[Front page] [Contents] [Previous] [Next] |
Ecotoxocicological assessment of antifouling biocides and non-biocidal antifouling paints
3. Sea-Nine
3.1 Physico-chemical properties
3.2 Biodegradation of DCOI in the aquatic environment
3.2.1 Primary degradation in seawater
3.2.2 Mineralization and metabolites in aerobic sediment
3.2.3 Mineralization and metabolites in anoxic sediment
3.2.4 Transformation and fate of DCOI in a harbour
3.3 Bioaccumulation and aquatic toxicity
3.3.1 Bioaccumulation
3.3.2 Toxicity towards aquatic organisms
3.4 Risk assessment of DCOI
This chapter contains an ecotoxicological assessment of 4,5-dichloro-2-n-octyl-4-isothiazolin-3-on (DCOI), which is the active substance in Sea-Nine 211.
3.1 Physico-chemical properties
Table 3.1 summarizes the physico-chemical properties of DCOI.
Table 3.1
Physico-chemical properties of DCOI.
| CAS No. | 64359-81-5 |
| Synonyms | 4,5-dichloro-2-n-octyl-4- isothiazolin-3-on 4,5-dichloro-2-n-octyl-3(2H)- isothiazolone RH-5287 |
| Classification | - |
| Molecular formula | C11H17Cl2NOS |
| Molar weight | 282.23 |
| Water solubility (20°C) | 6.5 mg/L1 |
| Vapour pressure (25°C) | 7.4 · 10-6 mm Hg1 |
| Octanol-water partition coefficient (log Kow) | 2.8 (measured)2 |
| Organic carbon-water partition coefficient (log Koc) | 3.2 (measured)3 |
| 1 | Shade et al. 1993 | 2 | Jacobson 1993 | 3 | Howard 1991. |
3.2 Biodegradation of DCOI in the aquatic environment
3.2.1 Primary degradation in seawater
Several studies have been made of the degradation of DCOI in the aquatic environment. It is stated that abiotic processes progress with half-lives of 9-12.5 days for hydrolysis and 13.4 days for photolysis. Biological processes are, however, of greater importance to the transformation of DCOI. Studies described by Shade et al. (1993) have shown that DCOI (10 µg/L) is transformed with a half-life of 11 hours in seawater with 7 × 104 bacteria/mL (total number of bacteria determined by counting in a microscope). Parallel tests with seawater samples with a lower number of bacteria (<1,000 bacteria/mL) resulted in longer half-lives for DCOI (Shade et al. 1993). These tests are not considered relevant as the biological activity of the seawater was unrealistically low. In a recent study, the transformation rate of DCOI (10 µg/L) was determined in seawater from the pleasure craft harbour of Jyllinge. The study demonstrated that 7.1% of the DCOI added remained after 72 hours at a temperature of 12°C (Jacobson and Kramer 1999). On the basis of the measured concentrations of DCOI (Jacobson and Kramer 1999), the biological half-life may be estimated at 14 hours at 12° C (Appendix 1, Section 2.4.1).
3.2.2 Mineralization and metabolites in aerobic sediment
Transformation of DCOI
The aerobic half-life of DCOI is very short in marine systems with sediment and
seawater. Analyses of samples from laboratory tests with sediment and seawater showed that
DCOI was rapidly transformed into other chemical compounds. In bottles with a dosage of
0.05 mg/kg, less than 6% of the radioactivity added was intact DCOI at sampling on the
first day of the test (day 0). At a dosage of 1 mg/kg, approx. 3.5% of the 14C
added was intact DCOI at sampling on day 1. In reality, the insignificant part of the
parent compound recovered on day 0 represented a sampling after one hour as the
preparation of the samples for analysis took approx. one hour. The very rapid
transformation of DCOI makes it impossible to calculate an exact half-life, which is,
however, for certain less than one hour (Lawrence et al.
1991a).
Mineralization and metabolites
During the 30-day test period, [14C]DCOI was partially mineralized as 22%
(0.05 mg/kg) and 8.7% (1 mg/kg) of the radioactivity added was transformed into 14CO2
at 25°C. DCOI is primarily transformed into polar metabolites
and into compounds that are not extracted from the sediment (Table 3.2). A comparison with
HPLC chromatograms of 15 potential metabolites did not result in an unambiguous
identification of the metabolites observed in the sediment tests. The most polar
metabolite had the same analytical retention time as n-octyl malonamic acid (C8H17NHC(=O)
CH2CO2H) and at least two other metabolites were linear structures
in which the isothiazolone ring was broken (Lawrence et al.
1991a). Assessed on the basis of analyses of 15 known standards, more cyclic structures
were formed at the initial primary degradation of DCOI. It is considered likely that the
metabolites present in the tests after 30 days were linear compounds. The two metabolites
found at the analysis of the sediment samples after 30 days were both more polar than the
isothiazolone standards used. The rapid primary degradation of DCOI (more than 94%
transformation after 1 hour) indicates a rapid reaction involving a chemically unstable
bond, e.g. the N-S bond in the isothiazolone ring (Lawrence et al.
1991a).
A positive identification of three metabolites was achieved in a later study in which a microbial enrichment culture proved suitable for achieving higher concentrations of metabolites (Mazza 1993). The culture was enriched after dosing aquatic sediment with DCOI (5 mg/kg). A comparison between HPLC chromatograms of metabolites formed in the enrichment culture and in sediment showed that the products were almost identical. By use of the enrichment culture and more analytical methods (i.a. HPLC and GC/MS), two essential metabolites were identified as N-(n-octyl) malonamic acid and N-(n-octyl) acetamide. Furthermore, a third quantitatively less important product N-(n-octyl) b hydroxypropionamide, which is probably formed at anaerobic degradation, was identified.
Table 3.2
Aerobic biodegradation of [14C]DCOI (0.05 mg/kg), polarity and
distribution of metabolites in sediment and seawater. Data from Lawrence et al.
1991a.
Time (days) |
% of 14C added |
||||
DCOI |
Polar substances* |
Non-polar substances** |
CO2 |
Non-extractable substances |
|
0 |
5.1 |
41.1 |
1.2 |
0.0 |
62.0 |
1 |
- |
44.7 |
0.65 |
0.55 |
62.2 |
2 |
- |
27.6 |
1.3 |
3.3 |
55.3 |
5 |
- |
27.0 |
0.3 |
8.1 |
66.8 |
9 |
- |
23.9 |
0.7 |
8.2 |
59.0 |
15 |
- |
22.3 |
- |
8.4 |
56.5 |
20 |
- |
24.8 |
- |
9.1 |
78.0 |
26 |
- |
20.3 |
- |
14.2 |
67.0 |
30 |
- |
13.1 |
- |
21.9 |
63.5 |
| -, | not detected | * | more polar than DCOI | ** | less polar than DCOI. |
The sediment from the aerobic biodegradation tests (Lawrence et al. 1991a) was further characterized as regards bound metabolites. Sediment samples sampled at the start of the tests and after 30 days were characterized by extraction with methylene chloride/methanol followed by extractions with HCl and NaOH (Kesterson and Atkins 1992a). Relatively water-soluble metabolites that are extracted with HCl, constituted <0.1% of the radioactivity added. Metabolites in the NaOH extract were further divided into fulvic acid and humic acid fractions containing 1.2% and 5.1%, respectively, of the 14C added after 30 days. The metabolites that were not extracted by these procedures were probably bound to humin or clay and constituted 45% of the 14C added after 30 days (Kesterson and Atkins 1992a). The results showed that the stable metabolites from DCOI were mainly bound to humic acid, humin and clay minerals in the sediment.
Studies with Danish sediments
The aerobic biodegradability of DCOI was examined by use of a clayey sediment (0.83
µg DCOI/g) and a sandy sediment (0.033 mg DCOI/g), which had
both been incubated with their respective seawater (Appendix 2). Both sediments and their
respective seawater had been collected at two localities in the Sound. The mineralization
of [2,3-14C]DCOI into 14CO2 constituted 13% of the 14C
added in the clayey sediment and 24% of the 14C added in the sandy sediment
after 42 days’ incubation at 15°C (Figures 3.1 and 3.2).
The examination of the distribution of 14C in the clayey sediment at the
termination of the test after 42 days showed that 48% of the 14C added was
bound to humic acids, humin and clay minerals. These substances are expected to be little
bioavailable. In the water phase of the test system or in the form of hydrolyzable
compounds and fulvic acids, the substances more easily soluble in water altogether
constituted 17% of the 14C added after 42 days. Tests were made with glucose in
order to examine the effect of the low concentration and the other experimental conditions
on the mineralization of a readily biodegradable substance. The mineralization of glucose
constituted 52% of the 14C added in the clayey sediment and 60% of the 14C
added in the sandy sediment after 42 days. Methods and results are described in detail in
Appendix 2.
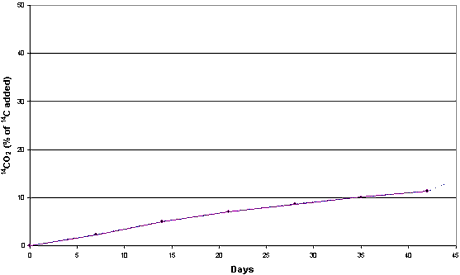
Figure 3.1
Mineralization of [14C] DCOI (0.83 µg/g) in clayey sediment and
seawater from the Sound (sediment LS). Aerobic conditions. Dotted curve represents 14CO2
released by acidification.
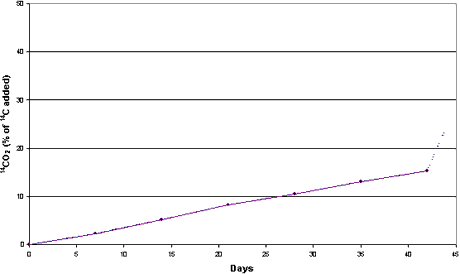
Figure 3.2
Mineralization of [14C] DCOI (0.033 µg/g) in sandy sediment and
seawater from the Sound (sediment SS). Aerobic conditions. Dotted curve represents 14CO2
released by acidification.
In the study with the clayey sediment, water and sediment samples were sampled at the start of the incubation and after 28 and 42 days. Chemical analyses of DCOI and metabolites in these samples were made by Rohm and Haas (Spring House, Pennsylvania). The water samples turned out to have a low content of 14C (2.5-6% of the 14C added), which did not allow a more detailed characterization of metabolites. The analyses of the sediment samples from the same test showed that DCOI was transformed into compounds more polar than the parent compound and that a considerable part of the radioactivity added resisted extraction from the sediment (Table 3.3).
Table 3.3
Aerobic biodegradation of [14C]DCOI into metabolites and carbon dioxide
in seawater and clayey sediment from the Sound. HPLC analyses were only performed with
sediment samples (sediment LS).
Time |
DCOI1 |
Polar sub- |
Non-polar substan- |
CO2 |
Non- |
% of 14C added |
|||||
0 |
0.37 |
46.4 ± 6.5 |
- |
0 |
43.7 ± 4.6 |
28 |
- |
20.4 ± 0.60 |
- |
8.7 ± 0.35 |
51.0 ± 13.6 |
42 |
0.80 ± 0.92 |
18.5 ± 1.7 |
- |
13 ± 0.52 |
49.2 ± 21.9 |
1 |
determined by HPLC co-chromatography with DCOI standard; |
| 2 | more polar than DCOI |
| 3 | less polar than DCOI |
| SD, | standard deviations of three replicates |
| -, | not detected |
3.2.3 Mineralization and metabolites in anoxic sediment
Transformation of DCOI
As was the case under aerobic conditions, DCOI was rapidly transformed into other chemical
compounds in anoxic sediment. In the tests, only 2.0% (0.05 mg/kg) and 2.2% (1 mg/kg) of
the radioactivity added were intact DCOI at sampling on the first day of the test (day 0).
As the first sampling in reality represents a 1-hour sample, it can be demonstrated with
certainty that the half-life of DCOI was less than 1 hour (Lawrence et al.
1991b).
Mineralization and metabolites
[14C]DCOI (0.05 mg/kg) was only less mineralized in the anoxic sediment as the
formation of 14CO2 constituted between 6.7 and 9.5% of the
radioactivity added throughout the entire test period of 365 days (Table 3.4). This level
was attained after 61 days’ incubation at 25°C. The
comparative share, which was mineralized in the parallel test with a dosage of 1 mg/kg,
constituted between 5.3% and 8.2% of the 14C added in the period from day 61 to
day 365 (Lawrence et al. 1991b). The products formed by the
degradation of DCOI were related to standards of 15 potential metabolites. The results
show that, after 29 days, at least three metabolites more polar than DCOI had been formed.
Although it cannot be excluded that one of these products resembles the parent compound,
it is considered most likely that linear structures are in question. Furthermore, two
metabolites less polar than DCOI were demonstrated. The identity of these non-polar
substances cannot be established with certainty as they could not be related to any of the
standards used. Table 3.4 shows that the quantitatively most important metabolites from
DCOI are polar compounds. The polar metabolites are presumably composed of more linear
compounds.
Table 3.4
Anaerobic biodegradation of [14C]DCOI (0.05 mg/kg), polarity and
distribution of metabolites in sediment and seawater. Data from Lawrence et al.
1991b.
Time (days) |
% of 14C added |
||||
DCOI |
Polar substances* |
Non-polar substances** |
CO2 |
Non-extractable substances |
|
0 |
2.0 |
13.3 |
0.9 |
0.0 |
47.1 |
14 |
-A |
25.3 |
2.1 |
1.1 |
41.4 |
29 |
- |
23.0 |
1.5 |
4.0 |
41.6 |
61 |
-A |
18.7 |
3.8 |
8.4 |
40.1 |
90 |
- |
18.6 |
2.5 |
7.6 |
58.5 |
120 |
- |
12.6 |
1.3 |
9.5 |
47.6 |
180 |
- |
12.6 |
2.2 |
8.5 |
48.9 |
270 |
- |
8.7 |
1.0 |
8.5 |
66.7 |
365 |
- |
*** |
*** |
6.7 |
44.0 |
| - | not detected (however: A, low conc. detected, probably artifact) |
| * | more polar than DCOI |
| ** | less polar than DCOI |
| *** | sample lost |
Water-soluble metabolites from the transformation of DCOI constituted between 3.6% and 9.3% of the radioactivity added throughout the entire test period. Metabolites that were bound to the sediment and could not be extracted with methylene chloride/methanol constituted a constantly high part of between 40% and 67% of the 14C added (Table 3.4). Further extraction with HCl and NaOH showed that relatively water-soluble metabolites constituted <0.1% while fulvic and humic acids constituted 0.6% and 3.6%, respectively, of the 14C added after 365 days. Metabolites that were still bound to the sediment, probably to humin or clay, constituted 30% of the 14C added (Kesterson and Atkins 1992b). The formation of metabolites binding to humic acids, humin and clay minerals in the sediment is in agreement with the results in the aerobic biodegradation tests (Kesterson and Atkins 1992a).
Studies with Danish sediments
The anaerobic biodegradability of DCOI (0.83 µg/g) was examined by use of a clayey
sediment and its seawater (Appendix 2), which was also used in the aerobic tests (cf.
Section 3.2.2). Sediment and seawater was incubated under anaerobic sulfate-reducing
conditions, which are normally prevalent in coastal marine sediments. The mineralization
of [2,3-14C] DCOI into 14CO2 constituted 14% of the 14C
added after 56 days’ incubation at 15°C (Figure 3.3).
The examination of the distribution of 14C in the clayey sediment at the
termination of the test after 56 days showed that 45% of the 14C added was
bound to humic acids, humin and clay minerals. Altogether, water-soluble substances in the
water phase of the test system and hydrolizable compounds and water-soluble fulvic acids
constituted 7% of the 14C added after 56 days. The mineralization of glucose,
which was included as a readily biodegradable reference substance, constituted 59% of the 14C
added after 56 days. Methods and results are described in detail in Appendix 2.
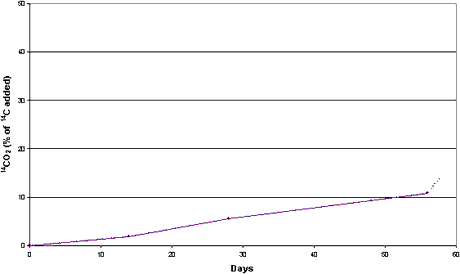
Figure 3.3
Mineralization of [14C] DCOI (0.83 µg/g) in clayey sediment and seawater
from the Sound (sediment LS). Anaerobic conditions. Dotted curve represents 14CO2
released by acidification.
Water and sediment samples from the tests were sampled at the start of the test and after 28 and 56 days. Chemical analyses of DCOI and metabolites in the water samples were made by Rohm and Haas (Spring House, Pennsylvania).
The analyses of water samples sampled at the termination of the test after 56 days showed that 4.0 ± 2.4% of the 14C added was present in the form of compounds with the same HPLC retention time as DCOI. In the same water samples, polar compounds constituted 13.7 ± 3.0% of the 14C added. The sediment samples were not analyzed as they contained 3-4 times less radioactivity than the sediment samples from the aerobic tests (Table 3.3).
3.2.4 Transformation and fate of DCOI in a harbour
An investigation of the spread and removal of DCOI was carried out in the vicinity of a freshly painted ship and of another ship that had been painted a couple of months earlier. Both ships were lying in Korsør Harbour where the investigations were made on 26 and 27 October 1998. Those days, the temperature of the water was approx. 10°C and varied very little according the depth of the water (Steen et al. 1999). The wind was southwesterly (between approx. 240 and 255° on 26 October and approx. 200° on 27 October). The wind velocity was approx. 8-10 m/sec with wind blasts of up to 15 m/sec on 26 October and a little more on 27 October (Danish Meteorological Institute 1999). The entrance of Korsør Harbour points in a north-easterly direction why the water must be expected to have been pressed out of the harbour.
The concentration of DCOI in the water phase was measured along two transects: one perpendicular to the direction of the ships and the other in north-easterly direction, i.e. in the wind direction. Most of the samples were taken on 26 October. The samples were taken over a relatively short period of time (approx. 5 hours) and the measured concentrations can thus only be considered valid for the day in question. The highest concentrations measured of DCOI were <300 ng/L close to the ship’s side (£1 m) and decreased to <50 ng/L at a distance of approx. 30 metres from the ship. The concentration of DCOI in a distance of 2 metres from the ships (along the transect and perpendicular to the ships) varied very little according to the water depth why the vertical mixing was considered to be total.
Steen et al. (1999) have made model calculations in which Korsør Harbour was modelled as a one-dimensional box, in which the flow in and out of the harbour was neglected and in which the dispersion coefficient was varied between approx. 0.004-0.03 m2/s. This interval is stated to be the end points of the expected variation interval of the dispersion coefficient of the harbour. Apart from the spread, a first order disappearance kinetics is assumed for DCOI. The simulations were made with three different rate constants for this first order process: 0 day-1, 1 day-1, and 1 hour-1. As a result of the winds on 26 and 27 October, the dispersion must presumably have been high in the basin. By way of comparison it may be mentioned that the horizontal dispersion coefficient in Danish coastal waters typically varies between 0.04 and 5 m2/s (Harremoës and Malmgren-Hansen 1989). As regards the two transects, the best correlation between the measured and the calculated DCOI concentrations was achieved by use of a rate constant of disappearance of between 1 hour-1 and 1 day-1.
With the rate constant, 1 hour-1, a good correlation was achieved between measured and calculated values close to the ships for one transect while the concentrations of the other transect was underestimated at distances of more than approx. 8 m. For both transects, the calculated concentrations are lower than the measured concentrations at larger distances from the ships (approx. 30 m). When assuming a rate constant of disappearance of 1 day-1, the calculated concentrations are higher than the measured concentrations close to the ships for both transects but lower than the measured concentrations farther away (approx. 60 m). There are thus indications that the rate constant of the disappearance of DCOI close to the ships is higher than the corresponding constant farther away from the ships. On this basis, the rate constant of disappearance of the whole basin is considered to be between 1 hour-1 and 1 day-1, which corresponds to a half-life of between approx. 0.69 and 16.6 hours. This half-life includes biological and abiotic transformation as well as processes like sorption to suspended matter, sedimentation, potential vertical mixing and potential imperfection in the calculation of the dilution in the harbour.
3.3 Bioaccumulation and aquatic toxicity
3.3.1 Bioaccumulation
Studies on the bioaccumulation of DCOI in fish are available but not in other types of organisms (e.g. mussels). The ability of DCOI to bioaccumulate in fish has been examined in laboratory tests over 28 days by use of [14C]DCOI. Two studies including chemical analyses of water and tissue samples have been made (Forbis et al. 1985; Derbyshire et al. 1991). In all tests, [14C]DCOI was continuously added to a flow-through system. Chemical analyses showed that, at the final part of the tests, the concentration of DCOI was considerably lower than the nominal concentration (e.g. 4.5% and 0.55% of the 14C added after 21 and 28 days, respectively (Leak 1986)) while DCOI was hardly measurable in the second test (Derbyshire et al. 1991). Presumably, the principal part of the remaining 14C activity in the water represented one or more polar metabolites.
The BCF values found (measured as radioactivity) were more or less identical in the two studies. The BCF values were 130-200 for muscle tissue, 700-1,100 for internal organs and 600 for the whole fish (Forbis et al. 1985; Derbyshire et al. 1991). The chemical analyses demonstrated that only 1% of the radioactivity in the fish was intact DCOI (Leak 1986). In connection with the study by Derbyshire et al. (1991), HPLC as well as TLC was used for identifying 14C labelled substances accumulated in the tissue of the fish. These studies indicate that it was most likely a question of substances without an isothiazolone ring structure and that the substances were built into the protein of the fish. The results indicate that DCOI was transformed in the water, after which it was mainly polar and probably linear compounds that were taken up in the fish. This assumption is confirmed by the biodegradation of DCOI (cf. Section 3.2.2; Lawrence et al. 1991a). It is thus considered likely that the measured BCF values should rather be related to metabolites of DCOI but as only a few of these metabolites are identified, the importance of the recorded bioaccumulation of labelled 14C cannot be assessed.
3.3.2 Toxicity towards aquatic organisms
Aquatic organisms
The toxicity of DCOI has been examined in standard laboratory tests with a number of
aquatic organisms living in fresh water and in seawater:
Fresh water:
| Selenastrum capricornutum, green algae (Forbis 1990) | |
| Daphnia magna, crustacean (Burgess 1990; Ward and Boeri 1990) | |
| Oncorhynchus mykiss, rainbow trout (Shade et al. 1993) | |
| Lepomis macrochirus, bluegill sunfish (Shade et al. 1993) |
Seawater:
| Skeletonema costatum, green algae (Debourg et al. 1993) | |
| Mysidopsis bahia, mysid, crustacean (Boeri and Ward 1990) | |
| Penaeus aztecus, brown shrimp, crustacean (Heitmuller 1977) | |
| Cyprinodon variegatus, sheepshead minnow, fish (Shade et al. 1993) | |
| Paralichthys olivaceus, Japanese flatfish, fish (Kawashima 1997a) | |
| Pagrus major, red sea bream, fish (Kawashima 1997b) | |
| Crassostrea virginica, oysters (Roberts et al. 1990) |
Furthermore, tests with one mussel and protozoans are quoted by Shade et al. (1993) and Debourg et al. (1993), respectively. In some of the tests, problems with maintaining a constant exposure concentration have been reported and not all results have been calculated on the basis of measured concentrations (see below). The result of these irregularities is an overestimation of the effect concentrations - resulting in an underestimation of the toxicity of the substance.
The results, which are compiled in Appendix 4, show that there was no big difference in the sensitivity of freshwater and marine organisms. Table 3.5 summarizes the effects on the different groups of organisms.
Table 3.5
Ecotoxicological data on effects of DCOI on aquatic organisms (see Appendix 4 for
detailed data).
Taxonomic group |
End point |
Exposure time |
Results |
Algae |
EC50 |
4-5 |
0.0139-0.036 |
Crustaceans |
EC/LC50 |
2-4 |
0.0047-1.312 |
Crustaceans |
NOEC* |
21 |
0.00063 |
Fish |
LC50 |
4 |
0.0027-0.030 |
Fish |
NOEC |
35 |
0.006 |
Molluscs |
EC/LC50 |
2-4 |
0.0019-0.850 |
Protozoans |
100% effect |
? |
5 |
| * | The highest concentration at which no effects were observed (NOEC, No Observed Effect Concentration). |
The results from the algal tests performed (Forbis 1990) are calculated on the basis of the nominal concentration. The report on one of the tests shows that the concentration of DCOI decreased during the whole test period. Only 48% of the nominal concentration was left after 48 hours and, at the end of the test after 72 hours, it was only possible to measure the substance in the test vessels containing the highest concentration (Forbis 1990). The EC50 values stated are thus too high.
A 21-day reproduction test with daphnids (Ward and Boeri 1990) was conducted in such a way that is difficult to draw certain conclusions. This is due to the use of various concentrations of a solvent in relation to the addition of DCOI and to large variation in the data. The NOEC value stated represents the lowest concentration tested but the way in which the test has been conducted does not exclude that effects of DCOI may have occurred at this concentration as the effect may be dimmed as an unintentional result of the solvent. As the result of this test is the lowest NOEC value found in the tests, this value forms the basis of the calculation of PNEC for DCOI.
N-(n-octyl) malonamic
acid
The acute aquatic toxicity of N-(n-octyl) malomanic acid, which is an important metabolite
from the transformation of DCOI (cf. Section 3.2.2), has been investigated in tests with
fish and daphnia. The toxicity of N-(n-octyl) malomanic acid was tested in static tests
and the calculations are based on measured mean concentrations of the substance. The
effect concentrations for N-(n-octyl) malomanic acid are given for daphnia (48 h): EC50 =
260 mg/L, NOEC = 16 mg/L (Sword and Muckerman 1994b) and for rainbow trout (96 h): LC50 =
250 mg/L, NOEC = 160 mg/L (Sword and Muckerman 1994a). In the daphnia test, there is large
variation in data and the basis of the calculation of the result is not clearly defined.
Daphnids lying on the bottom of the test vessels do not seem to have been included as
"immobile", which they should according to the method description used. The
actual EC50 is estimated to be in the interval of 90-160 mg/L rather than 260 mg/L as
stated in the report (Sword and Muckerman 1994b).
Even with the above reservations, it must, however, be concluded that N-(n-octyl) malomanic acid is several orders of magnitude less toxic than DCOI.
In connection with the investigations of N-(n-octyl) malomanic acid, QSAR calculations have been made of the toxicity of this metabolite and some substances with similar structure, which are important metabolites from the microbial transformation of DCOI. The results are given in Table 3.6.
Table 3.6
QSAR calculations of the toxicity and the potential bioaccumulation of four probable
metabolites from the transformation of DCOI.
Substance |
Calculated EC50 |
Calculated EC50 |
Calculated |
N-(n-octyl) malonamic acid |
172 |
199 |
1.9 |
N-(n-octyl) acetamide |
102 |
115 |
2.0 |
N-(n-octyl) oxamic acid |
140 |
160 |
1.9 |
N-(n-octyl)-b -hydroxypropionamide |
261 |
Not determined |
Not determined |
| * | From Sword and Muckerman 1994b. |
| ** | Personal comm., Andrew Jacobson, Rohm and Haas Company. |
The results in Table 3.6 indicate that the probable metabolites from the transformation of DCOI are neither particularly toxic nor bioaccumulative in aquatic organisms.
Sediment-living
organisms
Results of a 10-day test with the marine sediment-living crustacean, the amphipod Ampelisca
abdita are available: LC50 = 320 mg/kg and NOEC = 6.9 mg/kg dry weight (Putt 1994).
The test was made with 14C-labelled DCOI and the concentrations were measured
as radioactivity. At the end of the test, approx. 90% of the 14C activity was
attached to the sediment while the remaining part was distributed in the ratio of approx.
8:2 of pore water to the water above the sediment. No chemical analyses were made and the
authors draw attention to the fact that the measured radioactivity is probably owing to
metabolites and not to DCOI.
Algal communities
Acute and chronic effects of DCOI have been examined on communities of natural
phytoplankton (planktonic algae) and epipsammon (micro algae living on grains of sand).
Acute and chronic effects of DCOI on communities of phytoplankton have been found at a
concentration of DCOI of 0.0003 mg/L (the lowest concentration in which effects were
observed, Lowest Observed Effect Concentration, LOEC) (Arrhenius 1997). The acute effect
of DCOI was a stimulation of the activity of the algae while the chronic effect was an
adaptation to DCOI in a few days. Inhibition of the photosynthesis occurred at higher
concentrations (EC50: 0.05-0.1 mg/L (95% confidence interval)). The study concludes that
the effect of DCOI was still significant at the end of the test after 7 days. Communities
of epipsammon were extremely tolerant to DCOI and the effect concentrations were several
orders of magnitude higher than those for phytoplankton.
Effects of degradation of
DCOI on aquatic toxicity
Laboratory tests have been made in which the effects of degradation on the toxicity
towards aquatic organisms were tested for a number of antifoulants including DCOI, Irgarol
1051 and Diuron (Callow and Finlay 1995; Callow and Willingham 1996). In these tests, the
substances were incubated in seawater, seawater enriched with of marine bacteria and in
sterilized seawater. Changes in the toxicity as the result of degradation of the active
substances were tested towards marine bacteria (counting of colonial bacteria), diatoms (Amphora
coffeaeformis) and crustaceans (Artemia salina). The degradation tests were
started at concentrations of the substances causing 80% effect on the algae (EC80 = 0.5
mg/L for DCOI) so that a potential decrease of the toxicity of the solutions could be
traced. The results showed that the toxicity practically did not decrease in sterilized
seawater, and that the transformation of the active substance into metabolites with low
toxicity progressed most rapidly in the bacteria-enriched seawater. The diatom test showed
that e.g. the toxicity of DCOI had been considerably reduced (from approx. 80% to approx.
20% inhibition) after two weeks in natural and in bacteria-enriched seawater and that
tests incubated for 4, 6 and 8 weeks in these two types of seawater caused 10% or no
significant inhibition (Callow and Finlay 1995). The half-life of the toxicity of DCOI was
calculated at 8.5 days in natural seawater and 3 days in bacteria-enriched seawater
(Callow and Finlay 1995).
The relation between degradation and sorption of DCOI and the acute toxicity towards the marine crustacean Acartia tonsa has been examined in the present study. The tests were performed in systems with the sandy sediment and its seawater from the Sound (Appendix 2), which was also used in the biodegradation tests. DCOI was added in a concentration of 100 µg/kg to the sediment-seawater systems. Water phase and sediment were separated 20 min. after dosing and use of the water phase in tests with A. tonsa caused a mortality corresponding to 35% of the test organisms. Stationary incubation in the dark at 20-25°C resulted in the fact that there were no mortal effects on A. tonsa after day 1 (Figure 3.4). Similar results were achieved when the sediment-water systems were incubated in the light at an intensity corresponding to 340 µmol/m2 × s. Measurements made by VKI in the Sound show that, in the period from May to October 1998, the average light intensity was 420 µmol/m2 × s in a depth of approx. 1 metre. The light intensity used was thus approx. 80% of the mean value calculated on the basis of the measurements in 1998. The test results with A. tonsa show that DCOI sorbs to sediment or is transformed into metabolites with a considerably lower toxicity than the parent compound. The methods used are described in detail in Appendix 3. A parallel test was made with zinc pyrithione (cf. Section 4.4).
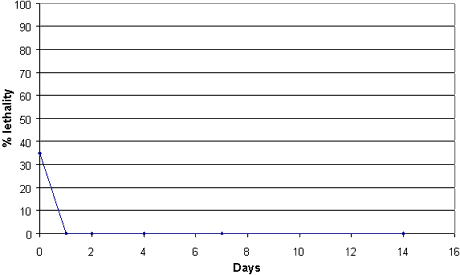
Figure 3.4
Effects of degradation of DCOI (100 µg/kg) dosed to sediment and seawater on the
acute toxicity to Acartia tonsa (test performed in the dark).
3.4 Risk assessment of DCOI
Calculation of exposure
concentrations (PEC)
In order to calculate the exposure concentrations (PEC, Predicted Environmental
Concentration), a model was established, based on principles normally used for exposure
assessments (EC 1996). The exposure assessments were made for two scenarios:
| A pleasure craft harbour (on the basis of the conditions in the pleasure craft harbour of Jyllinge) | |
| A busy navigation route (on the basis of the conditions at the Kronprins Frederiks Bro, Frederikssund) |
The model and the two scenarios are described in detail in Appendix 1. For the parent compound and the most essential metabolites, the following exposure concentrations were calculated for each of the two scenarios:
| PEC (water column) | |
| PEC (sediment) | |
| PEC (sediment-pore water) |
The three exposure concentrations were defined as the steady-state concentration of the sub-environment in question. I.e., the concentration which the calculated concentrations eventually approach when a continuous leaching of the parent compound to the water environment is simulated. The calculations of PEC have been made by use of realistic worst-case scenarios, which means that the parameters used in the model are based on realistically conservative assumptions, which results in the fact that, in practice, the calculated PEC values are seldom exceeded. The model used is not validated towards measured concentrations in harbour environments or navigation routes. More of the assumptions that form part of the simulation are of vital importance to the result of the calculations:
| The background concentrations for both the parent compound and the metabolites were
assumed to be zero. |
| 70% of the pleasure craft was assumed to have been painted with paint containing DCOI. |
| The leaching rate of DCOI from bottom paints was calculated at 13 mg/m2/day
in harbours and 25 mg/m2/day when sailing (Appendix 1). |
| The primary biological transformation of DCOI into the expected metabolite N-(n-octyl) malonamic acid was assumed to proceed with a half-life of 14 hours in surface water at a temperature of 12°C. |
The biological half-life of DCOI of 14 hours, which was assumed in the simulation, is established on the basis of an experimentally determined half-life in seawater at 12°C (Jakobson and Kramer 1999). The half-life of DCOI in seawater and not in seawater and sediment was chosen as the result of the simulation is exposure concentrations at a continuous leaching of DCOI to seawater after steady state was achieved. When the pleasure craft are taken out of the water at the end of the sailing season, DCOI will probably be rapidly eliminated as DCOI is either transformed in the water phase or sorbs to the sediment, in which it is transformed with a very short half-life (cf. Sections 3.2.2 and 3.2.3).
The exposure concentrations calculated for DCOI and its metabolites are approx. 50 times higher in the pleasure craft harbour than in the busy navigation route outside the harbour (Table 3.7).
Table 3.7
Calculated exposure concentrations (PEC) for DCOI and metabolites at steady state.
Scenario |
Substance |
PEC (water) |
PEC (sediment, pore water) |
PEC (sediment, sorbed) |
mg/L |
mg/L |
mg/kg |
||
Pleasure craft harbour |
DCOI |
0.52 |
0.0015 |
0.12 |
N-(n-octyl) malanomic acid |
1.98 |
0.83 |
2.32 |
|
N-(n-octyl) beta hydroxy- propionamide |
0.020 |
0.14 |
0.43 |
|
N-(n-octyl) acetamide |
0.050 |
0.084 |
2.42 |
|
Other compounds |
0.10 |
|||
Navigation route |
DCOI |
0.0061 |
0.00002 |
0.0014 |
N-(n-octyl) malanomic acid |
0.040 |
0.011 |
0.031 |
|
N-(n-octyl) beta hydroxy- propionamide |
0.00071 |
0.0019 |
0.0058 |
|
N-(n-octyl) acetamide |
0.0018 |
0.0013 |
0.039 |
|
Other compounds |
0.0040 |
Calculation of Predicted No-Effect Concentration (PNEC)
The Predicted No-Effect Concentrations (PNEC) are estimated for DCOI and N-(n-octyl)
malonamic acid. The other stable metabolites from the transformation of DCOI are
considered to have aquatic toxicity corresponding to that of N-(n-octyl) malonamic acid.
The available studies on the aquatic toxicity of DCOI are considered representative and the data include long-term studies with fish and crustaceans. The algal test may be interpreted as a short-term as well as a long-term test (EC 1996).
For DCOI, three NOEC values from long-term tests (fish, crustaceans and algae) are available, including the groups of organisms most sensitive in short-term tests (fish). On this basis, PNEC is calculated by dividing the lowest NOEC value, which is 0.00063 mg/L for crustaceans (Ward and Boeri 1990), by an assessment factor of 10 (EC 1996). This results in a PNEC of 0.00006 mg/L = 0.06 mg/L for DCOI. As already mentioned in Section 3.3.2, no unambiguous NOEC value can be derived from this long-term test with crustaceans (Ward and Boeri 1990). If this study is ignored, the results from one single long-term study with fish are available, in which NOEC was 0.006 mg/L. In this case, an assessment factor of 100 is applied, which results in a PNEC value calculated at 0.00006 mg/l = 0.06 µg/L, which is identical with the above calculated value.
Calculation of PNEC for N-(n-octyl) malonamic acid is based on the lowest effect concentration. As data primarily originates from short-term tests, an assessment factor of 1,000 is used for the lowest effect concentration. For N-(n-octyl) malonamic acid, the lowest reported LC50 = 250 mg/L for rainbow trout while the value for daphnia (EC50 = 260 mg/L) as already mentioned above is a moot point. For the calculation of a PNEC for N-(n-octyl) malonamic acid, an LC50 value of 90 mg/L (towards daphnids) is used as this value is considered the actual effect concentration in the tests performed (cf. Section 3.3.2). This results in a PNEC value of 0.09 mg/L = 90 µg/L. The calculated PNEC for N-(n-octyl) malonamic acid is assumed to be representative of the other metabolites from the transformation of DCOI. The two calculations of PNEC are shown in Table 3.8.
Table 3.8
Calculation of PNEC for DCOI and N-(n-octyl) malonamic acid.
| Substance | Lowest effect concentration |
Value |
Assessment factor |
PNEC |
| DCOI | Long-term test NOEC crustaceans |
0.63 |
10 |
0.06 |
| N-(n-octyl) malonamic acid | Short-term test EC50 crustaceans |
90,000 |
1,000 |
90 |
Risk quotient
On the basis of the above calculated PEC (water) values for DCOI and its metabolites
given in Table 3.7 and PNEC values for DCOI and N-(n-octyl) malonamic acid, the risk
quotients Rq = (PEC/PNEC) can be calculated as shown in Table 3.9.
Table 3.9
Calculation of risk quotients (Rq) for DCOI and its metabolites.
Substance |
PNEC [µg/L] |
Pleasure craft harbour |
Navigation route |
||
PEC |
Rq |
PEC |
Rq |
||
DCOI |
0.06 |
0.52 |
8.7 |
0.0061 |
0.10 |
Metabolites |
90* |
2.2 |
0.02 |
0.047 |
0.0005 |
* N-(n-octyl) malonamic acid
The stated risk quotients are calculated on the basis of realistic worst-case scenarios (Appendix 1), which are i.a. based on the assumption that 70% of the pleasure craft are painted with a bottom paint containing DCOI. On the basis of the assumptions made in the simulation and of the calculated PEC values, it is considered likely that a risk of chronic ecotoxic effects within the pleasure craft harbour may exist as, presumably, DCOI will constantly be applied by leaching from bottom paints. The risk quotient for DCOI out of the harbour is less than 1 and here, the risk of ecotoxic effects is considered to be low.
Within as well as out of the pleasure craft harbour, a very small risk of ecotoxic effects of metabolites from the transformation of DCOI is considered to exist.
[Front page] [Contents] [Previous] [Next] [Top] |