AMAP Greenland and the Faroe Islands 1997-20017 The Human Health Effects Biomarker Program7.1 Introduction7.1.1 Endocrine disruption 7.1.2 Exposure to chemical mixtures 7.1.3 POPs, gene polymorphism and hormone metabolism 7.1.4 Effect on hormone receptor numbers 7.1.5 The AMAP Human Health Effects Monitoring Programme 7.2 Methods 7.2.1 Determination of xenohormone activities in serum. 7.2.2 Determination of the sum of serum dioxin-like activities 7.2.3 Determination of estrogen metabolites in urine 7.2.4 Determination of inflammatory cytokines in serum 7.3 Results 7.3.1 Determination of xenohormon and dioxin-like activities in serum samples 7.3.2 Measurement of cytokine levels in serum 7.3.3 Determination of the ratio of estrogen metabolites in urine 7.3.4 Genotyping and gene expression 7.4 Discussion and Perspectives 7.5 References Eva Cecilie Bonefeld-Jørgensen 7.1 IntroductionHuman exposure to environmental contaminants is ubiquitous and is not restricted to individuals who live next to industries or waste disposal sites. Everyone carries a burden of POPs and heavy metals in their body. Persistent organochlorine compounds, such as dioxins/furans, polychlorinated biphenyls (PCBs) and certain pesticides, e.g., toxaphene and DDT/DDE, accumulate in body fat; meHg accumulates in organs, Pb in bones, etc. Environmental contamination is a global issue and POPs are transported to the Arctic by atmospheric and oceanic currents. Because of the lipophilic and persistent nature of POPs, bioaccumulation and biomagnification’s occur in the marine food web and in some freshwater predatory fish and piscivorous birds. Although far distant from the major pollution sources, some populations living in regions north of the Arctic Circle display a greater body burden of POPs than people living in industrialized regions, largely due to their reliance on a traditional diet that includes species high up in the marine food chain (see section 2 - 4 in this rapport) [Asplund et al., 1994; Dewailly et al., 1999; Dewailly et al., 1992; Dewailly et al., 1994; Jensen and Clausen, 1979]. Use of several organochlorine compounds, such as DDT and PCBs, was restricted or banned in most countries in the 1970s. Even though their concentrations in the environment have been slowly declining over the past 30 years, these compounds are still the most abundant persistent organochlorine contaminants found in wildlife and in human tissue and milk samples in the Arctic region. While concentrations of several organochlorine contaminants are decreasing, the continual introduction of ’new compounds’, such as brominated flame-retardants, into the environment has generated new concerns [Rahman et al., 2001]. In addition, attention has recently shifted from chronic diseases and reproductive endpoints to effects that are induced following exposure during the sensitive period of in utero development. Of particular concern are effects on development resulting from prenatal exposure to endocrine disrupting compounds. The burden of POPs in Arctic peoples has been monitored for some years. In 1997, the Alta Declaration extended AMAP’s mandate to cover assessment of the combined effects of environmental stressors. The AMAP Phase I assessment report (AMAP, 1998) gave an overview of the classical toxicology of contaminants. Only recently a programme for measuring the potential biological effects of these contaminants has been established: The AMAP Human Health Effects Monitoring Programme (Table 1). Body burden data alone are not enough to allow the health risks associated with exposure to environmental contaminants in Arctic peoples to be assessed. Furthermore, laboratory studies of the effects of single chemicals or chemical mixtures in laboratory animals and cell cultures cannot fully elucidate the human health risks. Integration of epidemiological and effect-biomarker studies on humans from exposed populations in the Arctic is needed in order to obtain information about the real health risks resulting from exposure to the accumulated mixtures of contaminants in the Arctic. The broad category of human health effects that are suspected to result from exposure to environmental contaminants include cancer, birth defects, effects on the reproductive and the neuro-endocrine-immune systems, altered metabolism, and specific organ dysfunction. Table 1 presents possible effect biomarkers that may be useful to include in epidemiological studies. By integration of epidemiological and biomarker effect studies it will be possible to establish a connection between traditional toxicological studies and new methods designed to study the potential of chemicals to interfere with the normal homeostasis by exerting endocrine-disrupting effects. As mentioned, most efforts have over the past decade been focused on the characterization of exposure of Arctic peoples to contaminants. Epidemiological studies have been conducted in which clinical endpoints, such as psychometrics, neuropsychological parameters, infection incidence, bone density, and sexual maturation among others, were the main focus (see chapter 5 -6 in this rapport). However, in order to detect the early biological changes preceding disease, knowledge about the mechanism of action of toxicants is required. Thus, biomarkers of effect need to be validated and used as concluded at the International Conference on Artic Development, Pollution and Biomarkers of Human Health in Anchorage, Alaska, May 2000. Effect biomarkers are early biological responses of the organism to an external toxic stress. Since the overall weight of evidence at the epidemiological level for adverse endocrine-related human health effects is not strong, further studies including validated biomarkers in epidemiological studies may help in identifying the possible relevant associations between exposure to contaminants and detrimental health effects in Arctic populations. 7.1.1 Endocrine disruptionIn order to establish consensus on the scope of endocrine disruption, to facilitate the identification of active chemicals, and to underpin future regulatory control it is essential to agree on a precise definition of an endocrine disrupting compound, The International Programme on Chemical Safety and the US EPA’s Endocrine Disrupter Screening and Testing Advisory Committee (EDSTAC) in 1998 proposed the following working definition. “An endocrine disrupter is an exogenous chemical substance or mixture that alters the structure or function(s) of the endocrine system and causes adverse effects at the level of the organism, its progeny, populations, or subpopulations of organisms, based on scientific principles, data, weight-of-evidence, and the precautionary principle”. Thus, the term ’endocrine disrupters’ covers all kinds of exogenous interfering chemicals; including synthetic chemicals and synthetic and naturally occurring hormones. Exposure can occur via food intake (especially high fat products) drinking water, pharmaceuticals, etc. The ability of a chemical to affect humans or wildlife depends on factors such as structure, concentration, bioavailability, degradation/metabolism, uptake, etc. The observed potency of a chemical is, therefore, very dependent on concentration and the system applied for testing. This can result in several classifications for any one single chemical, for example as carcinogenic, teratogenic, toxic, and endocrine disrupter, depending on which characteristic of the chemical is studied. Many of the compounds suspected of endocrine disrupting activity are known to be toxic (in some cases acutely toxic) at higher concentrations. They were therefore banned or controlled in some countries, either on this basis or because of their persistence and capacity to bioaccumulate in biota. Chronic low dose exposure and the subsequent bioaccumulation of lipophilic POPs with long biological half-lives are of special concern. These POPs may over time bioaccumulate to a critical level capable of eliciting an effect. Moreover the ’life-long’ duration of exposure, together with increasing environmental levels, may maximize the likelihood of induction of effects. Such factors must be taken into account in sub-chronic, chronic and/or multi-generation tests. Because of the complexity of the endocrine system, and the complex nature of human epidemiological studies, animal studies and in vitro screening methods are widely used for toxicity evaluation and risk assessment. To date, no clear-cut evidence for adverse endocrine-related human health effects has been obtained at the individual or population level. However, data from studies on wildlife species, studies on laboratory animals, and biomarker studies in vitro have strengthened the need for further research to address the uncertainty and alleviate concerns. Taking a precautionary approach, the weight of evidence would suggest that exposure levels seen in the Arctic have some potential for adverse effects on human health. Studies on wildlife populations have documented adverse effects that correlate with exposure to one or more putative endocrine modulating chemicals [Safe, 2000]. Adverse developmental and reproductive effects have been primarily linked to POPs and alkylphenols derived from alkylphenol ethoxylate surfactants used in industrial detergents. In many instances, it has been difficult to assign causality because of the complexity of environmental contaminant mixtures and the level of exposure during critical developmental windows. However, lower concentrations of POPs in the Great Lakes region were correlated with dramatic improvements in reproductive success and significant increases in an array of predatory birds in the Great Lakes basin [Tremblay and Gilman, 1995]. The range of toxicological effects that estrogenic chemicals can produce is illustrated by work on the synthetic estrogen diethylstilbestrol (DES). DES was used pharmaceutically from the late 1940s to the early 1970s to prevent abortions and pregnancy complications in women. However, studies found DES exposure to correlate with increases in abortions, neonatal death and premature birth, and an increase in the incidence of vaginal adenocarcinoma in young women who were exposed in utero [Herbst et al., 1971], and in utero exposed men had 4 times higher abnormalities of the reproductive tract compared with controls [Gill et al., 1979]. The abnormalities included cryptorchidism and hypospadias, and reduced sperm concentration and quality, although reduced fertility was not observed in these men [Wilcox et al., 1995]. Not all the effects of DES are ascribed to its binding to the estrogen receptor and recent studies have shown that several endocrine disrupting compounds induce their effects via different receptors and signaling pathways [Andersen et al., 1999; Andersen et al., 2002; Bonefeld-Jorgensen et al., 2001; Bonefeld-Jorgensen et al., 1997; Vinggaard et al., 1999a; Vinggaard et al., 2000; Vinggaard et al., 1999b]. The convergence of several lines of inquiry was crucial for the rapid growth of interest in the issue of endocrine disruption in the 1990s. A number of worrying trends related to human male reproductive health had been reported globally, including decline in semen quality parameters and increases in the incidence of testicular cancer, hypospadias and cryptorchidism. At the same time, adverse trends in the reproductive health of wildlife in some regions outside the Arctic had also been noted and correlated with exposure to environmental contaminants, and in some cases specific chemicals were implicated. Evidence was also emerging from a variety of experimental studies that many widely used chemicals, distributed extensively in the environment, had the ability to bind and activate estrogen receptors. Although their affinity for the receptor was weak compared with the natural ligand their activity was regarded as sufficient to support a working hypothesis that environmental chemicals might be damaging the reproductive health of human and wildlife populations by interfering with sex hormone activities. Behind this concern was the suspicion that chemicals acting through hormone receptors might mimic the natural hormones and have profound effects at very low concentrations. The conjunction of threat both to human and wildlife populations led to responses from international organizations (including AMAP), governments, and the chemical industry. The following general needs were identified.
The Endocrine Disrupter Screening and Testing Advisory Committee (EDSTAC, 1998) has developed a strategy for testing chemicals for endocrine modulating activity including an initial sorting of chemicals (based on existing data), priority setting (based on knowledge of exposure), and tier 1 screening and tier 2 testing, comprising:
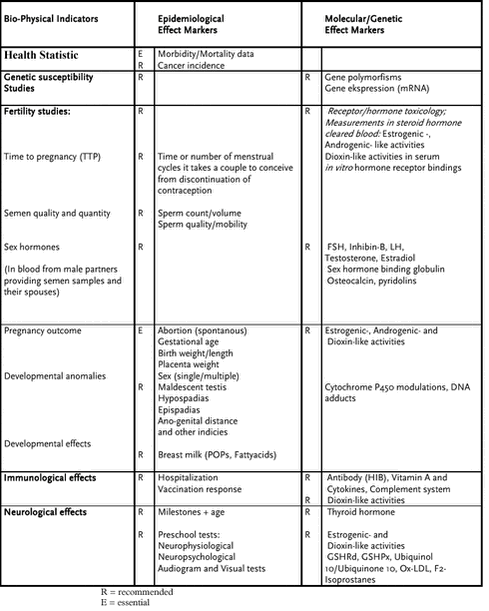
7.1.2 Exposure to chemical mixturesThere are a number of factors that complicate the toxicological evaluation of mixtures. First, it is important to remember that no test can evaluate all possible endpoints. However, existing methods in general include numerous endpoints that are sensitive to both strong and weak xenoestrogens such as the reproductive and developmental effects in humans and rodents of DES [Gill et al., 1979; Herbst et al., 1971; McLachlan, 1981; Wilcox et al., 1995] and DDT or chlordecone [Daston et al., 1997]. These endpoints, obtained by multi-generation studies in rodents, are sufficient to indicate a hazard. Subsequent decisions to further characterize the cellular and molecular steps in the hazard evaluation require mechanistic research for risk assessment, taking into account the possibility that the observed adverse effects may not be the most sensitive manifestation of toxicity. Second, two or more compounds may have additive effects as a result of acting via the same mechanism in concert. They may also elicit antagonistic, or synergistic (greater than additive) effects. Some studies have suggested synergistic responses of steroidal estrogens in vitro (yeast) and in vivo (turtle) [Arnold et al., 1997a; Arnold et al., 1997b]. However, estrogenic tests with mixtures of dieldrin and toxaphene in human breast cancer MCF-7 cells, yeast-based human estrogen receptor assays, and mouse uterus tests showed no apparent synergism [Ramamoorthy et al., 1997]. There are several other complications that must be taken into account when generalizing about what is known about the toxicity of single compounds and/or mixtures. A compound may have multiple sites of action and its toxicity may be mediated by different mechanisms. Many substances are biotransformed to metabolites (e.g., hydroxylated PCB metabolites) that may have a different biological activity than that of the parent compound. In addition, a single environmental contaminant may induce different effects depending on the organism’s age and reproductive state at the time of exposure. Lead is an example of a contaminant having little effect on neurobehavioral function in adults but irreversible effects on intelligence quotient (IQ) and behavior when exposure occurs in utero during the development of the nervous system [Carpenter et al., 1998]. It is known that developmental toxicity is dependent on highly susceptible periods of organogenesis, as demonstrated by prenatal exposure to, e.g., DES and thalidomide, and postnatal exposure to Pb, pesticides, methylmercury (see chapter 6 this rapport) and radiation [Selevan et al., 2000]. Toxicity scales have been developed for compounds that share a common mechanism of action. This concept was applied to mixtures of dioxin-like compounds that bind the aryl hydrocarbon receptor (AhR). The AhR is an intracellular ligand-dependent transcription factor expressed in most tissues of mammals. Dioxins and furans (polychlorinated dibenzo-p-dioxins, PCDDs; 217 polychlorinated dibenzofurans, PCDFs) as well as non- or mono-ortho chloro-substituted PCBs are ligands to the AhR [Birnbaum, 1995; Brouwer et al., 1999; Carpenter et al., 1998]. The activated ligand-receptor complex triggers the expression of enzymes including P4501A1, P4501A2, P4501B1, glutathione S-transferase, glucuronyl transferase, d -d aminolevulinate synthethase, epidermal transglutaminase, NAD(P)H: quinone oxidoreductase and aldehyde-3-dehydrogenase, which are involved in metabolism and detoxification of many POPs [Hahn, 1998; Safe and Krishnan, 1995]. A common practice in risk assessment is to calculate the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxic equivalents (TEQs) for mixtures comprising dioxin-like compounds. TEQs are calculated by multiplying the concentration of each dioxin-like compound by its Toxic Equivalency Factor (TEF), which corresponds to the relative potency of the specific compound in generating an AhR-mediated effect, in relation to that of TCDD, the most potent dioxin-like compound. Consequently, the classical TEQ/TEF risk assessment only accounts for potential dioxin-like properties of a mixture and not other relevant toxicological endpoints such as effects mediated via other receptors and biochemical pathways (e.g., interference with the sex hormones and thyroid hormone systems). For example, ortho-substituted PCBs are either weak ligands or do not bind at all to the AhR, therefore either very low or no TEF values are given for these compounds. Recently, however, it was reported that the three most highly bioaccumulated di-ortho substituted PCBs (CB138, CB153, and CB180) elicit the potential, in vitro, to interfere with cell proliferation as well as the function of the estrogen and androgen receptor [Bonefeld-Jorgensen et al., 2001]. These results emphasize that a full assessment of the toxicological potential of a chemical mixture is much more complex than can be deduced by the use of TEQ values alone. 7.1.3 POPs, gene polymorphism and hormone metabolismCentral to many of the influences on the biological system are effects that occur at the gene level. Genes regulate almost everything, including many aspects of hormonal production and the reproductive system, brain development and function, immune system balances, and organ physiology. A genetic disruption can, therefore, affect different organ systems, as a result of the extensive interaction among these systems; i.e., effects on one organ system may influence the function of other organs. During normal development, genes are activated and deactivated at different stages, often under the control of growth factors and hormones. Environmental factors interfere with these biologically balanced processes and may result in genetic dysfunction. Mutations in genes, inherited or induced by environmental factors, may thus result in reproductive effects, birth defects, and cancer. Gene polymorphism is known to exist between different ethnic groups, which can result in differences in tolerance e.g., to food components such as lactose [Harvey et al., 1998; Nei and Saitou, 1986]. In addition, gene polymorphism in metabolizing enzyme, e.g. in the P450 enzyme system, is suspected to influence susceptibility to environmental carcinogens, affecting the risk of cancer [Autrup, 2000; Coughlin and Piper, 1999; Morabia et al., 2000]. Genetic polymorphism and breast cancer risk has been extensively analyzed, and significant differences in genotype frequencies between cases and controls were found, including the aromatase cytochrome P450 (CYP19) gene which catalyses the conversion of androgens to estrogens [Dunning et al., 1999]. Recently, a study suggested an association between PCB concentrations and CYP1A1 gene polymorphism in women breast cancer patients compared to control groups [Moysich et al., 1999]. 218 Accumulation in fatty tissues and the potential of many persistent organochlorine contaminants to exert estrogenic/androgenic- or anti-estrogenic/anti-androgenic-like effects are hypothesized to promote the cancer process through the modulation of the estrogen receptor regulated responses [Wolff and Toniolo, 1995]. Therefore, to reject or verify the hypothesis future studies must include, in addition to the epidemiological investigation and burden of POPs, information on genetic polymorphisms and biomarkers related to the total impact of components with estrogenic (or anti-estrogenic), androgenic (or anti-androgenic), and dioxin-like activities. Currently, a pilot breast cancer study including these endpoints is being carried out in Greenland (E. Bonefeld-Jorgensen, personal comm., 2002). PCBs and dioxin are well known for their ability to induce certain iso-enzymes of P450 in mammalian liver via the AhR. Some of these enzymes, P4501A1, P4501A2, and P4501B1, are involved in estradiol metabolism and might disrupt hormone levels [Spink et al., 1992a; Spink et al., 1994; Spink et al., 1992b; Spink et al., 1998]. In vitro, several persistent organochlorine contaminants have been shown to increase the 16a-OHE1:2-OHE1 estradiol metabolite ratio; 16a-OHE1 is regarded as highly estrogenic while 2-OHE1 is a weak anti-estrogen [Bradlow et al., 1995]. Some studies have reported higher levels of the 16a-OHE1 metabolite in urine of breast cancer patients [Bradlow et al., 1995; Safe, 2000], whereas other studies did not observe this association [McDougal and Safe, 1998; Ursin et al., 1997]. Thus, inconclusive results exist and await further research. 7.1.4 Effect on hormone receptor numbersThe responsiveness of a tissue to a hormone depends on the density of receptors within its component cells. The number of receptors is determined by their rate of synthesis and catabolism, which is in turn controlled by complex feedback mechanisms involving hormone action. Some chemicals are shown to interfere with this regulation. For example, TCDD can act to decrease or increase the expression of the ER [Romkes et al., 1987], and compounds which bind to the ER (e.g., ICI 182,780, toxaphene, CB138 and several pesticides) influence receptor functions as well as the cellular level of ER mRNA [Andersen et al., 2002; Bonefeld-Jorgensen et al., 2001; Jensen et al., 1999]. 7.1.5 The AMAP Human Health Effects Monitoring ProgrammeAs previously stated, there are broad categories of health effects that may be linked to exposure to environmental contaminants. These include cancer, birth defects, decreased fertility, altered sex hormone balance, immune system defects, neurological effects such as reduced IQ and behavioral abnormalities, altered metabolism, and specific organ dysfunctions [Carpenter et al., 1998]. At AMAP Human Health Expert Group meetings held in Rovaniemi, Finland, January 2000 and Tórshavn, Faroe Islands, October 2000, a Human Health Effects Monitoring Programme was recommended to the eight Arctic nations. Table 7.1 Human Health Effect Monitoring Programme
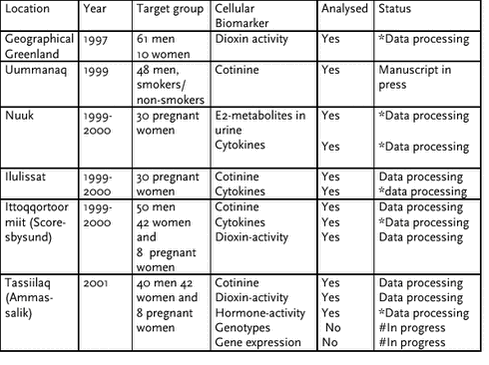
Effects on reproduction and development of dietary POP and heavy metal burden within the arctic. Requirement of parallel indicators: dietary questionnaire and indicators, and POP measurements carried out by laboratories with documented AQ/AC The AMAP Human Health Effects Monitoring Programme, (Table 7.1) includes several molecular biomarker endpoints for use in Arctic environmental health studies. Substances commonly found in the environment may have the potential to affect several organ systems. The diseases listed are identified on the basis of studies of both humans and animals, and in most cases these investigations were focused on a single contaminant. Several of these diseases, when found in a given individual, are difficult to ascribe to a particular exposure [Sharpe, 1993; Sharpe and Skakkebaek, 1993]. This is generally the case for cancer, reproductive effects (such as infertility, early birth, etc.), many of the endocrine modulators and nervous system actions. Others are clearly attributable to particular exposures, such as kidney disease following Cd exposure or the loss of particular neurons following MeHg exposure [Carpenter et al., 1998]. The main objective of the AMAP Human Health Effects Monitoring Programme is to characterize the impact of dietary exposure to POPs by monitoring biophysical indicators, and epidemiological and molecular/genetic effect markers (see Table 7.1). These effects studies are directed towards examining the hypothesis of xenobiotic interference with homeostasis of hormone functions, with a special focus on circumpolar populations. A number of persistent organochlorine contaminants exhibit estrogenic (and anti-estrogenic), androgenic (and anti-androgenic) and dioxin-like activities. Some bind to the estrogen receptor (e.g., DDT, toxaphene, CB126, CB153, CB180) [Bolger et al., 1998; Bonefeld-Jorgensen et al., 2001; Bonefeld-Jorgensen et al., 1997], and some bind to the androgen receptor (e.g., DDE, vinclozolin) [Bonefeld-Jorgensen et al., 2001; Crisp et al., 1998; Kelce et al., 1997; Kelce and Wilson, 1997] or bind to both receptors (e.g., metabolites of methoxychlor, CB138). Dioxins have been characterized as anti-estrogenic due to their AhR mediated interference with estrogen receptor activities [Kharat and Saatcioglu, 1996; Safe, 1994]. Because of the wide variety of endocrine disrupting effects possibly induced by mixtures of persistent organochlorine contaminants, there is a need to develop markers that integrate the effects of several chemicals on specific hormonal pathways and combine them with epidemiological studies that are also part of the AMAP Human Health Effects Monitoring Programme. The morbidity/mortality data and pregnancy outcomes are considered essential effect markers, whereas the other biomarkers listed in Table 1 are recommended measurements for inclusion within AMAP monitoring implementation plans. The biophysical indicators, and epidemiological and molecular/genetic effect markers included in Table 1 are, as far as possible, linked into the different studies. In addition, dietary questionnaires, relevant markers of a seafood based diet (e.g., n-3 fatty acid content in plasma phospholipids), should also be included in the studies, and laboratories performing these measurements must have documented quality assurance / quality control (QA/QC). Some studies listed in the Human Health Effects Monitoring Programme (Table 7.1) have already been initiated in some parts of the Arctic, while others are still in the planning phase. In the Faeroe Island the epidemiological approach has been in focus concerning immunological and neurological effects (see chapter 5 – 6, this rapport). In Greenland the part of the programme including the recommended molecular/genetic effect marker analyses have been initiated and some blood samples have been analyzed for the concerted action of xenohormone activities, the sum of dioxin-like activities and the level interleukin-1 beta (IL-1ß) as well as the level of estrogen metabolites in urine. These data will be described in the Results part of this section. 7.2 Methods7.2.1 Determination of xenohormone activities in serum.Sonnenschein and colleagues have devised a method for estimating human exposure to a complex mixture of xenohormones (environmental compounds with hormone-like activities) [Sonnenschein et al., 1995]. First, endogenous steroids are separated from persistent organochlorine contaminants in human plasma samples by high-performance liquid chromatography (HPLC) and the resulting fractions are tested for estrogenic activity using a proliferation assay with MCF-7 cells. Other investigators (see below 7.3.1) have further developed and applied the HPLC fractionation of human serum for separating endogenous hormones from xenohormones to obtain integrative measurements of estrogenic, androgenic, and dioxin-like effects of compounds using reporter-gene cell assays. The estrogenic data referred to in this rapport was obtained by exposure of MVLN cells, which carries a stable integrated ER reporter gene (ERE-CALUX), to the hormone free serum extracts. Upon cell exposure the estrogen receptor (ER) activated reporter gene (luciferase) activity was determined. Recently, a new assay for detection of xenoestrogens in serum upon eliminating the endogenous steroids by immuno-precipitation was reported [Natarajan et al., 2002]. 7.2.2 Determination of the sum of serum dioxin-like activitiesUsing the stable transfected mouse hepatoma cell line Hepa1.1 [Garrison et al., 1996; Ziccardi et al., 2000] carrying a Ah-receptor directed reporter gene (luciferase), we measured the sum of dioxin-like activity by exposure of the cells directly to serum and after hexane:ethanol extraction of the POPs from the fatty fraction of the serum (performed in Jean-Philippe Webers lab in Quebec). 7.2.3 Determination of estrogen metabolites in urineUrinary estrogen metabolite ratio, 2-hydroxyestrogen/16a-hydroxyestrone, were determined by the use of an enzyme immunoassay (ESTRAMETTM 2/16, Immuna Care Corporation, PA, USA)). Urine samples were collected and stored in tubes containing 1 mg/ml ascorbic acid. The samples was immediately placed on ice and then stored at –200C. The analyses were performed in accordance to the recommended protocol. 7.2.4 Determination of inflammatory cytokines in serumMeasurement of the cytokine interleukine-1 beta (IL-1ß) in serum was performed by the use of the chemiluminescence’s ELISA analyses following the protocol recommended by the manufacturer (R&D, London, UK). 7.3 ResultsIn table7.2 is summarized the cellular effect biomarker analyses performed on Greenlandic samples from 1997 to 2002. As given in table 7.2, all of the planned effect biomarker analyses but genotypes are performed and under data processing and manuscripts are under preparation for publication in international journals. Table7.2 Status of analyses of cellular effects biomarkers 2002
(*)Manuscript under preparation, (#) Analyses design and set up performed Concerning the effect biomarker analyses of samples from the geographical survey Greenland 1997 [Deutch and Hansen, 2000], measurements of the dioxin-like activities in serum were not originally planned since at that time we did not have the techniques available in the laboratory. However, because we think it is essential already now to start some kind of trend analyses, we decided to carry out the analyses. 7.3.1 Determination of xenohormon and dioxin-like activities in serum samplesUsing a further developed (performed at Dept. of Environ. Med., South Danish University, Odense) serum extraction method of Sonnenschein and cooperators [Sonnenschein et al., 1995] 100 serum samples (Ammassalik (Tasiilaq), Greenland 2000-2001) free of endogenous hormones have been analyzed for effects on the estrogen receptor (ER) trans-activity. We used the MVLN cell line to obtain the concerted action of the actual mixture of accumulated persistent organochlorine contaminants in these human samples on the ER hormone receptor activity. The results indicated that this hormone free serum fraction, containing the mixture of accumulated POPs, exerts an inhibitory effect on the normal estrogen hormone function mediated through the ER activity in human cells (Bonefeld-Jorgensen, manuscript in prep.). Another 71 serum samples from the Geographical Greenland (Geo) project (see chapter 4) and 100 serum samples from Ammassalik (Tasiilaq) have been analyzed for the sum of dioxin-like activities using the AH-receptor dependent AHR-CALUX, Hepa1.1 cell system. Approximately 30% of the samples from the Geo, analyzed by directly exposure of the responding reporter-gene cell line, elicited an increased dioxin-like activity compared to a reference control of pooled Danish serum samples (n = 10) with very low POP concentration (up to 100 times less) (see chapter 4.3.3, Table 4.3.16). The samples from Ammasalik await further analyses. Because it is known that various compounds in human serum can inhibit the activation of the AH- receptor and thus the AHR-CALUX system, we have analyzed the same samples for dioxin-like activities after extraction of the POPs from the fatty fraction of serum.) have been analyzed The results are currently under advanced statistical (SAS, SPSS) evaluation and the preliminary data suggests that the cellular effect measurements may be associated to specific PCB congeners and/or pesticides. Unfortunately, because of promising new information concerning the cellular effect of the POPs on human health, the data cannot yet be released for publication in the National Assessment Report of Greenland since the conclusion of the data may have far-reaching influence on diet recommendations and health policy in Greenland. Moreover, the manuscript of these new data is meanwhile under preparation, for publication in an international journal. 7.3.2 Measurement of cytokine levels in serumThe IL-1ß cytokine level has been measured in 29 serum samples from Ilulissat (1999-2000), 100 serum samples from Ittoqqortoormiit, Scorebysund (1999-2000), 30 serum samples from Nuuk (1999-2000). The results are currently being statistically evaluated for eventual associations to accumulated POPs and a manuscript is under preparation. 7.3.3 Determination of the ratio of estrogen metabolites in urineIn total 70 urine samples (both sexes) from inhabitants in Ammassalik and 24 urine samples from pregnant women living in Nuuk have been analysed for the ratio of the estrogen metabolites 2-hydroxyestrogen/16a-hydroxyestrone. For references 10 urine samples from Danish women, selected for having a very low level of POPs in their blood, have been analyzed for the estrogen metabolites. The results are currently statistically evaluated for eventually association to accumulated POPs and a manuscript is under preparation. 7.3.4 Genotyping and gene expressionBlood samples (100) for DNA and RNA isolation and the following genotyping and gene expression have been colleted from inhabitants in Scoresbysund (2000) and Ammassalik (2001). These very advanced analyses are going to be performed on an on-line PCR apparatus (LightCycler, Roche). The analyses design and set up is performed for genes, which are relevant for metabolising POPs and the following analyses are under performance. 7.4 Discussion and PerspectivesThe effects on human health of environmental contaminants like POPs are long-term effects, which means that to obtain a clearly evidential association between accumulations in human tissues, effects on cellular biomarkers and negative health effects, trend analyses in more than one generation may be necessary. However, there is increasing evidence of adverse trends in human reproductive health, most notably testicular cancer and female breast cancer, whereas the decrease in sperm counts apparent from some studies is still being discussed. Although, causal links between effects and exposure to environmental chemicals have still not been firmly established. Environmental chemicals have been focused on because of their capacity to interfere with hormone activities and hence their possible relation to trends in hormone related health effects. In wildlife, there is more convincing evidence of links between environmental exposure and endocrine disruption. This strengthens the concerns about endocrine modulation by environmental chemicals in humans. Because the developing fetus is particularly susceptible to exposure to environmental chemicals, and because there are many different effect targets, evaluation in terms of both lifetime effects (generations) and effects on organs (time to dysfunction) is complicated. Much research and monitoring are still required, and there is a need to develop, refine, and validate test methods that can accurately predict the effect of chemicals on human health. Importantly, it should be stressed that to elucidate the risk of these environmental contaminants, the future research must include an integration of the three disciplines 1) monitoring, 2) epidemiological analyses, and 3) cellular/genetic biomarker effect analyses. 7.5 ReferencesAndersen HR, Andersson AM, Arnold SF, Autrup H, Barfoed M, Beresford NA, Bjerregaard P, Christiansen LB, Gissel B, Hummel R, Jorgensen EB, Korsgaard B, Le Guevel R, Leffers H, McLachlan J, Moller A, Nielsen JB, Olea N, Oles-Karasko A, Pakdel F, Pedersen KL, Perez P, Skakkeboek NE, Sonnenschein C, Soto AM, et al. (1999): Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect 107:89-108. Andersen HR, Vinggaard AM, Rasmussen TH, Gjermandsen IM, Bonefeld-Jørgensen EC (2002): Effects of currently used pesticides in assays for estrogenicity, androgenicity and aromatase activity in vitro. Toxicol Appl Pharmacol 179:1-12. Arnold SF, Bergeron JM, Tran DQ, Collins BM, Vonier PM, Crews D, Toscano WA, McLachlan JA (1997a): Synergistic responses of steroidal estrogens in vitro (yeast) and in vivo (turtles). Biochem Biophys Res Commun 235:336-42. Arnold SF, Vonier PM, Collins BM, Klotz DM, Guillette LJ, Jr., McLachlan JA (1997b): In vitro synergistic interaction of alligator and human estrogen receptors with combinations of environmental chemicals. Environ Health Perspect 105 Suppl 3:615-8. Asplund L, Svensson BG, Nilsson A, Eriksson U, Jansson B, Jensen S, Wideqvist U, Skerfving S (1994): Polychlorinated biphenyls, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (p,p‘-DDT) and 1,1-dichloro-2,2-bis(p-chlorophenyl)-ethylene (p,p‘-DDE) in human plasma related to fish consumption. Arch Environ Health 49:477-86. Autrup H (2000): Genetic polymorphisms in human xenobiotica metabolizing enzymes as susceptibility factors in toxic response. Mutat Res 464:65-76. Birnbaum LS (1995): Developmental effects of dioxins and related endocrine disrupting chemicals. Toxicol Lett 82-83:743-50. Bolger R, Wiese TE, Ervin K, Nestich S, Checovich W (1998): Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect 106:551-7. Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM (2001): Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology 158:141-53. Bonefeld-Jorgensen EC, Autrup H, Hansen JC (1997): Effect of toxaphene on estrogen receptor functions in human breast cancer cells. Carcinogenesis 18:1651-4. Bradlow HL, Davis DL, Lin G, Sepkovic D, Tiwari R (1995): Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: a biologic marker of breast cancer risk. Environ Health Perspect 103 Suppl 7:147-50. Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, Schantz S, Winneke G (1999): Characterization of potential endocrine-related health effects at low- dose levels of exposure to PCBs. Environ Health Perspect 107 Suppl 4:639-49. Carpenter DO, Arcaro KF, Bush B, Niemi WD, Pang S, Vakharia DD (1998): Human health and chemical mixtures: an overview. Environ Health Perspect 106 Suppl 6:1263-70. Coughlin SS, Piper M (1999): Genetic polymorphisms and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 8:1023-32. Crisp TM, Clegg ED, Cooper RL, Wood WP, Anderson DG, Baetcke KP, Hoffmann JL, Morrow MS, Rodier DJ, Schaeffer JE, Touart LW, Zeeman MG, Patel YM (1998): Environmental endocrine disruption: an effects assessment and analysis. Environ Health Perspect 106 Suppl 1:11-56. Daston GP, Gooch JW, Breslin WJ, Shuey DL, Nikiforov AI, Fico TA, Gorsuch JW (1997): Environmental estrogens and reproductive health: a discussion of the human and environmental data. Reprod Toxicol 11:465-81. Deutch B, Hansen JC (2000): High human plasma levels of organochlorine compounds in Greenland. Regional differences and lifestyle effects. Dan Med Bull 47:132-7. Dewailly, Mulvad G, Pedersen HS, Ayotte P, Demers A, Weber JP, Hansen JC (1999): Concentration of Organochlorines in Human Brain, Liver, and Adipose Tissue Autopsy Samples from Greenland. Environ Health Perspect 107:823-828. Dewailly E, Nantel A, Bruneau S, Laliberté C, Ferron L, Gingras S (1992): Breast milk contamination by PCDDs, PCDFs and PCBs in arctic Québec: A preliminary assessment. Chemosphere 25:1245 - 1249. Dewailly E, Ryan JJ, Laliberte C, Bruneau S, Weber JP, Gingras S, Carrier G (1994): Exposure of remote maritime populations to coplanar PCBs. Environ Health Perspect 102 Suppl 1:205-9. Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF (1999): A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev 8:843-54. Garrison PM, Tullis K, Aarts JM, Brouwer A, Giesy JP, Denison MS (1996): Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam Appl Toxicol 30:194-203. Gill WB, Schumacher GF, Bibbo M, Straus FH, 2nd, Schoenberg HW (1979): Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. J Urol 122:36-9. Hahn ME (1998): The aryl hydrocarbon receptor: a comparative perspective. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 121:23-53. Harvey CB, Hollox EJ, Poulter M, Wang Y, Rossi M, Auricchio S, Iqbal TH, Cooper BT, Barton R, Sarner M, Korpela R, Swallow DM (1998): Lactase haplotype frequencies in Caucasians: association with the lactase persistence/non-persistence polymorphism. Ann Hum Genet 62:215-23. Herbst AL, Ulfelder H, Poskanzer DC (1971): Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 284:878-81. Jensen BL, Skouv J, Lundholt BK, Lykkesfeldt AE (1999): Differential regulation of specific genes in MCF-7 and the ICI 182780-resistant cell line MCF-7/182R-6. Br J Cancer 79:386-92. Jensen GE, Clausen J (1979): Organochlorine compounds in adipose tissue of Greenlanders and southern Danes. J Toxicol Environ Health 5:617-29. Kelce WR, Lambright CR, Gray LE, Jr., Roberts KP (1997): Vinclozolin and p,p‘-DDE alter androgen-dependent gene expression: in vivo confirmation of an androgen receptor-mediated mechanism. Toxicol Appl Pharmacol 142:192-200. Kelce WR, Wilson EM (1997): Environmental antiandrogens: developmental effects, molecular mechanisms, and clinical implications. J Mol Med 75:198-207. Kharat I, Saatcioglu F (1996): Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin are mediated by direct transcriptional interference with the liganded estrogen receptor. Cross-talk between aryl hydrocarbon- and estrogen-mediated signaling. J Biol Chem 271:10533-7. McDougal A, Safe S (1998): Induction of 16alpha-/2-hydroxyestrone metabolite ratios in MCF-7 cells by pesticides, carcinogens, and antiestrogens does not predict mammary carcinogens. Environ Health Perspect 106:203-6. McLachlan JA (1981). In Herbst AL, Bern HA (eds): "Developmental Effects of Diethylstilboestrol (DES) in Pregnancy." New York:, pp 148. Morabia A, Bernstein MS, Bouchardy I, Kurtz J, Morris MA (2000): Breast cancer and active and passive smoking: the role of the N-acetyltransferase 2 genotype. Am J Epidemiol 152:226-32. Moysich KB, Shields PG, Freudenheim JL, Schisterman EF, Vena JE, Kostyniak P, Greizerstein H, Marshall JR, Graham S, Ambrosone CB (1999): Polychlorinated biphenyls, cytochrome P4501A1 polymorphism, and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 8:41-4. Natarajan K, Overstreet JW, Rogers JM, Denison MS, Chen J, Lohstroh PN, McConnell DS, Lasley BL (2002): Detection of xenoestrogens in serum after immunoprecipitation of endogenous steroidal estrogens. Environ Health Perspect 110:791-5. Nei M, Saitou N (1986): Genetic relationship of human populations and ethnic differences in reaction to drugs and food. Prog Clin Biol Res 214:21-37. Rahman F, Langford KH, Scrimshaw MD, Lester JN (2001): Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ 275:1-17. Ramamoorthy K, Wang F, Chen IC, Norris JD, McDonnell DP, Leonard LS, Gaido KW, Bocchinfuso WP, Korach KS, Safe S (1997): Estrogenic activity of a dieldrin/toxaphene mixture in the mouse uterus, MCF-7 human breast cancer cells, and yeast-based estrogen receptor assays: no apparent synergism. Endocrinology 138:1520-7. Romkes M, Piskorska Pliszczynska J, Safe S (1987): Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on hepatic and uterine estrogen receptor levels in rats. Toxicol Appl Pharmacol 87:306-14. Safe S, Krishnan V (1995): Cellular and molecular biology of aryl hydrocarbon (Ah) receptor- mediated gene expression. Arch Toxicol Suppl 17:99-115. Safe SH (1994): Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol 24:87-149. Safe SH (2000): Endocrine disruptors and human health--is there a problem? An update. Environ Health Perspect 108:487-93. Selevan SG, Kimmel CA, Mendola P (2000): Identifying critical windows of exposure for children‘s health. Environ Health Perspect 108 Suppl 3:451-5. Sharpe RM (1993): Declining sperm counts in men--is there an endocrine cause? J Endocrinol 136:357-60. Sharpe RM, Skakkebaek NE (1993): Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? [see comments]. Lancet 341:1392-5. Sonnenschein C, Soto AM, Fernandez MF, Olea N, Olea Serrano MF, Ruiz Lopez MD (1995): Development of a marker of estrogenic exposure in human serum. Clin Chem 41:1888-95. Spink DC, Eugster HP, Lincoln DW, 2nd, Schuetz JD, Schuetz EG, Johnson JA, Kaminsky LS, Gierthy JF (1992a): 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1A1: a comparison of the activities induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in MCF-7 cells with those from heterologous expression of the cDNA. Arch Biochem Biophys 293:342-8. Spink DC, Hayes CL, Young NR, Christou M, Sutter TR, Jefcoate CR, Gierthy JF (1994): The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estrogen metabolism in MCF-7 breast cancer cells: evidence for induction of a novel 17 beta-estradiol 4-hydroxylase. J Steroid Biochem Mol Biol 51:251-8. Spink DC, Lincoln II DW, Johnson JA, Dickerman HW, Gierthy JF (1992b): Stimulation of 17 -beta- etradiol metabolism in MCF-7 breast cancer cells by 2,3,7,8-tetrachlorodibenzo-P-dioxin. Chemosphere 25:87 - 90. Spink DC, Spink BC, Cao JQ, DePasquale JA, Pentecost BT, Fasco MJ, Li Y, Sutter TR (1998): Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis 19:291-8. Tremblay NW, Gilman AP (1995): Human health, the Great Lakes, and environmental pollution: a 1994 perspective. Environ Health Perspect 103 Suppl 9:3-5. Ursin G, London S, Stanczyk FZ, Gentzschein E, Paganini Hill A, Ross RK, Pike MC (1997): A pilot study of urinary estrogen metabolites (16alpha-OHE1 and 2-OHE1) in postmenopausal women with and without breast cancer. Environ Health Perspect 105 Suppl 3:601-5. Vinggaard AM, Breinholt V, Larsen JC (1999a): Screening of selected pesticides for oestrogen receptor activation in vitro. Food Addit Contam 16:533-42. Vinggaard AM, Hnida C, Larsen JC (2000): Environmental polycyclic aromatic hydrocarbons affect androgen receptor activation in vitro. Toxicology 145:173-83. Vinggaard AM, Joergensen EC, Larsen JC (1999b): Rapid and sensitive reporter gene assays for detection of antiandrogenic and estrogenic effects of environmental chemicals. Toxicol Appl Pharmacol 155:150-60. Wilcox AJ, Baird DD, Weinberg CR, Hornsby PP, Herbst AL (1995): Fertility in men exposed prenatally to diethylstilbestrol. N Engl J Med 332:1411-6. Wolff MS, Toniolo PG (1995): Environmental organochlorine exposure as a potential etiologic factor in breast cancer. Environ Health Perspect 103 Suppl 7:141-5. Ziccardi MH, Gardner IA, Denison MS (2000): Development and modification of a recombinant cell bioassay to directly detect halogenated and polycyclic aromatic hydrocarbons in serum. Toxicol Sci 54:183-93. |