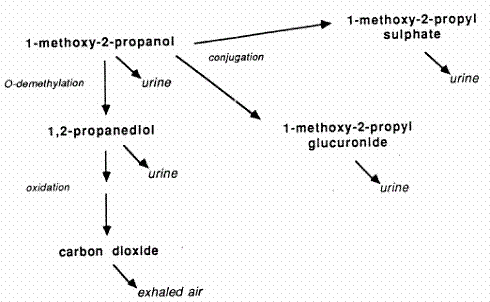
Appendices 1-18 to: Report on the Health Effects of Selected Pesticide Coformulants89 Toxicokinetics89.1 Absorption89.2 Distribution 89.3 Metabolism and elimination 89.4 Mode of action 89.1 AbsorptionThe toxicity data of 2PG1ME indicate uptake by all routes of exposure. In anaesthetised rats exposed to 1000 ppm 2PG1ME (3750 mg/m3), the respiratory uptake was 87% (Stott & McKenna 1984 – quoted from A&H 1990). 89.2 DistributionExposure by inhalation of rats over 6 hours to 300 -3000 ppm 2PG1ME (equivalent to 1125-11250 mg/m3) resulted in a continuous increase in blood concentration, which ranged from 109-2133 mg/g. The blood/air partition coefficient of 2PG1ME was 12400 in one study and 403 in another study (Johanson & Dynesius 1988 and Stott & McKenna 1984, respectively – quoted from A&H 1990). Oral administration to rats of 0.09 or 0.78 g/kg 14C-labelled 2PG1ME resulted in recovery of 92-98 % of the radioactivity. Forty-eight hours after dosing, the liver had the highest percentage (1.4 %) of the radioactive dose. The fat/blood partition coefficient was 0.09, indicating that 2PG1ME does not accumulate in adipose tissue. There were no indications of accumulation of radioactivity in other main body tissues, or in testes. (Miller et al. 1983). 89.3 Metabolism and eliminationRats were given an oral dose of 0.09 g/kg b.w. 14C-labelled 2PG1ME. Sixty-six percent of the dose was recovered in the expired air, 63% of which as carbon dioxide. Urinary excretion accounted for 11%, while 0.9% of the radioactivity was found in faeces. The predominant urinary metabolite was the glucuronic acid conjugate of 2PG1ME, followed by the sulphate conjugate, propylene glycol and unchanged 2PG1ME. The urinary recovery increased to 19-25% after exposure to a dose of 0.78 g/kg b.w. (Miller et al. 1983). A metabolism scheme for 2PG1ME is given in Figure 1. In rats exposed by inhalation to 300 - 3000 ppm 2PG1ME (1125-11250 mg/m3) for 6 hours, the kinetic was non-linear. The half-time in the blood was 2.4 hr after the lowest exposure level and 15.7 hr after the highest, indicating saturation of the metabolic pathways. (Morgott & Nolan 1987– quoted from A&H 1990). Figure 1. Proposed metabolism for 2PG1ME (modified from A&H 1990). 89.4 Mode of actionGlycol ethers capable of giving rise to alkoxyacetic acids have been shown to exhibit bone marrow depression and developmental, testicular and immunological toxicity. 2PG1ME is a secondary alcohol which does not form methoxy propionic acid but is primarily metabolised to carbon dioxide. No propylene glycol ethers being secondary alcohols have been shown to cause developmental toxicity. (ECETOC 1995).
|