Cross-flow filtration of fruit juice
Contents2. Theory 4. Pre-treatment 5. Microfiltration 6. Scaling 7. Fouling and cleaning 9. Ultrasounds 10. Feasibility studies
PrefaceAuthors Parts of the project
Project partners
The project was carried out in the period: Marts 1998 – February 2000 and was financed by the Danish Ministry of Environment and Energy and the industrial partners. We would like to use this opportunity to thank all partners involved for a good co-operation throughout the project. Engelsk forord, litteraturliste, bilag m.v.
SummaryCurrent filtration of a wide variety of process fluids from the Food, Pharmaceutical and Chemical industries is accomplished using filter aids. These filter aids have excellent filtration qualities, however there are some disadvantages involved in their use:
The general aim of this project is to develop and implement a system to filter various types of process fluids. The applied aim of this project is to develop a filtration system to clarify juice keeping and / or improving the quality of the juice. The two main quality demands in juice processing are to preserve the organoleptic quality and clarify the juice for storage. Current filtration of a wide variety of juices is accomplished using filters aids. However, a final polishing is achieved by means of ultrafiltration. Membrane technology is currently a "proven technology" within a few main areas, i.e. food and dairy industry, water purification and treatment of liquid fluent streams, and it is presently being introduced into a wide variety of other applications. The recent development of membrane technology will strongly influence the way industry evaluates separation processes in the immediate future. The advantages of using membrane technology in the beverage industry are related to economy, working conditions, environment and quality (Hägg, 1998):
Cross flow microfiltration is one of the most recent developments in membrane technology, and it is replacing a number of traditional clarification and sterilisation operations in a wide variety of industries (Forbes, 1987). This technique is today used with success in some applications in the pharmaceutical and the biotechnological industry in Europe when purifying products of high value and low volume. The increase of disposal and purchase costs will make it economically feasible to invest in alternative filtration methods for several industry sectors with a large use of filter aids, such as the brewing and the beverage industries, and cross-flow microfiltration can be one possible alternative. Furthermore, some studies have indicated some losses in flavour when clarifying apple juice by means of ultrafiltration compared to microfiltration, and other advantages of microfiltration are better efficiency and shorter processing periods (Wu et al., 1990). This new technology could offer a competitive and attractive alternative to the filtration technique that currently dominates the market because of its efficiency and because it is harmful to public health and environment. The project was divided in five parts:
The research institutions and companies involved in the project were:
The investigations carried out during this project indicated that Filtomat thread filters could be successfully used to produce black currant juice of excellent quality, since these can replace the vacuum filter. Further microfiltration was required when filtering sour cherry juice. Good performance and high fluxes were achieved with the Filtomat thread filters (pre-treatment) and the polymeric membranes from X-Flow (cross flow microfiltration). Furthermore, the quality of the juice obtained was improved since running at low temperatures avoids the precipitation of polyphenols, which results in undesired turbidity. The feasibility studies confirmed the potential of this new technology, since it results in lower production costs and it is also better for the environment when compared to the technology used nowadays. When applying this technology at larger scale, the shadow effect did not result in complications. However, the pressure distribution and the control of the backshock should be further investigated. It has also been demonstrated during this project that the backshock technique and high power ultrasounds had a positive effect on the microfiltration performance and efficiency, and that these were feasible methods to reduce fouling when filtering sour cherry juice. The composition of the juices investigated during this project is quite complex and these contain several components that could disturb the filtration and the cleaning processes. Some studies have verified that the nature of the foulants is complex and that the role of the sugars in membrane fouling should be further investigated. Furthermore, it has been proven that the cause for pore blocking was the amount of juice filtered, and not the concentration.
1. IntroductionGeneral aim The general aim of this project is to develop and implement a system to filter process fluids from the Food, Pharmaceutical and Chemical industries in order to substitute the use of filters aids, i.e. kieselguhr, Applied aim The applied aim of this project is to develop a filtration system to clarify juice keeping and / or improving the quality of the juice. Kieselguhr filtration The mineral-based Kieselguhr filter is the most widely used filtration technique, because it meets required turbidity standards while maintaining good taste levels. However, this technique involves significant costs (DE filters cost between 60,000 and 100,000 Euro per year to small and medium-sized fruit juice manufacturers), unhealthy (the handling of DE powder during the replacement of the filter involves dust inhalation that can cause lung diseases), and polluting (because of the deposition of sludge or "filter cakes" after use). Some European governments are considering banning the use of Kieselguhr because of the dangers it represents to public health and to the environment. Moreover, the disposal of DE is becoming more and more costly as a result of the harmonization policy of the European Commission with regard to disposal costs. For instance, while disposal costs in Germany range between 30 and 120 Euro per ton of disposed materials, these costs will be set to a level of 600 Euro per ton in the coming years. European brewing and beverage industries are thus exploring alternative filtration techniques. Membrane filtration Membrane technology is currently a "proven technology" within a few main areas i.e., food and dairy industry, water purification and treatment of liquid effluent streams and this technology is presently being introduced into a wide variety of other applications. There is clearly a very positive trend for the development of industrial membranes, which will strongly influence the way industry evaluates separation processes in the immediate future. This new technology will offer a competitive and attractive alternative to the filtration technique that currently dominates the market because of its efficiency and because it is harmful to public health and environment. Usually process solutions using integrated hybrid membrane systems will often be the best solution to a specific industrial separation problem. The lifetime of the membrane material is crucial for the integration of membrane modules in industrial process streams which will usually transport fairly large amounts of gas and liquids at pressures and temperatures where the durability of the membrane materials over time are not yet fully exploited. If the membranes have to be replaced too often or if the membrane is easily damaged, the solution may be too expensive. Membrane technology can work as well or better than the existing technology regarding product quality, energy consumption and environmental issues. The costs of this technology are not currently at a level, which will make the implementation attractive for all applications, but this is on its way. Membrane technology demands that basic research within material science is coupled to the understanding of problems related to the specific industrial process where the membrane module is to be integrated. Too often research at laboratory scale shows promising results, but a stronger involvement of industry is necessary in order to develop the membrane to commercial level and to promote the incorporation of membrane modules in a process together with other unit operations. The argument for doing so is the obvious advantages of this technology: cleaner and simpler process solutions, less chemical additives and lower energy consumption. The demand on industry for better environmental solutions and cleaner technology is also pushing the development and implementation of membrane technology.
2. Theory2.1 Sour cherry and Black currant juices 2.1 Sour cherry and Black currant juices 2.1.1 Cherries and cherry processing 2.1.1.1 Origins and botanical data The cherry is part of the family Rosaceae and belongs to the genus Prunus. Other members of this genus include apricot, nectarine, almond, peach, and plum. Cherries have traditionally been used for a wide variety of food and beverage products in Europe, and with European migration this tradition has spread to North America and to many other parts of the world (Kaack, 1990). The red cherry (Prunus cereus L.), also know as sour, tart or pie cherry, is a drupe fruit and originated in the territory between Switzerland and the Adriatic Sea on the west of the Caspian Sea and northward on the east (Hedrick, 1914). Although there are 270 named varieties of red cherries, only a few are grown for commercial purposes, and therefore are well known. Based upon the colour of juice in the fruits, these can be divided into two groups. Those giving a colourless juice are known as Amarelles while those with a darker colour and a reddish huice are known as Morellos. The Morellos are more acid and sour (Webster & Looney, 1996). Nutrition Nutritional studies have shown that red or sour cherries are a good source of Vitamin A, calcium, iron, potassium, and phosphorous. More detailed nutritional information is given in Table 2.1. Table 2.1. Nutritional Analysis of 100 g Fresh Sour Cherries (Cash et al., 1989).
Cyanidin-3-rutinoside and cyanidin-3-glucoside are important anthocyanin pigments in Morello sour cherry (Hong & Wrolstad, 1990). 2.1.1.2 Anatomy, physiology and composition Although characteristics of berry and stone fruits differentiate, the anatomy and physiology of these fruits are similar. Three different tissues can be differentiated: exocarp or skin, mesocarp or fruit flesh, and the endorsperm or section of seeds or stones. Skin The skin provides protection and often contains small amounts of valuable juice. Fruit flesh The mesocarp consists of very big cells, which contain nearly all characteristic and desired components. Seeds The seeds or stones usually contain large quantities of tannins. Therefore, destruction during processing is avoided. An exception is the production of cherry juice where the destruction of approximately 10% of the stones is desired for sensoric enhancement (Hamatscheck et al., 1995). Sour cherry juice contains a lot of small compounds such as phenols, proteins and different kinds of polysaccharides. All these compounds make the filtration of sour cherry juices difficult. Phenols The phenols in sour cherry juice are mainly hydroxycinnamic acids and anthocyanins (Macheix et al., 1990). The anthocyanins are responsible for the red/purple colour of the juice (Grassin and Fauquembergue, 1996). The amount of phenols in the juice depends on the species, the ripeness of the berries, place of growth, and method of production (Lee, 1992). 2.1.1.3 Production figures Both the sweet and the sour cherry are deciduous trees originating around the
Caspian and the Black Seas, and therefore thrive best in areas with a temperate or
Mediterranean-type climate. Cherries are known to be produced in significant quantities in
more than 40 nations of the world (Webster & Looney, 1996). 2.1.1.4 Harvesting, Handling and Processing The principal red cherry varieties are self-fertile when the conditions are proper (warm weather, adequate bee activity, required pollen tube growth, and proper nutrition). In selecting the proper location for a red cherry orchard, it is necessary to consider which sites are more susceptible to spring frosts, since cherries are very vulnerable to freezing three to four weeks before full bloom. Winter temperature should not go below –26°C, and the mean temperature for June, July, and August should be about 15°C. The blossoming period is sometime between mid-April and mid-May, depending on the location. The fruit ripens in late June to early July and may be harvested as late as mid-August in the more northern regions. Time of harvest Harvesting of cherries should be carried out at the optimum maturity stage for each
commodity.
Harvesting For many years, the harvesting and sorting of red cherries relied on workers, however, nowadays, the machines have simplified and speeded the harvesting operation as well as improved the processing. Mechanical harvesting is successfully carried out with the help of a trunk shaker. This machine shakes each trunk with a few firm movements loosening the fruits and dropping them into inclined frames. In this way, the fruits roll onto conveyor belts and then to tanks of cold water in order to be washed, to remove spray and to promote firming. Sorting As a first step of processing, the cherries go through an eliminator, where foreign matter and defective, undersized and immature fruit is removed. The next step is the sorting and an electronic sorter has taken the place of hand sorting. Once graded, the cherries move through mechanical pitters in order to separate them from the pits. From there, the cherries move along belts and are inspected again for blemishes, loose pits and foreign matter. Cherries will be processed differently according to the final product. Sour cherries are primarily grown for use in processing; they are used for juice and other beverages, nectar, jams and jellies, yoghurt, and are canned and frozen whole (usually pittet) or further prepared as fillings for use in a wide variety of bakery products (Poll, 1986). Small quantities of sour cherries are dehydrated and in some countries speciality wines are made from sour cherry juice. Furthermore, there is a considerable interest in using anthocyanin pigments from cherries as natural sources of food colorants (Chandra et al., 1993). Cold pressing frozen cherries Juices from berries and stone fruits are an important product of the fruit juice industry as beverage products and as ingredients for the soft drink, spirit and candy production (Hamatscheck et al., 1995). The cherry juice is the only deciduous fruit yielding bright red juice, but its strong flavour results in a limited commercial use compared to orange, grape and other popular juices. Consumers preferred when diluted or blended with another juice such as apple juice. The most important prerequisite for the production of a high quality product is an optimal ripe, unimpaired, sound and clean raw fruit (Schobinger et al., 1987), and the two main quality demands in juice processing are to preserve the organoleptic quality and clarify the juice for storage. Good quality Good quality cherries should be used to produce juice: overripe or spotted cherries produce a juice with a higher benzaldehyde flavour which is considered undesirable; mushy fruit can result in a juice with so much suspended material that filtering becomes difficult and the juice will become cloudy during storage. On the other hand, less mature fruit results in a juice, which is low in sugar, poor in colour and lacks some soluble components that contribute positively to the flavour. Assessing the suitability of raw fruit for juice processing mainly involves determining juice-soluble solids, acidity and anthocyanin-content. However, the incidence of cracking and decay, uniform maturity and the estimation of the yield of juice by pressing are also very important considerations. Juice yield depends mainly on the percentage of fruit flesh. In industrial sour cherry production, the berries are milled after washing and sorting. Then, a mashing treatment is necessary to increase the juice yield and extract other fruit components such as colour and flavour. Next comes the pressing stage. Applied pressure is very important: the recommended procedure is to start with a low pressure and gradually increase. There are three methods for pressing cherries for juice: hot pressing, cold pressing and cold pressing of frozen cherries. Hot pressing Hot pressing is the simplest procedure. Pitted fruit is heated to about 66.5°C and the cherries are pressed before they cool. The resulting juice is strained, chilled, allowed to settle, and then filtered. Juice is a deep red colour since the heating extracts a greater level of the pigments present. However, the flavour is more similar to that of canned cherries rather than fresh fruit. Cold pressing Cold pressing yields a juice not rich in colour, but that has a flavour similar to fresh cherries. In this procedure the cherries are coarsely ground or pulped to aid in the extraction of the colour. The ground fruit is then pressed and the expressed juice is flash heated, cooled to inactive enzymes and reduce the microbiological load, clarified and filtered. Cold pressing frozen cherries combines the best features of the hot and cold press methods. A dark juice is obtained, and its flavour resembles the fresh cherries. The cherries are either pitted or crushed and then frozen with or without sugar added. Prior to pressing they are thawed to a temperature of 4.5-10°C. After pressing, the juice is clarified and filtered (Tressler & Joslyn, 1971). Centrifugation Berry fruits can also de-juiced by means of a decanter after crushing. Centrifugal force is the effective principle, whereby liquid and solids are separated due to the different density. Separators and decanters are an indispensable integral part of modern technologies for the production of berry and stone juices since they completely avoid product looses and improve quality. Decanters are an alternative to conventional pressing techniques and both methods are comparable regarding yield and sensoric quality. However, a slight increase in phenolic compounds is observed when using the decanter (Hamatscheck et al., 1995). The prior removal of the stones is not required in case of juice extraction with the decanter. Depending on the pre-treatment of the fruits and the technology applied for phase separation, the recovered juices contain a certain quantity of cloud particles, which will be partly or totally removed during the further processing stages. After the pressing stage, the cloudy juice leaving the press is screened to remove any coarse material. Then, an enzyme treatment is necessary for viscosity reduction and better clarification (Baumann, 1981). Aroma development can be enhanced or suppressed during processing. With enzyme- or acid-catalysed conversion of amigdalins, occurring in the seeds and the fruit flesh, glucose, hydrocyanic acid and benzaldehyde are released. The latter is a very important aroma component in cherry. Because of these reactions, a pleasant cherry flavour can be obtained if about 20% of the stones are crushed. This is a practice often followed in the production of juices and wine. Enzymatic treatment In most cases, enzymatic decomposition of pectins is of advantage for an economic juice recovery. For juice extraction from fruits and vegetables, the cell walls have to be ruptured at least at one point. In practice, a combination of mechanical and enzymatic procedures achieve this, and in individual cases, this is supported by heat temperature treatment. During depectinisation, pectic enzymes hydrolyse pectin and, thereby, reduce the viscosity of the juice, making it more easily filtered. In addition, since pectin is a high molecular weight colloid, which acts as a protective colloid in suspending the particles in sour cherry juice, these particles are released when it is hydrolysed and settle to leave the supernatant juice clear. Thus, the amount of gelatine used during the later clarification can be decreased. There are two possibilities for using enzymes in juice processing: mash treatment and juice clarification (Höhn, 1996). Because of the low content of pectins in sour cherry, enzyme treatment before pressing is not always necessary. However, it is possible to obtain better quality and higher yield by treatment with pectin-degrading enzymes at 40-50°C (Baumann & Gierschner, 1974). Adding pectolytic enzymes to the mass prevents the formation of a pectin gel and increases the juice yield dramatically. The enzymes that are used for processing berry fruits have to meet special requirements, which are caused by the production technology and the character of the raw material. The mash is usually heated after crushing and special tubular heat exchangers are used for this production step. Heating the mash has several effects:
Cherry juice is generally clarified and filtered (Sahin & Bayindirli, 1993). Clarification In the clarification of sour cherry juice, the amount of gelatine used is very
important as its use other than in low quantities may cause colour loss. During
clarification, suspended particles in the juice settle. The reason for this precipitation
action is the charge difference between the colloidal material in the juice and the
clarifying agent. Settling of suspended particles makes the juice clearer and more easily
filtered. Current filtration of a wide variety of juices is accomplished using the minerals Diatomaceous Earth and Perlite (filter aids). The semi-concentrate is normally polished by means of ultrafiltration (McLellan, 1996). If enzymatic treatment of the mash is carried out before phase separation, juice yield and clarification efficiency are increased. Subsequently, the product is de-pectinised, de-aromatised, and pre-concentrated. During industrial processing, a small amount of anthocyanin is degraded but, more
importantly, the juice contains much less benzaldehyde and cyanide (from the crushed
stones) than is found in the macerated fruit (Table 2.3). Only about 18% of the
benzaldehyde and cyanide is extracted to the juice.
2.1.2 Black currants and processing Black currants contain large amounts of ascorbic acid (vitamin C), pectin and phenols. Pectin, which is primarily found at the cell wall, has the ability to form stable gels with water and enzyme treatment is therefore necessary to achieve an optimal pressing of the berries (Pilnik & Voragen, 1991). Phenols The phenols in black currants are mainly anthocyanins, which are responsible for the red/purple colour (Grassin & Fauqembergue, 1996). The amount of phenol in the juice is depending on the black currant species, the ripeness of the berries, place of growth, and method of production (Lee, 1992). 2.2 Membrane processes Membrane technology is still evolving and finding more and more applications in food and pharmaceutical processes (Mulder, 1991) and the development of membranes will strongly influence separation processes in the future. Various pressure driven membrane processes can be used to concentrate, sterilise or purify aqueous solutions. Development of membrane technology Membrane technology can work as well or better than the existing technology regarding product quality, energy consumption and environmental issues. The costs of this technology are not currently at a level, which will make the implementation attractive for all applications, but this is on its way. Membrane technology demands that basic research within material science is coupled to the understanding of problems related to the specific industrial process where the membrane module is to be integrated. Too often research at laboratory scale shows promising results, but a stronger involvement of industry is necessary in order to develop the membrane to commercial level and to promote the incorporation of membrane modules in a process together with other unit operations. The argument for doing so is the obvious advantages of this technology: cleaner and simpler process solutions, less chemical additives and lower energy consumption. The demand on industry for better environmental solutions and cleaner technology is also pushing the development and implementation of membrane technology (Hägg, 1998). Basic principles In membrane separations, each membrane has the ability to transport one component more readily than other because of differences in physical and/or chemical properties between the membrane and the permeating components. Furthermore, some components can freely permeate through the membrane, while others will be retained. The stream containing the components that permeate through the membrane is called permeate and the stream containing the retained components is called retentate. Driving force Transport through the membrane occurs as a result of a driving force acting on the individual components in the feed and usually the permeation (selective transport) rate through the membrane is proportional to the driving force. As it is seen in Table 2.4, membrane processes can be distinguished according to the type of the driving force that ensures the transport through the membrane. Table 2.4. Classification of membrane processes according to their driving forces (Mulder, 1991). 2.2.1 Microfiltration Microfiltration is the membrane process, which most closely resembles conventional coarse filtration. The characteristics of microfiltration are shown in Table 1.5. Table 2.5. Summary of Microfiltration (Mulder, 1991).
Dead-end vs. Cross-flow filtration There are two possible methods of operation in filtration processes: Dead-end and Cross-flow filtration. The simplest method used is the Dead-end filtration, where the feed flow is perpendicular to the membrane surface. It is forced through the membrane, which causes the retained particles to accumulate and form a type of cake layer at the membrane surface. The thickness of the cake increases with filtration time. The permeation rate decreases, therefore, with increased layer thickness. However, to reduce fouling, Cross-flow microfiltration is generally used. The feed flows parallel to the membrane surface and part of the retained solutes accumulates. The feed composition inside the module changes as a function of distance in the module, while the feed stream is separated in two: permeate (filtrated product) and retentate (unfiltrated product) stream. 2.2.2 Membranes General A membrane can be defined as an interphase that separates components according to their structure. In a more general way, a membrane is a permselective barrier through which fluids and solutes are selectively transported when a driving force is applied across it. The first membranes were produced in Germany in 1920 and used as filter for bacteria at laboratory scale. Since then, the membrane technology has been significantly developed. Nowadays, membranes are manufactured from a wide range of materials and they can offer a good selectivity, a high permeability and a considerable chemical stability. Structure The structure of the employed membrane is chosen according to the particles or molecular size, shape and chemical properties of the feed solution. Membranes can be classified as either biological or synthetic according to their nature. Regarding to morphology or structure, solid synthetic membranes can be classified as symmetric or asymmetric. Symmetric structure In the symmetric membranes, the diameter of the pores is almost constant through cross
section of the membrane. Furthermore, the entire membrane thickness causes resistance to
mass transfer acting as a selective barrier. In asymmetric membranes, the pore size at the surface is smaller, so only the thin top
of the layer determines the selective barrier. Large particles will not enter in the body
of the membrane. In this way, the plugging of the membrane is avoided. These membranes
combine the high permeation rate of a very thin membrane with the high selectivity of a
dense membrane. 2.2.3 Factors that influence the permeate flux during filtration 2.2.3.1 Transmembrane pressure Transmembrane pressure (TMP) is the driving force of the pressure driven membrane processes, and it is defined as the pressure difference between the retentate and the permeate side:
The permeate flux increases with the TMP, but the flux decreases with increasing resistance of the membrane. The relation between the flux and the membrane resistance is given by the Hagen Poiseuille´s equation:
Permente flux The permeate flux increases with the TMP but the relation between them is only linear when the feed is pure water. If the feed is another product, the flux becomes independent of the pressure and mass transfer controlled when the pressure increases above the level, where the concentration polarisation layer reaches a limiting concentration (see Fig.2.1). Figure 2.1.Generalised correlation between operating parameters and flux, indicating the areas of pressure control and mass transfer control (modified from Mulder, 1991). Gel layer The gel layer model explains the flux independence from the pressure, where the main
responsible for the limiting flux is the formation of a gel layer of a fixed
concentration. If the feed is pure water, this kind of plotting can be used to measure the Water permeability, which is an indication of the cleanness of the membrane. This phenomenon occurs because the membrane is free of any type of particles when pure water is used as feed. 2.2.3.2 Linear or cross-flow velocity Linear velocity is the velocity at which the feed flows across the membrane. For a tubular membrane the linear velocity can be defined as the relation between the feed flow (or retentate velocity) and the cross flow area of the membrane:
where Fret is the retentate flow [ m3/s] Acout is the cross-flow area [ m2] A higher flow rate tends to remove the deposited material and, consequently, reduces the hydraulic resistance through the membrane and, in this way, the obtained permeate flux will be higher. Higher feed flow rates also reduce the concentration polarisation phenomena by increasing the mass transfer coefficient. 2.2.3.3 Temperature Higher temperatures benefit higher permeate fluxes, since the viscosity will be lower and the diffusion higher. However, it is essential not to pass over certain limits, because high temperatures denature proteins and enhance microbial growth during processing. 2.2.4 Flux decline reasons During an actual separation, the membrane performance can significantly change with time, and often a typical flux-time behaviour may be observed: the flux through the membrane decreases over time. This behaviour is mainly due to the concentration polarisation and fouling. Mechanisms These two phenomena are aspects of the same problem, which is the build-up of retained components in the boundary layer of the membrane-solution interface. Both phenomena induce additional resistances on the feed side to the transport across the membrane, and at the same time, they are responsible for the gradual reduction of the permeate flux through the membrane, and for the change of the selectivity of the process. Concentration polarisation is a reversible phenomenon, while fouling is irreversible
and can be caused by several mechanisms: adsorption, pore blocking and/or formation of a
gel layer. The extent of these phenomena is strongly dependent on the type of membrane processes involved and the feed employed. The flux decline is very severe in Microfiltration and in Ultrafiltration and, very often, the process flux is less than 5% of that for pure water. Figure 2.2 shows a schematic representation of the various resistances that can arise during a separation process. Figure 2.2. Overview of various types of resistance towards mass transport across a membrane (Mulder, 1991). The flux through the membrane can be described as: Flux = driving force / (viscosity * total resistance) or:
Resistances, concentration polarisation and adsorption 2.2.4.1 Concentration polarisation Concentration polarisation describes the concentration profile of the solutes in the liquid phase adjacent to the membrane resulting from the balance between different transport phenomena (general convection and back diffusion). As it has been named before, this is a reversible mechanism that disappears as soon as the operating pressure has been released. Concentration polarisation is responsible for the decreasing flux in Cross-flow microfiltration during the first 15 seconds of operation. Accumulation of solutes When a driving force acts on the feed solutions (solvent and solutes), solutes are
partially retained while the solvent permeates through the membrane. This means that the
membrane has a certain retentivity for the solutes, whereas the solvent can pass more or
less freely. Thus, the concentration of the solutes in the permeate (cp) is
lower than the concentration in the bulk (cb), and this fact is the basic
principle of membrane separations. As the membrane is to some degree impermeable for the
solutes, the retained solutes can accumulate at the membrane solution interface and their
concentration will gradually increase. These components can only be transported back to
the bulk solution by diffusion and turbulent flow caused by the tangential flow along the
membrane surface. This phenomenon of surface accumulation is called concentration
polarisation. Figure 2.3. Schematic representation of hollow fibre and concentration polarisation profile for a normal asymmetric membrane and a reverse asymmetric membrane, where (a) skin layer, (b) porous layer, (J) permeate flux, (Cp.) concentration of the solute in the permeate, (Cb) concentration of the solute in the bulk solution, and (Cm) concentration of the solute at the surface of the skin layer (Guerra et al., 1996). 2.2.4.2 Membrane fouling One of the major problems in the application of membranes in the industry is fouling and this aspect will be reviewed in a forthcoming section. 2.3 Juice filtration The two main quality demands in juice processing are to preserve the organoleptic quality and clarify the juice for storage. Current filtration of a wide variety of juices is accomplished using filters aids, i.e., Diatomaceous Earth (DE) and Perlite, because it can meet required turbidity standards while maintaining good flavour levels. However, a final polishing is achieved by means of ultrafiltration. Filter aids DE or Kieselguhr is a natural substance derived from the cell walls of certain microscopic algae. Deposits of DE are found in various locations, including the US, England, and France. After mining and processing, DE can be supplied in a variety of grades. Perlite or volcanic silicate is an ore of volcanic rock containing silica. When crushed and heated, perlite expands to become a light, fluffy powder and is then suitable as a filter aid (Munroe, 1995). These filter aids have excellent filtration qualities, however they involve significant costs, can not be cleaned, and are discarded after use. As a result, it is a consumable in many filtration processes. DE filters cost between 60,000 and 100,000 Euros per year to small and medium-sized fruit juice manufacturers. In the last years 9000 tons of filter aids have been used annually in Denmark. Disadvantages of filter aids The use of filter aids in filtration has no known harmful effects. However, there are some disadvantages involved in their use (Casani et al., 1999):
The handling and disposal involve risks of inhalation, with specific health risk of silicosis, a disease of the lungs caused by inhaling siliceous particles. As a result, a number of health authorities want to reduce or eliminate the use of these filter aids by finding economic substitutes, and some European governments are considering banning the use of Kieselguhr (Russ, 1992 and Bridge, 1987). Disposal costs The main driving force in substituting kieselguhr filtration with new filtration methods is that the costs of disposal of kieselguhr sludge will increase dramatically in the years to come in Europe. The European Community causes this trend through harmonisation of the cost of disposal from country to country. Today the costs of disposal in Germany range between 30 and 120 Euros per ton of disposed materials, and these costs will be set to a level of 600 Euros per ton in the coming years (Kvistgaard & Jensen, 1994). For more than 30 years, centrifuges have been an integral part of the technology applied in fruit juice processing for the separation of insoluble solids (Hamatscheck & Schöttler, 1994). However, the use of centrifuges results in high energy requirements and costs. Membrane technology Membrane technology is currently a "proven technology" within a few main areas, i.e. food and dairy industry, water purification and treatment of liquid fluent streams, and it is presently being introduced into a wide variety of other applications. The recent development of membrane technology will strongly influence the way industry evaluates separation processes in the immediate future. Membrane technology can work as well or better than the existing technology regarding product quality, energy consumption and environmental issues. Advantages of membrane technology The advantages of using membrane technology in the beverage industry are related to economy, working conditions, environment and quality (Hägg, 1998):
Ultrafiltration Nowadays, ultrafiltration is still the dominating membrane separation technique for clarification of clear juices (McLellan, 1996). Hollow fibre ultrafiltration membranes (cutoff 50,000 or 100,000) produced by Romicon, Inc., Massachussets, USA, have been successfully used to clarify apple juice. The apple juice presented an excellent quality. The UF membrane holds back essentially all the polysaccharide materials such as pectin and starch, which are responsible for cloud and sedimentation (Short, 1983). However, some studies have indicated some losses in flavour when clarifying apple juice by means of ultrafiltration compared to microfiltration. Furthermore, other advantages of microfiltration are better efficiency and shorter processing periods (Wu et al., 1990). Microfiltration Microfiltration is also being used for the clarification and biological stabilisation of some beverages, and improvement in the Microfiltration membranes has led to improvement of bacterial and hygienic qualities. Cross flow microfiltration is one of the most recent developments in membrane technology, and it is replacing a number of traditional clarification and sterilisation operations in a wide variety of industries (Forbes, 1987). This technique is today used with success in some applications in the pharmaceutical and biotechnological industry in Europe when purifying products of high value and low volume. The increase of disposal and purchase costs of filter aids will make it economically feasible to invest in alternative filtration methods for several industry sectors with a large use of filter aids, such as the brewing and the beverage industries, and cross-flow microfiltration can be one possible alternative. 2.4 Fouling & Cleaning Fouling may be observed in membrane filtration as serious flux decline. Fouling is very complex and difficult to describe theoretically. Even for a given solution it will depend on physical and chemical parameters such as concentration, temperature, pH, ionic strength and specific interactions. Membranes are used to remove wide variation of substances from different process streams. However, membrane fouling is the main factor reducing the applicability of the membrane processes. The degree of membrane fouling determines the frequency of cleaning, lifetime of the membrane, and the membrane area needed, and this will have a significant effect on the cost, design and operation of membrane plants (Speth et al., 1998). With concentration polarisation phenomena, the flux at a finite time is always less than the original value. When steady-state conditions have been achieved, a further decrease in flux will not be observed and the flux will become constant as a function of time. Polarisation phenomena are reversible processes, but in practice, a continuous flux decline can often be observed. Such continuous flux decline is the result of membrane fouling. Fouling should be expected from any feed stream and, it comprises the matter that has left the liquid phase (retained particles, colloids, emulsions, suspensions, macromolecules, salts, etc.) to form a deposit on the membrane surface (adsorption and constriction) or inside of the pores (blocking). Depending on the size of the particle and the membrane pore size different cases of fouling can occur, giving different flux declines (see Figure 2.4). Figure 2.4. Fouling schematics: Case A- pore narrowing and
constriction, Case B-pore Part of the fouling can be defined as permanent or irreversible, which means that requires mechanical or chemical cleaning to restore the membrane properties. Another fraction of fouling may be non-permanent or reversible, when the deposited material is swept away by cross-flow, just after the pressure difference has been released. In normal asymmetric membranes the fouling cake layer or boundary layer is formed on top of the membrane. After some time the cake layer will be responsible for the separation. The resistance that this layer offers to the permeate stream is very dependent on the dynamic conditions during cross-microfiltration, such as linear velocity and transmembrane pressure. In reverse asymmetric membranes the cake layer or the boundary layer is formed inside the support layer. Fouling substances, foulants, can be divided into five categories:
Biofouling is fouling, in which the main reason for the flux decline and operational problems is caused by accumulation of microorganisms. 2.4.1 Fouling analysis Flemming et al. (1997) presented a procedure for analysis of the fouled membrane. The procedure is initialized with an optical inspection of the membrane, followed by microscopically inspection in order to get more detailed information about the structure of the fouling layer. Then, a defined area of the fouling layer is scraped off and the total amount of organics is determined. Finally, a part of the material is suspended in water and more specific analysis of the organic foulants are carried out. The problem in analysing the foulants is that most of the methods used are destructive. Thus, the analysis must be done either before or after the filtration test, because after analysis the membrane is destroyed. 2.4.2 Methods to reduce fouling The decline of the flux is prejudicial to the economics of a given membrane operation and, for this reason, measures must be taken to reduce this. It is possible to minimise fouling to some degree using one or more of the following alternatives:
2.4.2.1 Backflush and Backshock techniques Fouling is due to the deposition of solids on the membrane surface and within its pores, retaining partly the macromolecules, and affecting membrane performance. The fouling layer can be reduced by maintaining a shear at the membrane surface, which drags the suspended or oversized particles in a direction normal to the permeate flow. This requires high cross-flow velocities or backflushing techniques. High cross-flow velocities require greater energy consumption and can create transmembrane pressure gradients along the membrane. Backflushing Backflushing is an in situ method of cleaning the membrane by periodically reversing the transmembrane flow. In this way, the stationary concentration polarisation profiles are disturbed and the fouling layer is removed from inside the membrane and from the membrane surface. The backflushing medium can be the permeate, another liquid or gas, but if the permeate flow is used for flushing, it results in a loss of permeate against an increased flux. Backshock Jonsson and Wenten (1994) and Wenten et al. (1995) introduced the novel "Backshock" technique to reduce the loss of permeate during the backflushing and optimises the operation time during the filtration process. In this technique, the permeate flow is reversed for a short period of less than a second in order to avoid or reduce fouling or concentration polarisation problems, allowing the use of low linear velocities, which reduces the cost of running the process. Backshock and reversed asymmetric membranes Jonsson and Wenten (1994) also reported the advantages in using the Backshock technique on reverse asymmetric membranes. In this type of membranes, the fouling deposited inside the porous structure is less compact and thicker than that formed on the surface of normal asymmetric membranes. The resulting fouling layer presents less resistance as long as the porous layer is not completely filled up. If the cake is not removed from the porous layer, the permeate flux will approach to zero. Therefore, it is important to apply a very frequent backflushing in order to remove the cake and to avoid compacting the porous layer. When the backshock technique is applied, the induced concentration profile across the porous layer for the reversed asymmetric membranes will permit a steady state with 100% protein transmission even if the skin layer is very selective. The advantages of this arrangement are that even at linear velocities as low as 1 m/s and with an appropriate pore size, it is possible to limit the extend of concentration polarisation and achieve highly stable fluxes. 2.4.2.2 Chemical cleaning Chemical cleaning is the most important for reducing fouling, with a number of chemicals being used separately or in combination. The concentration of the chemical and the cleaning are also very important relative to the chemical resistance of the membrane. In Table 2.6 several cleaning agents from three different companies are presented as well as their composition and function. The selection, combination and concentration of the cleaning agents will depend on the composition of the process fluid that is being filtered and on the type of membrane. Table 2.6. Cleaning agents from Scan Diversey, NovoDan and Henkel-Ecolab.
2.4.2.3 Ultrasounds Background Membrane resistance control in cross-flow microfiltration by the use of high-power
ultrasound acting on the membrane, has been reported by E.S.Tarlton and R.J.Wakeman on
china-clay and anatase and by Yutaka Matsumoto, on bakers yeast and Bovine Serum Albumin
BSA. Yutaca Matsumoto recorded the important observation that major improvement in performance of the filtration can be obtained when the trans-membrane pressure is shifted to 0 (Bar) during ultrasonic irradiation.
3. Juice ProductionThe steps and the process parameters followed during the production of cherry and black currant juice at Vallø Saft are shown in appendix 1 and 2. The production process of these two types of juices is quite similar, and it is done according to the following procedure:
Crushing
Spiraflow
Enzyme treatment
Pressing
Pasteurisation
Clarification
Filtration of sludge
Pressure filter and ultrafiltration
Pasteurisation
Evaporation
In order to control the process, different analyses and measurements should be performed during production (appendix 3). A batch should meet all the quality requirements after each step.
4 Pre-treatment4.1 Introduction 4.1 Introduction Process solutions using integrated hybrid membrane systems will often be the best solution to a specific industrial separation problem (Hägg, 1998). In this project two processes are being studied: Dead-end microfiltration with polymeric thread filters and Cross-flow microfiltration both with polymeric and ceramic membranes. The first process is intended to be used as a pre-treatment step and the filters have a pore size of 3, 5 or 10 mm. Aim The aim of this project is to substitute the filtration step with membrane technology combined with Filtomat thread filters. In this work the effect of some microfiltration parameters on the pressure during filtration, and the retention/transmission of proteins, sugar, turbidity causing compounds, phenols and colour is studied. Quality requirements Sour cherry and black currant juices (final product) should follow the quality parameters listed below according to Vallø Saft A/S (Denmark):
4.2 Materials and Methods 4.2.1 Sour cherry and black currant juice The experiments were performed using sour cherry and black currant juice supplied by Vallø Saft A/S (Denmark). This juice was produced according to the flow diagram shown in appendix 1 and 2, and obtained after the second and final centrifugation step (supernatant). The juice was stored at -20oC before use. Sour cherries (Prunus cerasus L.) named "Stevnsbær" were used to produce the juice. The species of black currant (Ribes nigrum) used was Ben Lemond. The berries were harvested in summer 1998 in Denmark. The characteristics of the centrifuged juices used in this work are shown in Table 4.1. Table 4. 1. Characteristics of centrifuged (twice) sour cherry and black currant juice.
Centrifuged sour cherry juice contains several different particles: phenols, proteins and different kinds of polysaccharides. Phenols and proteins can combine in complexes, which can dissipate. Phenols alone can also dissipate but only at low temperatures. Centrifuged black currant juice contains several different particles: phenols, proteins and different kinds of saccharides. Phenols and proteins can combine in complexes, which can dissipate. Phenols alone can also dissipated but only at low temperatures. 4.2.2 Filtomat thread filter Filtomat thread filters of 0.01 m2 from Filtration Ltd. (Israel) and supplied by Gustav Fagerberg A/S (Denmark) were used to run the experiments. Construction These filters or cassettes (Fig. 4.1) consist of a thread made of polyester wound round a plastic support. Two outlets (permeate) are located at the underside of the plastic support and these are connected to a plastic tube, which goes all the way through the support. This tube has pores, where the filtrate is collected and led to the permeate tank. The cassette is inserted into a closed container (filter housing). Figure 4.1. Schematic representation of a Filtomat thread filter. 1. Plastic support, 2. Thread layer, 3. Permeate outlet. The thread can be wound with different degrees of tightness and this will result in different pore sizes. The pore sizes of the filters used in this work were 3, 5 and 10 mm. These numbers are statistical and highly dependent upon external parameters such as water turbidity (NTS), water quality (PPM) and type of suspended solids. A sample is checked from each production batch, and the size of the sample depends upon the size of the production batch (source Filtration Ltd.). 4.2.3 Experimental set-up The microfiltrations were run in a flexible filtration system assembled at the Department of Biotechnology at the Technical University of Denmark. The experimental set-up is illustrated in Figure 4.2.
Figure 4.2. Schematic draw of the experimental set-up. A. Feed tank, B. Feed pump, C. Flowmeter, D. Two-way valve, E. Manometer, F. Filter housing, G. Filtomat thread filter, H. Frequency converter. Operation The feed tank and the juice were temperated before each experiment. A gear pump delivered the feed flow, which was measured by a flowmeter. The dead-end filtration can be controlled using 4 valves. Before filtration valves 1 and 4 were closed leading to the filling of the filter housing with juice. This was necessary to avoid air bubbles, which could influence the results. Valves 1 and 3 were closed during filtration. Valve 1 was open, valve 2 was closed and the gear pump stopped to stop the filtration. Manometers placed at the retentate and permeate side measured the pressure difference over the filter during filtration. 4.2.4 Equipment The following equipment was used during the experiments: Thermometer
4.2.5 General guidelines for the experiments The general aim of the experiments was to examine the potential of Filtomat thread filters and to study the effect of some microfiltration parameters on the pressure during filtration, and the retention/transmission of proteins, sugar, turbidity causing compounds, phenols and colour.
Experimental design MODDE (modelling and design) is a PC-Windows program for the generation and evaluation of statistical experimental designs (Umetri AB, 1992-1995). This statistical program was used to plan the factorial experiment and to evaluate the results in order to find out which factors had a real influence on the responses (Screening). Using factorial experiments has several advantages:
A 23 factorial experiment was designed varying the temperature, the flow,
and the pore size of the filters.
*= The high level of the flow for sour cherry juice was 70 and 80 for black currant juice. Table 4.2. Experimental design.
The filters were cleaned by flushing with water under the tap. The flow was 540 l/h and the filters were cleaned for 3 min. (1½ min. on each side). 4.2.6 Data treatment and Analysis 4.2.6.1 Data treatment All data was treated using the statistical program MODDE in order to evaluate which factors (temperature, flow and/or pore size) had an influence on the responses listed below.
4.2.6.2 Analysis 4.2.6.2.1 Pressure D TMP The pressure difference TMP was measured with the manometers described in the section on Materials and Methods, and calculated as shown in the theory section. TMP is a measurement for the membrane state. There are two phenomena, which can explain the increase of the TMP when running a filtration: a cake is being formed on top of the membrane, and / or the process fluid that is being filtered has reached a viscosity (up-concentration, temperature changes) which makes it necessary to increase the driving force in order to press the filtration medium through the membrane. TMP2 TMP was measured at the beginning (TMP1) and at the end of thefiltration (TMP2). The difference between these was chosen as a response (D TMP).The response TMP2 can give an indication of how easy it is to filter sour cherry juice under the given conditions. 4.2.6.2.2 Water permeability The water permeability (WP) gives an indication of the state of the thread filters, and it is defined as the amount of ultrafiltered water (V) passing through the surface of the filter (Afilt) in a certain time (t) and at a certain pressure difference (TMP). The water permeability is calculated by the equation: Wp (l/m2/h/bar) = V (l) * 3600 (sec/h) / Afilt (m2) / t (sec) / TMP (bar) All filters had previously been used between 3 and 5 times. The water permeability was
measured for these filters before filtration and for new filters with the same pore sizes.
4.2.6.2.3 Protein content The total protein content before and after each filtration was determined with a Macro-N (Foss Electric, Denmark). 4.2.6.2.4 Sugar content The sugar content was analysed using a Refractometer. 4.2.6.2.5 Turbidity The turbidity was analysed using a Nephla laboratory turbidity photometer conforming to DIN EN 27027 and ISO 7027. All samples were diluted to a oBrix of 3 before the measurements were performed. 4.2.6.2.6 Total Phenol content Principle Phenolic substances are oxidised by the Folin-Ciocalteu reagent, which contains a mixture of phosphotungstic acid (H3PW12O40) and phosphomolybdic acid (H3Pmo12O40). The reagent becomes partly reduced resulting in the production of the complex molybden-tungsten blue, which is measured spectrophotometrically at 765 nm (Singleton and Rossi, 1965). Equipment Perkin Elmer l 2 UV/VIS
spectrophotometer Reagents olin-Ciocalteu’s phenol reagent from Merck diluted 1:10 with double distilled
water Procedure 1.0 ml Folin-Ciocalteu’s phenol reagent diluted 1:10 with double distilled water was added to 0.2 ml sour cherry juice. 0.8 ml of 7.5 % (w/v) sodium carbonate was added to develop the colour and the mixture was mixed on a whirl mixer. The mixture was then left for 30 min. with caps on. After mixing again with a whirl mixer the absorbance was read at 765 nm, using double distilled water for background correction. If the absorbance exceeded 1, the sour cherry juice was diluted with double distilled water, and the assay was carried out again. The concentration of total phenols was calculated from the standard curve obtained by subjecting known amounts of gallic acid solutions (0, 5, 10, 20, 40, 60, 80, 100 mg/l gallic acid) to the same treatment as the sour cherry juice and blank samples. Results were expressed in gallic acid equivalents (GAE). 4.2.6.2.7 Colour The colour of the juice was measured in two different ways for sour cherry juice:
Vallø Saft method Colour measurement (Vallø Saft) Principle The anthocyanins are responsible for the red/purple colour of the juice (Grassin and Fauquembergue, 1996). The red colour is measured with a spectrophotometer at 520 nm where red coloured liquids have the maximum absorption. The value should be between 0.700 and 1.500 after diluting according to this assay for this harvest. Equipment Perkin Elmer l2 UV/VIS spectrophotometer Reagents Trisodium citrate dihydrate, pure, Merck Procedure A buffer of pH 3 was prepared by mixing 18 g of Trisodium citrate dihydrate with 55.5 g
Citric acid monohydrate in 1 litter double distilled water. After mixing, the absorbance of the diluted sample was measured against the buffer at
520 nm. pH differential method Principle Equipment Perkin Elmer l 2 UV/VIS spectrophotometer Reagents Sodium acetate trihydrate, p.a. Merck Procedure The two buffers are prepared as described below and afterwards adjusted to the exact pH: pH 1.0 buffer: 125 ml of 0.2 N Potassium chloride (136 g/l) pH 4.5 buffer: 400 ml of 1 M Sodium acetate (136 g/l) A juice sample is first diluted with the pH 1.0 buffer. The absorbance read at 510-540 nm should be lower than 1 and preferably between 0.4 and 0.6. The sample is diluted to the same degree with the pH 4.5 buffer. Scanning the two diluted samples from 350 nm to 700 nm gives two curves which maximum adsorption is between 510-540 nm. The absorbance at 700 nm should for each curve be 0 if the juice sample does not contain any haze. Subtracting the value read at 700 nm from the maximum absorbance is a correction for haze. The difference in the maximum absorbance will be proportional to the anthocyanin content, which can be calculated with the following equation based on the Lambert-Beer’s law: C (mg/l) = A/e L * MW *103 * Dilution Factor Where C is the concentration of the major anthocynin pigment in the fruit
The concentration of the major anthocynin pigment in a fruit is representing the concentration of the anthocynin pigment in the fruit. In sour cherry cyanidin-3-rutinoside is the major anthocynin pigment. It should be emphasised that the pH differential method is a measure of the monomeric anthocyanin pigments and results may not seem to be correlated with the colour intensity of the juice samples as they are judged visually. The colour was measure at 640 nm using a Perkin Elmer l 2 UV/VIS spectophotometer for black currant juice. All samples were diluted to a ° Brix of 3 before the measurements were performed. 4.3 Results and discussion 4.3.1 Sour cherry juice Since the area of the filters is 0.01 m2, the flux in these experiments was between 2000 l/h/m2 (low level of the flow: 20 l/h) and 7000 l/h/m2 (high level: 70 l/h). Water permeability Table 4.3 shows the results for the Water permeability measurements for all filters before filtration at a flow of 30 l/h and the standard deviations (SD). Table 4.3. Water permeability (Wp) at a flow of 30 l/h.
At this flow there were no significant differences between the Water permeability for the new Filtomat thread filters with a pore size of 5 and 10 m m. The same phenomenon was observed for the used filters with a pore size of 5 and 10 mm. Lower values were obtained for the filters with a pore size of 3 mm and it can be deduced that the TMP increases when the pore size of the filter decreases from 10 mm or 5 mm to 3mm. The new filters of all pore sizes had lower Wp values than the same filters
after they have been used 3 to 5 times. An explanation for this could be that the threads
in the used filters have loosened. This result emphasises the importance of ‘breaking
in the filters’ before use to avoid unnecessary uncertainties about the results. The results of the screening experiments run with sour cherry juice are shown in Table 4.4. Table 4.4. Results for the screening experiments run with sour cherry juice
Changing the levels of the three factors had a slight effect, when considering the
values of the quality parameters for the different experiments. Effect of the factors Table 4.5 shows the effect of the factors and their interactions on the responses: - An empty box shows that the factor does not have a significant effect on the response, - * Means that the factor has a significant effect on the response when analysing the data with a 95% confidence level, - ** Means that the factor has a significant effect on the response when analysing the data with a 99% confidence level, - *** Means that the factor has a significant effect on the response when analysing the data with a 99.9% confidence level,
Table 4.5. Effect of the factors on the responses. The effect in changing each factor from the low level to the high level will in the
following be considered. The increase on turbidity when performing at higher temperatures was probably not caused by the transmission of phenols or proteins since the temperature factor had no significant effect on either of the responses. The turbidity could be caused by b -glucans released by the fungus Botrytis cinerea when processing the berries. This fungus, which is related to the contamination of red berries, secrets a beta 1,3-1,6 linked glucan. This gum dramatically reduces the filterability and the clarity of the juice (Grassin and Fauquembergue, 1996). The increase on the flow from 20 to 70 l/h only affected the response TMP2. TMP2 increased with increasing flow. D TMP and TMP2 were lower when filtering with
bigger pore sizes, indicating that more particles passed through the filter. The factor
pore size had however no significant effect on any of the other responses. The interaction between the temperature and the pore size of the filters had a significant effect on the colour measured following the method from Vallø Saft; at higher temperatures and bigger pore sizes, more colour passed through the filter. The interaction between the flow and the pore size of the filters had a significant effect on the colour measured following the method from Vallø Saft; at higher flows and bigger pore sizes, less colour passed through the filter. This result is not logical and it indicates that this method (Vallø Saft) may not be reliable. Comparison to juice filtered at Vallø Saft Table 4.6 shows the comparison of sour cherry juice filtered at Vallø Saft following the production process used in industry and juice obtained at the Department of Biotechnology (DTU) by using Filtomat thread filters (experiment 7). Experiment 7 was run at high flow, at low temperature and through the 10 mm filter. This experiment was chosen since higher fluxes result in lower costs and low temperatures reduce microbial growth during processing keeping the quality of the juice for a longer period. Table 4.6. Quality parameters for juice produced at Vallø Saft A/S and at the Department of Biotechnology.
All quality parameters for the juice filtered with Filtomat thread filters were higher
than the ones for the ultrafiltered juice from Vallø Saft. The ultrafiltered juice from Vallø Saft had been frozen before the analyses were run which could influence the results. When cherry juice is frozen, phenol-protein complexes precipitate which could explain why the colour is lower and the turbidity is higher than the quality limits set by Vallø Saft. Turbidity is not an independent parameter when measured in sour cherry juice. When the sour cherries are crushed, 25 % of the stones are mashed and chemical compounds like benzaldehyde or hydrogen cyanide are liberated. Benzaldehyde is an aromatic oil which increases the value of the turbidity. A clear filtered juice could therefore have a turbidity of 20-40 FNU (source Vallø Saft). 4.3.2 Black currant juice Since the area of the filters is 0.01 m2, the flux in these experiments was between 2000 l/h/m2 (low level of the flow: 20 l/h) and 8000 l/h/m2 (high level: 80 l/h). The results of the preface screening experiments are shown in Table 4.7. The protein content, sugar content, phenols and colour were found more or less in the same amount as in the centrifuged juice, whereas the turbidity was reduced three times by filtering the centrifuged juice. The turbidity, the sugar content and the colour for all the experiments were within the quality limit, which means that the quality of all filtered juices was excellent. It is clear that the use of Filtomat thread filters improves the quality of the juice when filtering the centrifuged black currant juice from Vallø Saft A/S. Table 4.7. Results for the preface screening experiments run with black currant juice.
Effects of the factors Table 4.8. Effect of the factors on the responses
The effect in changing each factor from the low level to the high level will be
considered in the following. When the filtration flow was increased from 20 l/h to 80 l/h, the final TMP increased as well as the turbidity in the filtered juice. Probably some particles are pressed through the filter when the flow is increased. The increase on the turbidity when running at higher temperatures and flows was probably not caused by the transmission of phenols and proteins, since these factors have no significant effect on either of the responses. When Filtomat thread filters with a pore size of 10 mm were used, more particles could pass through and that explains why the turbidity and the phenol content were higher. The interaction between the temperature and the pore size of the filters had a significant effect on the final TMP (TMP2) and the transmission of the colour. Looking at the results related to the chemical composition of the juice (Table 4.7: protein, sugar, phenol and colour), no real differences are observed when changing the level of the factors. When the turbidity values are considered, no real differences are observed either, even though they statistically differ from each other. All the values are also under 5 FNU, which means that the quality of the juice is excellent. The opposite phenomenon is observed for the response D TMP. The four experiments carried out at a high flow (80 l/h) resulted in higher D TMP, but flow was not found to have a significant effect on D TMP. This result could indicate a correlation between flow, TMP and fouling. Comparison to juice filtered at Vallø Saft Table 4.9. Quality parameters for juice produced at Vallø Saft A/S and at the Department of Biotechnology.
Protein, sugar, phenol and colour were found in the same range for both juices. Turbidity was slightly lower for the juice produced at the Department of Biotechnology. The results show that a microfiltration after this pre-treatment should not be necessary. 4.4 Conclusions The conclusions drawn from these screening experiments are:
4.5 Filtration of large amounts of juice prior to centrifugation 4.5.1 Objectives Aim The general aim of these experiments was to investigate if it was possible to replace the vacuum filter with a Filtomat filter or a Cross filter when processing black currant and cherry juice. The juice used was taken directly after the pressing step or after the clarification step. 4.5.2 Materials and Methods The experimental set-up was the same as the one used in the previous experiment with black currant and cherry juice. The black currant and cherry juice were filtered either through a Filtomat filter (20 mm) 0,01 m2 or a Cross filter (25 mm) 0,02262 m2. The temperature was 22oC, and the flow was kept constant at 15 l/h using either a flowmeter or a frequency converter. 4.5.3 Results All results are presented in Table 4.10 and 4.11. Filtrating pasteurised cherry juice after the pressing step or cherry juice after the clarification step was not possible. This was due to the large particles. Filters with a bigger pore size should be used either in a single formation or in series. Table 4.10. Results for sour cherry juice.
Filtrating black currant juice after the clarification step was possible with the filters used, but not profitable. Filters with a bigger pore size should be used either in a single formation or in series. Table 4.11. Results for black currant juice.
1 It was not possible to measure the flow because of the large particles. The flow was controlled using a frequency converter. The experiments was terminated at a TMP > 0,75 bar.2 The experiment was terminated at TMP > 0,75 bar. 3 Since the juice easily went though the Filtomat filter (20 mm) and Cross filter (25 mm), Filtomat filter with a smaller pore size (3 mm) was used. When filtrating black currant juice after the pressing step, Filtomat filters with a smaller pore size (3 mm) was used since the juice easily went though the Filtomat filter (20 mm) and Cross filter (25 mm). The filtration of black currant juice after the pressing step was further investigated using a response surface model (CCF). The volume chosen for each of these experiments was 20 l, the filter
used was a Filtomat filter 0,01 m2 (3 mm), there was
no circulation and samples were collected after 2, 8, 16 and 20 l. The two factors
investigated in this experiment and their responses were:
The experimental design is shown in Table 4.12. Table 4.12. Experimental design.
A new filter was used for each experiment, and the water permeability was tested prior to used, to ensure that this variable did not have an effect on the filtration performance (Appendix 4). Effect of the factors The effect of the factors on the responses is shown in Table 4.13. Table 4.13. The effect of the factors on the responses after
filtrating
The effect of the factors on the responses should have been evaluated after filtrating 20 l. However, it was impossible to hold the temperature at 4oC during the whole experiment. Therefore, the effect was evaluated after filtrating 16 l. Appendix 11 shows that the temperature raised rapidly after approx. 16 l had been filtrated. This is also seen in Appendix 5. Changing the temperature from the low to the high level had a significant influence on the TMP (-**), FNU (***) and Phenol (*), whereas changing the flow from the low to the high level had a significant influence on TMP (-**) and FNU (***). For TMP and FNU, the effect of the factors was more significant when filtrating larger amounts of juice. For example, flow and temperature did not have a significant influence on the FNU after 2 l had been filtrated, after 8 l and 16 l the factors had a significant influence on level 95 % and 99,9% respectively, for the turbidity (FNU). This phenomenon was not observed for the responses protein and phenol. During filtration an inner cake was created on the Filtomat filters, and this improved the filtration. The content of phenol, protein and FNU in the permeate decreased with increased amount of juice filtrated. An example of this is shown in table 4.14. The rest of the results are found in appendix 12, where 1,2, and 3 stands for 2 l, 8 l, and 16 l filtrated juice. Table 4.14. Effect of the inner filter cake.
The change of TMP as a function of the filtrated volume for the different flows (15 l/t, 30 l/t, 45 l/t) and temperatures (4 oC, 13 oC, 22 oC) is shown in Appendix 5, 6 and 7. The change of TMP as a function of the time for the different flows (15 l/t, 30 l/t, 45 l/t) and temperatures (4oC, 13 oC, 22 oC) is shown in appendix 8, 9 and 10. The diagrams indicate, that the lower the flow, the higher the TMP was. The TMP also increased faster at the lower flow especially at low temperatures. An explanation for this phenomenon is that at higher flows, the membrane is more opened, and larger particles can therefore be pressed though. The inner cake will not be created in the same extent at high flows, and the resistance in the filter will therefore be smaller. The diagrams also indicate, that at a higher temperature the resistance in the filter is smaller, and the reason for this is that the solubility of the particles has changed with the temperature. The experimental results back up these theories (appendix 12). 4.5.4 Conclusion It is not possible to filtrate pasteurised cherry juice after the pressing step or
cherry juice after the clarification step through Filtomat filters with a pore size of 20 mm and through a Cross filter with a pore size of 25 mm. It is not profitable to filtrate black currant juice after the clarification step
through a Filtomat filter with a pore size of 20 mm and through
a Cross filter with a pore size of 25 mm. Table 4.15. Quality comparison between vacuum filter and Filtomat filter.
5. Microfiltration5.1 Microfiltration of Cherry Juice with Polymeric Membranes 5.1 Microfiltration of Cherry Juice with Polymeric Membranes 5.1.1 Objectives The aim of this work was to find out the suitability of polymeric membranes for the filtration of ultrafiltered sour cherry juice. 5.1.2 Materials and Methods 5.1.2.1 Sour cherry juice The experiments were performed using UF-sour cherry juice supplied by Vallø Saft A/S (Denmark). This juice was produced, according to the flow diagram shown in the previous section on Juice Production, from sour cherries (Prunus cerasus L.) named ‘Stevnsbær’. The cherries were harvested in Denmark in August 1998. 5.1.2.2 Membrane modules Membrane modules of 0.0094 m2 with reverse asymmetric structure (RAS) were used to run the experiments
Figure 5.1. Membrane module (Department of Biotechnology, Technical University of Denmark). Figure 5.1. Membrane module (Department of Biotechnology, Technical University of Denmark). These modules were assembled at the Department of Biotechnology of the Technical University of Denmark using hollow fibres and epoxy glue. The characteristics of the membrane modules are shown in Table 5.1. Table 5.1. Characteristics of the membrane modules. Characteristics of the membrane modules.
Each module consisted of 8 hollow fibres made of polyethersulfone-polyvinylpyrrolidone (PES-PVP) and supplied by X-Flow (Almelo, The Netherlands). The pore sizes of the fibres used in this experiments are shown in Table 5.2. Table 5.2. Characteristics of the hollow fibres. M2 is an abbreviation for hydrophobic.
5.1.2.3 Experimental set-up The microfiltrations tests were run in a mini-flexible rig built and assembled at the Department of Biotechnology of the Technical University of Denmark. The experimental set-up is illustrated in Figure 5.2. Figure 5.2. Schematic drawing of the experimental set-up. 5.1.2.4 Equipment The following equipment was used during the experiments:
5.1.2.5 Tests and procedures 5.1.2.5.1 Leakage test or Forward flow test (FF) The membrane module was tested before use for a possible leakage and pore characterisation. The test was accomplished by using the Palltronic FFE03 system. The leakage test is based on the forward flow principle and performed in the form of a pressure hold test. Pressurised air was applied to the wetted filter (800 mbar in 120 seconds) and the test equipment measured the decrease on pressure as a result of airflow through the filter. The test was used as an indication for a damaged membrane, ineffective seals, or a leak in the system. 5.1.2.5.2 Water permeability As mentioned in the previous section on Pre-treatment, the water permeability (Wp) gives an indication of the membrane condition, therefore it should be measured before and after each experiment as well as after each cleaning step. The water permeability was measured at dead-end mode. This means that the direction of the water is from the retentate side to the permeate side. Ultrafiltered water obtained within 1 minute was collected from the permeate side (permeate water flow). If the membrane is new or clean, the TMP has to be as low as 0.1 bar (max. 0.2 bar) when measuring the Wp in order to get the proper value, but not lower than this value in order to be out of the range of the manometer’s error (+/- 0.04 bar). If the membrane is partly or completely blocked, the TMP should not be below 0.4-0.8 bar. The FF test was performed and the Wp was measured according to the following procedure:
The Wp was calculated by using the equation shown in the section on Membrane Processes. 5.1.2.6 Start and filtration procedure Before filling the tank with juice, the system was run at the decided parameters with UF-water until the system had been stabilised. When the system was stable, the juice was added to the feeding tank and recirculated without coming in contact with the filter until its temperature was 2°C. At this point the filtration could be started.
5.1.2.7 Cleaning procedure After each filtration the system was rinsed with distilled UF water and the Wp
measured. All the valves in the cleaning system were closed and the cleaning procedure described below was followed:
The pH of the flushed water after cleaning was determined to ensure that the cleaning solutions had been completely removed from the filtration system. 5.1.2.8 Analyses The different responses were analysed or measured following the same procedure as shown in the section on Pre-treatment. 5.1.2.9 Data treatment Noted data and data from the PC logging were compared, selected, adjusted and the TMP and the permeate flux were calculated. TMP and permeate versus time was presented in a graph (Excel 5.0 for Windows) for each experiment. 5.1.3 Experiments & Results Several experiments were run in order to optimise the microfiltration efficiency and this was done by:
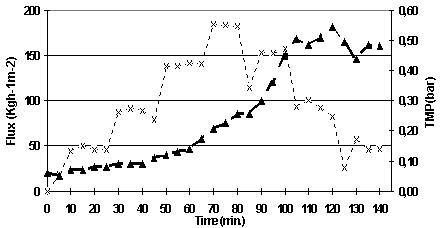
A selection of these experiments will be presented in this section. 5.1.3.1 Maximum permeate flux Seven experiments were run in order to study the microfiltration performance and to determine the maximum permeate flux when filtering UF sour cherry juice with a MF08 membrane. The most representative experiment will be presented in this section.
The permeate flux was set at 50 l/h/m2 at the beginning of the experiments and it was increased 50 l/h/m2 every 20 minutes until reaching a maximum. After that, the flux was decreased in the same way to 50 l/h/m2 in order to determine whether fouling is stopping the filter. The TMP was 0.06 bar at the beginning of the experiment and this was 1.21 bar at the end of the experiment. This means that the membrane had been fouled. A layer might have been deposited on top of the membrane resulting in an increase on the pressure difference. The maximum flux achieved was 350 l/h/m2. However, fluxes up to 500 l/h/m2 were reached in other experiments run at the same conditions with the same type of membrane. The Wp was 24500 l/m2/h/bar and 830 l/m2/h/bar at the end of the experiment. Further experiments should be run in order to determine whether the decrease on the flux is caused by fouling or concentration polarisation phenomena. 5.1.3.2 Backshock effect No backshock Several experiments were run in order to determine whether backshock had a positive effect on the microfiltration performance when filtering UF sour cherry juice with a MF08 RAS membrane. The three most relevant experiments will be presented in this section.
The first experiment was run without backshock for 20 minutes, and at that moment the TMP had already increased to 0.46 bar (Figure 5.3). Figure 5.3. Permeate flux and TMP versus time for the experiment run without backshock. This means that the membrane was getting fouled rapidly in these conditions. Therefore, backshock was applied from that moment and every 20 minutes for 0.01 seconds during 5 minutes. Backshock every 20 min. The results for these experiments are shown in Table 5.3. Table 5.3. TMP, permeate flux, and duration for the experiments.
As it is observed in Figure 5.4, a significant decrease on the TMP was observed when backshock was applied, which means that backshock partly removed the layer that had been formed on top of the membrane. This experiment was stopped after 65 minutes and the TMP was 0.18 bar at that moment. The permeate flux was 50 l/h/m2 during the first 20 minutes and it increased to approximately 100 l/h/m2 when the first backshock was applied staying stable on that level for the rest of the experiment.
Figure 5.4. Permeate flux and TMP versus time for the experiment run with backshock every 20 minutes. Constant backshock The permeate flux for the experiment run with frequent backshock (every 3 seconds for 0.01 s) was approximately 200 l/h/m2. However, it was quite unstable during the whole experiment. The experiment was running for 550 minutes and the TMP was 0.35 bar at the end of the experiment (Figure 5.5).
Figure 5.5. Permeate flux and TMP versus time for the experiment run with frequent backshock. These experiments have shown that backshock has a positive effect on the microfiltration performance. This technique cleans periodically the membrane avoiding and / or reducing further deposition of compounds on top of the membrane. Furthermore, backshock results in higher fluxes. 5.1.3.3 Effect of backshock frequency Several experiments were run in order to study the effect of the backshock frequency on the microfiltration performance when filtering UF sour cherry juice with a MF08 membrane. The three most representative experiments will be presented in this section.
The results for these experiments are shown in Table 5.4 and Figures 5.6, 5.7 and 5.8. Table 5.4. TMP and duration for the experiments run at different backshock frequencies.
The two first experiments were running for 360 minutes, whereas the experiment run with backshock every 5 s was only running for 200 minutes. 1 sec. backshock interval Figure 5.6. Permeate flux and TMP versus time for the experiment run with backshock every second. 3 sec. backshock interval The permeate flux was more stable for the experiment run with backshock every 3 s and the TMP increased constantly during the whole experiment from 0.07 to 0.33 bar. Figure 5.7. Permeate flux and TMP versus time for the experiment runwith backshock every 3 seconds. 5 sec. backshock interval Figure 5.8. Permeate flux and TMP versus time for the experiment run with backshock every 5 seconds. It has been demonstrated that the backshock frequency has a significant effect on the filtration duration. The backshock frequency should be between 1 and 3 seconds. However, both flux and TMP were more stable when applying backshock every 3 seconds. 5.1.3.4 Pore size Several experiments were run in order to test MF05 RAS and to compare the microfiltration performace with the one obtained when running with a MF08 RAS membrane. The three most representative experiments will be presented in this section.
Two concentration experiments were run; one at a flux of 150 l/h/m2 and the other at 300 l/h/m2. The third experiment was run in order to determine the maximum permeate flux. This was done by setting the flux at 50 l/h/m2 at the beginning of the experiment and increasing it 50 l/h/m2 every 20 minutes until a maximum was reached. After that, the flux was decreased in the same way to 50 l/h/m2 in order to determine whether fouling was stopping the filter. The results of these experiments are shown in Table 5.5 and Figures 5.9, 5.10 and 5.11. Table 5.5. TMP and duration for the experiments on MF 05.
The TMP increased to 0.49 bar in 50 minutes when filtering UF sour cherry juice through a MF05 membrane at a flux of 300 l/h/m2, whereas it was 0.33 bar after 360 minutes when filtering under the same conditions with a MF08 membrane. Figure 5.9. Permeate flux and TMP versus time for the concentration experiment at 300 l/h/m2. As it can be seen in Figure 5.10, the TMP increased pronouncedly after 60 minutes (flux 150 l/h/m2), and this increase became even more obvious when the flux was increased to 200 l/h/m2. This indicates that the maximum permeate flux for this membrane has been reached. The maximum permeate flux is a lot lower than the one found for the MF08 membrane.
Figure 5.10. Permeate flux and TMP versus time for the determination of maximum flux experiment. When running at 150 l/h/m2, the concentration experiment could be run for 150 minutes (three times more than at 300 l/h/m2). The TMP increased significantly after 90 minutes, but this was due to some problems in controlling the permeate pump via computer.
Figure 5.11. Permeate flux and TMP versus time for the concentration experiment at 150 l/h/m2. It can be concluded that significant lower fluxes are achieved when filtering UF sour cherry juice with MF05 than with MF08. MF05 can be used at the same conditions as MF08. However, more experiments should be run in order to find the optimal conditions. 5.1.3.5 Study of flux decline for MF05 Aim The aim of these experiments was to find out at which ° Brix the MF05 membrane could run for four different permeate fluxes. This study will lead to the cause for flux decline (concentration polarisation or fouling). Conditions 10 litres of UF water were added to the feed tank and cooled down to 3° C, and the permeate pump was set to a flux of 50, 100, 200 or 300 l/h/m2. After that, 1 litre of sour cherry concentrate with a °Brix of 64% was added and the °Brix was measured after 15 minutes at the permeate and the retentate sides. 0.5 l of the same concentrate was added every 15 minutes and the sugar concentration was measured. The experiments were stopped when the TMP was approximately 0.5 bar.
The TMP and the °Brix results for the four different fluxes are shown in Figure 4.12. The TMP increased slowly for 105 minutes when running at 50 l/h/m2
(23°Brix). The TMP increased more significantly after that and the filter blocked after
165 minutes. Figure 5.12. TMP and °Brix versus time for the different permeate fluxes. It can be concluded that MF05 can filter at 23°Brix when running at 50 l/h/m2, at 11.4°Brix when running at 100 and 200 l/h/m2, and at 7°Brix when running at 300 l/h/m2. These results showed that the cause for filter blocking was not the °Brix concentration but the amount of juice going through the membrane. No concentration polarisation phenomena were observed. 5.1.4 Study of flux decline for MF08 Aim The aim of these experiments was to find out at which ° Brix the MF08 membrane could run for four different permeate fluxes. This study will lead to the cause for flux decline (concentration polarisation or fouling). Conditions 10 litres of UF water were added to the feed tank and cooled down to 3° C, and the permeate pump was set to a flux of 50, 100, 200 or 300 l/h/m2. After that, 1 litre of sour cherry concentrate with a °Brix of 64% was added and the °Brix was measured after 15 minutes at the permeate and the retentate sides. 0.5 l of the same concentrate was added every 15 minutes and the sugar concentration was measured. The experiments were stopped when the TMP was approximately 0.5 bar.
The TMP and the °Brix results for the four different fluxes are shown in Figure 5.13. The TMP increased slowly for 45 minutes when running at 50 l/h/m2 and at 100
l/h/m2 (14.2°Brix). The TMP increased more significantly after 165 minutes
when running at 50 l/h/m2 and after 180 minutes when running at 100 l/h/m2.
The filters blocked at that time. Figure 5.13. TMP and °Brix versus time for the different permeate fluxes. It can be concluded that MF08 can filter at 14.2°Brix when running at 50 and 100 l/h/m2, at 12°Brix when running at 200 l/h/m2, and at 7°Brix when running at 300 l/h/m2. These results showed that the cause for filter blocking was not the °Brix concentration but the amount of juice going through the membrane. No concentration polarisation phenomena were observed. 5.1.5 Conclusions The conclusions drawn from these experiments are:
5.2 Microfiltration of Cherry Juice with Ceramic Membranes 5.2.1 Objectives The aim of this work was to find out the suitability of new ceramic membranes for the microfiltration of ultrafiltered sour cherry juice. The effect of backshock during filtration was tested and some of the substances fouling the membrane were analysed. 5.2.2 Materials & methods In the following sections, the filtration and cleaning procedures, the conditions used in different filtration tests and the analyses run on the juice samples as well as the analyses made on a used membrane will be presented. The filtration equipment used was a membrane filtration rig with backshock possibilities built at the Department of Biotechnology of The Technical University of Denmark (description found in the section on Microfiltration of Cherry Juice with Polymeric Membranes). The membranes used in the filtration tests were tubular ceramic membranes manufactured by Haldor Topsøe (Denmark). These were unmodified and modified a -alumina membranes with a maximum pore size of 0.5 µm. The membrane area was 52.6 cm² and the cross-section area tube was 35.2 mm². 5.2.2.1 Filtration procedure Filter tests Filtration tests were run according to the following procedure: Before each filtration test, a Forward Flow test was run with Palltronic equipment in order to check the integrity of the membrane. After that, the permeability of ultrafiltered water was measured at dead-end filtration and at three different pressures. Filtration procedure The actual filtration test was started with ultrafiltered water in order to get the pumps calibrated and to let the adjusted filtration conditions to stabilize. Water filtration took approximately 30 minutes. Then, the permeate and the retentate valves were closed in order to keep the membrane cell pressurized during the change from water to cherry juice. Pumps were stopped and the water was discharged into sewer. The feed tank was filled with juice and pressurized to 0.5 bar. Water and a small amount of juice was discharged from the retentate line, after which a feed sample was taken. Filtration was started by circulating the juice through the by-pass until this was cooled down to 3-4ºC. After that, the retentate and the permeate valves were opened and the filtration was started. During filtration, the permeate flux was measured every five minutes, and samples were taken from permeate and retentate every 20 minutes. The filtration test was stopped when the transmembrane pressure got too high. After each filtration test, the equipment and the membrane were rinsed at a high linear velocity with ultrafiltered water. Usually, the pressure on the retentate side was around 1 bar during rinsing. The pressure on the permeate side depended on the degree of fouling on the membrane, and this was therefore different for each test. At the beginning, the rinsing was conducted through all the lines, but after the retentate line was cleaned, the rinsing was continued at dead-end mode in order to clean the membrane and permeate line. Rinsing was continued until the water coming from the permeate line appeared clean. After rinsing, the permeability of ultrafiltered water was measured following the same procedure as before the filtration test. 5.2.2.2 Cleaning procedure Table 5.6. Cleaning agents and conditions used for the cleaning of membranes used in the microfiltration of cherry juice.
5.2.2.3 Filtration test A list of the filtration tests and the conditions applied is presented in Table 5.7. The temperature in the feed tank was 4 ºC, the pressure in feed tank 0.3 bar, the linear velocity 0.5 m/s, and the backshock flow 2.5 l/h for all tests. The filtration tests were run keeping the permeate flux constant. In tests 2, 3, 4, and 6, the permeate flow was increased step-wise every 20 minutes until the transmembrane pressure was too high, and then decreased step-wise every 10 minutes. The change on the transmembrane pressure during the filtration test was also measured. In test 5, the permeate flow was kept constant during the whole test, and the increase on the transmembrane pressure during filtration was also measured. Filtration test 6 was run without backshock. In filtration test 1, the membrane broke after 10 minutes of filtration and no results will be presented. Table 5.7. Filtration conditions used in microfiltration tests with cherry juice.
5.2.2.4 Juice analysis Colour, turbidity, sugar content, and total phenols were analysed from feed,
retentate and permeate samples. 5.2.2.5 Analysis of the fouled membrane Membrane nr. 3 (tests 3 and 4) was analysed after running the filtration test for membrane foulants. Total organics were analysed from the membrane by incinerating this one at 550ºC for 2 h. The protein content was analysed by means of Liquid Chromatography after extraction with HCl. Phenol content The total phenol content was extracted from the membrane with acetone, and the extract was analysed following the same method as described before for the juice samples. SEM pictures Pictures from the membrane surfaces and the cross-sections were taken with a Scanning Electron Microscope in order to see how the fouling layer looked like. Some pictures were also taken from membrane ceramic 4 (test 5) after cleaning, and these pictures were compared with those taken from membrane 3. 5.2.3 Results and discussion This chapter presents the fluxes and the permeate quality data from microfiltration of cherry juice trials run with ceramic membranes, as well as a short description of the fouling substances analysed from a used membrane. 5.2.3.1 Permeate fluxes and permeabilities Broken membranes The ceramic membrane used in test 1 broke after 10 minutes of filtration, and therefore, no results from this test will be presented. The membrane used in test 2 broke after cleaning, and the membrane used in test 4 during the filtration test. The membrane used in test 5 also broke after cleaning. It seems that the membranes used in these filtrations were extremely fragile and broke down very easily either during assembling (gluing), during the filtration tests or cleaning, since they could not stand any kind of bending. One reason that might explain this phenomenon during backshock filtrations could be the distribution of the pressure on the membrane during backshock. It is likely that pressure shock exerts only to one part of the membrane causing the same effect as when the membrane is bent. The transmembrane pressure versus flux curves for tests 2, 3, and 4 are presented in Figure 5.14. It is expected that when the permeate flow increases, the transmembrane pressure increases. If the membrane is fouled during filtration, the transmembrane pressures achieved when increasing the flux should be lower than those achieved when decreasing the flux. In test 2 and 3, when the permeate flux was increased, the transmembrane pressure increased. However, when the permeate flux was decreased, higher flux values resulted in lower transmembrane pressures than when the flux was increased. This is not in accordance with the theory. An explanation for this unexpected behavior could be the accuracy of the measurements or it could have something to do with the way the permeate is pumped into the backshock system. It is difficult to demonstrate the fouling on the membrane by looking at Fig. 5.15. However, the water permeabilities after the filtration tests were lower than those before the tests (Table 5.8.). This means that the membranes were partly fouled during filtration.
Figure 5.15. Permeate fluxes versus transmembrane pressures during microfiltration of cherry juice with ceramic membranes. Backshock of 0.010s every 3 s. Linear velocity 0.5 m/s. Table 5.8. Water permeabilities before and after filtration.
The same membrane was used in tests 3 and 4. However, the membrane was cleaned with cleaning agents between these filtration tests. The permeability after cleaning was much lower than before cleaning, which indicates that the membrane was fouled already after cleaning. The reason for this behaviour was that the ultrafiltered water used for the integrity testing and the permeability measurements had been standing in a pool for a long period of time and some biological growth had taken place. Moreover, the ultrafiltration system used for the treatment of tap water had not been properly cleaned and particles that fouled the membrane leaked through the filter. Therefore, the membrane was already fouled before the actual test was started and this is confirmed by the higher transmembrane pressures in test 4 compared to those from tests 2 and 3. In Figure 5.16. the behaviour of the transmembrane pressure during a 6 h filtration is presented. As it is observed, when the permeate flux was kept at approximately 236 l/m² h, the transmembrane pressure increased from 0.35 bar to 0.56 bar. This means that, although backshock was being applied, the membrane had fouled during the test, and that the backshocks could not totally stop the fouling. The variations in flux were probably caused by the backshocks. Figure 5.16. Permeate flux and transmembrane pressure (TMP) during microfiltration of cherry juice. Filtration conditions: backshock of 0.010 s every 3 s and linear velocity 0.5 m/s. The effect of backshock on microfiltration of cherry juice can be seen in Figure 5.17. It is evident that when operating at transmembrane pressures higher than 0.5 bar, the filtration run with backshock resulted in better fluxes than the filtration run without backshock. In the filtration run without backshock and above a transmembrane pressure of 0.5 bar, the flux-pressure curve starts to deviate from linear and the increase in the pressure does not result in an increase on the flux as much as at lower pressures. It seems that there exists an optimum in the correlation flux-pressure, and that the highest flux without backshock can be achieved at a pressure of approximately 1.25 bar.
5.2.3.2 Permeate quality The results of the different analysis for the feed, the retentate and the permeate samples are presented in Table 5.9. for all filtration tests. The data presented in the Table corresponds to samples collected at the end of each test for the permeate and the retentate and at the beginning of the test for the feed. Typically, the concentrations in the retentate and the permeate varied during the test, as it is observed in Figure 5.18., in which the colour of the retentate and the permeate samples collected during test 3 are presented as a function of the time. In most cases, at the beginning of the test, the concentrations in the permeate were much lower, and, therefore, the retentions were higher, since the ultrafiltered tap water used during the stabilisation of the filtration conditions diluted the permeate. At the end of the test, when the permeate side of the equipment was filled with permeate, the retentions were much lower, usually below 25%. In test 5, which was run for 6 h at a constant flux, the retentate and permeate values started to stabilise after 3 h of filtration. Taking into account that the quality requirements for sour cherry juice are: a turbidity lower than 10 FNU, a sugar content of 11-15ºBrix, and a colour content between 0.70-1.50, the quality of the permeate was not good enough in many tests. As it is observed in Table 5.10, only the quality requirements set for sugar and colour were achieved except for a few exceptions, whereas the turbidity in most cases was too high (18-19 FNU). Retentions of sugars were between 6 and 18% at the end of the filtration tests. Retentions of the phenols were negative in tests 3 and 5, which means that the concentrations of phenols were higher in the permeate than in the retentate. In tests 4 and 6, where the membranes were more fouled during the test, the retentions of phenols were 10-25%. This means that when backshock was applied, the membrane retained only small amounts of sugars and phenols. This did not have a significant effect on the quality of the juice. The retentions of colour were 0-11 % and those of turbidity 10-16%, except in test 6 (without backshock), where the retention of turbidity was 97%. The high retention of turbidity in test 6 was most likely due to the fouling of the membrane. Feed concentrations in test 5 were exceptionally low. One possible explanation for this is that the retentate line was not properly emptied from water when the feed sample was taken, and the feed sample was therefore diluted. Table 5.9. Analysis data from test microfiltrations of cherry juice run with ceramic membranes.
Figure 5.18. Colour of permeate and retentate during the microfiltration of cherry juice with ceramic membrane (Test 3). Filtration conditions: backshock of 0.010 s every 3s, linear velocity 0.5 m/s, and permeate flux 89-625 l/(m² h). 5.2.3.3 Membrane foulants The pictures taken from the used, and the used and cleaned membrane showed that there was fouling at both sides of the membrane. The cleaning procedure could not remove all the foulants, and some of them could be seen still after cleaning forming a sponge like structure. Since the filtration was run from inside to outside (from the support layer to the skin layer), it is understandable that the foulants could be found at both sides of the membrane. The amount of total organics in the ceramic membrane nr.3 was 1.93 mg/g incinerated membrane, whereas it was 0.13 mg/g incinerated membrane for the clean and unused membrane. The total amount of proteins in the used membrane was 217 m g/g dry membrane and 1.07 m g/g dry membrane for the unused membrane. In the chromatograms of the used membrane, the most abundant amino acids were glycine, aspartic acid, proline, serine and glutamic acid, whereas these were glycine, glutamic acid, serine, aspartic acid and histidine for the unused membrane. The peak of cysteine could be clearly detected for the used membrane, but not for the unused membrane. The total amount of phenols in the used membrane was 228 m g/g dry membrane and 72 m g/g dry membrane in the unused membrane. The total amount of proteins and phenols in the used membrane was only 0.45 mg/g, which was only 23% of the amount of total organics in the sample. Therefore, a large portion of the organic foulants stayed undefined. One of the main components of cherry juice, the sugars, were not analysed, and its role in the fouling formation is therefore unknown. 5.2.4 Conclusions The membranes used proved to be extremely fragile. The brittle character of the membranes made them very difficult to handle, and they did not seem to suit to backshock filtration. However, it is obvious that the use of backshock improved the filtration performance, and much higher permeate fluxes could be achieved at lower transmembrane pressures than in filtration tests run without backshock. The 6 h filtration test run with backshock showed that the transmembrane pressure increased during filtration. This means that the membrane fouled during filtration. When backshock was not applied, an optimum in flux-pressure curve was observed, above which the increase on the transmembrane pressure resulted in a decrease on the flux. Microfiltration of sour cherry juice did not have a significant effect on the colour, the sugar content, or the total phenols. The quality requirements for sugar content and colour were achieved except for a few exceptions, whereas turbidity was in most cases too high, and further treatment will therefore be needed. The Scanning Electron Microscopy pictures taken from the used and the used and cleaned membrane showed that there was fouling on both sides of the membrane. The cleaning procedure could not remove all the foulants. Furthermore, most of the organic foulants stayed undefined.
6 Scaling6.1 Transparent housing Filtration experiments with other medias have shown difficulties in 1m2 scale. To investigate the problems, a flexible experimental set-up has been established, for filtration of water. 6.1 Transparent housing A transparent filter house has been made, to be able to follow the flow inside, by adding colour to the water. Colour experiments Experiments with colour added to the water used for BackShock, and the BackShock device placed at the inlet end of the filter did not produce any useful information. When the BackShock device was moved to the middle of the filter, more information was revealed. From the middle to the outlet all the water was coloured, but the colour did not move towards the inlet end at all. When the colour was added to the feed water instead, it could be seen what was happening. The feed passes the membrane to the permeate side, here it flows outside the fibres to the outlet end, where it passes the membrane once again. Because of the high water permeability of the filter the resistance is lower going this way, than flowing in the fibres. In a filtration situation with a partly fouled filter, this effect will be reduced. It should however be taken into account. 6.2 Shadow effect It has been proposed, that the fibres closer to the BackShock-inlet would absorb all the introduced liquid, leaving little or nothing for the rest of the bundle. To investigate this, selected fibres of a filter have been supplied with small tubes in one end. The tubes should collect the water leaving that end of the filter. The volumes collected seemed to point to a small shadow effect when using low BackShock pressures. The effect dissapeared when the BackShock pressure was increased. 6.3 Pressure measurements A filter house was modified to have six ports instead of two. This allowed for more detailled pressure measurements to be undertaken. Pressure transducers were placed at the ports and before and after the filter. The transducers were connected to a computer running a data acquisition program. The pressure peaks for 1m2 filters show up much higher than for smaller filters. This indicates an increased resistance against moving the bigger volumes needed. 6.4 The BackShock device The traditional BackShock device consisteded of a rubber tube inside a steel house. The device was connected directly to the filter, in a way that allowed the permeate to enter the inside of the rubber tube. The BackShock is performed by closing the permeate outlet and applying compressed air to the outside of the rubber tube. The tube will collapse, pressing the permeate in it out through the filter. Unfortunately this approach leaves no way of controlling the volume of permeate flushed. If a constant BackShock time and pressure is used, the volume will be high when the filter is still clean and permeable. When the filter fouls the permeability will decrease, which in turn will decrease the volume of permeate flushed. In other words; when the demand is still low, a high volume will be flushed, but when the demand is high, only a small volume is flushed. After the BackShock, the compressed air is removed. This leads to a dramatic pressure drop at the permeate side. This will cause the permeate to rush back into the BackShock device to refill it. The high flow that will exist for a short while after the BackShock, will drag the removed dirt back into the pores, thereby undoing the BackShock. 6.5 Conclusion Colour experiments have shown that the water takes a shortcut through the membrane to the permeate side and back again. There is no reason to worry about the shadow effect. Pressure measurements show significant problems trying to flush with the volumes needed for bigger filters. To be able to scale up the BackShock technique, it is nessecary to solve some problems in controlling the BackShock. Other projects will continue development of the ideas from this project.
7. Fouling and cleaning7.1 Fouling analysis (ceramic membranes) Aim The main objective of this work was to study the possible foulants originating from sour cherry juice, and the effects of different cleaning agents on the membrane structure. The scheme for the analysis of membrane foulants presented in Figure 6.1 is in accordance with the one presented by Flemming et al. (1997). The effect of different cleaning agents on the membrane structure was evaluated by extracting a membrane in strong cleaning agents, looking to the scanning electron microscopy (SEM) pictures, and comparing them to the pictures from the original, unextracted membrane. Figure 7.1. Scheme for the analysis of the used membrane. Juice compounds Optical inspection Optical inspection of the membrane can significantly help in finding possible foulants, since the colour of the fouling can give an idea of the foulants. For example, microbial pigments, humic substances and iron can cause brownish colour to the membrane (Flemming et al., 1997). The optical inspection of the membrane used for the filtration of cherry juice showed that membrane was red. The red colour of the juice is caused by the polyphenols, especially anthocyanins. SEM Scanning electron microscopy shows how the fouling layer looks like, where the foulants are situated, and how thick the fouling layer is. EDAX The EDAX study could give information of the possible inorganic foulants. However, this method was not applied, since it was assumed that fouling was mainly caused by organic substances. Incineration Incineration of the membrane gives the amount of total organics on the membrane. The specific organic foulant groups, such as proteins and polyphenols, can be studied by extracting the membrane in order to remove the foulant from the membrane, and the analysing the extract for the different fouling species. 7.2 Sample treatment The samples used in these studies were:
The used membrane was broke at the end of the filtration test after which it was frozen until cutting for the analysis. The cleaned membrane was broken after cleaning, and it was air dried until the cutting. 7.2.1.1 Effect of cleaning agents on unused membrane The effect of strong phosphoric acid and hydrogen peroxide on the unused membrane was studied. Small pieces were cut from the membrane and sunk into the cleaning agent. They were kept in the cleaning agent over night, rinsed with distilled water, dried in the oven at 105ºC for 4 h, and then cooled down in dessicator. Phosphoric acid was more difficult to remove from the membrane, and the rinsing was repeated after drying. In Table 7.1 the cleaning agents, concentrations and the treatment times used in the tests are presented. Table7.1. Cleaning agents, concentration and treatment times.
7.2.1.2 Sample treatment for Scanning Electron Microscopy (SEM) and other analysis Cutting For all the analysis, small pieces (approximately 1 cm long) were cut from the membrane
with a blade. 7.2.2 Results 7.2.2.1 Scanning electron microscopy (SEM) Fourteen samples were cut or mashed into pieces. However, some of the samples proved to be unsuitable for the analysis: the membrane treated with phosphoric acid was not dry enough and the cross-section samples were too smooth. In Table 7.2 the list of the samples studied is presented. Table 7.2. Membrane samples studied with the SEM. Cer_is means ceramic membrane, inside of the tube and Cer_os ceramic membrane, outside of the tube, Cer_cs means ceramic membrane, cross-section.
SEM pictures of the skin and support layer of the unused membrane showed that the grain size of the support material was much bigger than that of the membrane skin layer. The skin layer of the studied membrane was outside of the tube. On the support layer some particles, which probably are dust, could be seen on the top of the bigger membrane particles. From the cross-section pictures, the thickness of the membrane layers was determined. The support layer was approximately 1.5 cm thick and the skin layer 63 mm. The SEM pictures of the membrane treated with hydrogen peroxide (40 vol.-%, 19 h) and phosphoric acid (80 %, 24h) showed that in phosphoric acid treated membrane some particles were etched more together than in peroxide treated membrane. It also seemed that the phosphoric acid treatment eroded the grains, so that the spaces between some of the grains were bigger. When comparing the peroxide treated membrane to the unused membrane, it was obvious that hydrogen peroxide treatment did not have any visible effect on the membrane structure, but that phosphoric acid may have changed the surface structure slightly. However, the changes were not so significant, and it was difficult to say whether they really were caused by the acid or by normal differences in the membrane surface. The exposure time should probably have been longer in order to be able to make more reliable conclusions. The cross-sections of treated membranes did not show any significant visible differences. Moreover, no visible differences were observed when the cross-sections of the treated membranes were compared to that of the untreated membrane. The cross-section pictures of the membrane used in juice filtration showed a significant difference in the thickness of the skin layer compared to that of the unused membrane. The thickness of the skin layer of the membrane used in the juice filtration was only from 8.0 mm to almost zero. SEM pictures of the skin layer of the membrane used in cherry juice filtration tests showed that in some places the big particles from the supporting structure came through the skin layer. Moreover, the material that formed the skin layer seemed to be much smaller in particle size than the material in the unused membrane. The first assumption when looking the skin layer of the used membrane was, that the small particles on top of the membrane were foulants. However, this probably was not the case as the cross-section picture showed. There are two possible explanations for the differences in the skin layer: either the manufacture of the membrane skin layer had failed, or the backshock had damaged the skin layer. The first option is the most likely. The fouling layer, if it existed, was difficult to see from the skin layer of the membrane, partly since during the cutting of the membrane pieces, the dust was attached to the surface of the membrane. However, rests from the juice could be observed on the support layer. Grain particles could be distinguished on the support layer. However, the sharpness of the grain borders had vanished. The bigger magnification showed that particles were covered with some kind of a gel layer. The SEM pictures of the membrane used for the filtration of the cherry juice and then cleaned with both acidic and alkaline cleaning agents showed that the cleaning agents had removed part of the fouling layer. The fouling was more evident in the cleaned than in the used membrane, partly because this membrane had not been cut with a blade and therefore there was no dust attached to the membrane. From the cross-section pictures of the used and cleaned membrane, some clusters of small particles could be seen in between the bigger particles. These small particle clusters could be either foulants or the material which was supposed to form the skin layer, but which instead had filtrated inside the supporting structure. 7.2.2.2 Total organics The water content of the piece of used membrane was measured by weighting this before and after drying. The membrane was dried in the oven at 105ºC for 4 h before been placed in the dessicator for 2 h. The amount of water on the membrane was 0.2201 g. The weight of the dry membrane was 1.6093 g. The membrane piece was stored in the dessicator for 21 days until it was incinerated in the oven at 550ºC for 2 h. The membrane was weighted again before incineration and its weight was 1.6075 g. This means that that 0.0018 g were lost during the storing in the dessicator. During incineration, the following program was used: First, the temperature was increased 5ºC/min to 150ºC and this temperature was held for 1/2 h. Then, the temperature was increased 5ºC/min to 550ºC and then held for 2 h. After that, the temperature decreased 10ºC/min to 20ºC. Membrane pieces (both unused and used) were placed into the oven when the temperature was 130ºC. The used membrane looked yellow when it was taken out of the oven, but after cooling in dessicator, it was white. The unused membrane was white when it was taken out of the oven. In Table 7.3 the results from the drying and the incineration with calculated weight losses are presented. Table 7.3. The weights of the unused and used membrane before and after drying. Abbreviations: D m weight loss, n.m. not measured.
The water content in the used membrane was 13.7% and the organic content 0.19%, which means that the amount of water was 137.2 mg/g of incinerated membrane and that of organics 1.93 mg/g incinerated membrane. The loss of matter during storing was 1.12 mg/g of incinerated membrane. The amount of total organics in the unused membrane was only 0.13 mg/g of incinerated membrane. The amount of water in the membrane after use was high. However, the total amount of organics in the used membrane was not that high. 7.2.2.3 Protein analysis One piece of dried and unused membrane, and one piece of dried and used membrane were analysed for amino acid content. The analyses were made according to the following procedure: membrane pieces were hydrolysed in 1 ml 6N HCl, 50 m l phenol and 50 m l DTDPA in a big beaker, and then washed in another beaker with 400 m l 0.1M HCl. After that, the sample pieces were discharged. Both the hydrolysing solution and the washing solution were then dried, and the amino acids were redissolved. The amino acids from the hydrolysing solution were redissolved in 1000 m l of a buffer and those from the washing solution in 100 m l of the same buffer. Then, the amino acids were determined by Liquid Chromatography. The most abundant amino acids in the used membrane were glycine, aspartic acid, proline, serine and glutamic acid, whereas in the unused membrane were glycine, glutamic acid, serine, aspartic acid and histidine. The peak of the TP (thiopropionic) cysteine could be detected clearly in the used membrane, but not in the unused membrane. The total amount of proteins in the different samples is presented in Table 7.4. The total amount of proteins for the used membrane was 217 m g/g membrane and 1.07 m g/g for the unused membrane. The results show that the hydrolysis did remove most of the proteins but not all. Furthermore, a significant amount of proteins was also found. Since washing was done only once there exists a small possibility that some of the proteins stayed in the membrane. Table 7.4. Total amount of proteins in different samples.
7.2.3 Analysis of phenols First, dried sample pieces of unused and used membrane were weighed. The weight of the unused membrane piece was 1.5888 g and that of the used membrane 1.6071 g. Then, samples were crushed into small pieces in a mortel in order to increase the surface area for extraction. The powdered sample was put into a glass bottle and the mortel was rinsed four times with approximately 10 ml of 70 vol.-% acetone and poured into the same bottle. Acetone and the powdered sample were well mixed and left over night (at least 14 h) to extract. Before analysis, samples were centrifuged at 10 000 rpm for 10 min in order to separate the membrane particles from the extractant. The supernatant was analysed for total phenols according to the following procedure (Singlenton et al., 1965): 1 ml of Folin-Ciocalteu’s phenol reagent, diluted 1:10 with double distilled water was added to 0.2 ml of phenolic extract. 0.8 ml of 7.5% (w/v) sodium carbonate was added to develop the colour and everything was mixed. The mixture was then left for 30 min with caps on. After mixing again, the absorbance was read at 765 nm, using double distilled water for background correction. The concentration of total phenols was calculated from a standard curve obtained by subjecting known amounts of gallic acid solutions (0, 5, 10, 20, 40, 60, 80 and 100 mg/l gallic acid) to the same treatment as the test samples. Results were expressed in gallic acid equivalents (GAE). The equation for the standard curve was c(mg/l GAE) = 94.276× ABS-2.2123. The results from the measurement are presented in Table 7.5. The total amount of phenols in the used membrane was 228 m g/g membrane and 72 m g/g in the unused membrane. Table 7.5. Absorbances and concentrations of phenols.
7.2.4 Summary and conclusions In this work an unused, modified ceramic membrane, a used ceramic membrane, and a cleaned ceramic membrane were studied in order to find out some of the membrane foulants and the effect of some cleaning agents on the membrane. The analysis of foulants was done by microscopic means and by analysing some specific groups of substances, which may cause fouling, as well as by determining the total amount of organics on the membranes. The effect of the cleaning agents on the membrane was studied by microscopic means. Microscopic studies were done by Scanning Electron Microscopy (SEM).The SEM pictures of unused and used membranes showed a significant difference in the thickness of the skin layers. It seems that the manufacture of the skin layer did not succeed in the membranes used in juice filtrations, and that the particles meant to form the skin layer were filtrated inside of the supporting structure. The SEM pictures of the membranes treated with hydrogen peroxide and phosphoric acid did not show visible differences. However, the phosphoric acid treated membrane seemed to have a slightly different surface structure, but it is difficult to conclude whether the differences were caused by the acid or whether they were just part of normal variations of the membrane structure. The pictures taken from the used and the used and cleaned membrane showed that there was fouling on both sides of the membrane. The cleaning could not remove all the foulants, and some of them could be seen after cleaning forming a sponge-like structure. Since the filtration was made from inside to outside (from support layer to skin layer), it is understandable that the foulants could be found at both sides of the membrane. The amount of water and total organics on the membrane was determined by drying the membrane at 105ºC and then incinerating it at 550ºC. The amount of water in the used membrane was 137 mg/g of incinerated membrane after two hours of evaporation. However, some substances (1.12 mg/g incinerated membrane) were evaporated from the membrane during storing in the dessicator. The amount of total organics in the used membrane was 1.93 mg/g incinerated membrane, whereas in the cleaned membrane this value was 0.13 mg/g incinerated membrane. Proteins were analyzed from the unused and used membrane by degrading them into amino acids with HCl and analysing the amino acids by Liquid Chromatography. The total amount of proteins in the used membrane was 217 m g/g dry membrane and in unused membrane 1.07 m g/g dry membrane. In the chromatograms of the used membrane the most abundant amino acids were glycine, aspartic acid, proline, serine and glutamic acid, whereas in the unused membrane the most abundant were glycine, glutamic acid, serine, aspartic acid and histidine. The peak of the TP Cysteine could be detected clearly in the used membrane, but not in the unused membrane. The total amount of phenols was analysed from the unused and used membrane by
extracting them with 70 vol.-% acetone (14 h), and then determining them from the
extract with a colorimetric method. The total amount of phenols in the used membrane was
228 m g/g dry membrane and in the unused membrane 72 m g/g dry membrane. Table 7.6. Summary of the foulant analysis. Abbreviations: mmembr weight of the dry membrane sample, mfoul weight of the foulants, minc.membr., weight or calculated weight of the incinerated membrane sample, mfoul/minc.membr. , weight of the foulants per weight of the incinerated membrane.
The procedure used for the analysis of the fouled membrane proved to be good. However, some improvements can still be done. Over 70% of the organic foulants remained undefined, and it might be a good idea to analyse the sugars, which were one of the main components of the juice, and to do some microbiological analysis in order to check out the importance of biofouling. Moreover, the possible inorganic foulants could be analysed by EDAX.
8. Chemical cleaningThe filtration system and the filters were rinsed with distilled UF water after each filtration test. Two cleaning procedures were tested with polymeric membranes from X-flow. The first cleaning procedure was based on a combination of two cleaning agents supplied by Scan Diversey (Divos 2 S and Divos 124). Divos 2S is a strong acid liquid cleaning agent based on H3PO4 and it is used to remove inorganic contaminates. Divos 124 is a strong alkaline liquid cleaning agent based on KOH, tetrasodium-EDTA and anionic tensed, and it is used to emulsify fat and proteins. This procedure did not recover successfully the polymeric membranes. The second cleaning procedure was based on a combination of several cleaning agents supplied by Henkel-Ecolab. The cleaning procedure is described in the section on Microfiltration of Cherry Juice with Polymeric Membranes. The agents used had different functions, i.e., emulsification and degradation of fat and proteins, disinfection, removal of fat and inorganic particles, etc. Better results were obtained with this cleaning procedure. However, the results were not optimal and the membranes could not be completely recovered. In order to get some comparable and concluding results from the different filtration tests, a new filter was used for each experiment.
9. Ultrasounds9.1 Introduction 9.1 Introduction Separation processes are most important operations in a great deal of industries, especially food production. In fruit juice production separation operations are constituing the major part. High cost, due to energy consuming techniqes, cleaning, use of chemicals for floculation and sedimentation, the later now considered a health-risk and undesired impact to natural environment, has encouraged development of new microfiltration technology based on new types of membranes for liquid filtration. The most predominant problem in membrane filtering is fouling, which in filtration of cherry juice and similar fluids incorporates a chain of events, linked to a complex composition of ingredients as polysaccarides, cromo-phenols, fat, proteins, salt and small particles and clusters of aroma carrying molecules in water. Earlier experimental work on micro-filtration of cherry juice using reverse-asymmetric hollow-fibre-based filters has shown that low temperatur filtering is essential to obtain an aquired quality due to the fact that part of the phenols dissolve easily at normal temperature, and therefor unwantedly precipitate after cooling, when not filteret at low temperature (below approx. 8 deg.C). The chain of fouling is considerd to involve all of the well established processes; concentration-polarisation layer formation, building of gel-layer, forming of a sediment layer. Membrane resistance control in cross-flow microfiltration by the use of high-power ultrasound acting on the membrane, has been reported by E.S.Tarlton and R.J.Wakeman on china-clay and anatase and by Yutaka Matsumoto, on bakers yeast and Bovine Serum Albumin BSA. Both groups are concluding that high-power ultrasonic irradiation is efficient in controlling fouling in some cases, and state that the effect is due to local cavitational events.E.S.Tarlton and R.J.Wakeman also reported very promissing results from using electrostatic fields combined with ultrasound. Yutaca Matsumoto recorded the important observation that major improvement in performance of the filtration can be obtained when the trans-membrane pressure is shifted to 0 (Bar) during ultrasonic irradiation. 9.2 Experiments The two types of filters used are shown in sketches in Fig. 9.1., together with the overview of the filters, membranes and variations in experimental parameters in tabel 9.1. Figure 9.1. The two types of filters. Tabel 9.1. OVERVIEW OF FILTERS MEMBRANES AND VARIATION IN EXPERIMENTAL PARAMETERS
All filters have approximately the same 100cm2 active filter area. The cherry juice was standard food-grade, press-filtered and cleared to approximately
14.3 Brix (215 FNU) and kept at 4 deg.C. 9.3 The experimental setup The instrumental setup of the experiments was very simple. Principles of the two experimental setups for filtering with reverse-asymmetric hollow-fiber membrane filter and for the yarn-wound filters respectively are shown below. The ultrasonic irradiation-cell was produced together with Reson A/S Denmark. The ultrasonic transducers were powered in 5 steps (0,37,74,100 and 180Watts) from a generator with the power load controlled by a pulsgated cut-off on 23kHz with 100Hz amplitude modulation, feeding a resonant load of four (60mmØ) transducers in parallel, (corresponding to a theoretical field of approx. 0.3-1.5Watt/cm sq. Where 0.3W/cm sq. is near and below cavitation limit in the actual case.) Coherent values of Trans-Membrane-Pressure difference (TMP), flow and temperature were recorded manually with regular intervals. *text in brakets is added after conf. Figure 9.2. Principle of experimental 1.
Figure 9.3. Principle of experimental 2.
Figure 9.4. Illustration of the irradiation. 9.4 Results A plot of Flow versus TMP (Fig.9.5.) shows a pressure driven behaviour for both types of filters. The three displayed time-series of Flow/TMP in Fig. 9.6. (a-c) present characteristic variations in Flow-rate correlated with the impact of ultrasound. Data for the time-series of the filtering using the low-flow octalobal yarn-wound
filter has been used to analyse the influence from the previous impact on this type of
filter. The result is presented in the last plot (Fig.9.7.), indicating a trend, that the
larger the power of the ultrasonic field is in the actual impact, compared to the power of
the last contained, previous event, the stronger the influence of the ultrasound will be.
9.5 Conclusions - In filtration of natural cherry juice, the filtration resistance is observed to decrease significantly for hollow-fibre membranes as well as for yarn-wound membranes when ultrasonic power is applied above cavitation level. - The observed decrease in filtration resistance enhance with increasing ultrasonic power. - The effect of the cavitation seems to depend on the level of the last irradiation. - The filter membranes are expected to be influenced only when cavitation takes place in the vicinity of the membrane surface and the fouling material. - There exists an upper limit of the ultrasound field having effect, expected to dependent on the fouling speed and filter design. Above and near this upper limit the filter membrane material is susceptible to fatigue, as no fouling protect the membrane surface - No upper limit could be reached with the present equipmemt for the yarn-wound membranes. However a limit of 0.7W/cm2, was found for the hollow-fiber membrane (polysulphone) - The standard quality parameter of clearity (Brix and Fnu-numbers) showed 3 x reduction using the hollow-fibre membranes. The high flux yarn-wound membrane resulted in 20% reduction, whereas the low-flux yarn-wound membrane resulted in 2.5 x reduction at low TMP (0.03Bar). - Based on subjective evaluation, no significant change in quality of smell or taste was found. However, the low-flux yarn-wound membrane seems to result in a small fade in taste. - High-power ultrasound, as a means to control fouling in microfiltration membranes seems be a theoretically feasible method. However, further investigation ( with special emphasis on materials in membrane and filter design, involving various types of membranes with different filter design techniques and applications), is needed to surmount the non-trivial technical difficulties in design of larger filters.
10. Feasibility studies10.1 The Method. In the Feasibility study the Filtomat and crossflow filtration technology will be compared with a traditional vacuumfilter and an ultrafiltration unit. The analyses of the different technologies will be concentrated partly on the effect they have on the environment and partly on their profitability. The Feasibility study is only connected to the first phase of this project. A financially foundation for the second phase has not yet been established. Objective According to the project description the feasibility study shall:
Compared systems The following three filtration systems’s effect on the environment and their profitability will be compared In this feasibility study. 1. A traditional filtration system based on pre-treatment consisting of a centrifuge and a Vacuumfilter using perlite. A pressurefilter with kieselguhr as a filteraid is used to the final filtration. This system is at the moment used daily at Vallø Saft A/S 2. A filtration system consisting of the Filtomat filtration technology for the pre-treatment of the juice and a 0,01 m2 crossflow microfiltration unit build at the Technical University of Denmark, Department of Biotechnology, Pilot Plant or the final filtration. Experiments with water on a 1 m2 crossflow microfiltration unit will also be conducted to examine possible shadoweffects 3. A filtration system consisting of the Filtomat filtration technology for the pre-treatment of the juice and a 0,01 m2 crossflow microfiltration unit build at the Technical University of Denmark, Department of Biotechnology, Pilot Plant or the final filtration. Experiments with water on a 1 m2 crossflow microfiltration unit will also be conducted to examine possible shadoweffects. Figure 10.1. The three filtration systems A cycle of live perspective will be used to compare the three different filtration systems. In the following the limits of the cycle of live perspective is defined. A general view of the exact parameters connected to the environment and economy will thereafter be presented. The comparison of the three different filtration systems will be based on these parameters. Phases of the study The effect the different filtration systems have on the environment will be considered in a cycle of live perspective. It will be relevant to examine the following phases in a filtration technology cycle of live:
Production of the filtration technology In relation to the phase: Production of the filtration technology, the feasibility study will focus on the investment costs (fixed costs) together with the transcription and lifetime of the technology. Operation and maintenance of the filtration technology The phase, Operation and maintenance of the filtration technology, is the primary area of the feasibility study. The focus will be on the maintenance and operational input and output for each of the specific filtration technologies. The output here is understood as the product and the by-products such as wastewater and normal waste. The quality of the juice is also a central point, since the new technology must produce a quality which is just as good or better than the one Vallø Saft is achieving by using the traditional pre-treatment filtration system together with either a pressure filter or a ultrafiltration unit. Dispose of the filtration technology Finally it shall be stated that it was not found relevant to focus on the phase: Dispose of the filtration technology since the project only consist of experiments concerning operation and maintenance. Phase two in the filtration technology cycle of live is therefore as mentioned the most important and through this the central questions of the feasibility study, will be answered 10.3 The different parameters used Below the parameters used in relation to the phases: Production of the filtration technology and operation and maintenance of the filtration technology are shown. The pre-filtration step and the final filtration step will be analysed separately. Table 10.1. Production of the filtration technology.
Table 10.2. Operation and maintenance of the filtration technology (input)
Table 10.3. Operation and maintenance of the filtration technology (output)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
[Front page] [Top] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||