemical (technical) types.
Fordelingskoefficienter og den modsvarende målte bioakkumulationsfaktor for 50 azofarvestoffer fordelt på 5 kemiske (tekniske) grupper.
| Structural type | Partition coefficient (log Kow) |
Bioaccumulation Factor ( in fish) (log BCF) |
| Acid | -3.3-0.01 |
0-0.7 |
| Basic (only one) | -1.0 |
-0.3 |
| Direct (only one) | <<0 |
0.2 |
| Disperse | 2.2-5.5 |
0-1.76 |
| Reactive | -2.2- -0.4 |
-0.2-0.7 |
| Ref.: Anliker et al. (1981). | ||
The survey shows, with a few exceptions, that the very hydrophilic (ionic) dyes have a log BCF of - 1 to 1, although from the log Kow lower log BCFs may have been predicted. This is explained by the adherence of dyes to the outside of the fish or to the intestine. None of the dyestuffs bearing at least one charged group has showed a log BCF larger than 1. It has been demonstrated that disperse dyes do not bioaccumulate in fish even though their log Kow values were larger than 3. The molecular weight was relatively high, between 450 to 550 g/mol, making the transport across membranes difficult.
These findings have been confirmed in other studies. The partition coefficients of 21 reactive dyes were very low (log Kow < 0) and none of these dyes have showed any tendency of bioaccumulation in the flow-through tests (MITI-standard) in the carp. (ETAD, 1991). In Carrassius sp., which was exposed to 2 mg/l and 0.2 mg/l for 42 days, the BCFs were less than 1.1 and less than 11, respectively (IUCLID).
ICI has carried out eight-weeks accumulation studies on the Carp (Cypri- nus carpio) (MITI standard). The results indicate that neither the 30 water-soluble2 nor the 12 disperse dyes2 with exposure levels up to 10 mg/l were accumulated. For the soluble dyes the accumulation factor was below the detection limit. The low accumulation of the soluble dyes may be expected, and the low accumulation factors found for the disperse dyes may be due to their relatively high molecular weight (typically 300-500) or because their absolute fat solubility is relatively low (Brown, 1987).
Anliker and Moser (1987) have investigated the melting point, the log Kow, solubility in water and n-octanol and the log BCF in fish for 8 disperse dyes (nitroazobenzene and phenylazopyridone types):
|
360 to 546 g/mol | ||
|
117 to 225 0C | ||
|
n.d. | ||
|
81 to 2,430 mg/l | ||
|
2.5 to 5.4 (majority above 3) | ||
|
0.3 to 1.76 |
They found that the high Kow suggested strong bioaccumulation tendencies, but the bioaccumulation was below 100. It was hypothesised that this behaviour may be due to their pronounced aggregation tendency, making transport across membranes difficult. The findings of Opperhuizen et al. (1985) support this. Their results indicated that for extremely hydrophobic chemicals with an effective cross section over 9.5 Å, a lack of uptake into biota (fish) can be expected, as membrane permeation seems practically impossible.
A study of 75 disperse dyes, even highly lipophilic ones, by Anliker (1986) and a later study by Anliker et al. (1988) on 23 disperse dyes3, including highly lipophilic ones, confirmed the above mentioned observations.
Similar results have been reported for the BCFs of chlorinated aromatic amines in guppies (Poecilia reticulata). The experimentally demonstrated values of BCF are significantly smaller that the calculated values of BCF (Wolf et al., 1992).
The azo compound (not dye) 3,3´,4,4´-tetrachloroazobenzene (TCAB), a common contaminant from 3,4 dichloroaniline based herbicides and of agricultural soils, has been tested (short-term) on the aquatic snail Indoplanorbis exustus by Allison and Morita (1995a). They found that even at detrital exposures of 2,500 ppm, the maximum level only reached 287 ppb (whole body basis). The authors above (1995b) also came to the same result in the Japanese Medaka (Oryzias latipes). The fish inhabit still waters and paddy fields and their drains. The study showed that TCAB is bioadsorbed and to some extent bioaccumulated in the fish. The contaminant was administered through the food.
Apart from the above stated findings, only a small amount of data was found in the literature and databases on the log Kow and the log BCF for specific azo dyes included in the present survey. The results of the literature study are presented in Table 5.9.
Table 5.9
Partition coefficient and bioconcentration factor for azo dyes used in Denmark.
Fordelingskoefficient og biokoncentrationsfaktor for azofarvestoffer anvendt i Danmark.
| Structural type | Partition coefficient (log Kow,, est.) |
Bioconcentration factor ( in fish) (log BCF) |
Reference |
| Acid Orange 10 | -4.6 |
0 (est.) |
HSDB |
| Acid Red 114 | - |
1.6-1.9 |
MITI |
| Acid Yellow 23 | - |
-0.54-0.48 |
MITI |
| Direct Black 38 | 2.0 |
1.3 (est.) |
HSDB |
| Direct Blue 1 | - |
0.3 (est.) |
HSDB |
| Disperse Blue 79 | 4.79 |
4.09 (est.) |
Yen et al., 1990 |
| Solvent Red 24 | -0.54-1.04 |
MITI |
|
| Solvent Yellow 1 | 2.62 |
1.76 (est.) |
HSDB |
| Solvent Yellow 2 | 4.05 |
3.25 (est.) |
HSDB |
| Solvent Yellow 3 | 3.92 |
2.29-2.75 (est.) |
HSDB |
Compared to the findings of Anliker et al. (1981) shown in Table 5.8, the log BCF for Acid Red 114* is above the range reported, whereas Acid Yellow 23* is in agreement. The remaining dyes are incomparable, as they are based on estimates rather than actual experimentally measured values of BCF. The solvent dyes were not included in the Anliker et al. (1981) study.
However, the calculated log BCF value for Disperse Blue 79* is in agreement with the findings of Anliker et al. (1981), whereas Direct Black 38* is twofold higher than reported by the above authors.
Metabolites
Both measured and estimated log BCFs for the cleavage products of the dyes, i.e. the 22 potentially carcinogenic aromatic amines, are according to ECDIN and HSDB (1998) below 3. The highest estimated log BCF has been found for 4-o-tolylazo-o-toluidine (2.75) (HSDB, 1998). The majority lies in the range of 1.5 to 2.0. The lowest values (<1.47) have been reported for o-anisidine (0.85), o-toluidine (1.2) and 4,4´-methylenedi-aniline (1.1). The log BCF values for the aromatic amines indicate that there is a risk of biomagnification for a great majority of the metabolites.
Summary
For the compounds with log BCFs larger than 3, there is a high risk of bioaccumulation, whereas for compounds with log BCFs between 1.47 and 3, the risks of biomagnification and secondary poisoning are important. For compounds with log BCF values below 1.47, there is no immediate concern with regard to bioaccumulation (Franke et al., 1994).
When looking at the values of the dyes included in the present survey, it is indicated that Acid Red 114 may bioaccumulate in fish, whereas the remaining ionic dyes do not seem to have any significant bioaccumulation potential. However, the estimated log BCFs for the non-ionic dyes, i.e. disperse and solvent, indicate a potential risk of bioaccumulation.
The estimated values for log BCF are generally to high which several authors have found. Therefore, the evidence of the risk bioaccumulation of the non-ionic azo dyes must be further validated, taking the potential barrier of uptake into account, as a result of the high molecular size of these compounds.
Generally, the cleavage products of the azo dyes, i.e. the aromatic amines, have a potential for bioaccumulation, too.
Aquatic compartment
Monitoring data
Only a few monitoring studies of environmental levels of dyes have been found, and data from Denmark have not been obtained.
In a study conducted by the US EPA, effluents from 25 textile industries were measured. The average TOC was measured to 276 mg/l (range 55 to 1,120 mg/l). The dyestuff itself has not been measured, but it is estimated that the dye contributes between 2 and 10% of the TOC and COD indicating worst case levels of 5.5 to 112 mg/l. However, the typical dye concentration lies in the range of 10 to 50 mg/l. Decolourised effluents contain less that 1 mg/l dye, and the TOC contribution of dyestuff following the primary and biological treatment stages is normally considerably less than 0.5 mg/l. In the same study, effluents from a tannery were measured, and the raw effluent contained 22 to 56 ppm dyestuff (Clarke & Anliker, 1980).
The following Table 5.10 and Table 5.11 summarise additional data regarding environmental monitoring of dyes in water and sediment.
Table 5.10
Monitoring data of dye concentrations in water.
Moniteringsdata for farvestofskoncentrationer i vand.
| Compound | Concentration |
Location/Reference | ||
Treated sewage effluent (ppb) |
River (ppb) |
Reservoir for drin- king water (ppb) |
||
| Acid Blue 1a | 12.3 |
1.7 |
0.6 |
Thames, Lee, UK (Brown & Anliker, 1988) |
| Fluoroscent whiteningb | 0.8-8 |
n.d (detection limit 0.01). |
European river (Anliker, 1986) | |
| Acid Yellow 219b | 120 |
22 |
5 |
Coosa River, US (Brown & Anliker, 1988) |
| Acid Orangeb | 10 |
Coosa River, US (ETAD, 1992b) |
||
| Acid Redb | 2 |
Coosa River, US (ETAD, 1992b) |
||
| Disperse Blue 26b,c 1985 1986 |
9.95 3.8 |
Yamaska River, Canada (ETAD, 1992b) |
||
| Disperse Red 60b,c 1985 1986 |
3.3 n.d. |
Yamaska River, Canada (ETAD, 1992b) |
||
| Disperse Blue 79*c 1985 1986 |
17.1 3.1 |
Yamaska River, Canada (ETAD, 1992b) |
||
| Solvent Yellow 1* | 522,7 |
Organics and Plastic industry, US (HSDB) | ||
| Most common acid dyesb | 20 |
Coosa River , US (Anliker, 1986) |
||
| a: Not an azo dye compound. b: The chemical class of the dye was not stated. c: Improved waste water treatment was installed between the two years. |
||||
Table 5.11
Monitoring data of dye concentrations in sediment.
Moniteringsdata for farvestofskoncentrationer i sediment.
| Compound | Sediment |
Suspended solids |
Location/ reference |
mg/kg dw |
mg/kg dw |
||
| Dyesb | 0.1-3 |
Coosa River, US (Brown & Anliker, 1988) | |
| No individual synthetic dyesb | n.d. (detection limit 0.05) |
Rhine, (Brown & Anliker, 1988) |
|
| Disperse Blue 26c 1985 1986 |
1.1 2.9 |
4.6 6.7 |
Yamaska River, Canada (ETAD, 1992b) |
| Disperse Blue 79*c 1985 1986 |
1.5 4.2 |
0.8 3.3 |
Yamaska River, Canada (ETAD, 1992b) |
| a: Not an azo dye compound. b: The chemical class of the dye was not stated. c: Improved waste water treatment was installed between the two years. |
|||
Estimation of PEC for the aquatic compartment
In most industrialised countries only about 20% or less of the release from processes will reach open water due to effective adsorption in the primary and the biologic treatment stages (Clarke & Anliker, 1980).
However, in the present calculation of PEC effluent, stp, two scenarios will be presented.
The estimation of PEC effluent, stp is based on the following assumptions:
| The processing industries do not treat waste water in agreement with TGD (1996). |
| Between 40 and 80% of the azo dyes are adsorbed in the sewage treatment plant (STP) (Clarke & Anliker, 1980). Resulting in a worst case scenario of an adsorption of 40% (60% release to the effluent) and a best case scenario of an adsorption of 80% (20% release to the effluent). |
| Adsorption is the only removal route of azo dyes in the STP, i.e. there is no abiotic or biotic degradation. |
Furthermore, a standard STP scenario, in compliance with TGD (1996), is used. According to this standard the values presented in Table 5.12 are standard characteristics of a STP:
Table 5.12
Standard characteristics of a sewage treatment plant.
Standardkarakteristika for et rensningsanlæg.
| Parameter | Symbol | Unit | Value |
| Capacity of local STP | Capacity stp | [eq] | 10,000 |
| Amount of wastewater per inhabitant | Waste inhab | [lxd-1xeq-1] | 200 |
| Surplus sludge per inhabitant | SURPLUSsludge | [kgxd-1xeq-1] | 0.011 |
| Concentration susp. matter in influent | SUSPCONCinf | [kgxm-3] | 0.45 |
Ref.: TGD (1996).
The calculation of PEC influent, stp is simplified and based on the equation below:
PEC influent, stp = Release wastewater /Waste inhab ´ Capacity stp ´ 365
The calculation of PEC effluent, stp is simplified and based on the assumptions mentioned above. In addition, the PEC effluent, stp for the processing industry is corrected for the number of sites present in Denmark , i.e. 40 sites for textile colouring and 1 site for leather dyeing. For the use, the number of inhabitants in Denmark (approximately 5 millions) is normalised to the capacitystp.
PEC effluent, stp = PEC influent, stp ´ (1- adsorption factor/ (number of sites) or inhabitants in Denmark (Table 5.13).
PEC surface water = PEC effluent, stp ´ dilution factor
According to the TGD (1996), the dilution factor is 10.
Table 5.13
Estimated PECeffluent, stp and PECsurface water for azo dyes.
Estimeret PECudløb, stp og PECoverfladevand for azofarvestoffer.
Re- |
PEC influent, stp |
PEC |
PEC |
PEC |
PEC |
|
t/year |
mg/l |
mg/l/site or inhab. |
mg/l/site or inhab. |
mg/l/site or inhab. |
mg/l/site or inhab. |
|
| Processing | Worst case |
Best case |
Worst case |
Best case |
||
| Textile | 70 |
95.89 |
1.44 |
0.48 |
0.14 |
0.048 |
| Leather | 1 |
1.37 |
0.82 |
0.27 |
0.08 |
0.027 |
| Use | ||||||
| Textile | 72 |
98.63 |
0.12 |
0.04 |
0.012 |
0.004 |
| Leather | - |
- |
- |
- |
- |
- |
| Total | 143 |
- |
- |
- |
- |
- |
The PECsediment is calculated from:
PECsediment = PECsurface water adsorption factor
In Table 5.14, the PECsediment is presented.
Table 5.14
Estimated PECsediment for azo dyes.
Estimeret PECsediment for azofarvestoffer.
| Scenario | PEC surfacewater |
Adsorption factor |
PECsediment |
mg/l |
mg/kg |
||
| Worst case | |||
| Processing | |||
| Textile | 0.14 |
0.8 |
0.11 |
| Leather | 0.08 |
0.8 |
0.06 |
| Use | |||
| Textile | 0.01 |
0.8 |
0.008 |
| Leather | - |
0.8 |
- |
| Best case | |||
| Processing | |||
| Textile | 0.05 |
0.4 |
0.02 |
| Leather | 0.03 |
0.4 |
0.01 |
| Use | |||
| Textile | 0.004 |
0.4 |
0.002 |
| Leather | - |
0.4 |
- |
Concerning the concentration of azo dyes in the sludge, the estimation is based on that the production of sludge amounts to 170,000 tonnes dw/year in Denmark (Miljøstyrelsen, 1996b). The "worst case" of adsorbed azo dyes onto the sludge is 80% and the "best case" is 40%. The calculated concentration in sludge is based on the following equation:
PEC sludge = (Release ´ adsorption factor ´ 106/ Sludge rate)/(number of sites) or inhabitants.
Sludge rate = 170.000 tonnes/year
Table 5.15
Estimated PEC sludge for azo dyes
Estimeret PECslam for azofarvestoffer.
Release |
PECsludge |
PECsludge |
|
t/year |
mg/kg/site or inhab. |
mg/kg/site or inhab. |
|
| Processing | Worst case |
Best case |
|
| Textile | 70 |
8.24 |
4.11 |
| Leather | 1 |
4.70 |
2.45 |
| Use | |||
| Textile | 72 |
0.68 |
0.34 |
| Leather | - |
- |
- |
| Total | 143 |
- |
- |
The estimated PECsurface water for processing and use is in the range of 0.04 to 1.44 mg/l. According to the monitoring studies (Table 5.10) a range of 0.012 to 0.523 mg/l for treated sewage effluent has been found. If comparing the two, the estimated PECeffluent, stp is approximately 3 times higher. Compared to the concentrations found in river water, the estimated PECsurface water is 2 to 6 times higher which may be due to the dilution effect. The estimated PECsediment is, on the other hand, below the range of the monitored data (Table 5.11), namely 0.002 to 0.11 mg/kg dw.
Due to the lack of monitoring data of environmental concentrations of azo dyes in Denmark, it is not possible to validate the estimated PECs based on Danish data. The basic assumption, however, that the processing industries do not carry out waste water treatment prior to outlet (PECinfluent, stp) is unlikely, because most of these companies, if not all, are encompassed by a special section of the Danish Environmental Protection Law (chapter 5). Hence, their emissions are restricted and must be approved by the authorities. Subsequently, the companies are obliged to have some degree of waste water treatment prior to the outlet to the municipal STP.
Assuming that 40 to 80% of the dyes are removed from waste water before the outlet from the industry and likewise in the STP, this indicates that the actual PECeffluent, stp for the processing and use phase is more likely to be in the range of 0.024 to 0.864 mg/l and the PECsurface water in the range of 0.002 to 0.086 mg/l. These concentrations are within the same range, and for PECsurface water approximately 4 times higher compared to the findings in the aforementioned monitoring studies. This indicates at least for the best case scenario, that the estimated PECs may be realistic.
If it is estimated that the PECsurface water is too high, then the PECsediment has to be reduced in the same order of magnitude. Resulting in a concentration of 0.001 to 0.090 mg/kg from processing and use which is within the low range of the monitoring studies (Table 5.11).
If it is assumed that the companies carry out waste water treatment, the PECsludge, stp may also be reduced 2 to 5 times, depending on the degree of adsorption (40-80%) at the companies, and this results in a range of 1.18 to 5.62 mg/kg for processing and use.
Monitoring data
No data have been obtained concerning monitoring of azo dyes in the atmosphere or bound to particulate matter.
Estimation of PEC for the atmosphere
It has not been attempted to calculate the atmospheric PEC, but it is estimated that the PEC is very low, because volatilisation is highly unlikely for the azo dyes from both moist and dry surfaces. Furthermore, the release from the processing industry and from incineration is considered to be very low (approximately equal to 0).
Terrestrial compartment
Monitoring data
There are no direct route by which agricultural soils may become contaminated with synthetic dyes. In principle, it is possible that the disposal of sludge from sewage treatment plants, which receive dye-house effluents, may provide an indirect route of exposure. Although, there appear to be no reported data of the levels of dyestuffs on agricultural soils, estimates based on the principles elaborated by the OECD, would indicate a worst case level of 1 mg/kg (w/w of dry soil) (Brown & Anliker, 1988).
Furthermore, deposition of particulate matter may be a potential pathway for the terrestrial environment, but as stated above it is considered to be an unlikely pathway.
A practical demonstration has showed that sewage sludge contaminated with dyes, when held under simulated landfill conditions, does not release dyes into the leachate. The amine metabolites, which may be expected to be produced from these dyes, cannot be found in the leachate or interstitial water either (Brown & Anliker, 1988)
However, no data have been obtained on terrestrial monitoring of azo dyes.
Estimation of PEC for the terrestrial compartment
The sources of environmental releases of azo dyes in the terrestrial environment are waste disposal in landfills and sludge applied to agricultural soil.
It is estimated that the total amount of sludge per year in Denmark is 170,000 tonnes of dry weight. About 114,000 tonnes (67%) are used in agriculture and 20,000 tonnes (12%) are deposited in landfills. The rest (21%) is incinerated (Miljøstyrelsen, 1996b).
It is not known how many hectares of agricultural soil which are fertilised with sludge in Denmark. But according to the TGD (1996), the following characteristics of soil and soil uses are accepted:
Table 5.16
Standard environmental characteristics for soil.
Standard miljøkarakteristika for jord.
Depth of soil |
Rate of sludge application |
|
[m] |
[kgdwtxm-2xyear-1] |
|
| PEC localagr.soil | 0.20 |
0.5 |
Ref.: TGD (1996).
In section 2.3.4 of the TGD (1996), the standard environmental characte- ristics are defined, and on this basis it may be calculated that the density of the soil is 1.7 t/m 3. By application of the depth of soil of 0.2 m in accordance with the TGD (1996), it is estimated that the weight of soil per square meter is equal to 0.34 tonnes.
Subsequently, assuming that in a worst case scenario 80% of the azo dyes are adsorbed onto the sludge, and that in a best case scenario 40% are adsorbed onto the sludge, then the amount of azo dyes on the agricultural fields can be estimated from the following equation:
PECagri sludge = (release ´ adsorption factor ´ fraction to agriculture)/ (sludge amount/application rate) ´ soil weight.
Table 5.17
Estimated PECagri sludge for azo dyes.
Estimeret PECagri slam for azofarvestoffer.
Release |
PECagri sludge |
PECagri sludge |
|
tonnes/year |
mg/kg |
mg/kg |
|
| Processing | Worst case |
Best case |
|
| Textile | 70 |
0.484 |
0.242 |
| Leather | 1 |
0.007 |
0.003 |
| Use | |||
| Textile | 72 |
0.498 |
0.249 |
| Leather | - |
- |
- |
| Total | 143 |
- |
- |
The allocation of sludge to landfill disposal amounts to 20,000 tonnes (dw)/year. The contribution of sludge adsorbed azo dyes to the total amount of azo dyes in landfills may be calculated on the basis of the equation shown below:
Sludge amount to landfills = release ´ adsorption factor ´ fraction to landfills.
In a worst case scenario, the total contribution (processing + use) may be 13.5 tonnes per year, and in a best case scenario 6.70 tonnes per year, which is approximately 6% and 3%, respectively of the total amount of dyes deposited in landfills.
Thus, the total release of azo dyes to landfills may be estimated to approximately 240 tonnes per year in worst case and 233 tonnes per year in best case.
Assuming that the processing industry carries out waste water treatment, the PECagri sludge is reduced to the range of 0.2 to 0.3 mg/kg. The contribution from the use phase is unchanged with 0.25 to 0.5 mg/kg soil. Due to the lack of monitoring data, it is not possible to validate the calculated PECs. However, these concentrations are, compared to a worst case level of 1 mg/kg (w/w of dry soil) reported by Brown and Anliker (1988), lower.
The fate of products containing dyes released to landfills is uncertain, but there may be a potential release of dyes to soil from this compartment.
Aquatic compartment
Azo dyes
Reactive Black 5 (diazo) has a low toxic potential in aquatic organisms (fish LC50 100-500 mg/l; bacteria EC50 > 2,000 mg/l) as well as the hydrolysed dye (fish LC50 > 500 mg/l; Daphnia magna EC50 (48h) > 128 mg/l) (Hunger & Jung, 1991, IUCLID). Very little information is available on the aquatic toxicity of the hydrolysed reactive dyes, but their loss of ability to react with various groups of vital organic materials, such as proteins and DNA, reduces the potential hazard considerably (ETAD, 1991).
Spencer (1984) has examined the effect of Aquashade (a mixture of Acid Blue 9 and Acid Yellow 23*) on the oxygen consumption of the crayfish Orconectes propinquus and has not found any effect at a concentration of 1 mg/l at an exposure of five days.
A survey of available fish toxicity data on over 3,000 commercially available organic dyes by ETAD member companies indicated that about 98% have a LC50 greater than 1 mg/l, a concentration at which colouring of a river normally would be observable. The remaining 2% were acute toxic (LC50 < 1 mg/l). The latter, consisted of 27 different chemical structures including four Acid dyes, sixteen Basic dyes of which 10 were of the triphenylmethane (not azo) type. In only one case, the LC50 was as low as 0.01 mg/l (Clarke & Anliker, 1980). The LC50 for 59% was more than 100 mg/l (Anliker, 1986), indicating that 41% of the organic dyes are potentially toxic or toxic at levels in the range of 1 to 100 mg/l.
Many acid dyes, including azo dyes, exhibit high toxicity to fish but do not significantly inhibit algal growth (Clarke & Anliker, 1980).
Zhang et al. (1995) showed that azo dyes competitively inhibit COD utilisation or respiratory rates of biofilms at concentrations of 10 mg/l of Acid Orange 14. However, the inhibition effect was much less significant in biofilms, compared to a suspended activated sludge system. Furthermore, the results indicated that the aerobically non-biodegradable dyes, Acid Orange 10* and 14, were more toxic compared to biodegradable dyes such as Acid Orange 7* and 8.
Brown et al. (1981) reported the results of a study of possible inhibitory effects of dyes on aerobic waste water bacteria measured as respiratory rate. They tested both acid, direct, disperse, reactive, basic, vat, solvent and mordant dyes. The study indicated that 18 out of 202 dyes showed an IC50 less than 100 mg/l, including three dyes with an IC50 between 1-10 mg/l. These 18 dyes were all basic dyes. Unfortunately it was a mixture of chemical classes of dyestuffs, including azo dyes, so it is not possible to relate the results directly to specific azo compounds.
ICI found no adverse effects on the carp (Cyprinus carpio) exposed to less than 10 mg/l of 30 water soluble (ionic) and 12 disperse dyes for 8 weeks (Brown, 1987).
Dyes in the aquatic environment were reported to affect microbial populations and their activities. Azo dyes such as Basic Brown 4, Direct Brown 95*, Direct Black 80, Mordant Black 11, Acid Black 52, Direct Red 81* and Direct Yellow 106 were inhibitory to microbial oxidation processes in both activated sludge and stream water. The inhibition by the basic dyes were stronger than the inhibition by acid dyes when the pH was above the isoelectric point of the micro-organism. The inhibition was weakened by introduction of the functional groups methyl, nitro, sulpho or acid to the azo dye or by replacement of the benzene ring with a naphthalene ring. However, introduction of chlorine or bromine strengthened the observed inhibition (Chung & Stevens, 1993). The IC50 was not stated.
In an ADMI (American Dye Manufacturers Institute) study, the toxic effects of 56 selected dyes to the green alga Selenastrum capricornutum were examined. The growth of the algae was assessed after 7 and 14 days in the presence of 1 and 10 mg/l of dyes. 15 dyes (27%) strongly inhibited growth at a test concentration of 1 mg/l after 7 days of incubation (Brown & Anliker, 1988).
The following short term test results are available from a study by ETAD and presented on a seminar in 1992 (ETAD, 1992b). ETAD carried out an investigation of 47 dyes of different chemical dye classes. Even though the specific amount of azo dyes in the investigation is not stated, the results are shown in Table 5.18, Table 5.19, Table 5.20, Table 5.21, Table 5.22 and Table 5.23 below, in order to gain insight to the toxicity of the different chemical (technical) groups of dyes.
Table 5.18
Toxicity of Acid dyes, a total of 11 (ETAD, 1992b).
Syre farvestoffers toksicitet, ialt 11 (ETAD, 1992b).
| Test organism | End point | No. of |
Toxicity mg/l |
|||
results |
<1 |
1-10 |
10-100 | >100 | ||
| Zebra fish | 96 hr LC50 | 11 |
0 |
2 |
3 |
6 |
| Daphnia Magna | 48 hr EC50 | 9 |
0 |
0 |
6 |
3 |
| Alga | 72 hr EC50 | 9 |
2 |
3 |
3 |
1 |
| Bacteria | IC50 | 11 |
0 |
0 |
0 |
11 |
Table 5.19
Toxicity of Basic dyes, a total of 6 (ETAD, 1992b).
Basiske farvestoffers toksicitet, ialt 6 (ETAD,1992b).
| Test organism | End point | No. of |
Toxicity mg/l |
||||
| results | <1 |
1-10 |
10-100 |
>100 |
|||
| Zebra fish | 96 hr LC50 | 6 |
0 |
3 |
3 |
0 |
|
| Daphnia Magna | 48 hr EC50 | 6 |
5 |
0 |
1 |
0 |
|
| Alga | 72 hr EC50 | 6 |
2 |
4 |
0 |
0 |
|
| Bacteria | IC50 | 6 |
0 |
3 |
2 |
1 |
|
Table 5.20
Toxicity of Hydrolysed Reactive dyes, a total of 8 (ETAD, 1992b).
Hydrolyserede reaktive farvestoffers toksicitet, ialt 8 (ETAD, 1992b).
| Test organism | End Point | No. of |
Toxicity mg/l |
|||||||||
| results | <11 |
1 | 1-10 |
10-100 |
>100 |
|||||||
| Zebra fish | 96 hr LC50 | 8 |
0 |
0 |
0 |
8 |
||||||
| Daphnia Magna | 48 hr EC50 | 8 |
0 |
0 |
0 |
8 |
||||||
| Alga | 72 hr EC50 | 8 |
0 |
0 |
71 |
1 |
||||||
| Bacteria | IC50 | 8 |
0 |
0 |
0 |
8 |
||||||
1
Alga results are >10 mg/l.Table 5.21
Toxicity of Direct dyes, a total of 7 (ETAD, 1992b).
Direkte farvestoffers toksicitet, ialt 7 (ETAD, 1992b).
| Test organism | End point | No. of |
Toxicity mg/l |
||||||||
| results | <1 |
1-10 |
10-100 |
>100 |
|||||||
| Zebra fish | 96 hr LC50 | 7 |
0 |
0 |
0 |
7 |
|||||
| Daphnia Magna | 48 hr EC50 | 7 |
0 |
0 |
0 |
7 |
|||||
| Alga | 72 hr EC50 | 7 |
0 |
3 |
2 |
2 |
|||||
| Bacteria | IC50 | 7 |
0 |
0 |
0 |
7 |
|||||
Table 5.22
Toxicity of Disperse dyes, a total of 11 (ETAD, 1992b).
Disperse farvestoffers toksicitet, ialt 11 (ETAD, 1992b).
| Test organism | End point | No. of |
Toxicity mg/l |
||||||||||
| results | <1 |
1-10 |
10-100 |
>100 |
|||||||||
| Zebra fish | 96 hr LC50 | 11 |
0 |
0 |
2 |
9 |
|||||||
| Daphnia Magna | 48 hr EC50 | 10 |
0 |
2 |
2 |
6 |
|||||||
| Alga | 72 hr EC50 | 8 |
3 |
3 |
2 (3)1 |
0 |
|||||||
| Bacteria | IC50 | 11 |
0 |
0 |
0 |
11 |
|||||||
1
3 alga results are >10 mg/l.Table 5.23
Toxicity of Mordant dyes, a total of 3 (ETAD, 1992b).
Mordante farvestoffers toksicitet, ialt 3(ETAD, 1992b).
| Test organism | End point | No. of |
Toxicity mg/l |
||||||||||
| results | <1 |
1-10 |
10-100 |
>100 |
|||||||||
| Zebra fish | 96 hr LC50 | 3 |
0 |
0 |
0 |
3 |
|||||||
| Daphnia Magna | 48 hr EC50 | 1 |
0 |
0 |
0 |
1 |
|||||||
| Alga | 72 hr EC50 | 3 |
3 |
0 |
0 |
0 |
|||||||
| Bacteria | IC50 | 3 |
0 |
0 |
0 |
3 |
|||||||
It is indicated that the bacteria are less susceptible to the different classes of dyes compared to other test organisms. Among the tested dyes, the bacteria were only susceptible to basic dyes at concentrations below 100 mg/l, which is in agreement with the findings of Brown et al. (1981) and Chung and Stevens (1993).
From the tables it is indicated that the zebra fish is susceptible to (in declining order) basic dyes > acid dyes > disperse dyes at a level less than 100 mg/l. For the other chemical classes, hydrolysed reactive, direct and mordant dyes, the LC50 is above 100 mg/l. The susceptibility to acid and basic dyes for fish is in agreement with the findings of Clarke and Anliker, 1980.
The susceptibility of Daphnia resembles that of the zebra fish, but the order is different, basic > disperse > acid. The remaining chemical classes all show a LC50 above 100 mg/l. The study confirms the findings reported by Hunger and Jung (1991) and IUCLID that the reactive dyes and hydrolysed reactive dyes have a low toxic potential in aquatic organisms.
The alga is apparently the most susceptible organism, because it for all the tested dyes showed a susceptibility to the dye below 100 mg/l. The susceptibility was in the following declining order Mordant > Basic/acid/-disperse > direct > hydrolysed reactive dyes.
Based on literature and database studies, it was possible to obtain results of the LC50 for some of the azo dyes in use in Denmark, but in general there are only a few data available on effects through the normal sources (AQUIRE, IUCLID, HSDB, MITI, etc.). Furthermore, only data on various fish species were obtained, and it was not possible to obtain data on the basic, mordant and the disperse dyes which are used in Denmark.
In Table 5.24, the lowest effect concentrations for azo dyes used in Denmark are presented. The data indicate that various fish species are susceptible to acid and direct dyes at a level between 1 to 10 mg/l. The susceptibility regarding solvent dyes is in one instance below 1 mg/l. It is not known, if some of these azo dyes were included in the study by ETAD (1992b).
Table 5.24
The lowest effect concentrations for azo dyes used in Denmark.
Laveste effektkoncentrationer for azofarvestoffer anvendt i Danmark.
| C.I. name | Fish | Effect | Conc. |
| Organism | mg/l |
||
| Acid Blue 1131 | Pimephales promelas | LC50, 96 h | 4 |
| Acid Red 1142 | Cyprinus carpio | LC50, 48 h | 4 |
| Direct Blue 141 | Oncorhynchus mykiss | LC50, 24 h | 6 |
| Oncorhynchus tschawytchia | LC50, 24 h | 6 |
|
| Ptychocheilus oregonensis | LC50, 24 h | 10 |
|
| Solvent Yellow 11 | Oryzias latipes | LC50, 58 h | 0.7 |
| Solvent Yellow 31 | Leuciscus delineatus | EC50, 96 h | 2 |
1
AQUIRE.2
MITI.In addition to the figures shown in Table 5.24, one fish species (Oryzias latipes), exposed 48 hours to Acid Yellow 36, had a LC50 of 68 mg/l. For the remaining dyes, amongst them 5 acid, 6 direct and 2 solvent dyes, the LC50 was above and well above 100 mg/l. Apparently, the different almost exclusively fish species show very variable susceptibility. For further details, see Appendix 2.
Azo compounds
At exposure levels of 2,500 ppm of the azo compound 3,3´, 4,4´-tetrachloroazobenzene on diet, the mortality of the Japanese Medaka (Oryzias latipes) was significantly higher compared to the control group (Allison & Morita, 1995b). On the other hand detrital exposure levels of 2,500 ppm of the same compound did not appear to cause any harmful effects towards the aquatic snail (Indoplanorbis exustus) (Allison & Morita, 1995a).
Metabolites
Couch and Harshbarger (1985) presented an overview of the effects of carcinogenic agents on aquatic animals in experimental studies. They found reports on the effects of aminoazotoluene on fish, adult guppy and adult Medaka at dietary exposure levels of 120 mg/l and 600 mg/l, respectively. Further neoplasm in the liver was induced within 12 weeks and 24 weeks, respectively. The argument of the authors was that in the environment the susceptibility to xenobiotics may differ among different species. Subsequently, proliferation and cellular disorder are neoplasms, which may be caused by xenobiotics, viruses or an interaction of both.
Hermens et al. (1990) investigated the influence on enzyme induction (MFO P450) on the acute toxicity (96-hr LC50) of 4-chloroaniline (p-chloroaniline) to the rainbow trout (Salmo gairdneri). The 95-hr LC50 was from 11.0 to 14.0 mg/l, and the results showed no significant difference between prior induced trout (50 mg/kg Aroclor 1254) and not induced trout, suggesting that metabolic activation does not necessarily play a role in the acute toxicity of aromatic amines to fish.
Metabolic activation of aromatic amines has been shown in the phyla: Mollusca, Crustacea and Echinodermata, e.g. Mytilus edulis, Mytilus galloprovincialis, Carcinus maenas, Asterias rubens, resulting in mutagenicity to Salmonella typhimurium (Marsh et al., 1992).
Dumpert (1987) showed that p-chloroaniline has a lethal effect on the embryo of Xenopus laevis at a concentration of 100 mg/l. Its development is inhibited (teratogenic) at concentrations of 1 and 10 mg/l, respectively.
In a study of bacterial growth kinetics to in vitro toxicity assessment of substituted phenols and anilines, Nendza and Seydel (1990) demonstrated that these compounds were inhibitory, and that the toxic action was probably caused by damage to the bacterial cells. This was documented by decrease in growth rate and in change of the Na+/K+ ratio with an increase in the Na+ and a decrease in the K+ concentrations. Furthermore, the authors found a good agreement between growth kinetics of E.coli and fish tests (guppy and zebra fish) for phenols - a linear relationship between log 1/LD50 guppy and log 1/I50 E.coli.
p-aminoazobenzene (10.23 mg/l) was by Zissi and Lyberatos (1996) found to result in a decrease of 15% in the specific growth rate of Bacillus subtilis.
The lowest effect concentrations found for the restricted aromatic amines are presented in 5.25 below. No data were obtained on the naphthalene based amines.
Table 5.25
The lowest effect concentrations for some of the metabolites.
Laveste effektkoncentrationer for visse nedbrydningsprodukter.
| Name | Organism | Effect |
Conc., mg/l |
| 4-chloroaniline | Daphnia magna | EC50, 24 h | 0.061 |
| Lepomis macrochirus | LC50, 96 h | 2.01 |
|
| Pimephales promelas | LC50, 96 h | 121 |
|
| Salmo gairdneri | LC50, 96 h | 141 |
|
| Xenopus laevis | Teratogen | 11 |
|
| 4- aminobenzene | Oryzias latipes, juv | LC50, 24 h | 1.72 |
| Oryzias latipes, juv | LC50, 48h | 0.72 |
|
| o-anisidine | Daphnia magna | EC50, 48 h | 6.83 |
| Poecilia reticulata | EC50, 336 h | 18 |
|
| benzidine | Limoria lignorum, adult | LC100, 18 h | >0.052 |
| Oryzias latipes, juv | LC50, 24 h | 16.02 |
|
| Oryzias latipes, juv | LC50, 48 h | 10.52 |
|
| 1 Dumpert (1987). 2 ECDIN.3 Federal Ministry of the Environment, Youth and Family, Austria 1997. |
|||
As shown in Table 5.25, it has been reported that benzidine and 4-aminobenzene are acute toxic (LC50 < 1 mg/l) to some crustaceans and juvenile fish. The EC50 for Daphnia magna is as low as 0.06 mg/l for
4-chloroaniline. In general, the LC50 of 4-chloroaniline for various fish species is in the range of 12 to 46 mg/l which indicates potential toxicity. o-anisidine has a LC50 (336 h) of 165 ppm towards adult Poecilia reticulata. o-toluidine has a LC50 (336 h) of 81 ppm towards juvenile Poecilia reticulata. For further details, see Appendix 3.
Summary
No specific data were obtained on basic, reactive, mordant and disperse dyes for any of the dyes encompassed in the present survey.
But it may be concluded that some of the acid and basic dyes are acute toxic to toxic to aquatic organisms (fish, crustaceans, algae and bacteria), which also applies for at least some of the direct dyes, e.g. Direct Blue 14*. Reactive dyes (Reactive Black 5) generally have very high effect concentration levels (>100 mg/l) and are not considered to be toxic to aquatic organisms.
Furthermore, it is indicated that the non-ionic (disperse, mordant and solvent) dyes are toxic and potentially toxic. Solvent dyes may even be acute toxic to aquatic organisms. The mordant dyes may, according to the present findings, not exhibit any toxicity at levels below 100 mg/l.
Algae are generally susceptible to dyes, but the inhibitory effect is thought to be related to light inhibition at high dye concentrations, rather than a direct inhibitory effect of the dyes. According to ETAD (1994), this effect may account for up to 50% of the inhibition observed.
The effects of the substitutional pattern of the dyes are inconclusive, but it has been suggested that introduction of the functional groups; methyl, nitro, sulpho or acid, weakens the inhibition of bacteria, whereas introduction of chlorine and bromine strengthens the inhibition.
In general, it should be noted that toxicity data of chronic low-level exposures for most of the commercial dyes and their derivatives are lacking.
It is indicated, in general, that the effects of the metabolites to aquatic organisms, except for algae, are at levels where potential toxicity is recognised (LC50 < 100 mg/l). This applies for all of the three groups: anilines, benzidines and toluidines. No data were obtained for the naphthalenes.
Anilines and benzidines are both acute toxic and toxic depending on the specific species. The anilines seem to be more toxic to Oryzias latipes juv than benzidine. The findings of the toluidines indicate potential toxicity for various aquatic organisms.
PNEC - Dyes
Applying an assessment factor of 100 on the EC50 from respiration inhibition test (Table 5.20), the following PNEC is derived in accordance with TGD Part II, section 3.4:
PNECstp is in the range of 10 : g/l to 100 : g/l.
It should be noted, however, that it is not known if the observed effect is caused by azo dyes or other dye types. But the significance of possible inhibitory effects of azo dyes to the bacteria in the sewage treatment plant is of great importance, therefore, the estimate of PNECstp is included.
Short term data from each of the three trophic levels (alga, fish, daphnia) of the base set are available. Hence, according to TGD Part II, section 3.3.1 an assessment factor of 1,000 is applied at the lowest L(E)C50. However, as stated above it is not known, if the observed effect is caused by azo dyes in the case of algae and daphnia, cf. Table 5.18 and Table 5.19. But the lowest observed effect is observed for fish (Table 5.24) with a LC50 of 0.7 mg/l for Oryzias latipes, arriving at a PNEC of:
PNECaquatic organisms = 0.7 : g/l.
Metabolites
No data were obtained on bacterial inhibition of the metabolites.
Data of two trophic levels were obtained for the metabolites. The lowest observed effect is found in daphnia (Table 5.25) with a LC50 of 0.06 mg/l for Daphnia magna, arriving at a PNEC of :
PNECaquatic organisms = 0.06 : g/l.
No data were obtained on atmospheric exposure.
Terrestrial compartment
ETAD has organised a study of the possible effects of dyes on plant germination and growth. Four dyes were used and among them an acid dye of the azo type (C.I. 13155). All four dyes were incorporated into a seed compost at concentrations of 1, 10, 100 and 1,000 mg/l and germination and growth of three plant species (sorghum, sunflower, and soya) were assessed. No effects were observed on seed germination. With respect to the growth rate, there was no observed effects at a concentration of 100 mg/l. At a level of 1,000 mg/l, however, there was a variable growth depending on the dye and the species of the particular plants. After a growth period of 21days, the plant foliage was analysed. At the 1,000 mg/kg soil level the dyestuffs, among them the azo (C.I. 13155), were just detectable in the plant foliage (max. 2 mg/kg) (Brown & Anliker, 1988).
Chung et al. (1997) found out that growth of the soil living nitrogen-fixing bacterium Azotobacter vinelandii is inhibited by p-phenylene- diamine and 2,5-diaminotoluene, which are derivatives after azo reduc-tion of e.g. Basic Brown (C.I. 21010) and Direct Black 80. The nitroge- nous activity was also significantly inhibited at a concentration of 50 : g/ml. p-phenylene- diamine was found to be inhibitory to the growth of other common aquatic and soil bacteria.
PNEC - terrestrial
According to the TGD Part II section 3.6.2.2, an assessment factor of 1,000 should be applied for L(E)C50 short-termed toxicity tests for soil. Brown and Anliker (1988) have reported effects at a level of 1,000 mg/kg for plants, indicating a PNEC of :
PNECsoil = 1 mg/kg.
Risk characterisation
The PEC/PNEC ratios which can be derived with the available data are shown in Table 5.26.
Table 5.26
PEC/PNEC ratios for the aquatic and terrestrial compartments.
PEC/PNEC forhold for vand- og jordmiljø.
| Compartment | Site | PEC (mg/l) |
PEC/- |
||||
Worst |
Best |
Worst |
Best |
||||
| STP | Sludge | Processing | |||||
| Textile | 8.24 |
4.11 |
82.4 |
41.1 |
|||
| Leather | 4.70 |
2.45 |
47.0 |
24.5 |
|||
| Use | |||||||
| Textile | 0.68 |
0.34 |
6.8 |
3.4 |
|||
| Aquatic | STP effluent | Processing | |||||
| Textile | 1.44 |
0.48 |
2057 |
685 |
|||
| Leather | 0.82 |
0.27 |
1171 |
386 |
|||
| Use | |||||||
| Textile | 0.12 |
0.04 |
171 |
57 |
|||
| Sediment | Processing | ||||||
| Textile | 0.11 |
0.02 |
1.1 |
0.2 |
|||
| Leather | 0.06 |
0.01 |
0.6 |
0.1 |
|||
| Use | |||||||
| Textile | 0.008 |
0.002 |
0.1 |
0.02 |
|||
| Surf. water | Processing | ||||||
| Textile | 0.14 |
0.048 |
200 |
69 |
|||
| Leather | 0.08 |
0.027 |
114 |
39 |
|||
| Use | |||||||
| Textile | 0.012 |
0.004 |
17 |
8 |
|||
| Terrestrial | Agr. sludge | Processing | |||||
| Textile | 0.484 |
0.242 |
0.5 |
0.2 |
|||
| Leather | 0.007 |
0.003 |
<0.01 |
<0.01 |
|||
| Use | |||||||
| Textile | 0.498 |
0.249 |
0.5 |
0.3 |
|||
For substances with a PEC/PNEC ratio of less than 1 there is, according to TGD, no need for further testing and risk reduction measures beyond those which are already being applied. A ratio higher than 1, however, indicates a need for further information and/or testing or even a need for limiting the environmental risks.
It is indicated that there is a need for further testing and information with regards to the concentration of dye in the aquatic compartment, except for the sediment, because the PEC/PNEC is higher than 1. Whereas the PEC/PNEC ratios for the terrestrial compartment indicate, that there is no need for further testing and/or information.
Furthermore, it is indicated that there is a need of further information with regards to the concentration of dyes in the STP, because the PEC/PNEC is well above 1.
With reference to the assumptions and recalculation of the PECs, it is indicated that the PEC/PNEC ratios presented in Table 5.26 are to high.
Recalculation of the PEC/PNEC ratios indicates:
|
3.4 to 5 | (>1) | ||
|
34 to 1,234 | (>>1) | ||
|
3 to 123 | (>1) | ||
|
0.01 to 0.9 | (<1) | ||
|
0.5 to 0.8 | (<1) |
Summary
The survey indicates that there is a need for further information and testing in order to assess the environmental risk in the STP, the STP effluent and surface water, whereas the releases associated with sludge application in agricultural soil not seem to present any immediate concern.
Toxicity and Fate of Azo Pigments
Physico-chemical properties
General aspects
It was possible to obtain data for 14 out of the 51 pigments encompassed in the present survey (ECDIN; IUCLID; HSDB). The molecular weight of the pigments used in Denmark lies within the range of 293 to 818,51 g/mol, and the average value is 484 g/mol. Generally, the red and orange pigments have lower molecular weights than the yellow pigments.
Pigments have many physico-chemical properties in common with the disperse, solvent and mordant dyes with respect to molecular size and hydrophobity. They have extremely low solubility in water and in the application substrate, but unlike the disperse, solvent and mordant dyes, the pigments also, generally, exhibit a low solubility in organic solvents. For this reason they remain essentially in the solid state during the processing and when they are applied to the substrate (Clarke & Anliker, 1980).
However, some azo pigments are sufficiently soluble under analytical test conditions to yield detectable amounts of the restricted aromatic amines (i.e. greater than 30 mg/kg consumer goods). These azo pigments are included in the German restriction, and amongst them are e.g. Pigment Red 22*, Pigment Red 38 and Pigment Red 8* (ETAD et al., 1995).
Due to the low solubility of azo pigments, hydrolysis may not be an important feature of these pigments. Photolysis, on the other hand, may in principle be possible. Absorption maximum lies within the range of visible and UV-light, but its stability indicates that it will be a slow process.
Diarylide pigments are susceptible to thermal breakdowns at temperatures above 200 0C (ETAD et al., 1995).
The molar weights, melting points and solubility in n-octanol were experimentally measured, and the partition coefficients and solubility in water were estimated for 2 mono and 3 diazo pigments by Anliker and Moser (1987). In addition, data from IUCLID were obtained for 3 azo pigments. The results are given in Table 6.1 below:
Table 6.1
Examples of melting points, solubility and partition coefficients of pigments.
Eksempler på smeltepunkt, opløselighed og fordelingskoefficient for pigmenter.
| Compound | MW |
Melting point |
Log Kow (est.) |
Solubility in water (est.) |
Solubility in n-octanol (exp.) |
g/mol |
o C |
mg/l |
mg/l |
||
| Monoazo 1 | 340.33 |
256 |
3.82 |
0.2-2 |
9.40 |
| Monoazo 2 | 439.78 |
330 |
3.40 |
0.1-5 |
3.50 |
| Diazo 1 | 629.50 |
320 |
6.80 |
10-5-10-4 |
0.50 |
| Diazo 2 | 726.44 |
320 |
8.10 |
10-6-10-5 |
0.46 |
| Diazo 3 | 818.50 |
400 |
7.10 |
10-6-10-5 |
<0.50 |
| Pigment Red 53* | 398.81 |
- |
- |
1.3-2.2 |
- |
| Pigment Yellow 12* | 629.51 |
>350 |
5.00 |
n.s. |
- |
| Pigments Yellow 83* | 818.51 |
- |
>6.00 |
n.s. |
- |
n.s. = not soluble.
Data on vapour pressure are not available for most of the pigments. They are, however, large, complex molecules, which can be expected to have lower vapour pressures than disperse dyes, i.e. lower than 10-13 to 10-11 mmHg (Baughman & Perenich, 1988b).
Acute toxicity
Due to the experience with azo dyes, the toxicity of azo pigments has been extensively investigated.
Acute toxicity of azo pigments, as defined by the EU criteria for classification, is very low. In acute toxicity tests, the azo pigments show practically no acute toxicity (NPIRI, 1983).
Highly water insoluble lipophilic azo pigments have shown to be poorly absorbed in the gastrointestinal tract. Consequently, they are not discharged via urine but via unchanged faeces of laboratory animals (Herbst & Hunger, 1993).
Information about the acute oral toxicity including skin and eye irritation, is in the form of material safety data sheets available for many commercial important azo pigments. A great majority of the pigments is non- irritating if tested on skin and mucous membranes.
Despite a very broad application field, only very few azo pigments, e.g. Pigment Red 3*, 5* and 7 and Pigment Yellow 1* and 3, are known to cause occupational contact dermatitis in heavily exposed painters. However, only a few pigments have been tested in the clinic or in animal tests. (Ullmann, 5th Edition; Foussereau et al., 1982).
Reduction and cleavage of azo linkage in vivo, resulting in recognised carcinogens, were the main concern regarding azo dyes. The apparent generality of this metabolic pathway has prompted concern about the potential hazards associated with exposure to azo pigments.
An earlier work by Akiyama in the seventies seemed to show that rabbits are able to metabolise Pigment Yellow 13* to the component aromatic amine 3,3´-dichlorobenzidine. An extensive research on several animal species, inclusive primates, has strongly contradicted these results.
Of particular interest are azo pigments, which theoretically may release 3,3´-dichlorobenzidine. Pigment Yellow 12*, a diazo pigment based on 3,3´-dichlorobenzidine, seems to be a model compound, as it is most widely applied for toxicological studies of azo pigments. The oral and dermal absorptions and distribution of Pigment Yellow 12 were investigated in rats. After oral administration, the entire dose was accounted for in faeces. Furthermore, Pigment Yellow 12*, 13* and 17* were rather extensively investigated for hypothetical release of aromatic amines in vivo according to the three exposure routes: oral, dermal and inhalation. In no case any presence of the metabolic cleavage of the azo linkage was shown (Herbst & Hunger, 1993).
Water solubility is a prerequisite for absorption and metabolism in vivo. Azo pigments are not soluble in water and therefore, in practice, not available for metabolic activity. Consequently, directly excreted in the faeces without any absorption or participation in the enterohepatic circulation.
A majority of azo dyes requires metabolic reduction and cleavage of the azo linkage to component aromatic amines, to show mutagenicity in vitro test systems. Azo pigments, which are not available for metabolic activity, do not show mutagenic properties in vitro.
In the early eighties, Ames’ test was applied for testing of azo pigments, namely Pigment Yellow 1*, 12* and 74*, Pigment Orange 5* and 13* and Pigment Red 1*, 22*, 23, 48*, 49*, 53 and 75. With the exception of Pigment Orange 5* and Pigment Red 1*, which were found weakly positive, all of the tested pigments were negative (NPIRI, 1983).
In connection with the testing for carcinogenicity, two azo pigments, Pigment Red 3* and 53*, have been extensively tested for mutagenicity. Pigment Red 3* did not induce gene mutation in bacteria or sister chromatid exchange or chromosomal aberrations in cultured mammalian cells (IARC, 1993).
Pigment Red 53* was inactive in all studies for mutagenicity, in which the DNA damage in cultured mammalian cells and in rodents in vivo, the sister chromatid exchange and chromosomal aberrations in cultured mammalian cells and micronucleus test in rats, treated orally, were tested. The test also included assays for gene mutation in bacteria and cultured mammalian cells (IARC, 1993).
Based on the experiences with azo dyes, the probable carcinogenicity of azo pigments has been of main concern. Although epidemiological studies have not revealed any risks, several carcinogenicity studies have been carried out with azo pigments.
Dichlorobenzidine based pigments, e.g. Pigment Yellow 12*, 16* and 83* were investigated in long-term feeding studies in rats and mice. The daily dosage for rats were up to 0.6 g/kg body weight and for mice up to 2 g/kg body weight. No carcinogenic effects were observed. For Pigment Yellow 12*, two subsequent studies were carried out and both with negative results (Herbst & Hunger, 1993).
Pigment Red 3 is one of the most widely used red pigments for colouring of paints, inks, plastics, rubber and textiles. The pigment was tested for carcinogenicity in rats and mice. In those species only limited evidence for carcinogenicity was established. An overall evaluation of the pigment, carried out by IARC, stated that it cannot be classified as to its carcinogenicity to humans (IARC, 1993).
Pigment Red 53:1 is very widely used in cosmetic products and as drugs in some countries. Furthermore it is used in printing inks, coated papers, crayons, rubber etc. In experimental animals the pigment was tested in two studies in rats and one study in mice. In addition, a long-term skin painting study was carried out on mice. Only limited evidence for carcinogenicity was established in rats, but in mice no evidence for carcinogenicity was found. The pigment was inactive in a very broad spectrum of mutagenicity tests. An overall evaluation of the pigment, carried out by IARC, stated that the pigment is not classifiable as to its carcinogenicity to humans (IARC, 1993).
Problems of impurities
Impurities in pigments may be introduced via contaminated raw materials and/or intermediates used in the manufacturing process. Impurities are mainly found in trace amounts and encompass:
| heavy metals | |
| aromatic amines | |
| polychlorobiphenyls | |
| polychlorinated dioxins and/-furans ("dioxins") |
Heavy metals may be found as impurities of raw materials and/or intermediates. The following heavy metals have been found in pigments: antimony, arsenic, barium, lead, cadmium, chromium, mercury and selenium. Upper limits for the content of heavy metals in pigments are established within certain areas of application, e.g. toys and paints.
Aromatic amines used for synthesis of pigments may be found in trace amounts. The following aromatic amines have been found in pigments:
4-aminobiphenyl, benzidine, 2-naphthylamine and 2-methyl-4-chloro- aniline. Upper limits for the content of aromatic amines have been defined for certain areas of application, e.g. packaging material for foods.
Polychlorobiphenyls (PCB) and polychlorinated dioxins and furans may, due to various site reactions, be found in trace amounts in azo pigments deriving from chloroaniline or dichloro- or tetrachlorodiaminodiphenyl. Furthermore, pigments, which are manufactured in the presence of solvents like di- or trichlorobenzene may contain traces of PCBs, formed by site reactions too.
Exposure to azo pigments may entail exposure to the component aromatic amines due to:
| presence of aromatic amines as impurities (their intermediates). |
Exposure to aromatic amines is of greatest concern, as many of them are characterised by serious long-term effects.
Exposure to azo pigments may take place through inhalation and accidental ingestion. Absorption of azo pigments through the skin is doubtful, whereas impurities may be absorbed, e.g. aromatic amines.
In Denmark, occupational exposure to azo pigments may take place within manufacturing processes and some other industrial sectors, mainly manufacturing of paints and inks, colouring of plastics and printing. Furthermore, the exposure may take place in several hand-craft sectors, e.g. painting.
Non-occupational exposure to azo pigments may take place within a few areas, e.g. home decorating.
Summary
The acute toxicity of azo pigments is very low.
Only a few pigments have been linked to allergic contact dermatitis, and in all cases in extensively exposed painters. These pigments were among the earliest synthetic organic pigments and are now replaced with pigments of greater fastness to light.
Azo pigments are due to their very low solubility in water, in practise, not available for metabolic activity. Consequently, metabolic cleavage to the component aromatic amines has not been shown.
Azo pigments do not show carcinogenic potential neither in humans nor in experimental animals. However, the presence of aromatic amines as impurities in commercially available azo pigments or during the synthesis (manufacture) of pigments, may depend on the actual exposure and constitute a risk for human health.
There is a small but potential risk of exposure to potentially carcinogenic aromatic amines from azo pigments in Denmark. Occupational exposure may take place within the manufacturing process and in some industrial sectors, mainly manufacturing of paints and inks, colouring of plastics and printing. Furthermore, the exposure may take place in several hand-craft sectors, e.g. painting. Non-occupational exposure may take place within a few areas, e.g. home decorating.
Environmental fate and exposure
Releases to the environment
Measured data concerning the emissions of azo pigments to the environment in Denmark are not available. This applies both for the production phase and the processing and use phases.
There is a possible release of azo pigments to waste water effluent during the production phase from the one Danish manufacturer of pigments and the processing industries: print, paint, textile and leather. However, compared to the azo dyes, the emissions are lower from these processing industries. The contribution to waste water effluent from the plastic and paper industries is negligible.
It is assumed that there is no significant release of pigments to waste water during the use phase (consumption of end-products). The predominant release from this phase is to landfills.
A potential release route to the atmosphere may be from pigments bound to particular matter in soil/sludge from either landfills or agricultural fields fertilised with sludge or from incineration of waste and emissions from the processing industry. However, this release route may not be very important, due to the physical-chemical properties of the pigments. It is assumed that the atmospheric release route is negligible, i.e. approximately 0.
Agricultural fields fertilised with sludge may give rise to soil and groundwater releases of pigments. Landfills may provide another release route of pigments to these compartments.
The estimated Danish releases are summarised in Table 6.2 below. The preconditions for the estimates are given in chapter 4.
Table 6.2
Estimated environmental releases of azo pigments in Denmark.
Estimeret frigivelse af azopigmenter til miljøet i Danmark.
Waste water (Influent, stp) |
Landfills |
|||
Processing in tonnes |
Use in tonnes |
Use in tonnes |
||
| Production | 180 |
n.a. |
? |
|
| Processing | ||||
| Leather | >0 |
n.a. |
1 |
|
| Paint | >0 |
n.a. |
800 |
|
| Paper | n.a. |
n.a. |
30 |
|
| Plastic | n.a. |
n.a. |
50 |
|
>0 |
n.a. |
183 |
||
| Textile | 2 |
8 |
21 |
|
n.a. = negligible amount.
As for the azo dyes, impurities of the pigments as well as decomposition by reductive cleavage may result in transformation of the azo pigments into the degradation products, i.e. metabolites - aromatic amines - of which some are potentially carcinogenic. Estimation of the decomposition of azo pigments in the environment may be derived from knowledge of the azo pigments’ structural and molecular composition and a stoichiometric equation.
Metabolites
The aspect of the metabolites is discussed in connection with the azo dyes in chapter 5, section 5.3 and section 5.4.
Abiotic degradation
Hydrolysis is not considered to play any role in the degradation of pigments in the environment, due to their physico-chemical properties as highly hydrophobic substances. This is supported by a study on Pigment Yellow 83* of which hydrolysis was not detected in a 56-day experiment (IUCLID).
Photolysis of pigments is, in principle, possible. Stability of the pigments to visible and UV light are very high, therefore, only slow degradation may take place (Clarke & Anliker, 1980).
Subsequently, abiotic degradation of azo pigments may not be very probable.
Biodegradation
The pigments are practically insoluble and therefore considered essentially non-bioavailable (ETAD, 1989).
Biodegradation studies carried out on Pigment Yellow 17* showed that no anaerobic biodegradation occurred (ETAD et al., 1995). The rate limiting step for biodegradation by bacteria may be the uptake over the membrane, according to the findings of Opperhuizen et al. (1985), where it was shown that xenobiotics, with a cross section of more than 9.5 Å, are not able to pass the cellular membrane.
Furthermore, data on biodegradation of two other pigments included in the present survey: Pigment Red 53* and Pigment Yellow 12* indicated that no biodegradation took place in a 2-week study with sludge concentrations of 30 mg/l of the pigment (MITI). The same applies for Pigment Yellow 83* (IUCLID).
According to IUCLID, aerobic degradation by activated sludge may take place. In 15-day studies 40 and 81% of Pigment Yellow 83* and Pigment Yellow 12*, respectively, were degraded. However, it should be noted that the pigments were dispersed in, among other things, ethandiol.
The white-rot fungus Pycnoporus cinnabarinus has been able to decolourise the effluent from a pigment plant, up to 90% in 3 days. The biodegradation was by way of extracellular oxidases (Banat et al., 1996).
Summary
Biodegradation of azo pigments may be insignificant at least in relatively short term studies, indicating that they are not biodegradable, neither ready nor inherent. No data were found on long term studies and biodegradation. It is concluded that pigments are likely to persist in the environment.
Intracellular biodegradation of azo pigments which is considered to be the main degradation route of bacteria is not feasible for pigments due to the large molecular size. However, it seems that there is a potential for biodegradation by means of extracellular enzymes and when the pigments are dispersed in reagents.
Volatilisation
In principle, pigments like disperse and solvent dyes are potentially volatile, but as they are large, complex molecules, they can be expected to have low vapour pressures, i.e. lower than 10-13 to 10-11 mmHg. Another reason for volatilisation to be unlikely for the uncharged pigments is that the escaping tendency or fugacity that drive volatilisation is also the driving force for both sorption and bioconcentration (Baughman & Perenich, 1988b).
Adsorption
The pigments are highly hydrophobic and like the non-ionic dyes (e.g. disperse dyes), they adsorb strongly to sediment and soil.
Tests indicate that dyes adsorb 40-80% (Clarke & Anliker, 1980). Due to the physico-chemical properties of pigments (e.g. Log Kow,), it is assumed that pigments adsorb strongly which indicates an adsorption of at least 80 to 98%. According to the TGD (1996) an adsorption of approximately 92% may be expected.
Furthermore, pigments do not reach open waters to any significant extent due to the extremely low water solubility and molecular weight. The pigments may be found on soil/sediment/sludge fraction due to precipitation. (Clarke & Anliker, 1980).
As for the disperse dyes it may be expected that the sorption of pigments to sediment is dependent of the substitutional pattern of the chemical structure of the pigments, pH, the organic content of waste water as well as salinity. Sorption is favoured by decreasing pH and increased salinities (Weber, 1991; Pagga & Taeger, 1994).
Summary
No data were obtained on adsorption of pigments, but it is indicated that this route of removal is most important. It is assumed that pigments adsorb or precipitate 80 to 98% in the aquatic environment.
Products which are almost completely insoluble in water present particular experimental difficulties both in fish accumulation tests and by mea- surement of partition coefficients (Clarke & Anliker, 1980).
Anliker and Moser (1987) studied the limits of bioaccumulation of organic pigments in fish and their relation to the partition coefficient and the solubility in water and octanol for 2 azo pigments: a tetrachloroisoindoli- none type and a phenyl azo-2-hydroxy-naphthoicacid type. They found:
| Estimated solubility in water 10-9 and 10-7 mg/l, respectively. | |
| Solubility in n-octanol <1 and <0.1 mg/l, respectively. | |
| Estimated log Kow 10.5 and 10.1, respectively. | |
| Experimentally assessed log BCF 0.48 and 0.70, respectively. |
The high log Kow would suggest strong bioaccumulation tendencies, but no accumulation was observed in the fish for the pigments tested. The reason for this apparent inconsistency is the very limited fat (lipid) storage potential of these pigments, indicated by their low solubility in n-octanol and their large molecular size. In addition, the findings of Opperhuizen et al. (1985) indicate that a lack of uptake can be expected for extremely hydrophobic chemicals with an effective cross section larger than 9.5 Å (0.95 nm), like the pigments described, because the membrane permeation seems practically impossible.
Studies of bioaccumulation of pigments by Anliker et al. (1981) and Anliker et al. (1988) are in agreement with the above stated results. In the study of 1988 the two pigments examined had cross sectional diameters of 0.97 and 1.68 nm, respectively, and the corresponding log BCFs were 0.48 and 0.70 (MITI standard), respectively.
In addition, the low solubility effects are further enhanced, because the dissolution rates for extremely insoluble hydrophobic solids are usually very low causing that equilibration with water may take months or even years (Anliker et al., 1981).
Only a few experimentally assessed data on log BCF of the pigments encompassed in the present study, were available (Table 6.3).
Table 6.3
Bioconcentration factors for some azo pigments used in Denmark.
Biokoncentrationsfaktorer for nogle azopigmenter anvendt i Danmark.
| Structural type | Organism | Concentration | log BCF |
mg/l |
|||
| Pigment Orange 13 | Cyprinus carpio | 0.150 |
-0.12-0.75 |
0.015 |
0.45 |
||
| Pigment Red 53 | Cyprinus carpio | 0.700 |
-0.05-0.26 |
0.070 |
0.93-1.18 |
||
| Pigment Yellow 12 | Cyprinus carpio | 0.100 |
-0.42-0.51 |
0.010 |
0.38-0.73 |
||
| Ref.: MITI. |
By the bioaccumulation factor, it is indicated that the immediate concern for bioaccumulation of azo pigment may be very low.
Aquatic compartment
Monitoring data
Only negligible amounts of pigments reach the environment, owing to their extremely low water solubility (10-6 to 5 mg/l) and their application in mostly non-aqueous systems (Anliker, 1986). The loss of organic pigments to the environment is estimated to be 1% in the production and 1 to 2% during the processing (Clarke & Anliker, 1980).
No monitoring data of azo pigments were obtained in the aquatic compartment.
Estimation of PEC
In the present calculation of PEC effluent, stp, two scenarios will be presented. The estimation of PEC effluent, stp is based on the following assumptions
| The production and processing industries do not treat waste water. |
| Between 80 and 98% (adsorption and precipitation) of the azo pigments are adsorbed and/or precipitated in the sewage treatment plant (STP) (Clarke & Anliker, 1980). This results in a worst case scenario of 80% adsorption and precipitation (20% are released to the effluent) and a best case scenario of 98% adsorption and precipitation (2% are released to the effluent). |
| Adsorption to sludge and sediment is calculated based on a worst case of 98% adsorption and precipitation and a best case of 80% adsorption and precipitation. |
| Adsorption is the only removal route of the azo dyes in the STP, i.e. there is no abiotic or biotic degradation. |
Furthermore, a standard STP scenario in compliance with TGD (1996), is used. According to this standard, the following values are standard characteristics:
Table 6.4
Standard characteristics of a sewage treatment plant.
Standardkarakteristika for et rensningsanlæg.
| Parameter | Symbol | Unit | Value |
| Capacity of local STP1 | Capacity stp | [eq] | 10000 |
| Amount of wastewater per inhabitant | Waste inhab | [lxd-1xeq-1] | 200 |
| Surplus sludge per inhabitant | SURPLUSsludge | [kg x d-1 x eq-1] | 0.011 |
| Concentration susp. matter in influent | SUSPCONCinf | [kgxm-3] | 0.45 |
1
STP: Sewage Treatment Plant.Ref.: TGD (1996).
The calculation of PEC influent, stp is simplified and based on the equation below:
PECinfluent, stp = Releasewaste water /Wasteinhab. ´ Capacitystp ´ 365
The calculation of PEC effluent, stp is simplified and based on the assumptions mentioned above. In addition the PEC effluent, stp for the processing industry is corrected for the number of sites present in Denmark, e.i. 1 production site, 40 sites for textile colouring and 1 site for leather dyeing and for the use, the number of inhabitants in Denmark (approximately 5 millions) is normalised to the capacitystp.
PEC effluent, stp = PEC influent, stp ´ (1- adsorption factor)/(number of sites) or inhabitants in Denmark.
PEC surface water = PEC effluent, stp ´ dilution factor. According to the TGD (1996), the dilution factor is 10.
In Table 6.5, the estimated PECeffluent, stp and PECsurface water for azo pigments are presented.
Table 6.5
Estimated PEC effluent, stp and PECsurface water for azo pigments.
Estimeret PECudløb, stp og PECoverfladevand for azopigmenter.
Release |
PEC influent, stp |
PEC |
PEC |
PEC |
PEC |
|
t/year |
mg/l |
mg/l/site or inhab. |
mg/l/site or inhab. |
mg/l/site or inhab. |
mg/l/site or inhab. |
|
Worst case |
Best case |
Worst case |
Best case |
|||
| Pro- duction |
180 |
246 |
49.3 |
4.93 |
4.93 |
0.49 |
| Pro- cessing |
||||||
| Textile | 2 |
2.7 |
0.014 |
0.001 |
0.001 |
0.0001 |
| Use | ||||||
| Textile | 8 |
10.9 |
0.004 |
0.0004 |
0.0004 |
0.00004 |
| Total | 190 |
- |
- |
- |
- |
- |
The PECsediment is calculated from:
PECsediment = PECsurface water ´ adsorption factor.
In Table 6.6 the PECsediment is presented.
Table 6.6
Estimated PECsediment for azo pigments
Estimeret PECsediment for azopigmenter.
| Scenario | PECsurface water |
Adsorption factor |
PECsediment |
mg/l |
mg/kg |
||
| Worst case | |||
| Production | 4.93 |
0.98 |
4.83 |
| Processing | |||
| Textile | 0.001 |
0.98 |
0.00098 |
| Use | |||
| Textile | 0.0004 |
0.98 |
0.00039 |
| Best case | |||
| Production | 0.49 |
0.80 |
0.392 |
| Processing | |||
| Textile | 0.0001 |
0.80 |
0.00008 |
| Use | |||
| Textile | 0.00004 |
0.80 |
0.000032 |
Concerning the concentration of azo pigments in the sludge, the estimation is based on an annual production of sludge of 170,000 tonnes dry weight in Denmark (Miljøstyrelsen, 1996b). The worst case of adsorbed azo pigments to the sludge is 98%, and the "best case" is 80% of adsorbtion. The calculated concentration in sludge is based on the following equation:
PECsludge = (Release ´ Adsorption factor ´ 106/ Sludge rate)/(Number of sites) or inhabitants.
Sludge rate = 170.000 tonnes/year.
Table 6.7
Estimated PECsludge for azo pigments.
Estimeret PECslam for azopigmenter.
Release |
PECsludge |
PECsludge |
|
t/year |
mg/kg/site or inhab. |
mg/kg/site or inhab. |
|
Worst case |
Best case |
||
| Production | 180 |
1,037 |
847 |
| Processing | |||
| Textile | 2 |
0.29 |
0.24 |
| Use | |||
| Textile | 8 |
0.09 |
0.08 |
| Total | 190 |
- |
- |
The estimated PECeffluent, stp and PECsurface water are very high from the production of azo pigments in the range of 4.9 to 49.3 mg/l and 0.49 to 4.93 mg/l, respectively, whereas from the processing and use phases they are much lower in the range of 0.04 to 1 : g/l (PECsurface water).
Due to the lack of monitoring data of environmental concentrations of azo pigments, it is not possible to validate the estimated PECs, but the basic assumption that the manufacturing and processing industries do not carry out waste water treatment prior to outlet (PECinfluent, stp) is unlikely, because most of these companies, if not all of them, are encompassed by a special section of the Danish Environmental Protection Law (chapter 5). Hence, their emissions are restricted and must be approved by the authorities. Subsequently, the companies are obliged to have some degree of waste water treatment prior to the outlet to the municipal STP. This indicates that the estimated PECinfluent, stp generally is to high.
Furthermore, the pigments are only sparingly soluble in water and may rather quickly be bound to the particulate matter or sludge if subjected to waste water treatment.
This indicates that the actual PECeffluent, stp and PECsurface water for the production phase are more likely to be in the range of 1 to 9.9 mg/l and 0.1 to 1 mg/l, respectively. The latter is still very high, because there will be a visual colouring of the water above concentrations of 1 mg/l. Recalculating the PECeffluent, stp and PECsurface water for the processing and use phases in the same way, results in concentrations of 0.02 to 4 : g/l and 0.002 to 0.4 : g/l, respectively.
If it is assumed that the PECsurface water is to high, then the PECsediment has to be reduced in the same order of magnitude. Resulting in a concentration of 0.1 to 1 mg/kg from production and 0.002 to 0.4 : g/kg from processing. However, as shown in the monitoring studies on dyes, there may be significantly higher concentrations in the sediment compared to the water phase.
If it is assumed that the companies carry out waste water treatment and that 80% of the pigments are removed in this way, 20% may be released to the waste water outlet (worst case). The PECsludge, stp may be reduced to 212 mg/kg for production and 0.14 mg/kg for processing and use.
Monitoring data
No monitoring data of azo pigments were obtained in the atmosphere.
Estimation of PEC
It was not attempted to calculate the atmospheric PEC, but it is estimated that the PEC is very low, because volatilisation is highly unlikely for the azo dyes from both moist and dry surfaces. Furthermore, release from the processing industry and from incineration is considered to be very low (approximately equal to 0).
Terrestrial compartment
Monitoring data
No monitoring data of azo pigments were obtained in the terrestrial environment.
The sources of environmental releases of azo pigments in the terrestrial environment are waste disposal in landfills and sludge applied as fertiliser in agriculture.
Estimation of PEC
It is estimated that the total amount of sludge per year in Denmark is 170,000 tonnes dry weight. About 114,000 tonnes (67%) are used in agriculture and 20,000 (12%) are deposited in landfills. The rest is incinerated (21%) (Miljøstyrelsen, 1996b).
It is not known how many hectares of agricultural soil that are fertilised with sludge in Denmark. But according to the TGD (1996), the following characteristics of soil and soil use are accepted:
Table 6.8
Standard environmental characteristics for soil.
Standard miljøkarakteristika for jord.
Depth of soil |
Rate of sludge application |
|
[m] |
[kgdwtxm-2xyear-1] |
|
| PEClocalagr. soil | 0.20 |
0.5 |
Ref.: TGD (1996).
In section 2.3.4 of the TGD (1996), standard environmental characteristics are defined and on this basis it may be calculated that the density of soil is 1.7 tonnes/m3. By application of a depth of soil of 0.2 m in accordance with the TGD (1996), it is estimated that the weight of soil per square meter is equal to 0.34 tonnes.
Subsequently, assuming this, 98% of the azo pigments are adsorbed to the sludge in a worst case scenario and 80% are adsorbed to sludge in a best case scenario. The amount of azo dyes on the agricultural fields can be estimated from the following equation:
PECagri sludge = (release ´ adsorption factor ´ fraction to agriculture)/ (sludge amount/application rate) ´ soil weight.
Table 6.9
Estimated PECagri sludge for azo pigments.
Estimeret PECagri slam for azopigmenter.
Release |
PECagri sludge |
PECagri sludge |
|
t/year |
mg/kg |
mg/kg |
|
Worst case |
Best case |
||
| Production | 180 |
1.53 |
1.23 |
| Processing | |||
| Textile | 2 |
0.02 |
0.01 |
| Print (de-inking) | 50 |
0.65 |
0.43 |
| Use | |||
| Textile | 8 |
0.08 |
0.06 |
| Total | 190 |
- |
- |
The allocation of sludge to landfill disposal amounts to 20,000 tonnes dry weight per year. The contribution of sludge adsorbed azo dyes to the total amount of azo dyes in landfills may be calculated on the basis of the equation shown below:
Sludge amount to landfill = release ´ adsorption factor ´ fraction to land- fill.
In a worst case scenario, the contribution from the one production site may be 20.8 tonnes/year and from processing and use 0.23 tonnes/year. In a best case scenario, the values are 16.9 and 0.19 tonnes/year, respectively. This corresponds to approximately 2 and 0.02% of the total amount of pigments deposited in landfills from the manufacture of pigments and > 0.1% from the processing industries.
Thus, the total release to landfills may be estimated to approximately 1,021 tonnes/year (worst case) 1,017 tonnes/year (best case).
Assuming that 80% of the pigments are removed by the waste water treatment facility at the production and processing sites, the PECagri sludge from production may be reduced to 0.311 mg/kg soil and from processing to 0.003 mg/kg soil. The contribution from the use and de-inking phases are unchanged 0.07 and 0.65 mg/kg soil, respectively. However, due to the lack of monitoring data, it is not possible to validate the calculated PECs. But the concentration is in the same order of magnitude as the worst case level of 1 mg/kg for dyes, reported by Brown and Anliker (1988).
The fate of products containing pigments released to landfills is uncertain, but there may be a potential release of pigments to soil from this compartment.
Aquatic compartment
The possible inhibitory effects of dyes, including 3 pigments, on aerobic waste water bacteria have been studied by Brown et al. (1981). For Pigment Orange 34, Pigment Red 9* and Pigment Yellow 13*, the IC50 was above 100 mg/l measured as the respiratory rate. The experimental results for Pigment Red 9* indicate that only some of the bacteria appeared to be sensitive to the pigment, but this sensitivity extended over a rather large concentration range. The IC50 found by extrapolation was 350 mg/l.
According to IUCLID, Pigment Red 53* has an IC50 at 24 hours of more than 1,500 mg/l and Pigment Yellow 12* an IC50 of more than 2,000 mg/l.
Based on literature and database studies, it was possible to obtain LC50 data for a few azo pigments on various fish species. The data are listed in Table 6.10 below.
Table 6.10
Effect of azo pigments used in Denmark.
Effekt af azopigmenter anvendt i Danmark.
| C.I. name | Fish | Effect |
Concentration |
| Organism | mg/l |
||
| Pigment Orange 13 | Cyprinus carpio1 | LC50, 48 h |
100 |
| Pigment Red 53 | Cyprinus carpio1 | LC50, 48 h |
420 |
| Brachydanio rerio3 | LC50, 48 h |
>500 |
|
| Oryzias latipes3 | LC50, 48 h |
>420 |
|
| Brachydanio rerio3 | LC50, 96 h |
>500 |
|
| Pigments Yellow 12 | Cyprinus carpio1 | LC50, 48 h |
>420 |
| Leuciscus idus3 | LC50, 48 h |
>500 |
|
| Leuciscus idus3 | LC50, 96 h |
>100 |
|
| Pigment Yellow 83 | Phoxicus phoxicus1 | LC50, 48 h |
45 |
| Oncorhynchus mykiss1 | LC50, 48 h |
18 |
|
| Leuciscus idus1 | LC50, 48 h |
45 |
|
| 1 Data from MITI. 2 Data from AQUIRE.3 Data from IUCLID. |
Summary
Short term studies indicate that azo pigments do in general not give rise to immediate concern about toxicity, as the toxic effects are exhibited at levels above 100 mg/l. But the effect concentrations for Pigment Yellow 83 indicate that this pigment is potentially toxic (LC50 10 to 50 mg/l).
The very limited data availability on short term effects of pigments and the lack of long-term studies on effects, makes it difficult to draw general conclusions on the toxicity of azo pigments, but compared to the azo dyes, their toxicity to aquatic organisms is in general lower.
PNEC - aquatic
Applying an assessment factor of 100 on the EC50 from a respiration inhibition test (IUCLID), the following PNEC is derived according to TGD Part II, section 3.4:
PNECstp = 15 mg/l
Despite the fact that short term data from each of the three trophic levels (alga, fish, daphnia) were not obtained in the present survey, the assessment factor of 1,000, according to TGD Part II, section 3.3.1, is applied at the lowest LC50. The lowest observed effect is for fish (Table 6.10) , i.e. the LC50 of 18 mg/l for Oncorhynchus mykiss, arriving at a PNEC of :
PNECaquatic organisms = 18 : g/l.
No data were obtained on atmospheric exposure.
Terrestrial compartment
No data were obtained on terrestrial exposure.
Risk characterisation
The PEC/PNEC ratios which can be derived with the available data are shown in Table 6.11 and Table 6.12.
Table 6.11
PEC/PNEC ratios for the aquatic and terrestrial compartments from manufacture (production).
PEC/PNEC ratioer for vand- og jordmiljø fra fremstilling (produktion).
| Compartment | Site | PEC (mg/l or kg) |
PEC/PNEC |
|||
Worst |
Best |
Worst |
Best |
|||
|
STP sludge | 1,037 |
847 |
69 |
56 |
|
| Aquatic | STP effluent | 49.3 |
4.9 |
2,738 |
272 |
|
| Surface water | 4.93 |
0.49 |
273 |
27.2 |
||
| Sediment | 4.83 |
0.392 |
0.32 |
0.03 |
||
| Terrestrial | Agr. sludge | 1.53 |
1.23 |
- |
- |
|
Table 6.12
PEC/PNEC ratios for the aquatic and terrestrial compartments from processing and use.
PEC/PNEC ratioer for vand- og jordmiljø fra procesanvendelse og brugsfasen.
| Com- partment |
Site | Site | PEC (mg/l or kg) |
PEC/PNEC |
||
Worst |
Best |
Worst |
Best |
|||
|
STP sludge | Pro- cessing |
||||
| Textile | 0.29 |
0.24 |
0.019 |
0.016 |
||
| Use | ||||||
| Textile | 0.09 |
0.08 |
0.006 |
0.005 |
||
| Aquatic | STP effluent | Pro- cessing |
||||
| Textile | 0.014 |
0.001 |
0.8 |
0.06 |
||
| Use | ||||||
| Textile | 0.004 |
0.0004 |
0.2 |
0.02 |
||
| Surface water | Pro- cessing |
|||||
| Textile | 0.001 |
0.0001 |
0.06 |
0.006 |
||
| Use | ||||||
| Textile | 0.0004 |
0.00004 |
0.02 |
0.002 |
||
| Sedi- ment |
Pro- cessing |
|||||
| Textile | 0.00098 |
0.00039 |
0.0001 |
0.00003 |
||
| Use | ||||||
| Textile | 0.00008 |
0.000032 |
0.000005 |
0.000002 |
||
| Terrestrial | Agr. sludge | Pro- cessing |
||||
| Textile | 0.02 |
0.01 |
- |
- |
||
| Printing (De- inking) |
0.65 |
0.45 |
- |
- |
||
| Use | ||||||
| Textile | 0.08 |
0.06 |
- |
- |
||
For substances with a PEC/PNEC ratio of < 1, according to TGD, there is no need for further testing and no need for risk reduction measures beyond those which are already being applied, whereas a ratio > 1 indicates a need for further information and/or testing or even a need for limiting risks.
The PEC/PNEC ratios from the production of pigments are well above 1 (Table 6.11), indicating a need for further testing, whereas the ratios for processing and use are well below 1 (Table 6.12), indicating that there is no immediate (acute) risk.
With reference to the assumptions and recalculation of the PECs, it is indicated that the PEC/PNEC ratios presented in Table 6.11and Table 6.12 are to high. Subsequently, the PEC/PNEC ratios for production may be in the range:
|
0.01 to 14 | >1 | ||
|
56 to 556 | >>1 | ||
|
5.6 to 56 | >1 | ||
|
0.007 to 0.07 | <1 |
Recalculation of the PEC/PNEC ratios for processing indicates a range well below 1 which indicates that there is no immediate need for further testing.
Summary
Subsequently, the survey indicates that there is a need for further information and testing in order to assess the environmental risk associated with the manufacturing of azo pigments, whereas the releases associated with processing and use not seem to present any immediate concern.
Conclusion and Recommendation
Conclusions on the individual elements of the survey
Mass balance
In order to establish the mass balance of azo colorants in Denmark, it has been a prerequisite to base the estimations on assumptions. The number of assumptions means that the mass balance does not show the precise flow of azo colorants in Denmark, but at present, it is the best estimate for the total flow of colorants.
It is concluded that the results of the present survey may indicate the order of magnitude of the mass balance but not the exact figures/amounts.
Azo colorants may be subdivided in two groups: dyes and pigments. When looking into the ratio of consumption and use between the two groups of azo colorants, pigments clearly dominate the use of azo colorants in Denmark. They constitute approximately 66% of the colorants used and contained in imported products. Pigments are used in all industrial trades included in the survey. Pigments are also produced in Denmark, and it is assumed that the production amounts to approximately 18,000 tonnes p.a., and that approximately 90% are exported.
The survey indicates that dyes are, in contrast to pigments, almost exclusively used in the textile industry and is imported within textile products. The latter dominates and constitutes almost 75% of the total dye input to Denmark. However, it should be noted, that azo dyes may be used to a lesser extent in other industrial sectors. There is no direct production of dyes in Denmark, but several mixing houses manufacture dye formulations by the blending of different dyes.
It is concluded that pigments constitute the most significant part of the flow of colorants in Denmark, but at the same time, azo dyes constitute an important part (34%). Dyes are mainly associated with textiles but are used in other products/trades too. Thus, it is possible to distinguish between the two groups of azo colorants: pigments and dyes in the mass balance, and allocate their consumption and use among trades. However, based on the present findings it is not possible to qualify the distribution of the different technical (chemical) groups of dyes, except for textile and pigments. In addition, it is not possible to conclude on the consumption and use of individual azo colorants.
Because of the large number of more than 3,000 azo colorants, , the survey focused on colorants which according to the literature are in general use. Therefore, the individual colorants encompassed in the survey are not totally representative of the colorants used in Denmark.
The survey revealed that the major importers and manufacturers of azo colorants do not import or sell colorants, which are subject to restrictions in e.g. Germany. However, the restricted compounds may be present in textiles and leather products from e.g. Asia, Eastern Europe and South America. The imports from Asia alone account for 430 tonnes of azo dyes, primarily in textiles, and 40 tonnes of azo pigments in leather products. Thus, at least 20% of the azo dyes associated with imported goods, stem from regions where there may be a potential use of the restricted dyes. But it should be noted, that the possible content of problematic dyes and their cleavage products in imported goods has not been assessed, and whether the goods contain these dyes or not or to which degree is not known.
It is concluded that dyes contained in imported products, mainly textiles and leather, may contribute to a flow of azo dyes based on potentially carcinogenic aromatic amines in Denmark.
The survey indicates that the problematic azo dyes are being out-phased at least for the major manufacturers. Furthermore, there is world-wide a trend towards increased use of pigments and a decline in the use of dyes. The azo dyes are cheap but have relatively poor technical properties, e.g. light fastness, etc. Therefore, it may be speculated if there besides a general trend towards an increased use of pigments may be a market trend towards use of other chemical classes of colorants than azo colorants.
Dyes released to waste water constitute 6% of the total input of dyes. More than 50% of the dyes released to waste water originate from private households. The environmental release of pigments is lower, approxi- mately 1% of the total input.
It is concluded that there is a potential release of dyes and pigments to the environment. However, in order to make any final conclusions with regards to the environmental loads, the distribution between the disposal routes, i.e. waste water, landfill and incineration as well as recycling, need to be further investigated
Human toxicity
The acute toxicity of azo dyes is low, and the acute toxicity of azo pigments is very low. However, potential health effects are recognised for the dyes. The azo linkage of azo dyes, but not of azo pigments, may undergo metabolic cleavage resulting in free component aromatic amines. At least 22 of these are recognised as possible humans carcinogens. Therefore, the toxicity of azo dyes is mainly based on the toxicity (carcinogenicity) of the component amines.
Several studies have indicated that sulphonation of the parent dye inhibits the release of aromatic amines and therefore reduces the toxicity.
It is concluded that the toxicity of the parent compounds - the azo colorants - is low, however, some of the metabolic cleavage products, e.g. 22 component aromatic amines, are potentially carcinogenic.
The potential carcinogenic aromatic amines are those containing a moiety of: aniline, benzidine, toluidine or naphthalene. They are synthesis compounds/intermediates in the manufacture of some of the azo dyes and azo pigments and are represented in all chemical classes of azo colorants. In addition, they may be present as impurities.
It is concluded that, in principle, all the chemical classes of azo colorants may represent a potential toxicological risk, if the individual colorant is synthesised from one of the 22 aforementioned aromatic amines.
In Denmark, human exposure to aromatic amines may take place as a result of a breakdown of the colorants or due to impurities of the colorants during:
| Synthesis of azo pigments. | |
| Manufacturing of commercial formulations. | |
| Industrial uses: colouring of consumer goods, e.g. plastics, textiles, leather, printing, paint and lacquer, and paper. | |
| Consumption by end-users of products containing azo colorants, e.g. use of paints, wearing of textiles, etc. |
It is concluded that there is a small but possible risk of exposure to potential carcinogenic aromatic amines from azo colorants and coloured products in Denmark. However, to fulfil the risk assessment requires investigation of the production, manufacturing and processing technologies applied in Denmark as well as a closer examination of imported products and additional information on the content of impurities in formulations or products.
The sensitisation potential of azo colorants is rather low. However, sensitisation to azo colorants has been reported. Most reported cases, with relevance today, is related to the disperse azo dyes. Exposure to disperse dyes may take place during production of dyes and in the processing industry, predominantly textile. In addition, exposure may take place when wearing textiles, particularly those in close contact with the skin.
It is concluded that a few of the azo colorants are potentially allergenic, but it has been shown that sensitisation only is developed as a result of rather extensive exposure.
Environmental fate and toxicity
Due to the physico-chemical properties of the azo colorants, adsorption to soil and sediment is the primary fate of azo colorants in the environment, except for the ionic, acid and reactive dyes. It is indicated that biodegradation is the only degradation pathway for both dyes and their metabolites. Pigments, on the other hand, are not biodegradable.
Biodegradation of the dyes predominantly takes place in an anaerobic environment, whereas degradation of their metabolites takes place in an aerobic environment. The degradation of dyes varies from hours to several months or more, indicating that they are at least inherent biodegradable.
Substituents, like methyl, methoxy, sulpho or nitro groups reduce the biodegradability of the ionic dyes. The sulphonated metabolites may not be biodegradable either. The molecular size of the colorants may reduce the biodegradability too; this applies for e.g. disperse dyes, due to limited possibility of membrane uptake by the biota.
It is concluded that pigments and some of the dyes may accumulate in soil and sediment, due to limited bioavailability and because the prerequisite for biodegradation is the presence of an anaerobic environment. The degradation products may accumulate too, if they are not transported to the aerobic environment.
Furthermore, it is concluded that sulpho groups and other substituents may reduce the biodegradability of dyes and their metabolites. The molecular weight may reduce it too. Thus, a high degree of sulphonation and a high molecular size may, in addition, enhance the accumulation potential of the colorants and their metabolites.
With respect to bioaccumulation, it is indicated that the ionic dyes do not have a significant bioaccumulation potential in general, however, at least some acid dyes may bioaccumulate. The non-ionic dyes and pigments, on the other hand, have a high bioaccumulation potential indicated by high partition coefficients (log Kow ). Despite the high log Kow for pigments, experimentally assessed bioconcentration factors indicate that the immediate concern for bioaccumulation is very low. The metabolites, generally, have a potential for bioaccumulation.
It is concluded that azo colorants, with the exception of most ionic dyes, may have a potential for bioaccumulation, indicated by high partition coefficients, but due to limited bioavailability, e.g. molecular size, the bioaccumulation is generally low. The metabolites, on the other hand, have a potential of bioaccumulation.
Due to the lack of monitoring data of environmental concentrations of azo colorants in Denmark, it is not possible to validate the estimated predicted environmental concentration (PEC) with Danish data. The PEC estimates are based on the sewage treatment plant (STP) model applied in the TGD (1996) by the EU. The standard characteristics of this STP may be in accordance with the average Danish municipal STP. However, at least for industrial waste water treatment, the characteristics may not apply/corre- spond. Furthermore, the PEC estimates were carried out on the basis of the assumption that the processing industries do not carry out waste water treatment prior to outlet (PECinfluent, stp) which is unlikely, because most of these companies, if not all, are encompassed by a special section of the Danish Environmental Protection Law (chapter 5). Hence, their emissions are restricted and must be approved by the authorities. Subsequently, most of the companies are obliged to have some degree of waste water treatment prior to the outlet to the municipal STP. In accordance with this, the PECs have been modified "double" treatment, which reduces the PECs.
Another limitation of the PEC estimates is that all the Danish releases of colorants "are placed" in one sewage treatment plant. In order to compensate for this, the PECeffluent, stp has been normalised to represent the concentration per company or per Danish inhabitant.
Based on the above mentioned modifications, the PECsurface water has been estimated to be:
| 0.04 to 1.44 mg/l for dye processing and use. | |
| 0.1 to 1 mg/l for pigment production. | |
| 0.002 to 0.4 : g/l for processing and use of pigments. |
Even in this case, the PECs may be overestimated, because visual colouring of the water would be observed at levels above 1 mg/l, and the basic assumption that the degree of adsorption is in the range of 40 to 80% for dyes and 80 to 98% for pigments may be an underestimate. The size of the overestimate cannot be predicted at present.
Despite the fact of possible overestimation, the modified PECs for dyes in the aquatic environment are within the same range as concentration measures in monitoring studies abroad. However, it should be noted, that these studies, which are carried out in the US and Canada, are from confined areas where intensive textile dyeing takes place with a total use of dyes amounting to at least 3,500 tonnes p.a., in comparison to the total Danish input of 2,400 tonnes. For the pigments no monitoring studies have been found. Hence, it is not possible to validate these estimates further.
Even though, the PECs are uncertain, it is concluded that there is a release of azo colorants to the environmental compartments, especially to water and soil. The environmental exposure of water may take place as a result of outlet of colorants to waste water during production, processing and end-user consumption. There is a potential indirect exposure of agricultural soils through the application of sludge. Annually, approximately 1,300 tonnes of azo colorants are deposited in landfills and there is a potential release to soil and groundwater from landfills, but the fate of products containing azo colorants, deposited in landfills, is uncertain. It is concluded that predicted environmental concentrations may be established, which may indicate the environmental load, but validation to Danish conditions is not possible due to the lack of monitoring data in Denmark.
In addition, it is concluded that there may be accumulated substantial amounts of colorants in the environment, even though the emissions have been regulated for some years, due to the high accumulation potential of the colorants.
Generally, the availability of published data on the ecotoxicity of azo colorants is very sparse. Therefore, it was only possible to obtain data for a few of the azo colorants used in Denmark. However, short term studies indicate that some of the azo colorants in use are acute toxic (acid, basic and solvent dyes) to aquatic organisms and that others are toxic or potentially toxic (remaining dyes). Only reactive dyes are not considered to be toxic to aquatic organisms. In general, the pigments do not give rise to immediate concern about aquatic toxicity. However, it is indicated that some of them may be potentially toxic. The metabolites are potentially toxic to aquatic organisms, as well.
It is concluded that various azo colorants, representing all the chemical groups consumed in Denmark, may be potentially toxic to aquatic organisms. The metabolites are potentially toxic to aquatic organisms too. However, the limited data availability on ecotoxicity makes it difficult to draw definite conclusions.
The predicted no effect concentration (PNEC) for azo colorants used in Denmark in the aquatic compartment is low:
| Dyes: 0.7 : g/l. | |
| Pigments: 18 : g/l. |
The survey indicates that there is a need of further information, e.g. QSAR or testing, to assess the environmental risk of azo dyes in the STP sludge and the aquatic compartment, except for sediments indicated by PEC/PNEC ratios >> 1, whereas releases associated with sludge applied to soil not seem to present any immediate concern, indicated by PEC/PNEC < 1. As it was impossible to predict the concentration of dyes in landfill soils, the environmental risk for this compartment cannot be established at present.
With regards to azo pigments, the survey indicates that there is a need of additional information or testing in relation to the manufacture of pigments, indicated by PEC/PNEC ratios >> 1, whereas the exposures related to processing and use do not seem to present any immediate concern, indicated by PEC/PNEC ratios < 1.
It is concluded that processing and end-use of dyes as well as the manufacturing of pigments may pose an environmental risk for the microorga- nisms in the sewage treatment plant and for the aquatic compartment, except for sediments. However, this risk assessment is strictly preliminary, because of:
| the aforementioned limitations with regards to estimation of PEC. | |
| the lack of monitoring data of environmental levels (aquatic and terrestrial) of azo colorants in Denmark. | |
| the limited knowledge of consumption and use of individual azo colorants - quantities and specific compounds. | |
| the limited knowledge of the ecotoxicity of the specific compounds. | |
| the lack of long term low level exposure studies. |
Thus, carrying out a "true" risk assessment requires further investigation of the abovementioned parameters, in order to establish a more profound basis for the assessment.
Recommended areas for future investigations
Based on the findings and conclusions of the survey, especially with regard to the assessment of risk in relation to human health and environment, the following focus areas are recommended for future investigation.
The proposed actions are prioritised on the basis of a professional evaluation, taking the potential financial costs into account.
Mass balance
- It is recommended to elaborate further on the results of the mass balance in order to qualify the balance with regards to specific consumption and use, for:
| Azo colorants consumed by industrial end-users, e.g. the iron and steel industry. | |
| Dyes at group level and individual dyes. | |
| Dyes in the trades encompassed by the survey. | |
| Individual pigments. |
2) It is recommended to carry out further investigations on the distribution of dyes and pigments among the environmental disposal routes, i.e. waste water, landfill, incineration and recycling.
For priority 1 and 2, it is suggested that additional information/knowledge is obtained by interviews and questionnaires directed to experts, e.g. from the industry, in a two step approach. The first step may be collection of information of the most representative colorants of the different trades. The second step may, based on the findings of step 1, be collection of information about specific colorants.
3) It is recommended to investigate the general market trends for development and use of organic colorants.
As a feasible approach, it is suggested to conduct interviews with foreign and domestic experts.
4) It is recommended to carry out investigations on the possible content of azo dyes and the associated aromatic amines in imported textile and leather products.
Investigation of the imported goods may be carried out by random sampling and analysis of both large and small batches of textiles from Asia, Eastern Europe, Africa and South America. However, it is very costly to conduct a full monitoring program and, therefore, it is suggested to postpone these investigations, until the results of the more thorough mass balance are established.
Toxicity
1) It is recommended to investigate further on the molecular structure of the colorants used in Denmark and the possible toxicological effects of the other aromatic amines, i.e. besides the 22 well-known. It is known that other aromatic amines may be toxic, like aromatic amines with aryl moieties of anthracene, stilbene, phenanthrene, etc. It may be relevant to know, if azo colorants used in Denmark contain other problematic aromatic amines. In addition, further elaboration on the consumption of disperse dyes and investigation of substitutional options are recommended.
The prerequisite for investigations on molecular structures of colorants used in Denmark is additional knowledge of consumption and use derived from a mass balance study. The possible effects of other aromatic amines, than the 22 well-known, may be clarified through QSAR analysis and grouping.
With regard to investigation of options for substitution of the colorants with the problematic component amines and the disperse dyes, it is suggested, to await the results of a more detailed mass balance of consumption and use and the results of a more thorough analysis of market trends.
2) It is recommended to investigate further on the typical ratios of impurities associated with colorants and products containing colorants, especially associated with imported products.
Information on the typical ratio of impurities associated with colorants may be obtained from experts, e.g. from the industry, and knowledge about impurities associated with products may be obtained by random sampling and analysis of products. The latter may require substantial economic resources, and is suggested to await the results of the former.
3) It is recommended to carry out monitoring studies on occupational environment and to establish an overview of the applied technologies in the manufacturing and processing industries.
Monitoring studies of the occupational environment are quite costly and time-consuming. Therefore, the precondition for carrying these studies is additional knowledge of consumption and use of specific colorants.
Environmental fate and toxicity
1) It is recommended to carry out further investigations of:
| accumulation. | |
| bioaccumulation. | |
| biodegradation. | |
| ecotoxicity (short-term and long-term low level exposure). | |
| exposure routes. |
for the specific azo colorants used in Denmark, with special attention to molecular weight and substitutional pattern.
Additional knowledge of environmental fate and toxicity may be obtained by comparison of more detailed QSAR analysis and the available experimentally assessed data. In this way, it will be possible to address both the most problematic groups and individual colorants.
2) It is recommended to gather information or carry out monitoring studies of the potential releases and environmental concentrations of azo colorants in Denmark, in order to qualify the predictions and in order to establish a more detailed overview of the potential exposures.
With regards to exposure routes and assessment of the environmental risks, the prerequisite is further information of consumption, use and disposal of specific azo colorants in Denmark and to qualify the predicted environmental concentrations. This may be obtained by establishment of a more detailed mass balance and by gathering of information from the municipal authorities on allowed emissions or by actual monitoring studies. However, the latter may not be an economically feasible approach in a short term, and therefore, it is suggested to await the results, that may be obtained by the detailed mass flow analysis.
3) It is recommended to carry out experiments/model studies with regard to the decomposition and potential release of colorants deposited in landfills. Approximately 1,300 tonnes of azo colorants are annually deposited in landfills which may be released to soil and ground water.
Due to the relatively high costs of monitoring studies of landfill soil and leachate, it is suggested to limit the investigation to a thorough literature study of fate of azo colorants in landfills, possibly followed by computerised modelling of the fate of azo colorants incorporated in a product matrix.
References
Literature
| Allison G. and Morita M. (1995a). Bioaccumulation and toxic effects of elevated levels of 3,3’,4,4’-tetrachloroazobenzene (33’44’-TCAB) towards aquatic organisms. III: Bioaccumulation and toxic effects of detrital 33’44-TCAB on the aquatic snail, Indohiramakigai (Indoplanorbis Exustus). Chemosphere vol. 30, no. 2, pp. 233-242. |
| Allison G. and Morita M. (1995b). Bioaccumulation and toxic effects of elevated levels of 3,3’,4,4’-tetrachloroazobenzene (33’44’-TCAB) towards aquatic organisms. II: Bioaccumulation and toxic effects of dietary 33’44-TCAB on the Japanese Medaka (Oryzias Latipes). Chemosphere vol. 30, no. 2, pp. 223-232. |
| Anliker R., Clarke E. A. and Moser P. (1981). Use of the partition coefficient as an indicator of bioaccumulation tendency of dyestuffs in fish. Chemosphere vol. 10, no. 3, pp. 263-274. |
| Anliker R. (1986). Organic colorants - Interpretation of mammalian-, geno- and ecotoxicity data in terms of potential risk. Toxic Hazard Assessment of Chemicals, editor Richardson M. The Royal Society of Chemistry, London, pp. 166-187. |
| Anliker R. and Moser P. (1987). The limits of bioaccumulation of organic pigments in fish: Their relation to the partition coefficient and the solubility in water and octanol. Ecotoxicology and Environmental Safety 13, pp. 43-52. |
| Anliker R., Moser P. and Poppinger D. (1988). Bioaccumulation of dyestuffs and organic pigments in fish. Relationships to hydrophobicity and steric factors. Chemosphere vol. 17, no. 8, pp. 1631-1644. |
| Arcos J. C. and Argus M. .F. (1994). Chemical induction of cancer: structural bases and biological mechanisms. Academic Press. |
| Banat I. M., Nigam P., Singh D. and Marchant R. (1996). Microbial decolorization of textile-dye-containing effluents: A review. Biosource Technology 58, pp. 217-227. |
| Baughman G. L. and Perenich T. A. (1988a). Fate of dyes in aquatic systems: I. Solubility and partitioning of some hydrophobic dyes and related compounds. Environmental Toxicology and Chemistry (7), pp. 183-199. |
| Baughman G. L. and Perenich T. A. (1988b). Investigating the fate of dyes in the environment. American Dyestuff Reporter, pp. 19-22 and 47. |
| Baughman G. L. and Weber E. J. (1994). Transformation of dyes and related compounds in anoxic sediment: Kinetics and products. Environ. Sci. Technol. 28, pp. 267-276. |
| Boethling R. S., Gregg B., Frederick R., Gabel N. W., Campbell S. E. and Sabljic A. (1989). Expert systems survey on biodegradation of xenobiotic chemicals. Ecotoxicology and Environmental Safety 18, pp. 252-267. |
| Brown D., Hitz H. R., and Schäfer L. (1981). The assessment of the possible inhibitory effect of due-stuffs on aerobic waste-water bacteria. Experience with a screening test. Chemosphere vol. 10, no. 3, pp. 245-261. |
| Brown D. and Laboureur P. (1983a). The aerobic biodegradability of primary aromatic amines. Chemosphere vol. 12, no.3, pp. 405-414. |
| Brown D. and Laboureur P. (1983b). The degradation of dyestuffs: Part I, Primary biodegradation under anaerobic conditions. Chemosphere vol. 12, no. 3, pp. 397-404. |
| Brown D. (1987). Effects of colorants in the aquatic environment. Ecotoxicology and Environmental Safety 13, 139-147. |
| Brown D. and Hamburger B. (1987). The degradation of dyestuffs: Part III - Investigations of their ultimate degradability. Chemosphere vol. 16, no. 7, pp. 1539-1553. |
| Brown D. and Anliker R. (1988). Dyestuffs and the environment - A risk assessment. Risk Assessment of Chemicals in the Environment, editor Richardson M. The Royal Society of Chemistry, pp. 398-413. |
| Brown M. A. and DeVito S. C. (1993). Predicting azo dye toxicity. Critical Reviews in Environmental Science and Technology, 23(3), pp. 249-324. |
| Buljan J., Reich G., Ludvik J. (1997): Mass balance in leather proces- sing. |
| Cartwright R. A. (1983). Historical and modern epidemiological studies on populations exposed to N-substituted aryl compounds. Environmental Health Perspective 49, pp. 13-19. |
| Chung K.-T. (1983). The significance of azo-reduction in the mutagenesis and carcinogenicity of azo dyes. Mut. Res. 114(3), pp. 269-281. |
| Chung K.-T. and Stevens E. (1993). Degradation of azo dyes by environmental micro-organisms and helminths. Environmental Toxicology and Chemistry 12, pp. 2121-2132. |
| Chung K.-T., Chen S.-C., Zhu Y.-Y., Wong T. Y. and Stevens S. E. Jr. (1997). Toxic effects of some benzamines on the growth of azotobacter vinelandii and other bacteria. Environmental Toxicology and Chemistry 16(7), pp. 1366-1369. |
| Clarke E. A. and Anliker R. (1980). Organic dyes and pigments. Handbook of Environmental Chemistry, Springer Verlag. |
| Clayson D. B. and Garner R. C. (1976). Carcinogenic aromatic amines and related compounds. Chemical Carcinogens, American Chemical Society Monograph 173. |
| Couch J. A. and Harshbarger J. C. (1985). Effects of carcinogenic agents on aquatic animals: An environmental and experimental overview. Environ.. Carcinogenesis Revs. 3(1), pp. 63-105. |
| Cronin E. (1980). Contact dermatitis. Churchill Livingstone. |
| Danmarks Statistik (1997a). Udenrigshandelen fordelt på varer og lande 1997. Danmarks Statistik. |
| Danmarks Statistik (1997b). Forsyningsstatistikken. Danmarks Statistik. |
| Danmarks Statistik (1997c). Statistisk årbog. Danmarks Statistik. |
| Deutsche Forschungsgemeinschaft (1997). MAK- und BAT-Werte-Liste, Mitteilung 33. Deutsche Forschungsgemeinschaft. |
| Dieckmann M. S., Gray K. A. and Zepp R. G. (1994). The sensitised photo catalysis of azo dyes in a solid system: A feasibility study. Chemosphere vol. 28, no. 5, pp. 1021-1034. |
| Dumpert K. (1987). Embryotoxic effects of environmental chemicals: Tests with the South African clawed toad (Xenopus laevis). Ecotoxico- logy and Environmental Safety 13, pp. 324-338 |
| Eitel, Kurt (1988). DASD Färben von Leder. Umschau Verlag. |
| Erickson R. J. and McKim J. M. (1990). A simple flow-limited model for exchange of organic chemicals at fish gills. Environmental Toxicology and Chemistry (9), pp. 159-165. |
| ETAD (1989). An overview of their unique characteristics and the requirements for their safe handling and use. Ecological and toxicological association of dyes and organic pigments manufacturers, Switzerland. Azo Colorants, ETAD Information Notice no. 1, pp. 1-6. |
| ETAD (1991). Reactive Dyes: Mode of action and safe handling. ETAD Information Notice no. 3. |
| ETAD (1992a). Colorants and the environment. Guidance for the user. ETAD Information Notice no. 4. |
| ETAD (1992b). Environmental Hazard and Risk Assessment of Organic Colorants (including life-cycle Analysis). Materials for ETAD Seminar May 22, 1992 in Denmark. |
| ETAD (1994). Inhibition of algal growth caused by coloured test substances. Summary report. ETAD Project E 3023. |
| ETAD (1997). German ban of use of certain azo compounds in some consumers goods. ETAD Information Notice no. 6 (Revised July 1997). |
| ETAD, BCMA, EPSON and VdMI (1995): Safe handling of pigments. A publication of ETAD, BCMA, EPSON and VdMI.. |
| EU Commission (1996). Technical guidance documents in support of the commission directive 93/67/EEC on Risk Assessment for new notified substances and the commission regulation (EC) 1488/94 on risk assessment for existing substances. |
| Federal Ministry of Environment, Youth and Family and the Federal Chancellery (1997). Risk assessment - o-anisidine. Draft of 07.08.97. |
| Field J.A., Stams A.J.M., Kato M., and Schraa G. (1995). Enchanted biodegradation of aromatic pollutants in cocultures of anaerobic and aerobic bacterial consortia. Antoine van Leeuwenhoeck 67, pp. 47-77. |
| FitzGerald S. W. and Bishop P. L. (1995). Two stage anaerobic/aerobic treatment of sulfonated azo dyes. J. Environ. Sci. Health A30(6), pp. 1251-1276. |
| Foussereau J., Benexra C. and Maibach H. (1982). Occupational contact dermatitis. Clinical and chemical aspects, Munksgaard. |
| Franke C., Studinger G., Berger G., Böhling S., Bruckmann U., Cohors-Fresenborg D. and Jöhncke U. (1994). The assessment of bioaccumulation. Chemosphere vol. 29, no. 7, pp. 1501-1514. |
| Freeman H. S., Hinks D. and Esancy J. (1996). Genotoxicity of azo dyes: Bases and implications. J. Adv. Color. Chem. Ser. 4, pp. 254-292. |
| Heinfling A., Bergbauer M., Szewyk (1997). Biodegradation of azo and phthalocyanine dyes by Trametes versicolor and Bjerkandera adusta. Appl. Microbiol. Biotechnol. 48, pp. 261-266. |
| Herbst W. and Hunger K. (1993). Industrial organic pigments. Ecology, toxicology, legislation 5, pp. 567-577. |
| Hermens J. L. M, Bradbury S. P. and Broderius S. J. (1990). Influence of cytochrome P450 mixed-function oxidase induction on the acute toxicity to rainbow trout (Salmo gairdneri) of primary aromatic amines. Ecotoxicology and Environmental Safety 20, pp. 156-166. |
| Hunger K. and Jung R. (1991). On the toxicology and ecology of organic colorants. Chimia 45, pp. 297-300. |
| IARC Monograph 1 (1972). International Agency for Research on Cancer, pp. 80-86. |
| IARC Monograph 57 (1993a), pp. 203-12. |
| IARC Monograph 57 (1993b), pp. 259-67. |
| Jinqi L. and Houtian L. (1992). Degradation of azo dyes by algae. Environmental Pollution 75, pp. 273-278. |
| Kirk-Orthmer. Encyclopaedia of chemical technology. Third edition. John Wiley & Sons. |
| Knackmuss H.-J. (1996). Basic knowledge and perspectives of bioelimination of xenobiotic compounds. Journal of Biotechnology 51, pp. 287-295. |
| Marsh J. W., Chipman J. K. and Livingstone D. R. (1992). Activation of xenobiotics to reactive and mutagenic products by the marine invertebrates Mytilus edulis, Carcinus maenas and Asterias rubens. Elsevier Science Publishers, pp. 115-127. |
| Matsumoto M. R., Jensen J. N. and Reed B. E. (1995). Physico-chemical processes. Water Environment Research, 67(4), 419-432. |
| Mensink, Looye, van Westerhoven and Fluitman (1998). Prevention of carcinogenic azo dyes in the Netherlands. CREM Report no. 34A, The Netherlands. |
| Miljøstyrelsen (1991). Renere teknologi i den grafiske branche. Miljøprojekt 169. |
| Miljøstyrelsen (1993). Paradigma for materialestrømsanalyser. |
| Miljøstyrelsen (1994a). Kortlægning af ressourcehåndtering i tekstil vådbehandling. Miljøprojekt 268. |
| Miljøstyrelsen. (1994b). Produktion og miljøforhold i papirindustrien. Miljøprojekt 257. |
| Miljøstyrelsen (1995). Indsatsområder for renere teknologi i den grafiske branche. Miljøprojekt 284. |
| Miljøstyrelsen (1996a). Brancheorientering for farve- og lakindustrien. Brancheorientering 5. |
| Miljøstyrelsen (1996b). Analyseparametre for spildevandsslam til jordbrugsmæssig anvendelse. Arbejdsrapport 19. |
| Miljøstyrelsen (1996c). Status og perspektiver for kemikalieområdet. Et debatoplæg. Oplæg fra Miljøstyrelsen. |
| Miljøstyrelsen (1997). Environmental and health assessment of azo-colorants in textiles including toys. In the consumer situation. Draft from Miljøstyrelsen. |
| Miller J. A., Miller E. C. (1983). Some historical aspects of N-aryl carcinogens and their metabolic activation. Environmental Health Perspectives 49, pp. 3-12. |
| Milman H. A and Weisburger E. K. (1994). Handbook of Carcinogen Testing. Noyes Publications, New Jersey, USA. |
| MITI (1992). Biodegradation and bioaccumulation - data of existing chemicals based on the CSCL Japan. Chemicals Inspecting & Testing Institute, Ministry of International Trade & Industry, Japan. |
| Motschi, H. (1994). Assessment and management of environmental exposure to colorants. Chemical Safety, Richardson M. L., pp. 329-352. |
| Nendza M. and Seydel J. K. (1990). Application of bacterial growth kinetics to in vitro toxicity assessment of substituted phenols and anilines. Ecotoxicology and Environmental Safety 19, pp. 228-241. |
| Niimi A. J., Lee H. B. and Kissoon G. P. (1989). Octanol/water partition coefficients and bioconcentration factors of chloronitrobenzenes in rainbow trout (Salmo Gairdneri). Environmental Toxicology and Chemistry (8), pp. 817-823. |
| NIOSH (1980). Special occupational hazard review for benzidine-based dyes. National Institute of Occupational Safety and Health, US. |
| NPIRI (1983). Raw materials data handbook, Pigments. 4. National Printing Ink Research Institute. Lehigh University, Pennsylvania, US. |
| Opperhuizen A., Velde E. W. v. d., Gobas F. A. P. C., Liem D. A. K. and Steen J. M. D. v. d. (1985). Relationship between bioconcentration in fish and steric factors of hydrophobic chemicals. Chemosphere vol. 14, no. 11/12, pp. 1871-1896. |
| Pagga U. and Brown D. (1986). The degradation of dyestuffs: Part II Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere vol. 15, no. 4, pp. 479-491. |
| Pagga U. and Taeger K. (1994). Development of a method for adsorption of dyestuffs on activated sludge. Wat. Res. 28(5), pp. 1051-1057. |
| Parkinson Th. M. and Brown J. P. (1981). Metabolic fate of food colorant. Ann. Rev. Nutr. 1, pp. 175-2051. |
| Pasti-Grigsby M. B., Paszczynski A., Goszczynski S., Crawford D. L. and Crawford R. L. (1992). Influence of aromatic substitution patterns on azo dye degradability by streptomyces spp. and phanerochaete chrysosporium. Applied and Environmental Microbiology, pp. 3605-3613. |
| Paszczynski A., Pasti-Grigsby M. B., Goszczynski S., Crawford R. L. and Crawford D. L. (1992). Mineralization of sulfonated azo dyes and sulfanilic acid by Phenerochaete chrysosporium and Streptomyces chromofuscus. Applied and Environmental Microbiology 58(11), pp. 3598-3604. |
| Platzek Th. (1995). How serious is the actual health hazard caused by textiles? Melliand Sonderdruck, Berlin, Germany. |
| Produktregistret (1997/1998). |
| Razo-Flores E., Luijten M., Donlon B. A., Lettinga G. and Field J. A. (1997a). Complete biodegradation of the azo dye azodisalicylate under anaerobic conditions. Environ. Sci. Technol. 31, pp. 2098-2103. |
| Razo-Flores E., Luijten M., Donlon B., Lettinga G. and Field J. (1997b). Biodegradation of selected azo dyes under methanogenic conditions. Wat. Sci. Tech. 36(6-7), pp. 65-72. |
| Rendan (1996). Affaldshåndbogen ‘96/97. Rendan A/S. |
| RPA (July 1997). Analysis of the advantage and drawback of banning azo dyes and products treated with azo dyes. Risk and policy analysis ltd. Final Report for Directorate General III of the European Commission, Great Britain. |
| Seidenari S., Mamzini B. M., Schiavi M. E. and Motolese A. (1995). Prevalence of contact allergy to non-disperse azo dyes for natural fibres: A study in 1814 consecutive patients. Contact Dermatitis l33, pp. 118-122. |
| Seshadri S., Bishop P. L. and Agha A. M. (1994). Anaerobic/aerobic
treatment of selected azo dyes in wastewater. Waste Management 14(2), pp. 127-137. |
| Shauel G. M., Holdsworth T. J., Dempsey C. R. and Dostal K. A. (1991). Fate of water soluble azo dyes in the activated sludge process. Chemosphere vol. 22, no. 1/2, pp. 107-119. |
| Shu H.-Y., Huang C.-R. and Chang M.-C. (1994). Decolorization of mono-azo dyes in wastewater by advanced oxidation process: A case study of Acid Red 1 and Acid Yellow 23. Chemosphere vol. 29, no.12, pp. 2597-2607. |
| Shu H.-Y. and Huang C.-R. (1995). Degradation of commercial azo Dyes in water using ozonation and UV enhanced ozonation process. Chemosphere vol. 31, no. 8, pp. 3813-3825. |
| Sontag J. M. (1981). Carcinogenicity of substituted-benzenediamines (phenylenediames) in rats and mice. Journal of National Cancer Institute 66(3), pp. 591-602. |
| Spadaro J. T., Gold M. H. and Renganathan V. (1992). Degradation of azo dyes by the lignin-degrading fungus Phanerochaete chrysosporium. Applied and Environmental Microbiology 58(8), pp. 2397-2401. |
| Specht K. and Platzek T. (1995). Mittel zum Färben und Ausrüsten von Textilien-Anmerkungen zu gesundheitlichen und analytischen Aspekten. Deutsche Lebensmittel-Rundschau 11, pp. 352. |
| Spencer David F. (1984). Oxygen Consumption by the crayfish Orconectes propinquus (Girard) exposed to aquashade. Bull. Environ. Contam. Toxicol. 33, pp. 373-378. |
| SRI (Dec. 1993). Attractiveness of the textile dye business beyond the year 2000. |
| Ullmann: Encyclopaedia of industrial chemistry. 5th edition. VCH Verlagsgesellschaft, Weinheim, Deutschland. |
| Van Hoogen G. and Opperhuizen A. (1988). Toxicokinetics of chlorobenzenes in fish. Environmental Toxicology and Chemistry 7, pp. 213-219. |
| Webber T. G. (1979). Colouring of plastics. John Wiley & Sons. |
| Weber E. J. (1991). Studies of benzidine-based dyes in sediment-water systems. Environmental Toxicology and Chemistry 10, pp. 609-618. |
| Weber E. J. and Adams R. L. (1995). Chemical- and sediment-mediated
reduction of the azo dye disperse blue 79. Environ. Sci. Technol. 29, pp. 1163-1170. |
| Wolf W. d., Bruijn J. J. M. d., Seinen W. and Hermens J. L. M. (1992). Influence of biotransformation on the relationship between bioconcentration factors and octanol-water partition coefficients. Environ. Sci. Technol. 26, pp. 1197-1201. |
| Yen C.-P. C., Perenich T. A. and Baughman G. L. (1990). Fate of dyes in aquatic systems II. Solubility and octanol/water Partition coefficients of disperse dyes. Environmental Toxicology and Chemistry, pp. 981-985. |
| Yen C.-P. C., Perenich T. A. and Baughman G. L. (1991). Fate of commercial disperse dyes in sediments. Environmental Toxicology and Chemistry 10, pp. 1009-1017. |
| Zerbinati O., Vicenti M., Pittavino S. and Gennaro M. C. (1997). Fate of
aromatic sulfonates in fluvial environment. Chemosphere 35(10), pp. 2295-2305. |
| Zhang T. C., Fu Y.-C., Bishop P. L., Kupferle M., FitzGerald S., Jiang H. H. and Harmer C. (1995). Transport and biodegradation of toxic organics in biofilms. Elsevier Science B. V., pp. 267-285. |
| Zhou W. and Zimmermann W. (1993). Decolorization of industrial effluents containing reactive dyes by actionomycetes. Microbiology Letters 107, pp. 157-162. |
| Zissi U. and Lyberatos G. (1996). Azo-dye biodegradation under anoxic conditions. Wat. Sci. Tech. 34(5/6), pp. 495-500. |
Databases
| AQUIRE | Aquatic Toxicity Information Retrieval System, US EPA, Duluth, Montana. CD-ROM (1993). |
| CISDOC | The International Occupational Safety and Health Information Centre of the U. N. Labour Organisation (1998). |
| ECDIN | Environmental Chemicals Data and Information Network, EU. Commission of the European Communities, Environmental Institute Ispra, Italy (1993). |
| HSDB | Hazardous Substance Databases. US National Library of Medicine (1998). |
| HSELINE | Health and Safety Executive Line. Health and Safety Executive (1998). |
| IUCLID | International Uniform Chemical Information Database. European Chemicals Bureau, Ispra, Italy (1996). |
| NIOSHTIC | The National Institute for Occupational Safety and Health, US (1998). |
Appendix 1 Investigated Azo Colorants
See table HERE
Appendix 2 Effect Concentration of Azo Colorants Used in Denmark
See table HERE
Appendix 3 Effect Concentration of the Metabolites
See table HERE
Appendix 4A QSAR
QSAR estimations
In an environmental risk assessment, information of physicochemical properties and ecotoxicity is of basic need. For azo colorants, information of physico-chemical properties and environmental toxicity (ecotoxicity) is very often not exiting or unavailable.
When such data are not available, a possible way to estimate the necessary values is the use of estimation models. These models based on theories of comparable properties between analogous molecular structures are called quantitative structure-activity relationships (denoted QSARs). The models are derived from comparison between experimental values by mathematical variance analysis. The best fitted correlations are then used to develop a mathematical expression to estimate selected end-values of unknown substances.
During the research for the present survey, it was recognised that data on azo dyes necessary for the risk assessment were often unavailable and it was decided to perform estimations based on QSAR methods. The lack of experimental data means that more general QSARs had to be used. It may reduce the accuracy of estimations.
When applying QSAR, it should be taken into account that a QSAR is an estimation method and therefore, there is a certain probability that the estimate is poor even for well evaluated models. QSARs are no better than the data on which they are based. It should be noted that QSAR models, generally, only exist for discrete organic substances and not for more complex substances or reaction mixtures. This should be kept in mind when reading this report. However, this study has found that most literature data were also estimations and the result of experimental studies were so few that it was decided that the use of QSARs was acceptable and necessary for a first estimation. In the survey, QSAR estimations are performed on approximately 140 azo colorants. The estimations are focused on azo colorants used in Denmark.
The methods for deriving QSARs will not be described in this document as other sources exist which review the tremendous amount of literature on the subject (e.g. Lyman et al., 1982; Turner et al., 1987; Karcher & Devillier, 1990; Verhaar et al., 1995; Russom et al., 1997).
The appendix includes a presentation of experimental data, if available and QSAR estimation of:
| Melting point | |
| Boiling point | |
| Water solubility | |
| Vapour pressure | |
| Octanol-water partition coefficient, log Kow | |
| Soil adsorption coefficients, correlated to organic carbon content, Koc | |
| Bioaccumulation factor, log BCF for fish and earthworms | |
| Acute toxicity on fish, Daphnia and algae. |
QSAR and azo dyes
Evaluation of the validity of the latest accepted QSARs is performed by comparing experimental values from handbooks and databases (e.g. NPIRI (1983), HSDB (1993), ECDIN (1993) and the QSAR model estimates where no model input/calculations are changed.
The QSAR estimations are performed by programmes developed by Syracuse Research Corporation: MPBPVP, WSKOW, KOWWIN, HENRY, PCKOCWIN. The programmes are stand-alone programmes but can be run together using the Estimation Programs Interface (EPIWIN) as an interface.
Physico-chemical properties
Melting point
The melting point is an important parameter since it affects the solubility. Solubility controls toxicity by affecting the bioavailability of the substance and the possibility of being transported to the active site within an organism. Melting point tends to increase with molecular size, simply because the molecular surface area available for contact with other molecules increases (Dearden, 1991).
The melting point is estimated by Meylan and Howard (1994) by two different methods. The first is an adaptation of the Joback group contribution method for melting point and the second is a simple Gold and Ogle method suggested by Lyman (1985).
The computer programme MPBPVP by Meylan and Howard (1994) performs minor evaluations. If the values are close to the model averages, the two estimates are averaged, if not, the programme performs and decides which estimate is more likely to be accurate and presents a "suggested" melting point. Although, the suggested MPBPVP estimates are usually adequate for screening purposes, the overall accuracy is not outstanding. The accuracy of the "suggested" value was tested on a 666 compound data set containing a diverse mix of simple, moderately complex compounds and many pesticides and pharmaceutical compounds. MPBPVP estimates yielded a correlation coefficient (r2) of 0.73. However, even if the estimated melting points can only be used for screening purposes, it seems to be the best method currently available (Meylan & Howard, 1994).
With a few exceptions, the estimated values appear to be in agreement with the measured values. However, the origin of the literature values is not always stated and it was often uncertain whether the data were in fact measured or estimated values. Due to the uncertainty of the data origin, no attempt has been made to calculate correlations between the two. The ranges are presented in Table 1. The detailed data on the specific colorants are presented in Appendix 4B.
Table 1
Measured and estimated melting point (MP) ranges for azo colorants.
Målte og estimerede smeltepunkter for azofarver.
| Group | No. |
Measured MP, ºC |
No. |
Estimated MP, ºC |
| Acid azo dyes | 2 |
185, 582 |
18 |
315 - 350 |
| Basic azo dyes | 0 |
5 |
131 - 269 |
|
| Direct azo dyes | 2 |
>300 - 887 |
12 |
350* |
| Disperse azo dyes | 7 |
127 - 195 |
19 |
147 - 331 |
| Mordant azo dyes | 0 |
8 |
200 - 350 |
|
| Reactive azo dyes | 1 |
>180 |
8 |
350* |
| Solvent azo dyes | 2 |
116, 125 |
14 |
77 - 350 |
| Pigments (azo) | 38 |
255 - 380 |
56 |
195 - 350 |
| Total numbers | 82 |
140 |
*: One value indicates that all substances were estimated to have the same value.
The validity of the estimations on azo dyes could not be evaluated due to the lack of experimental data on melting points for azo dyes. For pigments, where the largest amount of literature values were observed, it was uncertain whether the data were experimental or estimated values. Generally, the method seems to be in agreement with the melting point values, when present and the estimated values acceptable.
Boiling point
The boiling point is defined as the temperature at which the vapour pressure of a liquid is equal to the pressure of the atmosphere on the liquid. For pure compounds, the normal boiling point is defined as the boiling point at one standard atmosphere of pressure on the liquid. Besides being an indicator for the physical state (liquid vs. gas) of a chemical, the boiling point also provides an indication of the volatility.
The boiling point is estimated by using the Stein and Brown (1994) method of group contributions that calculates boiling point (BP) of a compound by adding group increment values according to the relationship:
BP = 198.2 + å ni gi
where gi is a group increment value and ni is the number of times, the group occurs in the compound.
The resulting BP is then corrected by one of the following equations:
BP(corr.) = BP - 94.84 + 0.5577 BP - 0.0007705 (BP)2 [BP£ 700oK]
BP(corr.) = BP + 282.7 - 0.5209 BP [BP>700oK]
The Stein and Brown method was derived from a training set of 4,426 organic compounds. Besides the Stein and Brown method, no other estimation method exists that has been validated so extensively or accurately for diverse structures.
Other methods are described in Lyman et al. (1982) but are either not validated or are using a reduced number of chemicals.
A summary of the results of the estimations on azo colorants is presented in Table 2.
Table 2
Estimated boiling point (BP) ranges for azo colorants.
Estimerede kogepunkter for azofarver.
| Group | No. |
Measured BP,ºC |
No. |
Estimated BP, ºC |
| Acid azo dyes | 0 |
18 |
720 - 1,240 |
|
| Basic azo dyes | 0 |
5 |
382 - 628 |
|
| Direct azo dyes | 0 |
12 |
903 - 1,370 |
|
| Disperse azo dyes | 0 |
19 |
399 - 753 |
|
| Mordant azo dyes | 0 |
8 |
474 - 996 |
|
| Reactive azo dyes | 0 |
8 |
973 - 1,309 |
|
| Solvent azo dyes | 0 |
14 |
191 - 964 |
|
| Pigments (azo) | 0 |
56 |
474 - 1,251 |
|
| Total numbers | 0 |
140 |
The validity of the estimations could not be evaluated due to the lack of experimental data on boiling points for azo colorants. No experimental values on boiling points were found.
Solubility in water
The water solubility is one of the most important physico-chemical properties in ecological hazard assessment and exposure assessment, including environmental fate. The spatial and temporal movement (mobility) of a substance within and between the environmental compartments of soil, water and air depends largely on its solubility in water. The knowledge of the solubility in water is essential when estimating biological degradation, bioaccumulation, hydrolysis, adsorption and the octanol/water partition coefficient, log Kow. Most of the azo colorants are substances with low water solubility and therefore potentially slowly distributed by the hydrologic cycle, as they tend to have relatively high adsorption coefficients (i.e. high adsorption to soil and sediment).
As the term "insoluble" is frequently met in handbooks and datasheets on azo colorants, it must be emphasised that no organic chemical is completely insoluble in water. All organic chemicals are soluble to some extent. The range observed in azo dyes are usually between µg/l to g/l. In a few instances, it may be lower and some are infinitely soluble, i.e. totally miscible with water.
Several approaches to estimate water solubility have been developed (Lyman et al., 1982; Yalkowsky & Banerjee, 1992). Yalkowsky and Banerjee (1992) have reviewed most of the recent literature where a variety of estimation methods are available. After critical evaluation of the available methods in terms of range of applicability, accuracy, ease of use and strength of underlying theory, Yalkowsky and Banerjee (1992) concluded that only two methods could be considered for universal application:
| group activity coefficient techniques which include group contribution fragment methods. | |
| correlations based upon log Kow. |
The group activity coefficient method is demonstrated with the group contribution approach of Wakita et al. (1986) including the summation of all applicable fragment values. The fragment values are derived from experiments starting with small molecules and increasing the molecular structure with known atoms or functional groups and calculate the contribution from each change in the molecular structure. The Wakita method was fairly accurate for its training set which primarily consisted of hydrocarbons and simple monofunctional compounds.
At present, the most practical method to estimate water solubility involves regression derived correlations using log Kow. Most of the highly water soluble substances show low log Kow values. Several correlations have been developed depending on the chemicals used in the calculations. Eighteen different regression equations that correlate water solubility to log Kow have been found in the literature (Lyman et al. 1982; Isnard & Lambert, 1989).
Meylan and Howard (1994) have developed a QSAR model on water solubility where the water solubility in mol/l is estimated based on log Kow with and without a melting point. The first equation was developed based on a validation set of 85 substances with an experimental log Kow and water solubility values but with no available melting point. The second validation set included 817 compounds with measured water solubility and melting points. The Meylan and Howard equations are shown below:
| log S (mol/l) = 0.796 - 0.854 log Kow - 0.00728MW + cf |
| log S (mol/l) = 0.693 - 0.96 log Kow - 0.0092(MP-25) - 0.00314MW + cf |
where "MW" is the molecular weight, "MP" is the melting point and "cf" the correction factor. Knowledge of the melting point reduces the standard deviation and improves the correlation coefficient and this model should be used when a measured melting point is available. The melting point is only used for solids. The correction factor is applied to 15 structure types (e.g. alcohols, acids, selected phenols, amines, amino acids, etc). The calculations of the Meyland and Howard QSAR model can be performed on computer (WS-KOW, Syracuse, Meylan & Howard, 1995).
The water solubility was estimated using the equation:
log S (mol/l) = 0.796 - 0.854 log Kow - 0.00728 MW + cf (cf. above)
It was decided to use the equation based on log Kow and not to use the melting point, unless it was clearly stated to be measured.
Table 3
Measured and estimated water solubility (SOLW) ranges for azo colorants.
Målte og estimerede vandopløseligheder for azofarver.
| Group | No. |
Measured SOLW, mg/l |
No. |
Estimated SOLW, mg/l |
| Acid azo dyes | 2 |
>500, 80000 |
18 |
6´ 10-5 - 1´ 106 |
| Basic azo dyes | 0 |
5 |
0.6 - 139.2 |
|
| Direct azo dyes | 2 |
<100, 40000 |
14 |
9.2´ 10-7 - 2.2´ 105 |
| Disperse azo dyes | 11 |
6.3´ 10-4 - 1.2 |
19 |
8.5´ 10-7 - 10.3 |
| Mordant azo dyes | 1 |
>1000 |
8 |
1.7 - 527.1 |
| Reactive azo dyes | 1 |
>100000 |
8 |
22.1 - 98600 |
| Solvent azo dyes | 2 |
1.4, 33.5 |
14 |
5.1´ 10-8 - 20.5 |
| Pigments (azo) | 1 |
<0.004 |
56 |
8.7´ 10-15 - 8.4´ 105 |
| Total numbers | 20 |
142 |
For direct dyes, two measured values were found. For Direct Blue 1, a value of 40,000 mg/l was contradictory to the estimated value 3.5 ´ 10-6 mg/l. However the estimate was based on the acid and not the salt which would increase the water solubility substantially.
The estimated values are mostly close to the measured values when they were available. However, a few exceptions exist which could not be explained.
Vapour pressure
The vapour pressure is a chemical specific property which is important in evaluating the behaviour and fate of an azo colorant in the environment. Especially, the distribution into the environmental compartments; soil, air and water and its persistence in the compartments.
Numerous equations and correlations for estimating vapour pressure are presented in the literature. They normally require information on:
| the critical temperature | |
| the critical pressure | |
| the heat of vaporisation | |
| the vapour pressure at some reference temperature |
The modified Grain method is described in Lyman (1985). The method is a modification of the modified Watson method. It is applicable for solids, liquids and gases. The method converts super-cooled liquid vapour pressure to a solid phase vapour pressure. It is probably the best all-round vapour pressure estimation method currently available (Meylan & Howard, 1994) and is used by the US EPA in the PC-CHEM programme.
The computer estimations made by MPBPVP (Meylan & Howard, 1994) report three methods and a "suggested" value. The suggested vapour pressure for solids is the modified Grain estimate. For liquids and gases, the average of the Antoine and the modified Grain method is suggested. The Mackay method is not used as it is limited to its derivation classes: hydrocarbons and halogenated compounds (both aliphatic and aromatic). Using a data set of 805 compounds, a correlation coefficient (r2) of 0.941 and a standard deviation (sd) of 0.717 were observed.
A summary of the estimated vapour pressures is presented in Table 4.
Table 4
Estimated vapour pressure (VP) ranges for azo colorants.
Målte og estimerede damptryk (VP) for azofarver.
| Group | No. |
Measured VP, Pa |
No. |
Estimated VP, Pa |
| Acid azo dyes | 0 |
18 |
6.8´ 10-33 - 3.6´ 10-15 |
|
| Basic azo dyes | 0 |
5 |
3.4´ 10-12 - 2.6´ 10-4 |
|
| Direct azo dyes | 0 |
12 |
1.4´ 10-45 - 1.5´ 10-24 |
|
| Disperse azo dyes | 3 |
2.7´ 10-11 - 2.7´ 10-8 |
19 |
1.9´ 10-19 - 6.9´ 10-5 |
| Mordant azo dyes | 0 |
8 |
3.5´ 10-22 - 3.5´ 10-8 |
|
| Reactive azo dyes | 0 |
8 |
3.0´ 10-42 - 1.3´ 10-22 |
|
| Solvent azo dyes | 0 |
14 |
4.9´ 10-26 - 1.3´ 10-2 |
|
| Pigments (azo) | 0 |
56 |
2.6´ 10-33 - 3.5´ 10-8 |
|
| Total numbers | 3 |
140 |
The estimated vapour pressures could not be evaluated due to the few experimental results available. However, as expected the estimated vapour pressures were very low.
Henry’s Law constant
The partitioning between water and air is a physical property that is described by the Henry’s Law Constant, H. The magnitude of H provides an indication of which of the two phases a chemical will tend to partition into at equilibrium. Henry’s Law constant can be estimated from calculation and the bond contribution method.
The calculation method uses the equation:
H = vapour pressure ´ molecular weight / water solubility [Pa m3/mol]
QSARs estimations of H based on group and bond contributions are developed from experimentally measured log Kair-water values, when available. The methods of Hine and Mokerjee (1975) have been further developed and are now available in PC programme (HENRY in EPIWIN, Meylan & Howard, 1992, 1994).
Compounds with large structures which include many different types of bonds and groups may have significant inaccuracies in their estimations.
Two methods were applied: The calculated H and the bond estimation method.
A summary of the estimated Henry’s Law Constants is presented in Table 5.
Table 5
The calculated Henry’s Law Constant H and the structure estimated Hbond ranges for azo colorants.
Beregnet Henrys Lov Konstant,struktur estimeret Hbond for azofarver.
| Group | No. |
H calc., Pa m3/mol |
No. |
H bond, Pa m3/mol |
| Acid azo dyes | 18 |
1.0´ 10-28 - 4.0´ 10-16 |
0 |
|
| Basic azo dyes | 5 |
1.5´ 10-10 - 4.8´ 10-4 |
3 |
8.3´ 10-20 - 9.1´ 10-18 |
| Direct azo dyes | 12 |
1.1´ 10-37 - 1.0´ 10-22 |
1 |
3.0´ 10-39 |
| Disperse azo dyes | 19 |
2.1´ 10-14 - 2.0´ 10-3 |
19 |
1.3´ 10-22 - 2.7´ 10-9 |
| Mordant azo dyes | 8 |
5.4´ 10-22 - 5.7´ 10-9 |
3 |
1.4´ 10-14 - 2.0´ 10-11 |
| Reactive azo dyes | 8 |
3.0´ 10-44 - 3.7´ 10-21 |
0 |
|
| Solvent azo dyes | 14 |
6.9´ 10-14 - 2.3 |
13 |
5.7´ 10-12 - 5.5´ 10-5 |
| Pigments (azo) | 56 |
1.4´ 10-22 - 2.3´ 10-4 |
43 |
7.1´ 10-27 - 1.1´ 10-7 |
| Total numbers | 140 |
82 |
It was possible to use the bond contribution method for 82 of the 140 substances. The bond contribution estimation method generally resulted in lower values than the calculation method.
The estimated Henry’s Law Constant using both methods indicated H to be low for all evaluated substances. This indicate that evaporation from surface water is expected to be insignificant or negligible.
Octanol/water partition coefficient (Kow)
Hydrophobicity is one of the key parameters in QSARs for environmental endpoints. The property is usually modelled by the n-octanol/water partition coefficient (Kow) which is an established laboratory method to measure the hydrophobicity of a chemical. Kow has been found to be a good predictor for relatively non-specific processes. For instance, many distribution processes are found to be related to Kow, e.g. sorption to soil and sediment, partitioning into air and bioconcentration, and non-specific toxicity. This especially relates to non-polar organic chemicals. When more polar chemicals and more specific processes such as degradation, biodegradation and specific toxic interactions are the subject, other kinds of interactions (stereo-electronic) become more relevant.
The literature contains several methods for estimating log Kow. The most common method for the estimation of Kow is based on fragment constants. The fragmental approach is based on simple addition of the lipophilicity of the individual molecular fragments of a given molecule, i.e. atoms or larger functional groups. The most widely used fragment constant method was proposed by Hansch and Leo (1979) and initially computerised for use by Chou and Jurs in the CLOGP programme (Daylight Chemical Information Systems, New Orleans). Other methods have been developed but have, at present, not proven to be acceptable as a general estimation method (Meylan & Howard, 1995). Meylan and Howard (1995) have evaluated 10 different methods and concluded that the CLOGP and the AFC methods (cf. below) are the best comprehensive predictors currently available. A major problem with most fragment constant approaches is their inability to estimate log Kow for a structure containing a fragment that has not been correlated.
Meylan and Howard (1995) have developed a new fragment constant approach, the atom/fragment contribution (AFC) method which was developed by multiple linear regressions of reliable experimental log Kow values. The regressions were performed in two stages: The first regression correlated atom/fragment values with log Kow and the second correlated correction factors. The log Kow is then estimated by summing up the values from a structure.
In general, each non-hydrogen atom, e.g. carbon, nitrogen, oxygen, sulphur, in a structure is a core for a fragment, and the exact fragment is determined by what is connected to the atom.
The general equation for estimating log Kow of any organic compound is
log Kow = å (fini) + å (cjnj) + 0.229 (n=2351, r2=0.982, sd=0.216)
where å (fini) is the summation of fi (the coefficient for each atom or fragment) and times ni (the number of times, the atom/fragment occurs in the structure). å (cjnj) is the summation of cj (the coefficient for each correction factor) and times nj (the number of times, the correction factor occurs or is applied in the structure).
The AFC method developed by Meylan and Howard (1995) was applied. A summary of the estimated Log Kow values is presented in Table 6.
Table 6
The measured and estimated log Kow ranges for azo colorants.
Målte og estimerede log Kow for azofarver.
| Group | No. |
Measured log Kow |
No. |
Estimated log Kow |
| Acid azo dyes | 0 |
18 |
-10.5 - 5.9 |
|
| Basic azo dyes | 0 |
5 |
2.1 - 3.9 |
|
| Direct azo dyes | 0 |
14 |
-2.7 - 4.1 |
|
| Disperse azo dyes | 10 |
2.4 - >6 |
19 |
3.6 - 7.0 |
| Mordant azo dyes | 0 |
8 |
0.3 - 4.8 |
|
| Reactive azo dyes | 0 |
8 |
-7.8 - 8.2 |
|
| Solvent azo dyes | 2 |
3.4, 4.6 |
14 |
3.2 - 8.7 |
| Pigments (azo) | 0 |
56 |
-3.1 - 15.8 |
|
| Total numbers | 12 |
142 |
Only a few experimental octanol/water partition coefficients were available. However, when present, they were in good agreement with the estimated values. For instance, for disperse azo dyes, 10 experimental values and their estimated values had a correlation coefficient of 0.894. Two solvent dyes, Solvent Yellow 1 and 2, had experimental values of 3.41 and 4.58 and estimated values of 3.19 and 4.29, respectively.
The estimated octanol/water partition coefficients, log Kow, were in agreement with the experimental values. Therefore, the estimated log Kow values are used in the estimation of bioaccumulation factors and ecotoxicity. The results indicated that the azo dyes and especially the pigments include several compounds with high log Kow values.
Sorption
The sorption (adsorption/desorption) to soil and sediments is a determining factor for the mobility of chemicals. This property also contributes to the distribution among soil, sediment and water phases, volatilisation from soil surfaces and bioavailability. The extent of soil sorption and sediment is governed by a variety of physico-chemical properties of both soil and chemical, e.g. organic carbon content, clay content, humidity, pH value, cation exchange capacity, temperature, etc.
The sorption of non-polar substances may be regarded as a distribution process between the polar phase of the soil water and the organic phase of the soil component. The equilibrium constant of this partitioning between solid and solution phase constitutes the adsorption coefficient for soil and sediments. The sorption coefficient is defined, at a steady state, as:
Kd = Concentration sorbed to soil / Mean concentration in aqueous solution.
As the organic fraction is the principal interaction site for hydrophobic compounds, a partition coefficient normalised for the content of organic carbon (OC) is used to reduce the variance of sorption coefficients:
Koc = (Kd / OC%) ´ 100
The remaining variation may be due to other characteristics of soils (clay content, clay composition, surface area, cation exchange capacity, pH, etc.), the nature of the organic matter present and/or variation in the test methods. Numerous studies of the correlation of adsorption coefficient with these variables found that the organic carbon content usually gave the most significant correlations.
Other factors affect the measured value of Koc under actual environmental conditions besides the differences in laboratory procedures (Lyman, 1990):
| temperature. | |
| pH of soil and water. | |
| particle size distribution and surface area of solids. | |
| concentration of dissolved organic matter in water. | |
| non-equilibrium adsorption mechanisms or failure to reach equilibrium conditions. | |
| solids to solution ratio. | |
| loss of chemical due to volatilisation, degradation, adsorption to test flask walls etc. | |
| non-linear isotherm. | |
| time factor. |
The temperature may affect the measured values since adsorption is an exothermic process. Values of Koc usually decrease with increasing temperature.
Chemicals that tend to ionise are much affected by the pH. Weak acids and weak bases show the greatest sensitivity to pH changes in the range, normally met in soil and surface waters (pH 5 to pH 9).
The fine silt and clay fraction of soil and sediments may have a great tendency to absorb chemicals. The different clay fractions have different adsorptive capacities.
Non-equilibrium adsorption may occur when a chemical moves through an environmental compartment so rapidly that equilibrium conditions cannot be achieved.
Changes in the water content of soil or sediment will change the fraction of the chemical that is adsorbed. As the water content is lowered, the fraction adsorbed will increase as the concentration in solution does.
The chemical may be lost during the test due to volatilisation, degradation, adsorption to test flask walls etc., if this is not considered.
If the adsorption isotherm is non-linear, the reported value of Koc will depend on the range of chemical concentrations used in the tests.
The time for the chemicals to adsorb/desorb varies depending on conditions.
Several compilations of QSAR models for soil sorption are published in the literature. All of the available methods for estimating Koc involve empirical relationships with some other property of the chemical:
| water solubility | |
| octanol/water partition coefficient (Kow) | |
| bioconcentration factor etc. |
Most models are based on Kow because hydrophobic interactions are the dominant type of interactions between non-polar substances and soil organic carbon. However, chemicals with more polar groups may interact by other types of interaction. It is therefore obvious that not one single model accurately predicts soil sorption coefficients and that different models should be used depending on which class of chemicals that the specific compound belongs to.
The EU Technical Guidance Document (TGD, 1996) for risk assessment presents 19 equations to estimate log Koc in soil and sediment for different chemical classes. The 19 QSAR models were developed by Sabljic et al. (1995). The soil sorption data used in Sabljic et al. (1995) were determined for non-ionic species of respective chemicals and thus, the QSAR models presented in Table 7 will be applicable only for non-ionised chemicals:
Table 7
List of derived QSAR models for soil sorption with their chemical domains (Sabljic et al., 1995).
Liste over udledte QSAR modeller til estimering af adsorption med deres kemiske domæner (Sabljic et al., 1995).
| Chemical class | Regression equation | n |
r2 |
SE |
| Predominantly hydrophobics | log Koc = 0.81 log Kow + 0.10 | 81 |
0.89 |
0.45 |
| Nonhydrophobics | log Koc = 0.52 log Kow + 1.02 | 390 |
0.63 |
0.56 |
| Phenols, anilines, benzonitriles, and nitrobenzenes | log Koc = 0.63 log Kow + 0.90 | 54 |
0.75 |
0.40 |
| Acetanilides, carbamates, esters, phenylureas, phosphates, triazines, triazoles, and uracils | log Koc = 0.47 log Kow + 1.09 | 216 |
0.68 |
0.43 |
| Alcohols and organic acids | log Koc = 0.47 log Kow + 0.50 | 36 |
0.72 |
0.39 |
| Acetanilides | log Koc = 0.40 log Kow + 1.12 | 21 |
0.51 |
0.34 |
| Alcohols | log Koc = 0.39 log Kow + 0.50 | 13 |
0.77 |
0.40 |
| Amides | log Koc = 0.33 log Kow + 1.25 | 28 |
0.46 |
0.49 |
| Anilines | log Koc = 0.62 log Kow + 0.85 | 20 |
0.82 |
0.34 |
| Carbamates | log Koc = 0.365 log Kow + 1.14 | 43 |
0.58 |
0.41 |
| Dinitroanilines | log Koc = 0.38 log Kow + 1.92 | 20 |
0.83 |
0.24 |
| Esters | log Koc = 0.49 log Kow + 1.05 | 25 |
0.76 |
0.46 |
| Nitrobenzenes | log Koc = 0.77 log Kow + 0.55 | 10 |
0.70 |
0.58 |
| Organic acids | log Koc = 0.60 log Kow + 0.32 | 23 |
0.75 |
0.34 |
| Phenols and benzonitriles | log Koc = 0.57 log Kow + 1.08 | 24 |
0.75 |
0.37 |
| Phenylureas | log Koc = 0.49 log Kow + 1.05 | 52 |
0.62 |
0.34 |
| Phosphates | log Koc = 0.49 log Kow + 1.17 | 41 |
0.73 |
0.45 |
| Triazines | log Koc = 0.30 log Kow + 1.50 | 16 |
0.32 |
0.38 |
| Triazoles | log Koc = 0.47 log Kow + 1.405 | 15 |
0.66 |
0.48 |
n: Number of substances.
r2: Correlation coefficient.
SE: Standard error.
In Table 7, predominantly hydrophobics were in this context defined as compounds that only contain carbon, hydrogen and halogen atoms (i.e. C, H, F, Cl, Br, I). Nonhydrophobics are all the chemicals which are not defined as predominantly hydrophobic. It means that the definition was based on molecular structure and does not imply anything about lipophi- licity.
Of other methods, the first order molecular connectivity index (1c ) has been used successfully to predict log Koc for hydrophobic organic compounds (Sabljic, 1987). The calculations are performed by PCKOC, a part of the EPIWIN (Meylan & Howard, 1994).
The structure analysis method developed by Meylan and Howard (1995) and two QSARs from TGD were applied: The QSAR for predominantly hydrophobics and the QSAR for non-hydrophobics (cf. above). However, due to some limitations in their domain (cf. below), not all azo colorants could be estimated.
| Model: | Log Kow domain | Chemical domain |
| Hydrophobics | 1 - 7.5 | All chemicals with C, H, F, Cl, Br, and I atoms. |
| Nonhydrophobics | (-2.0) - 8.0 | All chemicals that are not classified as hydrophobics. |
A summary of the estimated log Koc values is presented in Table 8.
Table 8
Estimated log Koc ranges for azo colorants.
Estimerede log Koc for azofarver.
| Group | No. |
EPI |
No. |
QSAR 1 |
No. |
QSAR 2 |
| Acid azo dyes | 14 |
1.9 - 9.2 |
5 |
0.9 - 2.9 |
13 |
0.1 - 4.1 |
| Basic azo dyes | 3 |
4.1 - 5.1 |
5 |
1.8 - 3.2 |
5 |
2.1 - 3.0 |
| Direct azo dyes | 12 |
3.6 - 10.8 |
11 |
1.4 - 3.9 |
14 |
0.7 - 3.5 |
| Disperse azo dyes | 19 |
1.3 - 5.5 |
19 |
3.0 - 5.8 |
19 |
2.9 - 4.7 |
| Mordant azo dyes | 8 |
2.5 - 4.8 |
6 |
1.0 - 4.0 |
8 |
1.2 - 3.5 |
| Reactive azo dyes | 8 |
3.1 - 7.0 |
0 |
2 |
1.1, 1.2 |
|
| Solvent azo dyes | 14 |
2.7 - 8.5 |
9 |
2.7 - 4.7 |
14* |
3.2 - 3.9(5.6) |
| Pigments (azo) | 56 |
1.0 - 10.8 |
27 |
1.5 - 6.3 |
55** |
0.1 - 5.1(9.1) |
| Total numbers | 134 |
82 |
130 |
*: Including 3 values above log Kow 8.
**: Including 17 values estimated to be above log Kow 8. Their maximum value is included in the brackets.
The estimated adsorption coefficients based on structure analysis were generally above the QSAR estimations. In addition azo pigments with a log Kow value above the QSAR domain for nonhydrophobics appeared to be in general agreement with the results of the structure analysis.
Generally, substances with a log Koc below 2.7 may be considered potentially mobile. Except for the solvent dyes, all groups include compounds estimated to be potentially mobile. On the other hand, all groups also include compounds with estimated high adsorption potential.
The estimated adsorption coefficients log Koc indicate that the azo colorants range from compounds that could be classified as potentially mobile and with a low adsorption to immobile substances with a high adsorption. A case by case evaluation is necessary for evaluation of the adsorption.
Bioaccumulation
Bioaccumulation factor for aquatic organisms
The uptake of chemical substances into living organisms occurs mostly by direct adsorption but also along the trophic food web. The internal concentration, e.g. in fish, may increase by accumulation to a level causing toxic effects, even if the internal concentration remains below the critical limit (OECD, 1993b). The accumulated substance may then be passed on to other organisms higher up in the food web which were not directly exposed themselves.
The bioaccumulation in aquatic organisms is defined by the bioconcentration factor (BCF) which is the ratio between the concentration of the chemical in biota and the concentration in water at equilibrium.
Procedures for estimating the bioconcentration potential have been reviewed by e.g. Lyman et al. (1982), Connell (1988), Nendza (1991b), OECD (1993b). Comparison of non-ionic organic chemicals exhibiting substantial bioconcentration revealed several common characteristics. The bioconcentration potential of a chemical was directly related to its lipophilicity and inversely related to its water solubility, molecular charge and degree of ionisation (Lyman et al., 1982; Connell, 1988). In fish, the lipid tissue is the principal site for bioaccumulation and since n-octanol often is a satisfactory surrogate for lipids, linear correlations are usually observed between log BCF and log Kow. Most QSAR models on bioconcentration are based on log Kow. The simplest form of the relationships is based on the partition process of the lipid phase of fish and water:
BCF = a ´ Kow
(where a is the lipid fraction actually ranging from 0.02 to 0.20).
It is generally agreed that a linear relationship exists for chemicals, which are not biotransformed with a log Kow < 6. Veith et al. (1979) developed a linear model based on fathead minnows (Pimephales promelas) valid for log Kow < 6:
log BCF = 0.85 log Kow - 0.70 (n = 55, r2 = 0.90)
In the log Kow range above 6, the measured log BCF data tend to decrease with increasing log Kow.
For azo colorants, where several compounds have log Kow estimated to be above 6, three QSARs are used in the estimation of BCF. Two are recommended in the TGD (1996) and are used related to their domain, i.e. log Kow below or above 6. In addition, a model developed by Anliker et al. (1988) specifically for dyes was included (cf. Table 9).
Table 9
QSARs for estimation of BCF for fish.
QSAR ligninger til estimering af BCF for fisk.
| Model | Equation |
| Linear equation log Kow <6 | log BCF = 0.85 log Kow - 0.70 |
| Parabolic equation log Kow >6 | log BCF = -0.20 log Kow2 + 2.74 log Kow - 4.72 |
| Anliker et al.(1988) |
|
A summary of the estimated log BCF values is presented in Table 10.
Table 10
The measured and estimated log BCF ranges for azo colorants.
Målte og estimerede log BCF værdier for azofarver.
| Group | No. |
Experimental |
No. |
QSAR TGD |
QSAR Anl. |
| Acid azo dyes | 2 |
<-0.5 - 1.9 |
18 |
-9.3 - 4.3 |
-15.6 - -0.2 |
| Basic azo dyes | 0 |
5 |
1.1 - 2.6 |
0.7 - 1.8 |
|
| Direct azo dyes | 0 |
14 |
-3 - 3.3 |
-3.2 - 1.8 |
|
| Disperse azo dyes | 4 |
<-0.5 - 1.6 |
19 |
2.4 - 16.4 |
0.6 - 2.0 |
| Mordant azo dyes | 1 |
0.6 |
8 |
-0.4 - 3.4 |
-0.8 - 2.2 |
| Reactive azo dyes | 1 |
1.0 |
8 |
-7.1 - -0.4 |
-13.1 - -1.7 |
| Solvent azo dyes | 1 |
1.0 |
13 |
2.0 - 22.5 |
0.5 - 2.6 |
| Pigments (azo) | 0 |
56 |
-3.3 - 48.6 |
-4.8 - 2.5 |
|
| Total numbers | 9 |
141 |
QSAR TGD: Model equations from TGD.
QSAR Anl.: Model equations from Anliker et al. (1988).
The Anliker estimated log BCF values are significantly below the estimated log BCF values made by the other QSARs. None of the used azo colorants have a log BCF above 3 according to the Anliker model. Unfortunately, the test substances used to develop the QSAR could not be identified as any of the substances in this study. The main part, 23 out of 25 dyes, was disperse dyes and the remaining two pigments. The number of dyestuffs that were azo compounds was 20. The test method used to find BCF was a method specified by the Japanese authorities. No details on method are presented.
More detailed information on the test method and data for other azo groups should be included before a final evaluation of the Anliker model relative to the TGD models can be performed.
Aquatic toxicity
QSAR models on aquatic ecotoxicity
Within the aquatic ecotoxicology, QSAR models have been used to estimate biological effects of various chemical substances, and frequently, the octanol-water coefficient (log Kow) of a substance has been used to estimate the ecotoxicity potential of the substance to organisms.
Most of the literature on developing QSARs for toxicity estimations has assumed that compounds from the same chemical class should behave in a similar toxicological manner.
Base-line toxicity or the "minimum toxicity" is related to the hydrophobicity of the substance and is also referred to as non-polar narcosis. In absence of specific toxic mechanisms, the internal effect concentration is almost constant and a substance will then be as toxic as predicted by its hydrophobicity due to the relation with bioconcentration (McCarthy & MacKay, 1993). Indications of non-polar narcosis are the change of EC50 over time. A ratio EC50 (24 hours)/EC50 (96 hours) of approximately 1.0 is considered indicative of non-polar narcosis. Excess toxicity values, calculated by dividing predicted narcosis Type I EC50 values by the observed values, greater than 10 indicate that the substance does not act by non-polar narcosis (Russom et al., 1997).
The class consists of more polar chemicals such as phenols, esters and anilines. The mode of action of these substances is not very specific, but they are significantly more toxic than predicted by non-polar narcosis.
QSARs for acute and long term effects on fish, daphnia and algae are present for chemicals that act by non-specific mode of action (non-polar narcosis as well as polar narcosis).
The latest evaluation of current models in ecotoxicity resulted in the QSAR models mentioned in Table 11 and Table 12 (Verhaar et al., 1992, 1995).
Table 11
QSARs for non-polar narcosis.
QSAR for ikke-polær narkotisk virkende stoffer.
| Species | Regression equation | Statistics |
|||
| (mol/l) | n |
r2 |
SE |
||
| Fish Pimephales promelas |
log LC50 (96h) = -0.85 log Kow-1.39 | 58 |
0.94 |
0.36 |
|
| Daphnia: Daphnia magna |
log EC50 (48h) = -0.95 log Kow - 1.32 | 49 |
0.95 |
0.34 |
|
| Algae: Selenastrum capricornutum |
log EC50 (72-96h) = -1.0 log Kow - 1.23 | 10 |
0.93 |
0.17 |
|
Ref.: Verhaar et al. (1995).
TGD (1996).
The models were generated by linear regression analysis. The experimental data were generated according to the OECD test guidelines or comparable methods.
QSAR models for chemicals which act by polar narcosis (esters, phenols and anilines) are also available. The mode of action of these compounds is also not very specific, but they are significantly more toxic than predicted by non-polar narcosis.
Table 12
QSARs for polar narcosis (Verhaar et al., 1995; TG, 1996).
QSARs for polær narkotisk virkende stoffer (Verhaar et al., 1995; TGD, 1996).
| Species | Regression equation | Statistics |
||||
| (mol/l) | n |
r2 |
SE |
|||
| Fish Pimephales promelas |
log LC50 (96h) = -0.73 log Kow - 2.16 | 86 |
0.90 |
0.33 |
||
| Daphnia: Daphnia magna |
log EC50 (48h) = -0.56 log Kow - 2.79 | 37 |
0.73 |
0.37 |
||
The models were generated by linear regression analysis. The experimental data were generated according to the OECD test guidelines or comparable methods.
For classification purposes, the Danish Environmental Protection Agency has developed a model to estimate the minimum acute aquatic toxicity: QTOXMIN (Pedersen et al., 1995; Pedersen & Falck, 1997).
The QSAR equations recommended for classification are the same or almost the same as the equations recommended in TGD (1996) which will be used in the estimations of aquatic toxicity of azo colorants.
More recent developments in QSARs for ecotoxicity have been performed by Jay Niemelä in the Danish EPA (Niemelä, pers. comm., 1998). The results are not yet published, but Dr. Niemelä has kindly performed the calculations on fish for this project, and his results are presented in the appendix and the summary table below (Table 13). The Niemelä equations are interesting, because they are bilinear and thus overcome the problems of the wide range of log Kow. From studying the individual results, it was observed that the results from bilinear QSARs gave comparatively better results, and they are therefore considered to be most representative for the estimations of ecotoxicity to fish.
A summary of the estimated acute LC50-values for fish is presented in Table 13. Both results from non-polar and polar QSARs have been presented in the summary. Generally, the results from non-polar and polar QSARs are close or at least in the same order of magnitude for azo colorants. For detailed results refer to Appendix 4B.
Table 13 Fish toxicity.
The measured and estimated EC50 ranges (mg/l) for azo colorants on fish.
Målte og estimerede EC50 værdier for azofarver.
| Group | No. |
Measured EC50 mg/l |
No. |
Estimated EC50, non-polar |
Estimated EC50, polar |
No. |
Ecosar |
| Acid azo dyes | 7 |
4 - >1000 |
18 |
35 - 9´ 1012 |
22 - 9´ 1010 |
14 |
0.1 - 3´ 104 |
| Basic azo dyes | 0 |
5 |
8 - 169 |
4 - 48 |
2 |
0.8, 25 |
|
| Direct azo dyes | 6 |
6 - >180 |
14 |
3 - 6´ 106 |
2 - 6´ 105 |
3 |
0.9 - 163 |
| Disperse azo dyes | 5 |
>50 - >500 |
19 |
0.5 - 10.2 |
0.5 - 4.9 |
6 |
0.8 - 6.9 |
| Mordant azo dyes | 1 |
32 |
8 |
1 - 8600 |
0.7 - 1590 |
0 |
|
| Reactive azo dyes | 1 |
>500 |
8 |
2´ 104 - 1´ 1011 |
2000 - 2´ 109 |
0 |
|
| Solvent azo dyes | 3 |
0.7 - >100 |
14 |
0.4 - 66 |
0.2 - 194 |
8 |
0.004 - 7.8 |
| Pigments (azo) | 4 |
18 - 420 |
56 |
0.3 - 1´ 1010 |
0.2 - 2´ 1011 |
41 |
1´ 10-5 - 1´ 105 |
| Total numbers | 27 |
142 |
74 |
It should be noted that values above the water solubility indicate low acute toxicity.
Only a few experimental values were available for fish, and the results are mostly in accordance with the estimated. However, exceptions occur, e.g. in the disperse dyes where the estimated acute toxicities to fish are two orders of magnitude lower than the experimental values. Since the experimental data have not been studied, no explanation can be given.
For most of the substances the estimated acute toxicity was above the water solubility and may thus be considered of low acute toxicity to fish.
Many questions were raised by the estimation results and indicate that further investigation was necessary. However, a detailed discussion on the individual results was considered to be outside the scope of this survey.
For Daphnia, the QSARs of the TGD were used. Both non-polar and polar estimations were used and a summary of the acute 48-hour toxicities is presented in Table 14.
Table 14 Acute toxicity to Daphnia.
The measured and estimated EC50 ranges (mg/l) for azo colorants on Daphnia.
Målte og estimerede variationsbredder for azofarvers akutte toksicitet (EC50, mg/l) for dafnier.
| Group | No. |
Measured EC50 mg/l |
No. |
Estimated EC50, non-polar |
Estimated EC50, polar |
No. |
Ecosar |
| Acid azo dyes | 0 |
18 |
0.1 - 2´ 109 |
0.6 - 4´ 108 |
13 |
0.2 - 3´ 104 |
|
| Basic azo dyes | 0 |
5 |
4 - 118 |
4 - 30 |
2 |
1.1, 1.2 |
|
| Direct azo dyes | 0 |
14 |
1.3 - 2´ 107 |
3 - 6´ 104 |
3 |
0.6 - 50 |
|
| Disperse azo dyes | 0 |
19 |
0.01 - 4.5 |
0.1 - 5.0 |
6 |
1.0 - 7.7 |
|
| Mordant azo dyes | 0 |
8 |
0.4 - 9462 |
1.0 - 420 |
0 |
||
| Reactive azo dyes | 1 |
>800 |
8 |
1´ 104 - 6´ 1011 |
594 - 3´ 107 |
0 |
|
| Solvent azo dyes | 0 |
14 |
9´ 10-5 - 8.8 |
0.01 - 5.2 |
8 |
0.04 - 1.4 |
|
| Pigments (azo) | 0 |
56 |
4´ 10-11 - 1´ 106 |
4´ 10-6 - 8,100 |
40 |
2´ 10-4 - 1´ 105 |
|
| Total numbers | 1 |
142 |
72 |
It should be noted that values above the water solubility indicate low acute toxicity.
Only one experimental value was available for Daphnia, and the result was in accordance with the estimated value.
For most of the substances, the estimated acute toxicity was above the water solubility and may thus be considered of low acute toxicity to Daphnia.
Many questions were raised by the estimation results and indicate that further investigation was necessary. However, a detailed discussion on the individual results was considered to be outside the scope of this survey.
For algae, the QSAR of the TGD was used. A summary of the acute 72-hour toxicities is presented in Table 15.
Table 15 Acute effects on algae.
The measured and estimated EC50 ranges (mg/l) for azo colorants on algae.
Målte og estimerede variationsbredder for azofarvers akutte effekt (EC50, mg/l) for alger.
| Group | No. |
Measured EC50 mg/l |
No. |
Estimated EC50, non-polar |
No. |
Ecosar |
| Acid azo dyes | 1 |
1´ 104 |
18 |
0.1 - 5´ 1014 |
5 |
0.2 - 2´ 104 |
| Basic azo dyes | 0 |
5 |
3 - 112 |
1 |
0.97 |
|
| Direct azo dyes | 0 |
14 |
0.9 - 3´ 107 |
0 |
||
| Disperse azo dyes | 0 |
19 |
0.004 - 4.0 |
5 |
0.6 - 2.8 |
|
| Mordant azo dyes | 0 |
8 |
0.3 - 1´ 104 |
0 |
||
| Reactive azo dyes | 1 |
2.3 |
8 |
1´ 104 - 3´ 1012 |
0 |
|
| Solvent azo dyes | 0 |
14 |
4´ 10-5 - 7.5 |
2 |
0.2, 1.1 |
|
| Pigments (azo) | 0 |
56 |
1´ 10-11 - 3´ 107 |
18 |
0.001 - 7´ 104 |
|
| Total numbers | 2 |
142 |
31 |
It should be noted that values above the water solubility indicate low acute toxicity.
Only two experimental values were available for algae, and the results were not in accordance with the estimated values. Since the experimental data have not been studied, no explanation can be given.
For most of the substances, the estimated acute toxicity was above the water solubility and may thus be considered of low acute toxicity to algae.
Many questions were raised by the estimation results and indicate that further investigation was necessary. However, a detailed discussion on the individual results was considered to be outside the scope of this survey.
Appendix 4B QSAR
QSAR derived physico-chemical properties and effect concentrations
Colour index name and number, CAS number, molecular weight, measured and QSAR estimated values for 143 azo colorants.
CI: Colour Index
MW: Molecular Weight
MP: Melting Point
BP: Boiling Point
SOLW: Water Solubility
* Non-salt
See table HERE
Appendix 5 Molecular Structure
Molecular structure of selected azo dyes
| Colour index name and number, CAS number, molecular structure and chemical name of selected azo dyes. |
See table HERE
Appendix 6 Molecular Structure
Molecular structure of selected azo pigments
| Colour index name and number, CAS number, molecular structure and chemical name of selected azo pigments. |
See table HERE
|
[Front page] [Top] |