[Front page] [Contents] [Previous] [Next] |
Ecotoxocicological assessment of antifouling biocides and non-biocidal antifouling paints
4. Zinc pyrithione
4.1 Physico-chemical properties
4.2 Abiotic degradation
4.3 Biodegradation of zinc pyrithione in the aquatic environment
4.3.1 Mineralization and metabolites in aerobic sediment
4.3.2 Mineralization and metabolites in anoxic sediment
4.4 Toxicity to aquatic organisms
4.5 Assessment of zinc pyrithione and metabolites
4.6 Risk assessment of zinc pyrithione
4.1 Physico-chemical properties
Table 4.1 gives an overview of the physico-chemical properties of zinc pyrithione.
Table 4.1
Physico-chemical properties of zinc pyrithione (Olin 1997).
| CAS No. | 13463-41-7 |
| Synonyms | Bis(1-hydroxy-2[1H]- pyridinethionato-O-S)-(T4)zinc, Zinc Omadine |
| Classification (two products) | T, R22, R23, R41, R38 + Xn, R20/22, R36/38 |
| Molecular formula | C10H8N2O2S2Zn |
| Molar weight | 317.68 |
| Water solubility | 6.0 mg/L |
| Vapour pressure (25°C) | Not volatile, solid substance |
| Octanol/water partition coefficient (log Kow) |
0.97 |
| Organic matter/water partition coefficient (log Koc) | 2.9-4.0 |
4.2 Abiotic degradation
Photolysis
Zinc pyrithione is very rapidly transformed by photolysis. Experiments conducted under
sterile conditions with a light:dark cycle of 12:12 hours have shown that, under exposure
to light, the concentration of [pyridine-2,6-14C]zinc pyrithione in pH 9 buffer
was reduced to 33% of the radioactivity added in 15 min. Data from this study also
demonstrated that less than 5% of the 14C added occurred as zinc pyrithione
after 1 hour of exposure to light. Similar results have been achieved when photolysis of
zinc pyrithione was investigated by use of artificial seawater. In this study, the parent
compound constituted 45% of the radioactivity added after 15 min while, after 24 hours,
1.3% of the added dose occurred as zinc pyrithione. The estimated half-lives of the
photolytic transformation of zinc pyrithione was 13 min in pH 9 buffer and 17.5 min in
artificial seawater (Reynolds 1995a).
Hydrolysis
Hydrolysis of [pyridine-2,6-14C]zinc pyrithione has been investigated in
aqueous solution at pH 5, 7 and 9 and in artificial seawater at pH 8.2. In general, zinc
pyrithione was hydrolysis-stable at all of the pH values investigated (Reynolds 1995b).
4.3 Biodegradation of zinc pyrithione in the aquatic environment
Transformation of
zinc pyrithione
Experiments with freshwater and marine sediments have shown that the transformation of
zinc pyrithione in aquatic systems proceeds with an initial rapid rate followed by a
slower rate. This is the result of the distribution between the water phase and the
sediment, in which the degradation proceeds with different rates in the two
sub-environments. The resulting two-phased transformation is observed in fresh water as
well as in seawater and under both aerobic and anaerobic conditions (Ritter 1996,
1999a-e). The half-lives of removal of zinc pyrithione from the water phase via
degradation and sorption to the sediment were between 0.5 and 0.6 hours. For the aerobic
as well as the anaerobic systems, this removal resulted in <5% of the added dose
remaining in the water phase after 6 hours. In the following second phase, the removal of
sorbed pyrithione proceeded with a half-life of 4 days under aerobic conditions and 19
hours under anaerobic conditions, respectively (Ritter 1999a-e).
Zinc pyrithione reacts by transchelation in the presence of metals transforming zinc pyrithione into copper(II) pyrithione and other more stable metal-pyrithione complexes. The slower secondary transformation rate in studies performed at a low concentration of zinc pyrithione (0.05 µg/g) is probably due to the sorption of the metal-pyrithione complexes to the sediment (Ritter 1999a-e). In previous studies, in which a higher concentration of zinc pyrithione (3 µg/g) was used, the secondary transformation rate may be the result of the lower water solubility of copper(II)pyrithione being limiting to the transformation rate (Ritter 1996; Smalley and Reynolds 1996).
Zinc pyrithione is transformed to heterocyclic metabolites with one ring like omadine sulfonic acid and pyridine sulfonic acid. More other metabolites identified by Arch Chemicals are known to VKI but are given as NP1-NP5 in this project.
4.3.1 Mineralization and metabolites in aerobic sediment
The aerobic biodegradability of zinc pyrithione (3 µg/g) was investigated by use of water and sediments collected in freshwater and marine harbours in which maintenance of boats is carried out (Ritter 1996). Later investigations with seawater and sediment were made with both zinc pyrithione and copper pyrithione, which were added at a lower concentration of 0.05 µg/g (Ritter 1999a, b, d). In these studies, the degradation proceeded at the same rate and resulted in the same metabolites whether the pyrithione was added as the zinc or the copper complex. The greatest importance is attached to the results of the most recent experiments as the lower concentration of the parent compound results in more realistic mechanisms of sorption and degradation.
Mineralization and
metabolites
After 84 days of incubation at 25°C, the mineralization of [pyridine-2,6-14C]zinc
pyrithione (0.05 µg/g) to 14CO2 in seawater and sediment
constituted 0.44% of the 14C added (Ritter 1999a, b, d). A correspondingly low
mineralization was observed in the previous studies, in which zinc pyrithione was added at
a concentration of 3 µg/g (Ritter 1996). In the fresh water and sediment, the
mineralization of zinc pyrithione was higher as 12% of the 14C added was
transformed to 14CO2 after 30 days at 25°C (Ritter 1996).
The first stage of the aerobic degradation of zinc pyrithione is the formation of its disulfide, which is identified as omadine disulfide. In studies performed with zinc pyrithione at the concentration of 3 µg/g (Ritter 1996), omadine disulfide was formed as one of the most important metabolites. Omadine disulfide has almost the same chemical structure as zinc pyrithione and has been shown to be very toxic to aquatic organisms (Table 4.7). The presence of omadine disulfide was only transient as the further transformation of this metabolite caused omadine disulfide to constitute 2.8% of the radioactivity added after 30 days in the experiment with seawater and sediment (in the experiment with fresh water and sediment, the concentration of omadine disulfide was below the detection limit of 0.3 ng/g after 30 days). The demonstration of omadine disulfide in the studies, in which zinc pyrithione was added in 3 µg/g, is probably due to the kinetics of desorption and degradation at the concentration used, which is considered environmentally unrealistic. In the more recent experiments, in which the level of concentration was 0.05 µg/g (Ritter 1999a, b, d), omadine disulfide was not detected and omadine disulfide must thus be considered a transient metabolite in the biological transformation of zinc pyrithione into heterocyclic compounds with one ring. On the basis of the experiments made at a concentration of 0.05 µg/g, the most important metabolites from the aerobic degradation of zinc pyrithione are considered to be omadine sulfonic acid and pyridine sulfonic acid and two other metabolites called NP1 and NP2 (Table 4.2). NP2 was only demonstrated by extraction of the sediment with alkali. It is, however, not clear yet whether this metabolite was formed in the sediment before extraction or by a chemical reaction in the alkaline extract. Data from investigations of the transformation of copper pyrithione in anaerobic aquatic systems suggest that, most likely, NP2 was present in the sediment before the extraction (Ritter 1999a-e).
Table 4.2
Aerobic biodegradation of [14C]zinc pyrithione into metabolites and carbon
dioxide in seawater and sediment
(data from Ritter, 1999a, b, d).
Time (days) |
% of added dose |
||||||
Zinc |
NP1 |
Omadine sulfonic acid |
Pyridine |
NP2 |
Non- |
CO2 |
|
0 |
49.1 |
16.2 |
- |
- |
20.5 |
6.2 |
- |
1 |
9.8 |
25.8 |
- |
- |
40.7 |
14.2 |
0.01 |
3 |
11.7 |
36.0 |
- |
- |
22.0 |
22.2 |
0.02 |
7 |
1.5 |
41.5 |
4.2 |
4.5 |
17.3 |
24.8 |
0.05 |
14 |
1.6 |
26.4 |
17.2 |
6.8 |
13.5 |
28.5 |
0.08 |
21 |
3.0 |
5.6 |
33.7 |
10.2 |
10.5 |
31.8 |
0.16 |
30 |
1.1 |
7.2 |
35.3 |
11.8 |
8.2 |
27.8 |
0.23 |
42 |
1.8 |
8.0 |
28.6 |
11.9 |
8.0 |
34.3 |
0.33 |
63 |
1.2 |
- |
28.9 |
17.4 |
7.9 |
29.7 |
0.39 |
84 |
2.0 |
1.7 |
31.7 |
24.7 |
4.4 |
30.4 |
0.44 |
| -, | not detected |
A considerable part of the metabolites sorbed to the sediment and resisted extraction with acetonitrile followed by two extractions with 0,1 N KOH. The percentage of these non-extractable 14C labelled metabolites increased during the first fortnight and, in the period from day 14 to the end of the experiment after 84 days, it constituted approx. 30% of the 14C added. The total recovery of the radioactivity added varied between 93 and 99% (Ritter 1999a, b, d).
Studies with Danish
sediments
The aerobic biodegradability of zinc pyrithione (0.0037 µg/g) was examined in sediment
and seawater from the same two locations in the Sound as in the study of DCOI, including a
clayey and a sandy sediment (cf. Section 3.2.2 and Appendix 2). After 42 days’
incubation at 15°C, the mineralization of [pyridine-2,6-14C]zinc
pyrithione into 14CO2 constituted 2.8% of the 14C added
in the clayey sediment and 5.0% in the sandy sediment (Figures 4.1-4.2). The examination
of the distribution of 14C at the termination of the tests after 42 days showed
that metabolites from the transformation of zinc pyrithione were primarily water-soluble
compounds. In the test system with the sandy sediment, 65% of the 14C added was
recovered in the water phase of the test system while 22% was bound to hydrolizable
compounds and fulvic acids in the sediment. In the test with the clayey sediment, 32% of
the 14C added was recovered in the water phase while the sediment contained 38%
in the form of hydrolizable compounds and fulvic acids. Compared with this, metabolites
bound to humic acids, humin and clay minerals constituted a minor part of between 3.6%
(sandy sediment) and 16% (clayey sediment) of the radioactivity added. The results from
the chemical analyses (Table 4.2) show that the 14C remaining at the
termination of the test was metabolites and not intact zinc pyrithione. The metabolites
are considered to have a high bioavailability as the radioactivity occurred especially in
the form of water-soluble compounds. Methods and results are described in detail in
Appendix 2.
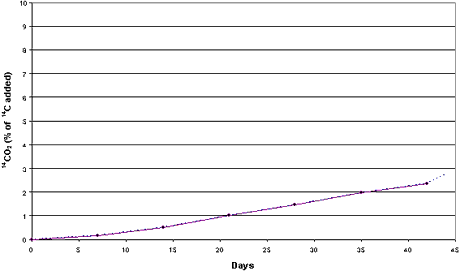
Figure 4.1
Mineralization of [14C] zinc pyrithione (0.037 mg/g)
in clayey sediment and seawater from the Sound (sediment LS) Aerobic conditions. Dotted
curve represents 14CO2 released by acidification.
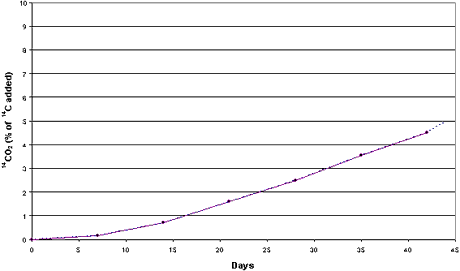
Figure 4.2
Mineralization of [14C] zinc pyrithione (0.037 mg/g)
in sandy sediment and seawater from the Sound (sediment SS) Aerobic conditions. Dotted
curve represents 14CO2 released by acidification.
Water and sediment samples from the tests were taken at the start of the tests and after 28 days. Chemical analyses of zinc pyrithione and metabolites were made by Arch Chemicals (Cheshire, Connecticut). These analyses showed that zinc pyrithione was mainly transformed into omadine sulfonic acid, pyridine sulfonic acid and NP1 in both sediments (Table 4.3).
Table 4.3
Aerobic biodegradation of [14C]zinc pyrithione into metabolites and carbon
dioxide in seawater and sediment from the Sound.
| Sample | Zinc |
NP1 |
Omadine |
NP2 |
NP3 |
NP5 |
Non- |
CO2 |
% of 14C added |
||||||||
| Day 0 | ||||||||
| LS, water 1 | 2.3 |
4.5 |
0.5 |
- |
- |
- |
- |
- |
| LS, sediment 1 | 34.6 |
6.2 |
3.6 |
17.0 |
4.6 |
2.6 |
13.3 |
|
| LS, total 1 | 36.9 |
10.7 |
4.1 |
17.0 |
4.6 |
2.6 |
13.3 |
|
| Day 28 | ||||||||
| LS, water 2 | - |
4.4 |
41.0 |
- |
- |
- |
- |
1.5 |
| LS, sediment 2 | 0.6 |
2.8 |
5.3 |
3.9 |
3.0 |
2.9 |
30.3 |
|
| LS, total 2 | 0.6 |
7.2 |
46.3 |
3.9 |
3.0 |
2.9 |
30.3 |
|
| Day 0 | ||||||||
| SS, water 1 | 19.5 |
22.7 |
- |
1.9 |
- |
3.8 |
- |
- |
| SS, sediment 1 | 11.1 |
4.1 |
1.2 |
3.4 |
1.9 |
1.1 |
11.7 |
|
| SS, total 1 | 30.6 |
26.8 |
1.2 |
5.3 |
1.9 |
4.9 |
11.7 |
|
| Day 28 | ||||||||
| SS, water 2 | - |
24.5 |
26.3 |
1.2 |
- |
- |
- |
2.5 |
| SS, sediment 2 | 4.1 |
0.7 |
4.3 |
1.5 |
- |
0.3 |
19.4 |
|
| SS, total 2 | 4.1 |
25.2 |
30.6 |
2.7 |
- |
0.3 |
19.4 |
|
| LS | clayey sediment |
| SS | sandy sediment |
| * | contained also NP4 |
| 1 | tests performed by Arch Chemicals |
| 2 | tests performed by VKI |
| - | not detected |
4.3.2 Mineralization and metabolites in anoxic sediment
The anaerobic biodegradability of zinc pyrithione (3 µg/g) was investigated by use of water and sediments collected in the same freshwater and marine localities as in the aerobic experiments (Ritter 1996). Later investigations with seawater and sediment were made with both copper pyrithione and zinc pyrithione, which were added at a concentration of 0.05 µg/g (Ritter 1999a, c, e). In the assessment of the fate of zinc pyrithione under anaerobic conditions, the greatest importance is attached to the most recent results from experiments carried out at the concentration of 0.05 µg/g (Ritter 1999a, b, d).
Mineralization and
metabolites
As was the case with the results from the aerobic biodegradation studies, the
mineralization of [pyridine-2,6-14C]zinc pyrithione to carbon dioxide was
negligible in anoxic marine sediment. After 182 days of incubation at 25°C, the formation
of 14CO2 constituted 0.9% of the 14C added (Ritter 1999a,
b, d).
In the previous studies, in which zinc pyrithione was added at a concentration of 3 µg/g, omadine disulfide was formed as a transient metabolite while an unsymmetrical disulfide of NP3 and 2-mercaptopyridine N-oxide was present throughout the entire test period of 91 days (Smalley and Reynolds 1996). The formation of these metabolites with two rings in considerable amounts (>10% of the radioactivity added) is probably the result of the kinetics of sorption and degradation at the concentration used. In the recent studies, in which the concentration of zinc pyrithione was 0.05 µg/g, neither omadine disulfide nor the unsymmetrical disulfide was detected (Ritter 1999a, b, d). The most important metabolite from the anaerobic transformation of zinc pyrithione added at a concentration of 0.05 µg/g was NP3 while lower concentrations of three other heterocyclic compounds with one ring (pyridine sulfonic acid, NP4 and NP5) were formed as a result of the further transformation of NP3 (Table 4.4). Small amounts of NP1 were formed immediately after the start of the test (<1% of the 14C added; day 3) but this metabolite was transformed into other compounds and could not be detected after 14 days (Ritter 1999a, b, d).
Table 4.4
Anaerobic biodegradation of zinc pyrithione into metabolites and carbon dioxide in
seawater and sediment (data from Ritter 1999a, b, d).
Time (days) |
% of added dose |
||||||
Zinc pyrithione |
NP3 |
NP4 |
Pyridine sulfonic acid |
NP5 |
Non- |
CO2 |
|
0 |
30.1 |
21.2 |
2.1 |
1.0 |
5.2 |
7.4 |
- |
1 |
4.7 |
62.0 |
3.5 |
- |
6.8 |
5.8 |
- |
3 |
0.3 |
74.5 |
3.0 |
0.1 |
3.1 |
7.9 |
- |
7 |
0.1 |
78.1 |
1.5 |
0.8 |
2.7 |
9.1 |
- |
14 |
- |
54.5 |
5.9 |
3.3 |
8.2 |
14.8 |
- |
22 |
- |
38.0 |
8.8 |
1.4 |
5.4 |
18.2 |
0.1 |
30 |
- |
32.9 |
7.4 |
2.0 |
4.7 |
19.2 |
0.3 |
63 |
- |
14.3 |
8.5 |
2.2 |
5.5 |
29.4 |
0.6 |
90 |
1.8 |
13.7 |
3.4 |
2.2 |
4.0 |
34.3 |
0.3 |
182 |
- |
2.3 |
7.9 |
4.3 |
1.2 |
52.7 |
0.9 |
-, not detected
A considerable part of the metabolites sorbed to the sediment and resisted the extraction with acetonitrile and alkali. The concentration of non-extractable metabolites sorbed to sediment gradually increased throughout the test and constituted 53% of the 14C added after 182 days. The total recovery of the radioactivity added varied between 90 and 102% (Ritter 1999a, b, d).
Studies with Danish
sediments
The anaerobic biodegradation of zinc pyrithione (0.037 mg/g)
was examined by use of the clayey sediment and its seawater (Appendix 2), which was also
used in the aerobic tests (cf. Section 4.3.1). Sediment and seawater were incubated under
anaerobic sulfate-reducing conditions, which are normally prevalent in coastal marine
sediments. The mineralization of [pyridine-2,6-14C]zinc pyrithione into 14CO2
constituted 3.5% of the 14C added after 56 days’ incubation at 15°C (Figure 4.3). Compared with the tests performed under aerobic
conditions, a larger part of the metabolites formed under anaerobic conditions was bound
to humic acids, humin and clay minerals in the sediment. This part constituted 39% of the 14C
added after 56 days’ incubation of the clayey sediment and its seawater. More
water-soluble metabolites in the water phase of the system or bound to hydrolizable
compounds and fulvic acids constituted, however, a considerable part of 35% in total of
the radioactivity added. As in the aerobic tests, zinc pyrithione was transformed into
metabolites (Table 4.5), of which several are considered to have a high bioavailability.
Methods and results are described in detail in Appendix 2.
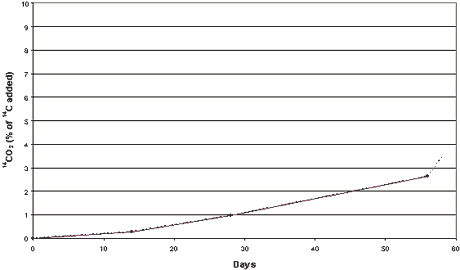
Figure 4.3
Mineralization of [14C] zinc pyrithione (0.037 mg/g)
in clayey sediment and seawater from the Sound (sediment SS) Anaerobic conditions. Dotted
curve represents 14CO2 released by acidification.
Water and sediment samples from the tests were taken at the start of the tests and after 28 days. Chemical analyses of zinc pyrithione and metabolites in these samples were made by Arch Chemicals (Cheshire, Connecticut). The results from the analyses performed showed that the quantitatively most essential metabolites under anaerobic sulfate-reducing conditions were the heterocyclic compounds with one ring, i.e. NP3 and NP5 (Table 4.5).
Table 4.5
Anaerobic biodegradation of [14C]zinc pyrithione into metabolites and carbon
dioxide in seawater and sediment from the Sound.
Time (days) |
Sample |
Zinc |
NP3 |
Omadine |
NP1 |
NP2 |
NP5 |
Non- |
CO2 |
% of 14C added |
|||||||||
0 |
LS, water 1 | 15.1 |
13.7 |
0.8 |
0.5 |
- |
- |
- |
- |
0 |
LS, sediment 1 | 19.0 |
18.9 |
6.3 |
3.3 |
1.1 |
2.7 |
11.0 |
|
0 |
LS, total 1 | 34.1 |
32.6 |
7.1 |
3.8 |
1.1 |
2.7 |
11.0 |
|
28 |
LS, water 2 | - |
0.7 |
10.2 |
- |
- |
21.2 |
- |
2.6 |
28 |
LS, sediment 2 | - |
21.2 |
3.2 |
- |
- |
1.3 |
25.7 |
|
28 |
LS, total 2 | - |
21.9 |
13.4 |
- |
- |
22.5 |
25.7 |
|
| LS | clayey sediment |
| * | contained also NP4 |
| 1 | tests performed by Arch Chemicals |
| 2 | tests performed by VKI |
| - | not detected |
4.4 Toxicity to aquatic organisms
Zinc pyrithione
The toxicity of the active substance zinc pyrithione has been investigated in standard
laboratory tests with a number of aquatic organisms living in fresh water (the green alga Selenastrum
capricornutum, the crustacean Daphnia magna, the fish rainbow trout (Oncorhynchus
mykiss) and fathead minnow (Pimephales promelas)) and in seawater
(the crustacean Mysidopsis bahia, the fish sheepshead minnow (Cyprinodon
variegatus) and the oyster (Crassostrea virginica)) (Boeri et al.
1993; 1994a-e; Ward et al. 1994a). Furthermore, five species of freshwater fish
were used at the same time in a single test (Olin 1997). Pimephales promelas
was also used in this test and the results for this species agreed with the results in the
standard test. Besides, P. promelas was the most sensitive of the five species.
Because of the lack of stability of zinc pyrithione when exposed to light, the tests were
carried out with subdued light and all tests - with the exception of the algal test - were
made with constant renewal of the test solution (flow through). By so doing, the exposure
concentration was successfully kept almost constant in all of the tests - even in the
algal test (Ward et al. 1994a), in which the medium cannot be renewed, and all
results are calculated on the basis of measured concentrations.
The results summarized in Appendix 5 show that the difference in sensitivity was not pronounced between the freshwater and the marine organisms. Algae are apparently the taxonomic group least sensitive to zinc pyrithione. Table 4.6 gives an overview of the toxicity of zinc pyrithione to various groups of organisms.
Long-term studies have been made with crustaceans (daphnids and small prawns) and fish (the most sensitive fish, Pimephales promelas, in a short-term test). In the studies with crustaceans, reproduction was examined and, in the study with fish, the development from egg to small fry was followed. The results in Table 4.6 indicate that fish are also the most sensitive group in long-term tests though the results with crustaceans and fish are of the same order of magnitude. The lowest NOECs are 0.0023 mg/L for crustaceans and 0.0012 mg/L for fish.
Table 4.6
Ecotoxicological data on effects of zinc pyrithione on aquatic organisms. All
concentrations are measured concentrations, tests with animals were flow-through tests
(see Appendix 5 for detailed data).
| Taxonomic group | End point |
Exposure time |
Result |
| Algae | EC50 |
5 |
0.028 |
| Algae | NOEC* |
5 |
0.0078 |
| Crustaceans | EC50 |
2-4 |
0.0036-0.0063 |
| Crustaceans | NOEC (reproduction) |
21 | 0.0023-0.0027 |
| Fish | LC50 |
4 |
0.0026-0.4 |
| Fish | NOEC |
32 |
0.0012 |
| Oyster | EC50 |
4 |
0.022 |
| * | The highest concentration at which no effects were
observed (NOEC, No Observed Effect Concentration). |
Metabolites
The toxicity of the three metabolites has been investigated in the laboratory. The results
of these tests are summarized in Table 4.7 together with the results from the tests with
zinc pyrithione.
Table 4.7
Summary of results from aquatic toxicity tests with zinc pyrithione and three metabolites.
All are short-term tests and the results are expressed as LC50 or EC50. Measured
concentrations for zinc pyrithione and omadine sulfonic acid.
| Taxonomic group | Zinc pyrithione |
Omadine |
Omadine sulfonic acid |
Pyridine sulfonic acid* |
| Algae | 0.028 |
0.14 |
36 |
29 |
| Crustaceans | 0.0036-0.0063 |
0.0064-0.013 |
>127-71 |
72- >122 |
| Fish | 0.0026-0.4 |
0.03-1.1 |
59- >137 |
57- >127 |
| Oyster | 0.022 |
0.160 |
99 |
86 |
*: Data from Olin 1997.
It applies to all four substances (in Table 4.7) that they have been tested with one freshwater alga (Selenastrum capricornutum), one freshwater crustacean (Daphnia magna), one marine crustacean (Mysidopsis bahia), two freshwater fish (Pimephales promelas and Oncorhynchus mykiss) and one sea fish (Cyprinodon variegatus) and furthermore, a shell deposition test with the oyster species Crassostrea virginica (marine). Furthermore, pyridine sulfonic acid was used in a long-term test with the fish Pimephales promelas (Boeri et al. 1999).
In the algal test with omadine sulfonic acid, the concentration of the substance fell during the test. The concentrations used for calculating the effect concentration are measured at the start of the test and the real EC50 is probably somewhat lower than the value stated in Table 4.7 (EC50: 36 mg/L) (Boeri et al. 1994g). In the other tests, the results are calculated as the average of the concentrations at the start and at the end of the test (Ward et al. 1994b, c, d; Boeri et al. 1994f, h, i). If this method of calculation is applied to the results of the algal test, an EC50 = 23 mg/L is achieved.
The results show that while zinc pyrithione and omadine disulfide were very toxic to aquatic organisms (L(E)C50 in the order of 3-300 µg/L), omadine sulfonic acid and pyridine sulfonic acid were considerably less toxic (L(E)C50 in the order of >20 mg/L) (Olin 1977). In a long-term study with fish eggs and larvae, pyridine sulfonic acid gave no effects at a concentration of 0.01 mg/L (Boeri et al. 1999). Algae were the group of organisms most sensitive to the last two substances.
Effects of degradation of zinc pyrithione on aquatic toxicity
A parallel test, like the one described in relation to DCOI (cf. Section 3.3.2), was
performed in order to examine the relation between degradation of zinc pyrithione and the
acute toxicity towards Acartia tonsa. The studies were made in the same way as
those of DCOI by use of sediment-seawater systems dosed with zinc pyrithione in a
concentration of 25 µg/kg. Water phase and sediment were separated 20 min after dosing.
The use of the water phase in tests with A. tonsa resulted in a lethality
corresponding to 100% of the test organisms. The test results showed that stationary
incubation in the dark or in the light (340 µmol/m2 • s) at 20-25°C resulted in the fact that no lethal effects on A. tonsa
were observed after one day (Figure 4.4). The rapid detoxification demonstrates that zinc
pyrithione was rapidly bound to the sediment or transformed to metabolites with
considerably lower toxicity than the parent compound as was the case in relation to DCOI
(cf. Section 3.3.2). The methods used are described in detail in Appendix 3.
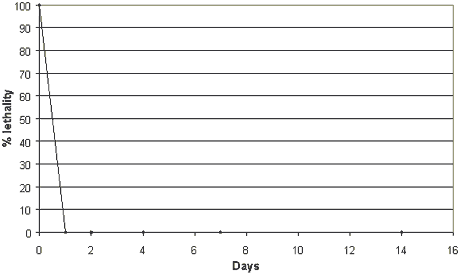
Figure 4.4
Effects of degradation of zinc pyrithione (25 µg/kg) dosed to sediment and seawater on
the acute toxicity to Acartia tonsa (test performed in the dark).
4.5 Assessment of zinc pyrithione and metabolites
Zinc pyrithione is transformed very rapidly in aquatic systems. Tables 4.2 and 4.4 show that, after incubation for less than 24 hours, the intact zinc pyrithione constituted less than half of the radioactivity added (day 0). It is assumed that zinc pyrithione is transformed via the structurally comparable omadine disulfide, which is rapidly transformed to heterocyclic compounds with one ring under environmentally realistic test conditions. The tests performed with zinc pyrithione showed that the quantitatively most important metabolites were omadine sulfonic acid and pyridine sulfonic acid under aerobic conditions and NP3, NP4, NP5 and pyridine sulfonic acid under anaerobic conditions (Tables 4.2-4.5). The heterocyclic compounds with one ring are all considered to be recalcitrant and stable in aquatic systems. The biological degradation of zinc pyrithione results in a quantitatively considerable formation of metabolites that sorb to the sediment. This appears from the fact that, at the end of the aerobic biodegradation test after 84 days, approx. 30% of the radioactivity added was sorbed to the sediment while, in the anaerobic test, approx. 50% of the 14C added could be recovered in the sediment after 182 days (Ritter 1999a, b, d).
The aquatic toxicity was investigated for omadine sulfonic acid and pyridine sulfonic acid, which were both considerably less toxic (L(E)C50 in the order of >20 mg/L) than zinc pyrithione and omadine disulfide (L(E)C50 in the order of 3-300 µg/L). Based on the chemical structure of the substances, the toxicity of the other metabolites with one ring is expected to be at the same level as the toxicity of omadine sulfonic acid and pyridine sulfonic acid. On this basis, the known stable metabolites from the transformation of zinc pyrithione under aerobic and anaerobic conditions are considered to have an aquatic toxicity that is between 1,000 and 10,000 times lower than the toxicity of zinc pyrithione (cf. Table 4.7). The metabolites sorbed to sediment are not yet identified. As these metabolites could not be extracted from the sediment with acetonitrile and KOH, they are considered to have a low bioavailability and thus a low toxicity to aquatic organisms.
4.6 Risk assessment of zinc pyrithione
Calculation of exposure
concentrations (PEC)
Exposure concentrations (PEC, Predicted Environmental Concentration) were calculated
for a pleasure craft harbour (Jyllinge) and a busy navigation route by use of
internationally recognized principles (EC 1996) as described in relation to DCOI (cf.
Section 3.4). The model and the two scenarios are described in detail in Appendix 1. For
parent compound and the most essential metabolites, the following exposure concentrations
were calculated:
| PEC (water column) | |
| PEC (sediment) | |
| PEC (sediment-pore water) |
The three exposure concentrations were defined as the steady-state concentration of the sub-environment in question. I.e., the concentration which the calculated concentrations eventually approach when a continuous leaching of the parent compound to the water environment is simulated. The model used is not validated towards measured concentrations in harbour environments or navigation routes. The exposure concentrations were calculated on the basis of the following assumptions:
| The background concentrations for both the parent compound and the metabolites were
assumed to be zero. |
| 70% of the pleasure craft was assumed to have been painted with paint containing zinc
pyrithione. |
| The leaching rate of zinc pyrithione from bottom paints was calculated at 21 mg/m2/day
in harbours and 41 mg/m2/day when sailing. |
| The average photolytical half-life of zinc pyrithione was calculated at 9.8 hours for
the pleasure craft harbour of Jyllinge and 6.6 hours for Kronprins Frederiks Bro (cf.
Appendix 1). It was not possible to quantify the influence of the presence of the pleasure
craft and the shadow effects from the pier on the amount of light falling on the surface
and calculations have thus been made with and without the inclusion of photolysis. |
| The primary biological transformation of zinc pyrithione into heterocyclic compounds with one ring was assumed to proceed with a half-life of 12 hours in surface water at a temperature of 25°C. |
The half-life for zinc pyrithione, which is assumed in the simulation, corresponds to a considerably slower transformation of zinc pyrithione than the initial removal of the substance from the water phase in studies with seawater and sediment (cf. Section 4.3). Compared with the removal of zinc pyrithione from the water phase (Ritter 1999a-e), a longer half-life was used in the simulation as aquatic systems with sediment make sorption possible and normally have a larger potential for biodegradation compared with the degradation potential in the surface water. The reason for using a half-life for transformation of zinc pyrithione corresponding to the expected transformation in surface water is that the result of the simulation is exposure concentrations at a continuous leaching of zinc pyrithione after steady state was achieved. When the pleasure craft are taken out of the water at the end of the sailing season, zinc pyrithione will probably be rapidly eliminated as the substance is either transformed in the water phase or sorbs to the sediment, in which it is transformed with a very short half-life (cf. Sections 4.2 and 4.3).
The exposure concentrations calculated for zinc pyrithione and its metabolites are approx. 50 times higher in the pleasure craft harbour than in the busy navigation route outside the harbour (Table 4.8).
Table 4.8a
Calculation of PEC for zinc pyrithione and metabolites at steady-state. Photolysis
included.
| Scenario | Substance | PEC |
PEC |
PEC (sediment, sorbed) |
| Pleasure craft harbour | Zinc pyrithione | 0.56 |
0.00056 |
0.089 |
| NP3 | 1.22 |
0.25 |
0.68 |
|
| NP4 | 0.099 |
0.19 |
0.078 |
|
| Pyridine sulfonic acid | 0.0080 |
0.068 |
0.040 |
|
| NP1 | 0.15 |
0.091 |
0.062 |
|
| Omadine sulfonic acid | 0.012 |
0.48 |
0.47 |
|
| Other compounds | 0.11 |
- |
- |
|
| Navigation route | Zinc pyrithione | 0.0053 |
0.00001 |
0.00090 |
| NP3 | 0.027 |
0.0028 |
0.0076 |
|
| NP4 | 0.0027 |
0.0022 |
0.00089 |
|
| Pyridine sulfonic acid | 0.00040 |
0.00077 |
0.00045 |
|
| NP1 | 0.0032 |
0.0011 |
0.00077 |
|
| Omadine sulfonic acid | 0.00046 |
0.0059 |
0.0058 |
|
| Other compounds | 0.0032 |
- |
- |
Table 4.8b
Calculation of PEC for zinc pyrithione and metabolites at steady-state. Photolysis not
included.
| Scenario | Substance | PEC |
PEC |
PEC |
| Pleasure craft harbour | Zinc pyrithione | 1.7 |
0.0013 |
0.21 |
| NP3 | 0.00006 |
0.54 |
1.5 |
|
| NP4 | 0.20 |
0.43 |
0.18 |
|
| Pyridine sulfonic acid | 0.016 |
0.15 |
0.090 |
|
| NP1 | 0.45 |
0.24 |
0.17 |
|
| Omadine sulfonic acid | 0.036 |
1.3 |
1.3 |
|
| Other compounds | 0.24 |
- |
- |
|
| Navigation route | Zinc pyrithione | 0.022 |
0.00002 |
0.0027 |
| NP3 | 0.00001 |
0.0072 |
0.019 |
|
| NP4 | 0.0059 |
0.0061 |
0.0025 |
|
| Pyridine sulfonic acid | 0.00088 |
0.0022 |
0.0013 |
|
| NP1 | 0.013 |
0.0042 |
0.0028 |
|
| Omadine sulfonic acid | 0.0019 |
0.022 |
0.021 |
|
| Other compounds | - |
- |
- |
Calculation of Predicted No Effect Concentrations (PNEC)
Predicted No Effect Concentrations (PNECs) are estimated for zinc pyrithione and
pyridine sulfonic acid. The other stable metabolites from the transformation of zinc
pyrithione are considered to have the same aquatic toxicity as pyridine sulfonic acid.
The available studies of the aquatic toxicity of zinc pyrithione are considered representative and the data material includes long-term studies with crustaceans and the most sensitive group of organisms, i.e. fish. The algal test may be interpreted both as a short-term test and as a long-term test (EC 1996).
For zinc pyrithione, data are interpreted as including three NOEC values from long-term tests (crustaceans, algae and fish), which includes the group of organisms that was most sensitive in the short-term test (fish). On this basis, PNEC is calculated by dividing the lowest NOEC value, which is 0.0012 mg/L for fish, by an assessment factor of 10 (EC 1996). This results in a PNEC of 0.0001 mg/L = 0.1 mg/L for zinc pyrithione.
The result from the long-term test carried out with fish and pyridine sulfonic acid (Boeri et al. 1999) is not considered applicable for calculation of PNEC. This is due to the fact that the study used only one concentration (0.01 mg/L) at which no effects were measured. The result does thus not give any indications of the concentration area in which effects may be expected. Calculations of PNEC for pyridine sulfonic acid are thus based on the lowest effect concentrations shown in Table 4.7. The algal test is the only test that may be considered a long-term test but this test alone is not adequate for making the calculations on the basis of NOEC (EC 1996). As all data were thus derived from short-term tests, an assessment factor of 1,000 is used with lowest effect concentration. For pyridine sulfonic acid, the EC50 value of 28.9 mg/L for algae (pyridine sulfonic acid) is used which results in a PNEC of 0.03 mg/L = 30 µg/L. The PNEC calculated for pyridine sulfonic acid is considered representative of the other stable metabolites from the transformation of zinc pyrithione.
Table 4.9 shows the two calculations of PNEC.
Table 4.9
Calculation of PNEC for zinc pyrithione and pyridine sulfonic acid.
| Substance | Lowest effect concentration | Value |
Assessment factor |
PNEC |
| Zinc pyrithione | Long-term test NOEC fish |
1.2 |
10 |
0.1 |
| Pyridine sulfonic acid | Short-term test EC50 algae |
28,900 |
1,000 |
30 |
Risk quotient
When transformation of zinc pyrithione by photolysis is included in the calculation of
PEC, the risk quotient is calculated on the basis of PEC (water) for zinc pyrithione and
the metabolites stated in Table 4.8. PEC (sediment, pore water) for the metabolites is
higher than the corresponding PEC (water) when photolysis is ignored. In this case, PEC
(water) for zinc pyrithione and PEC (pore water) for the metabolites are used for
calculating the risk quotient. As PNEC values for zinc pyrithione and pyridine sulfonic
acid are used, risk quotients (Rq = PEC/PNEC) are calculated as shown in Table 4.10.
Table 4.10
Calculation of risk quotients (Rq) for zinc pyrithione and its metabolites.
| Substance | PNEC [µg/L] |
Pleasure craft harbour |
Navigation route |
||
PECA |
RqA |
PECA |
RqA |
||
| Zinc pyrithione | 0.1 |
0.56 |
5.6 |
0.0053 |
0.05 |
| Metabolites | 30* |
1.6 |
0.05 |
0.037 |
0.0012 |
A
, upper value, photolysis included; lower value; photolysis not included.*, Pyridine sulfonic acid
The stated risk quotients are calculated on the basis of realistic worst-case scenarios (Appendix 1), which are i.a. based on the assumption that 70% of the pleasure craft are painted with a bottom paint containing zinc pyrithione. On the basis of the assumptions made in the simulation and of the calculated PEC values, it is considered likely that a risk of chronic ecotoxic effects within the pleasure craft harbour may exist as, presumably, zinc pyrithione will constantly be applied by leaching from bottom paints. The risk quotient for zinc pyrithione within the pleasure craft harbour is between 0.05 and 0.22 and here the risk of ecotoxic effect of zinc pyrithione is considered to be low. The risk quotient out of the pleasure craft harbour is probably closest to 0.05, in which photolysis has been included in the calculation of PEC as major shadow effects are not expected on a normal navigation route.
Within the pleasure craft harbour, a low risk of ecotoxic effects of stable metabolites from the transformation of zinc pyrithione is considered possible and this risk is considered very low in areas out of the pleasure craft harbour.
[Front page] [Contents] [Previous] [Next] [Top] |