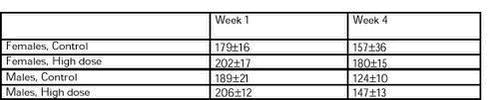| Front page | | Contents | | Previous | | Next |
In Vivo Investigation of Dietary Exposure to 5 Pesticides
4 Dose-response study of Chlorpyrifos
4.1 Objectives of the dose-response study of chlorpyrifos
4.2 Material and methods
4.2.1 Animals
4.2.2 Environment
4.2.3 Diet and water
4.2.4 Test and control substances
4.2.5 Pre-test procedures
4.2.6 Experimental procedures
4.2.7 Terminal procedures
4.2.8 Pathology
4.3 Results
4.3.1 Clinical signs, morbidity and mortality
4.3.2 Body weights
4.3.3 Relative food and water consumption
4.3.4 Functional observational battery (FOB)
4.3.5 Motor activity measurements
4.3.6 Eye examinations
4.3.7 Acetylcholinesterase activity
4.3.8 Pathology
4.4 Discussion and conclusion of the dose-response study
4.1 Objectives of the dose-response study of chlorpyrifos
The objectives of this study was to characterise the dose-response of chlorpyrifos and compare results with the NOAEL set by WHO based on the scientific literature.
4.2 Material and methods
The study design followed OECD guideline 407 ’repeated dose 28 day oral toxicity study’ with minor deviations.
4.2.1 Animals
Male and female rats, 24 of each sex, of the Brl:WIST Han@Mol strain were obtained from M&B A/S, Ry, Denmark. The animals were 4 weeks of age on arrival (on 29 March 2001). Groups of six animals per sex were formed by stratified randomisation. The group mean body weight was within 5 % of the overall mean for each sex. As up to 10% is acceptable, this is regarded as satisfactory. Males weighed between 44 and 66 g (overall mean 55.2 g), females weighed between 52 and 72 g (overall mean 61.6 g). The animals were approximately 5 weeks old at the start of dosing on 5 April 2001.
4.2.2 Environment
The animals were housed in a single, exclusive room, air-conditioned to provide a minimum of 10 air changes per hour. The temperature in the animal experimental room was 22±1°C, and the relative humidity was 55%±5%. Fluorescent lighting was controlled automatically to give a cycle of 12 hours light (0900 to 2100) and 12 hours dark. The animals were housed in pairs by sex in macrolon cages type III with Tapvei aspen wood bedding.
4.2.3 Diet and water
Diet was provided ad libitum. All rats received control group diet for 6 days until the start of the dose period. During the dose period, the rats received test diet according to dose group. Acidified tap water was provided ad libitum in nipple bottles (citric acid, pH=3). Drinking water was from the municipal water supply.
4.2.4 Test and control substances
The test substance, a brown solid, was chlorpyrifos, CAS No. 002921-88-2 supplied by Dow Chemicals. The purity of the test substance was 98.6%. The control substance and vehicle for the test substance was acetone. The test substance was administered orally in the diet. The animals received the substance in the diet for 28 days excluding the day of sacrifice.
4.2.4.1 Dose levels
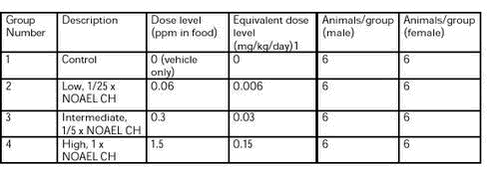
In Table 2 the selected dose levels are shown.
Table 2. Group number, group description in words and dose levels in ppm and mg/kg/day for amount of chlorpyrifos (CH) given to each dose group, and number of animals per group (male/female).
Click on the table to see: ‘‘Table 2‘‘
((mg/kg/day)1 )
4.2.4.2 Test substance formulation and preparation
Four test diets with added vehicle (acetone) and/or chlorpyrifos (0.06, 0.3, 1.5 mg/kg diet) were prepared by Altromin International, Germany. Diets were prepared once for the entire study. The test substance was added to powdered diet and made to pellets. The diets were stored in the original bags at room temperature.
4.2.4.3 Stability and homogeneity
From the initial study (see Appendix A) it was known that chlorpyrifos is stable for at least 28 days. In the present study, the concentration in week 4 was determined (see Table 3). The test diets were not analysed for homogeneity. Samples of 50 g of each test diet were obtained during week 4 of the dosing period. Each sample was analysed in duplicates. The achieved concentrations are shown in Table 3. The concentrations of pesticides in the diets were regarded as satisfactory.
Table 3. Intended and measured concentration of chlorpyrifos in the diet for each dose group (see Table 2).

Click on the table to see: ‘‘Table 3‘‘
4.2.5 Pre-test procedures
All animals were given a health inspection at arrival and observed daily during the 7-day acclimatisation period. The animals were assigned to treatment groups during the acclimatisation period using a randomisation procedure based on body weight.
Group body weight means and standard deviations were calculated and inspected to ensure there were no unacceptable differences between groups. The animals were individually identified by ear clipping (see Table 4). Cages were appropriately identified/ tagged with study information including study number and animal numbers.
Table 4. Group number, group colour code and animal identity numbers (ear clipping) for male and female rats.
4.2.6 Experimental procedures
4.2.6.1 Clinical signs, morbidity and mortality
All animals were observed twice daily in their cage for signs of adverse health or overt toxicity. A record was maintained of the clinical conditions for the study population as a whole. If adverse clinical signs were observed, an individual record was kept for the animals concerned. However, no adverse clinical signs were observed during the study.
4.2.6.2 Body weights
Individual body weights were recorded at weekly intervals. The last of these measurements was done in the morning on the day of sacrifice, and these body weights were used for calculation of relative organ weights.
4.2.6.3 Food and water consumption
The amount of food and water consumed by the animals in each cage was measured weekly.
4.2.6.4 Functional observational battery (FOB)
All animals were subjected to a battery of behavioural tests and observations (as listed below) during the fourth week of the dose period. At the time of testing, the observer was unaware of each animal’s dose level. Each animal was assigned a code number and moved to a new cage (1 animal/cage) by an independent person.
Observational measurements were performed before and upon removal from the home cage. The animal was placed into a square arena for 3 minutes. During this time, behaviour was evaluated as indicated (open field). Various manipulative tests were also done.
The following observations and measurements were done:
In the home cage: posture, eye opening, convulsions, and vocalisation.
During removal from the cage: reaction to handling, piloerection, lacrimation, salivation and eye opening.
In the open field arena: number of rears during 3 minutes, convulsions, gait, ease of movement, arousal, number of fecal boli and urine pools during 3 minutes.
Reflex testing: reaction to an object moved towards the face, to a push to the hindquarter, to a sudden noise, to a tail pinch, and the pupillary response to light.
Assessment of righting reflex, foot splay, grip strength of fore- and hind limbs. Measurement of body weight and temperature. Any other observations were recorded.
4.2.6.5 Motor activity measurement
Following FOB testing, the motor activity of each animal was assessed in an automated photocell activity recorder for 30 minutes. Activity counts were recorded in 5-minute intervals. Thus, data were obtained from six 5-minute motor activity intervals, called periods.
4.2.6.6 Eye examinations
Eye examinations were performed on all rats following FOB testing, before blood sampling. The assessment included pupillary response to light, and examination of the eye by direct observation, and following drug-induced (tropicamide) pupillary dilatation examination with a slit lamp and by indirect ophthalmoscopy.
4.2.6.7 Plasma acetylcholinesterase activity
Blood samples (1.0 ml intended) were drawn from all animals on the day before sacrifice. Samples were collected from the orbital sinus under CO2/O2 anaesthesia. Blood was collected in heparinized tubes and analysed for acetylcholinesterase activity. Samples were analysed on the day of sacrifice or refrigerated and analysed the following day. The analysis was performed at 37° C on a Cobas Mira auto analyser by use of a commercially available kit obtained from Boehringer Manheim applying Calibrator® Roche as the calibrator.
4.2.6.8 Brain acetylcholinesterase activity and protein concentration
At sacrifice the brain was removed and separated sagittally into two halves. The left half was used for measurement of brain acetylcholinesterase activity. The tissue was homogenized. A 2 ml sample of the homogenate was analysed for acetylcholinesterase activity and protein content. Relative brain acetylcholinesterase activity was calculated (units/g protein). Samples were analysed on the day of sacrifice or refrigerated and analysed the following day. The analysis was performed at 37° C on a Cobas Mira auto analyser by use of a commercially available kit obtained from Boehringer Manheim applying Calibrator® Roche as the calibrator. The protein analysis was performed on a Hitachi 912 auto analyser by use of a commercially available kit obtained from Roche applying CFAS® Roche as the calibrator.
4.2.7 Terminal procedures
4.2.7.1 Necropsy and macroscopic pathology
All animals were subjected to necropsy. The order of necropsy was balanced with respect to dose group and sex. The animals were euthanized by decapitation in CO2/O2 anaesthesia (on 3 May 2001). A full macroscopic examination was performed and all lesions were recorded.
4.2.7.2 Organ weights
The following organs of all animals were weighed: liver, kidneys, adrenal glands, testes, thymus and brain. The last of the weekly body weight
measurements, obtained on the day of sacrifice, was used for calculation of relative organ weights.
4.2.7.3 Histology
The following tissues were preserved in formaldehyde: the above-mentioned organs (from the brain the right half only) and in addition tissues with macroscopic lesions. It was planned to perform histopathology on the brain and on organs with macroscopic lesions and on organs with statistically significant deviation in relative weight.
4.2.7.4 Brain histology
Histological examination of the brains of group 1 and 4 was performed on slides stained with haematoxylin and eosin (HE). Brain tissue was examined for glial fibrillary acidic protein (GFAP) content by immunocytochemistry, using antibodies to the glial filament. GFAP staining is a commonly used method to examine the distribution of astrocytes and their hypertrophic response to neurotoxicants. The method allows for the direct visualization of astrocytes. The brains from control animals and group 4 were stained for GFAP and evaluated under light microscopy for expression of GFAP and distribution of astrocytes.
4.2.8 Pathology
Data evaluation
Data from treated animals were compared with control data. Males and females were evaluated separately.
No statistical analysis was done on data where it was obvious that no group difference was present (some FOB data, eye examination data2 , brain GFAP content).
Continuous data were evaluated with respect to conformity with normal distribution and variance homogeneity, and it was determined whether a parametric or a non-parametric test would be more suitable.
Two-way repeated measures analysis of variance was used for: body weight, relative food- and water consumption, and motor activity data.
Two-way analysis of variance was used for: organ weights, continuous FOB data. The analysis was ANOVA with Dunnett’s test for pair wise comparisons.
Non-parametric analysis of variance was used for: Acetylcholinesterase activity. The analysis was Kruskal-Wallis with Wilcoxon rank sum test for pair wise comparisons.
4.3 Results
4.3.1 Clinical signs, morbidity and mortality
No adverse clinical signs were observed, and no animals died during the study.
4.3.2 Body weights
All groups gained weight during the study period (Statistically significant effect of time). There was no statistically significant difference among groups within each sex.
4.3.3 Relative food and water consumption
All groups showed the normal decrease in relative food and water consumption during the study period (statistically significant effect of time). There was no statistically significant difference among groups within each sex. The relative food and water consumption (g food or water/kg bodyweight/day) in the control and high dose groups are shown in Table 5 and 6.
Table 5. Relative food consumption (g food /kg bodyweight/day) in week 1 and week 4 for females and males in control (group 1) and high dose (group 4) groups. See dose groups in Table 2.

Click on the table to see: ‘‘Table 5‘‘
Table 6. Relative water consumption (g water /kg bodyweight/day) in week 1 and week 4 for females and males in control (group 1) and high dose (group 4) groups. See dose groups in Table 2.
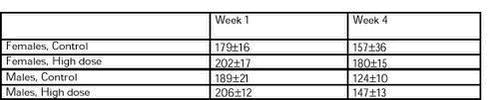
Click on the table to see: ‘‘Table 6‘‘
4.3.4 Functional observational battery (FOB)
No unusual behaviour was observed. The only statistically significant difference was a 16% reduced forelimb grip strength in females of the high dose group (1.5 ppm, group 4). No dose response was seen. However, it cannot be excluded that the reduced forelimb grip strength in females is substance related, since this was only seen in the high dose group.
4.3.5 Motor activity measurements
The variability in the data was large, which is commonly observed. Total motor activity during 30 minutes was unaffected by treatment. In all groups, a decline in activity over time was found reflecting the habituation to the novel situation. This decline was statistically significant. There was no difference between treatment groups in the repeated measures analysis of individual 5-minute activity periods.
4.3.6 Eye examinations
Because of technical failure of the eye examination utensils, an overall diagnosis could not be made in 7 males (0, 3, 3 and 1 animal at 0, 0.06, 0.3 and 1.5 ppm respectively) and in 10 females (1, 2, 3 and 4 animals at 0, 0.06, 0.3 and 1.5 ppm respectively), however all eyes were examined by inspection and pupil response to light was assessed. No treatment-related abnormalities were observed. Overall, the eye examination is considered inconclusive due to the lacking slit lamp and ophthalmoscopy data.
4.3.7 Acetylcholinesterase activity
4.3.7.1 Plasma acetylcholinesterase activity
No treatment-related difference was found. The plasma acetylcholinesterase level was approximately four times higher in females compared to males (see Figure 1). This is a normal finding.
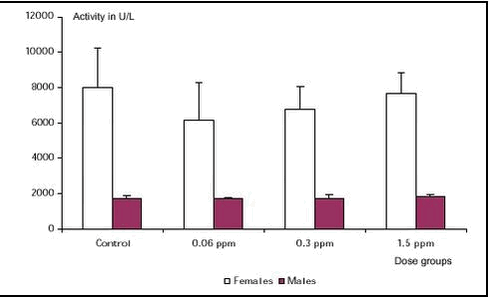
Figure 1. Plasma acetylcholinesterase activity, males and females for the 4 dose groups. Control (group 1), 0.06 ppm chlorpyrifos (group 2), 0.3 ppm chlorpyrifos (group 3) and 1.5 ppm chlorpyrifos (group 4).
4.3.7.2 Relative brain acetylcholinesterase activity
No treatment-related difference was found. The relative brain acetylcholinesterase level was the same in females and in males.
Acetylcholinesterase activities measured in plasma and brain in this study are erroneously about a factor 4 too high, because the calibration value for butyrylcholineesterase activity by mistake was programmed in the autoanalyser. Consequently, all values are about a constant 4 too high, but this does not influence the results of the study.
4.3.8 Pathology
4.3.8.1 Macroscopic pathology
At autopsy haemorrhages were found in the thymus of males and females in the groups 2, 3 and 4. These findings are normally related to euthanasia by CO2/O2 and decapitation. The few other macroscopic findings are considered spontaneous lesions unrelated to treatment.
4.3.8.2 Organ weights
No statistically significant treatment-related changes in absolute and relative organ weights were observed (group means of organ weights are presented in Appendix B)
4.3.8.3 Histopathology
As no organ weight changes were detected, no overall group histological examination was performed. One male rat in group 4 exhibited a changed bladder wall thickness (described grossly as muscular hypertrophy in the bladder), which was evaluated by microscopy to be a severe hyperplasia of the bladder epithelium.
4.3.8.4 Brain histology
No changes in colour intensity or distribution of astrocytes were seen in brains stained for GFAP in either control animals or in the high dose group (group 4). No histopathological findings were identified in brains stained with HE in either control animals or in group 4.
4.4 Discussion and conclusion of the dose-response study
The high level of toxicity in the initial study was unexpected, since a specific effect in the form of brain acetylcholinesterase inhibition was identified in all dose groups. The interest was focused on chlorpyrifos, the only acetylcholinesterase inhibitor among the five pesticides in the combination. The result could be explained as a true combination effect. However, since the dose of chlorpyrifos had been set on the basis of literature data, the possibility that chlorpyrifos had been administered at effective levels above those intended could not be excluded.
The objectives of the present study were to characterise the dose-response of chlorpyrifos and compare results with the NOAEL based on the scientific literature. Apart from a slight significant reduced forelimb grip strength in the high dose females, no effects were found in any dose group either on clinical signs, morbidity or mortality, body weights, food or water consumption, behaviour, pathology, plasma or brain acetylcholinesterase activity. Therefore it was concluded that the present study confirms the NOAEL of 1.5 ppm based on scientific literature. Furthermore the present study supports the hypothesis that the effects found in the initial study were related to the toxicity of the combination of the five pesticides and not to an unintended overdose of chlorpyrifos.
1 based on OECD guidance notes for analysis and evaluation of chronic toxicity studies 2000(24)
2 only data for visual inspection and pupillary response to light were collected from all animals. Data for slit lamp and ophthalmoscopy were not collected from 7 males and 10 females, and therefore these two endpoints were not considered for statistical analysis.
| Front page | | Contents | | Previous | | Next | | Top |
|